Abstract
Background
Despite the emergence of immune checkpoint inhibitors (ICIs) as first-line treatment for advanced hepatocellular carcinoma (HCC), there is an unmet need regarding subsequent treatments in patients that fail ICI. Regorafenib is a vascular endothelial growth factor receptor (VEGFR) inhibitor, which could increase programmed death-ligand 1 (PD-L1) expression in tumors and increase intra-tumoral CD8+ T-cell infiltration by normalizing the cancer vasculature and improving the efficacy of the programmed cell death protein 1 (PD-1) antibody. Thus, we evaluated the combination of regorafenib and a PD-1 inhibitor for advanced HCC patients that had failed combined tyrosine kinase inhibitors (TKIs) plus ICI.
Methods
Data of patients with advanced HCC who had failed combined TKIs plus ICI treatment and were afterwards treated with combined regorafenib plus a PD-1 inhibitor were reviewed. All patients had received PD-1 inhibitors as part of the first-line treatment and regorafenib every 4 weeks until disease progression, intolerable toxicities, or physician/patient withdrawal. The clinical data, previous treatment strategies, follow-up imaging results, and adverse events (AEs) during follow-ups were recorded. Common Terminology Criteria for Adverse Events (CTCAE) v. 5.0 was used to evaluate AEs and Response Evaluation Criteria in Solid Tumors (RECIST) v. 1.1 was used to evaluate response. The primary endpoint was safety, and the secondary endpoints were the objective response rate (ORR), progression-free survival (PFS), disease control rate (DCR), overall survival (OS), and duration of response (DOR).
Results
From November 15, 2020, to January 31, 2022, data of 17 patients with advanced HCC that met the criteria were reviewed. The cohort included 16 men and 1 woman with a median age of 54 years (interquartile range, 46 to 63 years). Sixteen patients had Child-Pugh class A (n=16, 94.12%) and one with class B (n=1, 15.9%) liver disease. Thirteen patients received second-line treatment, and the remaining patients received third-line treatment. All patients received at least 1 dose of PD-1 inhibitors. The median follow-up duration was 7.62 months. Twelve recipients experienced treatment-related AEs. The most frequent AE (≥5%) included fatigue (17.64%), diarrhea (17.65%), proteinuria (5.88%), bleeding gums (11.76%), and hypertension (11.76%). No grade-4 AE or new safety signals were identified. The ORR and DCR were 41.2% and 64.7%, respectively, and the median PFS was 5.09 months.
Conclusions
Regorafenib combined with PD-1 inhibitor is a promising regimen in treating patients with advanced HCC owing to its safety and effectiveness as well as low incidence of serious AEs with its use.
Keywords: Immune checkpoint inhibitors (ICIs), immune resistance, advanced hepatocellular carcinoma (advanced HCC), regorafenib, second-line treatment
Highlight box.
Key findings
• Regorafenib combined with programmed cell death protein 1 (PD-1) inhibitors exhibited benefits in terms of short-term therapeutic effect (objective response rate, disease control rate, and median progression-free survival) and also had a manageable safety profile in patients who progressed on immune checkpoint inhibitor (ICI)-containing regimens.
What is known and what is new?
• Tyrosine kinase inhibitors (TKIs) plus ICIs have shown clinical benefits as first-line treatment for patients with advanced hepatocellular carcinoma (HCC). However, there are insufficient data regarding the selection of treatment after disease progression in patients on ICIs.
• This study aimed to explore the efficacy of sequential treatment of regorafenib in patients who failed previous TKI plus ICIs treatment.
What is the implication, and what should change now?
• Regorafenib combined with PD-1 inhibitors might be an efficient option for ICI-pretreated patients with advanced HCC.
Introduction
Hepatocellular carcinoma (HCC) is one of the most malignant types of carcinomas (1). Each year, nearly 906,000 new HCC cases and more than 830,000 HCC-associated deaths occur worldwide (2), with China accounting for 50% of the total number of cases and deaths (3). Before 2021, approximately 50% of patients with HCC received systemic therapies, sorafenib, lenvatinib, or atezolizumab plus bevacizumab in the first line setting and regorafenib, cabozantinib, ramucirumab, nivolumab, or pembrolizumab in the second-line setting. Recently, immune checkpoint inhibitor (ICI) therapy, particularly programmed cell death protein 1/programmed death-ligand 1 (PD-1/PD-L1) inhibitors, has made a breakthrough in cancer treatment (4). Tumor cells may evade detection by the immune system by upregulation of cell-surface immune checkpoints, while checkpoint inhibitors function by unmasking these cells and potentiating immune responses against them and have been utilized in a growing number of HCC and other solid tumors (5). In addition, using adjuvant/neoadjuvant ICIs or other ICIs combination therapies can reduce HCC recurrence, eliminate micrometastatic occurrence and improve patients’ prognosis (6,7). Treatment of tyrosine kinase inhibitors (TKIs) plus ICIs has shown superior clinical benefits than TKI monotherapy as first-line treatment for patients with advanced HCC (8-11). However, the efficacy of the combination remains suboptimal, with the objective response rate (ORR) of lower than 40% and a median progression-free survival (PFS) of almost 6 months (12-14).
