Abstract