Achieving clinical benefits after treatment is switched to another PD-1 inhibitor following primary or secondary PD-1 inhibitor resistance is challenging and options are limited. One potential option is to maintain the immunotherapy regimen and change the TKI drug to regorafenib. Regorafenib is a vascular endothelial growth factor receptor (VEGFR) inhibitor, which could increase PD-L1 expression in tumors and increase intratumoral CD8+ T-cell infiltration by normalizing the cancer vasculature and improving the efficacy of the PD-1 antibody (15,16). Regorafenib is the only systemic treatment shown to provide survival benefit in HCC patients progressing on sorafenib treatment (17). All these provide the theoretical basis for the combined strategy of regorafenib and PD-1 inhibitors.
Limited studies have reviewed the safety and benefits of this treatment regimen. The aim of this study was to evaluate the safety and efficacy of combined regorafenib plus PD-1 inhibitor treatment, after the patient demonstrated progression of disease while on combination of other TKIs and a PD-1 inhibitor. We present this article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-590/rc).
Methods
Patient selection
We reviewed the electronic medical records of consecutive patients with advanced HCC who had failed previous treatment with TKIs (at least 1 TKI including apatinib, sorafenib, or lenvatinib) combined with PD-1 inhibitors and were treated with regorafenib and a PD-1 inhibitor (camrelizumab or sintilimab) from November 15, 2020, to January 31, 2022 in The First Affiliated Hospital of Nanjing Medical University. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of The First Affiliated Hospital of Nanjing Medical University (No. 2021-SR-434). Informed consent was obtained from all participants involved in the study.
HCC was diagnosed according to the European Association for the Study of Liver and American Association for the study of Liver Disease guidelines. The inclusion criteria for the study population were as follows: (I) age ≥18 years; (II) Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1; (III) radiographic disease progression on first-line treatment with apatinib, sorafenib or lenvatinib. Patients were excluded from this study if they: (I) participation in other interventional clinical studies during regorafenib administration; (II) unavailable data for study analysis; (III) presence of other primary solid tumors or hematologic malignancies.
Study design
As is standard practice at our institution, regorafenib was given orally at a dose of 40, 80, or 120 mg/m2/day, days 1–21) every 4 weeks. Standard doses of anti-PD-1 antibodies (camrelizumab 200 mg or sintilimab 200 mg) were administered intravenously every 3 weeks. Clinical and laboratory data were retrospectively collected from the eligible patients. The patients’ demographics were also collected.
According to standard practice, patients were followed-up every 6–8 weeks using computed tomography (CT) or magnetic resonance imaging (MRI) of the chest, abdomen, pelvis, and other known sites of disease. Vital signs were checked on day 1 of each cycle or more frequently as clinically indicated. Adverse events (AEs) were recorded from the time the patients received the medications up to 30 days after the last dose was administered. Clinicopathological data and follow-up information were collected using the electronic medical records and telephone interviews.
Patients’ responses were assessed according to the revised Response Evaluation Criteria in Solid Tumors (RECIST) v. 1.1 every 3 cycles. The primary endpoint was safety. The secondary endpoints were the ORR, which was defined as the proportion of patients with a best overall response of complete response (CR) or partial response (PR). Other secondary endpoints were PFS, the disease control rate (DCR), overall survival (OS), and the duration of response (DOR). PFS was defined as the time between the initiation of treatment and the observation of disease progression or death from any cause in patients with neoplastic disease. AEs were evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) v. 5.0.
Statistical analysis
Patients’ characteristics were summarized using descriptive statistical methods. Statistical analyses were performed using SPSS 17 (IBM Corp., Armonk, NY, USA). Statistical values are reported as medians. Safety and efficacy were assessed by the investigators according to CTCAE v. 5.0 and RECIST v. 1.1, respectively. PFS and OS were plotted according to the Kaplan-Meier method. Survival distributions were compared using the log-rank test. Multivariate analysis was performed using the Cox proportional hazards model. P<0.05 indicated statistical significance.
Results
Study population
Between November 15, 2020, and January 31, 2022, 17 patients were included in the study. The cohort included 16 men and 1 woman with a median age of 54 years (interquartile range, 46 to 63 years). Sixteen patients had Child-Pugh class A (n=16, 94.12%) and one with class B (n=1, 15.9%) liver disease. The ECOG performance status in patients was 0 (n=3, 17.65%) or 1 (n=14, 82.35%). Thirteen patients received second-line treatment, and the remaining patients received third-line treatment. All patients received at least 1 dose of PD-1 inhibitors. Demographic characteristics are shown in Table 1. More than half of the patients (52.94%) had Barcelona Clinic Liver Cancer (BCLC) stage B and the rest were BCLC stage C. The median follow-up duration was 7.62 months (range, 4.76–10.28 months). Patients received apatinib, sorafenib, or lenvatinib as the initial treatment, and they were also treated with camrelizumab or sintilimab until the end of follow-up (Table 1).
Table 1. Baseline demographics and clinical characteristics (n=17).
| Variables | Values |
|---|---|
| Age (years) | |
| Mean ± SD | 55.18±11.30 |
| Median (Q1, Q3) | 54.00 (46.00, 63.00) |
| Min, Max | 36, 77 |
| Baseline alpha-fetoprotein level (ng/mL) | |
| Mean ± SD | 3,163.40±9,469.88 |
| Median (Q1, Q3) | 184.00 (21.12, 1,210.00) |
| Min, Max | 1.29, 39,425 |
| White blood cell (109/L) | |
| Mean ± SD | 4.45±2.21 |
| Median (Q1, Q3) | 3.82 (2.96, 4.73) |
| Min, Max | 2.16, 10.39 |
| Red blood cell (1012/L) | |
| Mean ± SD | 3.97±0.53 |
| Median (Q1, Q3) | 3.76 (3.63, 4.38) |
| Min, Max | 3.34, 5.2 |
| Platelets (109/L) | |
| Mean ± SD | 93.65±49.17 |
| Median (Q1, Q3) | 78.00 (56.00, 131.00) |
| Min, Max | 32, 185 |
| Glutamic-pyruvic transaminase (U/L) | |
| Mean ± SD | 38.82±22.98 |
| Median (Q1, Q3) | 32.60 (18.70, 57.90) |
| Min, Max | 11.1, 89.9 |
| Glutamic oxalacetic transaminase (U/L) | |
| Mean ± SD | 47.75±25.63 |
| Median (Q1, Q3) | 38.10 (32.40, 64.40) |
| Min, Max | 17.8, 98.5 |
| Total bilirubin (μmol/L) | |
| Mean ± SD | 16.95±6.96 |
| Median (Q1, Q3) | 16.80 (13.90, 21.40) |
| Min, Max | 4.19, 27.1 |
| Albumin (g/L) | |
| Mean ± SD | 36.54±3.99 |
| Median (Q1, Q3) | 36.10 (33.80, 38.90) |
| Min, Max | 31, 46.7 |
| Des-gamma-carboxyprothrombin (mAU/mL) | |
| Mean ± SD | 3,084.94±6,810.71 |
| Median (Q1, Q3) | 254.00 (35.00, 2,004.00) |
| Min, Max | 16, 27,737 |
| Gender, n (%) | |
| Male | 16 (94.12) |
| Female | 1 (5.88) |
| Eastern Cooperative Oncology Group performance status, n (%) | |
| 0 | 3 (17.65) |
| 1 | 14 (82.35) |
| Aetiology of chronic liver disease, n (%) | |
| Hepatitis B | 14 (82.35) |
| Hepatitis C | 1 (5.88) |
| Unknown | 2 (11.76) |
| Liver function classification, n (%) | |
| 5 | 12 (70.59) |
| 6 | 4 (23.53) |
| 7 | 1 (5.88) |
| Barcelona Clinic Liver Cancer stage, n (%) | |
| B | 9 (52.94) |
| C | 8 (47.06) |
| Vascular invasion, n (%) | |
| No | 16 (94.12) |
| Yes | 1 (5.88) |
| Extrahepatic spread, n (%) | |
| No | 10 (58.82) |
| Yes | 7 (41.18) |
| Metastatic site, n (%) | |
| No | 10 (58.82) |
| Gut | 1 (5.88) |
| Lung | 3 (17.65) |
| Lymph node | 1 (5.88) |
| Adrenal gland | 1 (5.88) |
| Omentum | 1 (5.88) |
| Local treatment, n (%) | |
| Radiotherapy | 2 (11.76) |
| No | 15 (88.24) |
| Previous surgery, n (%) | |
| No | 3 (17.65) |
| Yes | 14 (82.35) |
| Previous TKI†, n (%) | |
| Apatinib | 15 (88.24) |
| Sorafenib | 4 (23.53) |
| Lenvatinib | 2 (11.76) |
| Previous PD-1, n (%) | |
| Camrelizumab | 16 (94.12) |
| Sintilimab | 1 (5.88) |
†, some patients were treated with second-line TKI before switched to regorafenib. SD, standard deviation; Q1, first quartile; Q3, third quartile; TKI, tyrosine kinase inhibitor; PD-1, programmed cell death protein 1.
Efficacy
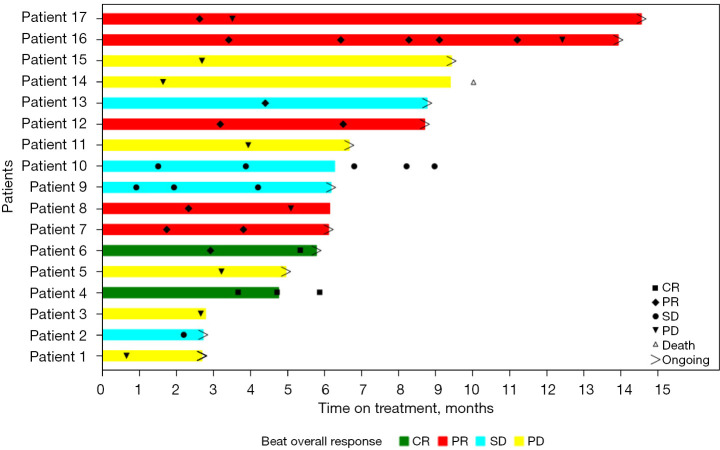
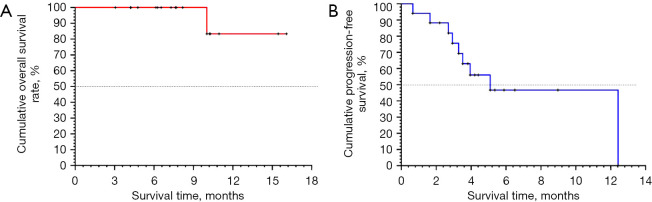
The combination of PD-1 inhibitors and regorafenib resulted in an ORR and DCR of 41.2% and 64.7%, respectively, and 75% of patients achieved tumor regression (including two patients with CR and five with PR, for an ORR of 41.2%). For patients receiving second-line treatment, the confirmed ORR and DCR were 46.2% and 69.2%, respectively. For patients without radiotherapy, the confirmed ORR and DCR were 33.3% and 60.0%, respectively. At data cutoff, 12 patients remained on treatment (Figure 1). The median PFS was 5.09 months [95% confidence interval (CI): 2.92–12.42 months]. The 15-month OS rate was 83.33% (95% CI: 27.31–97.47%). Median OS was not reached (Table 2 and Figure 2).
Figure 1.
Treatment was evaluated based on RECIST V.1.1 criteria. CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
Table 2. Efficacy of patients with advanced HCC treated with PD-1 inhibitors and regorafenib (n=17).
| Outcome | Values |
|---|---|
| Deaths, n (%) | 1 (5.88) |
| Survivors, n (%) | 16 (94.12) |
| 95% CI | 10.02, – |
| OS rate (%) | |
| 6 months (95% CI) | 100.00% (100.00–100.00%) |
| 9 months (95% CI) | 100.00% (100.00–100.00%) |
| 12 months (95% CI) | 83.33% (27.31–97.47%) |
| 15 months (95% CI) | 83.33% (27.31–97.47%) |
| Median PFS (months) | 5.09 (2.92–12.42) |
| ORR (%) | 41.2 |
| DCR (%) | 64.7 |
| Median follow-up time (months) (95% CI) | 7.62 (4.76–10.28) |
HCC, hepatocellular carcinoma; PD-1, programmed cell death protein 1; CI, confidence interval; OS, overall survival; PFS, progression-free survival; ORR, objective response rate; DCR, disease control rate.
Figure 2.
Kaplan-Meier estimates of OS and PFS among patients treated with regorafenib combined with PD-1 inhibitors. OS, overall survival; PFS, progression-free survival; PD-1, programmed cell death protein 1.
Safety
Twelve recipients experienced AE. The most common AE (≥5%) were fatigue (17.64%), diarrhea (17.65%), proteinuria (5.88%), bleeding gums (11.76%), and hypertension (11.76%). Three patients (17.64%) had grade 3 AE, including proteinuria, gastrointestinal bleeding, and peptic ulcers. No grade-4 AE or new safety signals were observed (Table 3).
Table 3. Safety and adverse event analysis in patients with advanced HCC treated with PD-1 inhibitors and regorafenib.
| Adverse events | Grade 1, n (%) | Grade 2, n (%) | Grade 3, n (%) |
|---|---|---|---|
| Fatigue | 2 (11.76) | 1 (5.88) | 0 |
| Diarrhea | 0 | 3 (17.65) | 0 |
| Hypertension | 2 (11.76) | 0 | 0 |
| Bleeding gums | 2 (11.76) | 0 | 0 |
| Proteinuria | 0 | 0 | 1 (5.88) |
| Red rash | 0 | 1 (5.88) | 0 |
| Oral mucositis | 0 | 1 (5.88) | 0 |
| Rash | 1 (5.88) | 0 | 0 |
| Hand/foot/skin reaction | 1 (5.88) | 0 | 0 |
| Hair loss | 1 (5.88) | 0 | 0 |
| No appetite | 1 (5.88) | 0 | 0 |
| Gastrointestinal bleeding | 0 | 0 | 1 (5.88) |
| Peptic ulcer | 0 | 0 | 1 (5.88) |
HCC, hepatocellular carcinoma; PD-1, programmed cell death protein 1.
Discussion
In this study, 52.94% of the patients had BCLC stage B HCC, while the remaining patients had BCLC stage C HCC. The median follow-up duration was 7.62 months (range, 4.76–10.28 months). The ORR and DCR were 41.2% and 64.7%, respectively, and the median PFS was 5.09 months (95% CI: 2.92–12.42 months). For patients treated with second-line therapy, the confirmed ORR and DCR were 33.3% and 60.0%, respectively, while the median OS and DOR were not reached. This demonstrated that regorafenib plus PD-1 inhibitors showed promising efficacy with favorable safety in ICI-tolerant patients with HCC, especially in the second-line setting. Moreover, the regimen featured a higher ORR than that reported in the RESORCE (Regorafenib for Patients with Hepatocellular Carcinoma Who Progressed on Sorafenib Treatment) trial (46.2% vs. 10.6%). To our best knowledge, this is the first study on therapy with regorafenib plus PD-1 inhibitor in patients with advanced HCC that develop progression of disease while on combined TKI and ICI.
Cancer immunotherapies, such as ICIs, which is capable of inducing the immune system to effectively recognize and attack tumors, can be affected by several parameters/predictors, including tumor mutational burden (TMB), epigenetic modifications, the degree of mismatch repair deficiency (MMR-D) and microsatellite instability (MSI) (18,19). Besides, ICIs can be used as neoadjuvant or adjuvant treatment approaches for HCC and other cancers (5). Combination therapies, including antiangiogenesis agents combined with ICIs, dual-ICI therapy, and targeted agents in conjunction with surgery or other locoregional therapies, have been extensively investigated (8,20-22). Combination therapy with TKI and ICIs can normalize tumor blood vessels and improve the efficacy of ICIs, increase the expression of PD-L1 in tumors, and increase the infiltration of CD8+ T cells in tumors, thus increasing the benefits of patients (16). In the ORIENT-32 trial, the combination of the PD-L1 inhibitor sintilimab with a bevacizumab biosimilar proved superior to sorafenib among 571 HCC patients. After a median follow-up of 10 months, median OS was 10.4 months in the sorafenib arm and was not reached in the combination arm [hazard ratio (HR) =0.57; 95% CI: 0.43–0.75; P<0.0001] (13). In the National Comprehensive Cancer Network (NCCN) standard, cabozantinib, which has potent activity against VEGFR 1–3 and the receptor tyrosine kinase MET, was assessed in the phase 3 randomized CELESTAL trial of 707 patients with advanced HCC who progressed on or after sorafenib. The median OS and PFS were significantly longer in patients who received cabozantinib (10.2 and 5.2 months, respectively) than in patients treated with placebo (8.0 and 1.9 months, respectively), and similar results were observed for the ORR (4% vs. 0.4%; P=0.009) (23). Sequential-line treatment of cabozantinib combined with nivolumab and ipilimumab was also evaluated. For patients treated with nivolumab and cabozantinib, the ORR and DCR were 17% and 81%, respectively. For patients who received nivolumab, ipilimumab, and cabozantinib, the ORR and DCR were 26% and 83%, respectively, but median OS was not reached (24). In the phase 3 KEYNOTE 240 trial, pembrolizumab did not achieve its prespecified combined endpoints, including OS and PFS in patients with advanced HCC (25).
In the phase 2 RESCUE trial, patients with advanced HCC were treated with camrelizumab and apatinib. The ORR assessed by the independent review committee in the first- and second-line settings were 46% and 25%, respectively. Additionally, the median OS was 20.1 months in the first line setting and 21.8 months in the second-line setting (26). However, most second-line treatments were based on first line TKI progression. Currently, there is no published study data on ICIs combined with TKIs as sequential therapy after immune resistance. Regorafenib is an effective second-line therapy for advanced HCC, but whether it can prolong the survival of patients who have failed first-line immunotherapy has not been extensively examined. Our study was thus conducted to clarify this uncertainty and provide a strategy to overcome secondary resistance. In our study, the median PFS in the regorafenib plus PD-1 inhibitor arm was 5.09 months (95% CI: 2.92–12.42 months), and 75% of patients had tumor remission. These results constitute the first retrospective evidence that sequential regorafenib administration with camrelizumab/sintilimab therapy can prolong median OS; specifically, patients with PD-1 inhibitor-refractory advanced HCC followed up for a median of 7.62 months in this study had an approximate 5.72-month extension in median OS. Our study supports the value of future exploration of combination treatment with regorafenib and PD-1/PD-L1 inhibitors in patients with ICI-resistant advanced HCC.
The increasing use of combination strategies (combining immunotherapy with traditional therapies such as chemotherapy or TKI) may improve the efficacy of cancer immunotherapy, but may also amplify immune related adverse events (irAEs) (27). Different immune microenvironments may drive tissue-specific irAE patterns. There are various irAEs in trials using ICI drugs. The endocrine, skin, and gastrointestinal systems are most commonly affected by irAEs (28,29). Other tumor dependent irAE spectra can be determined from ICI experimental data (30). The safety profile observed for this combination therapy was generally consistent with the use of each agent alone and the combination as previously reported (17,31), and no unexpected AEs were observed. Timely identification of AE, management with supportive therapy, and regorafenib dose reduction as needed might have facilitated treatment continuation, as only a minority of patients discontinued regorafenib or both study drugs.
In the current study, the switch over to regorafenib demonstrated response with the potential to overcome immune resistance in patients with advanced HCC. Encouraging results were obtained in the study of the administration of ICIs in combination with regorafenib, but the mechanism underlying this effect is unclear. Previous studies have proposed that regorafenib or its major human active metabolites M-2 and M-5 can inhibit the activity of RET, VEGFR1–3, KIT, PDGFR-alpha, PDGFR-beta, FGFR1, FGFR2, TIE2, DDR2, TrkA, Eph2A, RAF-1, BRAF, BRAFV600E, SAPK2, PTK5, and Abl at clinically achievable concentrations (32,33). Regorafenib is a commonly targeted drug in HCC and is recommended as a second-line drug for advanced HCC by most guidelines. By uniquely targeting CSF-1R to suppress immune escape, regorafenib has synergistic effects with PD-1 inhibitors (34-36). By blocking epidermal growth factor receptor (EGFR), regorafenib can break the crosstalk between the epidermal growth factor (EGF) pathway and the vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF) pathways (37). In addition, by targeting CXCL10, regorafenib could increase intratumoral CXCR3 CD8+ T-cell infiltration to inhibit tumor growth and prolong survival in patients with HCC (15). These findings indicated that regorafenib could overcome drug resistance. Further evaluation of the mechanism of regorafenib combined with PD-1 inhibitor sequential therapy is needed.
In China, 39.0–53.6% of patients with HCC have been diagnosed at an advanced stage and have lost the opportunity of radical treatment (38). At this time, immunotherapy combination therapy becomes extremely important. However, the ORR remains very limited for advanced HCC regardless of the modality of immunotherapy combination. Hence, the choice of TKI drugs is very critical for patients with advanced HCC, especially for those who need to receive second-line or third-line immunotherapy. In our study, we found that the confirmed ORR and DCR were 46.2% and 69.2% for patients receiving second-line treatment, respectively. At the same time, the results show that there are three cases (17.64%) in patients with grade 3 AE, but no cases of grade 4 AE. PD-1 inhibitor combined with regorafenib is a promising regimen in treating patients with advanced HCC owing to its safety and effectiveness as well as low incidence of serious AEs with its use. In the future study, we plan to conduct prospective, interventional studies with larger sample size to further confirm the efficacy and safety of PD-1 inhibitor combined with regorafenib. At the same time, we will involve finding relevant indicators that affect the prognosis and efficacy of patients with advanced HCC and screening biomarkers for patients who can benefit from PD-1 inhibitor combined with regorafenib in order to achieve personalized precision treatment for patients with advanced HCC.
The current study has several limitations. First, this study was insufficiently powered due to its small sample size and short follow-up duration. Additional cases will be added over time with continued follow-up. Second, the data from prospective trials of patients who progressed on ICI-containing regimens are scarce, and the synergistic effects and safety of these regimens must be further investigated in a randomized controlled trial.
Conclusions
The high efficacy of regorafenib combined with PD-1 inhibitor sequential therapy could be a new strategy for therapy of patients with advanced HCC following the failure of first-line treatment and may serve as a bridge to ICI therapy.
Supplementary
The article’s supplementary files as
Acknowledgments
We thank Joe Barber Jr, PhD, from Liwen Bianji (Edanz) (www.liwenbianji.cn) for editing the English text of a draft of this manuscript.
Funding: This research was funded by the Jiangsu Provincial Medical Innovation Center (No. CXZX202203) and the Jiangsu Provincial Medical Key Laboratory (No. ZDXYS202201).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of The First Affiliated Hospital of Nanjing Medical University (No. 2021-SR-434). Informed consent was obtained from all participants involved in the study.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-590/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-590/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-590/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-590/coif). The authors have no conflicts of interest to declare.
References
- 1.Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2021;7:6. 10.1038/s41572-020-00240-3 [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Colombet M, Soerjomataram I, et al. Cancer statistics for the year 2020: An overview. Int J Cancer 2021. [Epub ahead of print]. doi: . 10.1002/ijc.33588 [DOI] [PubMed] [Google Scholar]
- 3.Liu X, Qin S. Immune Checkpoint Inhibitors in Hepatocellular Carcinoma: Opportunities and Challenges. Oncologist 2019;24:S3-10. 10.1634/theoncologist.2019-IO-S1-s01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eggermont AMM, Blank CU, Mandala M, et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. N Engl J Med 2018;378:1789-801. 10.1056/NEJMoa1802357 [DOI] [PubMed] [Google Scholar]
- 5.Sahin IH, Khalil L, Millett R, et al. Neoadjuvant and adjuvant treatment approaches for hepatocellular carcinoma: future outlook. Chin Clin Oncol 2021;10:7. 10.21037/cco-20-248 [DOI] [PubMed] [Google Scholar]
- 6.Janjigian YY, Wolchok JD, Ariyan CE. Eradicating micrometastases with immune checkpoint blockade: Strike while the iron is hot. Cancer Cell 2021;39:738-42. 10.1016/j.ccell.2021.05.013 [DOI] [PubMed] [Google Scholar]
- 7.Wu JS, Hong TC, Wu HT, et al. Hepatic arterial infusion chemotherapy and immune checkpoint inhibitors, alone or in combination, in advanced hepatocellular carcinoma with macrovascular invasion: a single-centre experience in Taiwan. J Gastrointest Oncol 2023;14:849-62. 10.21037/jgo-22-858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang EL, Zhang ZY, Li J, et al. Complete Response to the Sequential Treatment with Regorafenib Followed by PD-1 Inhibitor in a Sorafenib-Refractory Hepatocellular Carcinoma Patient. Onco Targets Ther 2020;13:12477-87. 10.2147/OTT.S284092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J Clin Oncol 2020;38:193-202. 10.1200/JCO.19.01307 [DOI] [PubMed] [Google Scholar]
- 10.D'Alessio A, Cammarota A, Prete MG, et al. The evolving treatment paradigm of advanced hepatocellular carcinoma: putting all the pieces back together. Curr Opin Oncol 2021;33:386-94. 10.1097/CCO.0000000000000744 [DOI] [PubMed] [Google Scholar]
- 11.Llovet JM, Castet F, Heikenwalder M, et al. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol 2022;19:151-72. 10.1038/s41571-021-00573-2 [DOI] [PubMed] [Google Scholar]
- 12.Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med 2020;382:1894-905. 10.1056/NEJMoa1915745 [DOI] [PubMed] [Google Scholar]
- 13.Ren Z, Xu J, Bai Y, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2-3 study. Lancet Oncol 2021;22:977-90. 10.1016/S1470-2045(21)00252-7 [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Xu J, Shen J, et al. Update on overall survival (OS) of RESCUE: An open-label, phase 2 trial of camrelizumab (C) in combination with apatinib (A) in patients with advanced hepatocellular carcinoma (HCC). J Clin Oncol 2021;39:abstr 4076.
- 15.Shigeta K, Matsui A, Kikuchi H, et al. Regorafenib combined with PD1 blockade increases CD8 T-cell infiltration by inducing CXCL10 expression in hepatocellular carcinoma. J Immunother Cancer 2020;8:e001435. 10.1136/jitc-2020-001435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tai WT, Chu PY, Shiau CW, et al. STAT3 mediates regorafenib-induced apoptosis in hepatocellular carcinoma. Clin Cancer Res 2014;20:5768-76. 10.1158/1078-0432.CCR-14-0725 [DOI] [PubMed] [Google Scholar]
- 17.Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;389:56-66. 10.1016/S0140-6736(16)32453-9 [DOI] [PubMed] [Google Scholar]
- 18.Chowell D, Yoo SK, Valero C, et al. Improved prediction of immune checkpoint blockade efficacy across multiple cancer types. Nat Biotechnol 2022;40:499-506. 10.1038/s41587-021-01070-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pilard C, Ancion M, Delvenne P, et al. Cancer immunotherapy: it's time to better predict patients' response. Br J Cancer 2021;125:927-38. 10.1038/s41416-021-01413-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uson PLS, Junior, Liu AJ, Sonbol MB, et al. Immunotherapy and chimeric antigen receptor T-cell therapy in hepatocellular carcinoma. Chin Clin Oncol 2021;10:11. 10.21037/cco-20-231 [DOI] [PubMed] [Google Scholar]
- 21.Yang JD, Hainaut P, Gores GJ, et al. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol 2019;16:589-604. 10.1038/s41575-019-0186-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zou J, Huang P, Ge N, et al. Anti-PD-1 antibodies plus lenvatinib in patients with unresectable hepatocellular carcinoma who progressed on lenvatinib: a retrospective cohort study of real-world patients. J Gastrointest Oncol 2022;13:1898-906. 10.21037/jgo-22-643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benson AB, D'Angelica MI, Abbott DE, et al. Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2021;19:541-65. 10.6004/jnccn.2021.0022 [DOI] [PubMed] [Google Scholar]
- 24.Martini G, Ciardiello D, Paragliola F, et al. How Immunotherapy Has Changed the Continuum of Care in Hepatocellular Carcinoma. Cancers (Basel) 2021;13:4719. 10.3390/cancers13184719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finn RS, Ryoo BY, Merle P, et al. Results of KEYNOTE-240: phase 3 study of pembrolizumab (Pembro) vs best supportive care (BSC) for second line therapy in advanced hepatocellular carcinoma (HCC). J Clin Oncol 2019;37:abstr 4004.
- 26.Qin S, Ren Z, Meng Z, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol 2020;21:571-80. 10.1016/S1470-2045(20)30011-5 [DOI] [PubMed] [Google Scholar]
- 27.Ramos-Casals M, Brahmer JR, Callahan MK, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers 2020;6:38. 10.1038/s41572-020-0160-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tarhini A. Immune-mediated adverse events associated with ipilimumab ctla-4 blockade therapy: the underlying mechanisms and clinical management. Scientifica (Cairo) 2013;2013:857519. 10.1155/2013/857519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freeman-Keller M, Kim Y, Cronin H, et al. Nivolumab in Resected and Unresectable Metastatic Melanoma: Characteristics of Immune-Related Adverse Events and Association with Outcomes. Clin Cancer Res 2016;22:886-94. 10.1158/1078-0432.CCR-15-1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khoja L, Day D, Wei-Wu Chen T, et al. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol 2017;28:2377-85. 10.1093/annonc/mdx286 [DOI] [PubMed] [Google Scholar]
- 31.Granito A, Marinelli S, Forgione A, et al. Regorafenib Combined with Other Systemic Therapies: Exploring Promising Therapeutic Combinations in HCC. J Hepatocell Carcinoma 2021;8:477-92. 10.2147/JHC.S251729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao P, Wang Y, Kang X, et al. Dual-targeting biomimetic delivery for anti-glioma activity via remodeling the tumor microenvironment and directing macrophage-mediated immunotherapy. Chem Sci 2018;9:2674-89. 10.1039/C7SC04853J [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ettrich TJ, Seufferlein T. Regorafenib. Recent Results Cancer Res 2018;211:45-56. 10.1007/978-3-319-91442-8_3 [DOI] [PubMed] [Google Scholar]
- 34.Hume DA, MacDonald KP. Therapeutic applications of macrophage colony-stimulating factor-1 (CSF-1) and antagonists of CSF-1 receptor (CSF-1R) signaling. Blood 2012;119:1810-20. 10.1182/blood-2011-09-379214 [DOI] [PubMed] [Google Scholar]
- 35.Espinosa I, Beck AH, Lee CH, et al. Coordinate expression of colony-stimulating factor-1 and colony-stimulating factor-1-related proteins is associated with poor prognosis in gynecological and nongynecological leiomyosarcoma. Am J Pathol 2009;174:2347-56. 10.2353/ajpath.2009.081037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell 2000;103:211-25. 10.1016/S0092-8674(00)00114-8 [DOI] [PubMed] [Google Scholar]
- 37.Terme M, Tartour E, Taieb J. VEGFA/VEGFR2-targeted therapies prevent the VEGFA-induced proliferation of regulatory T cells in cancer. Oncoimmunology 2013;2:e25156. 10.4161/onci.25156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as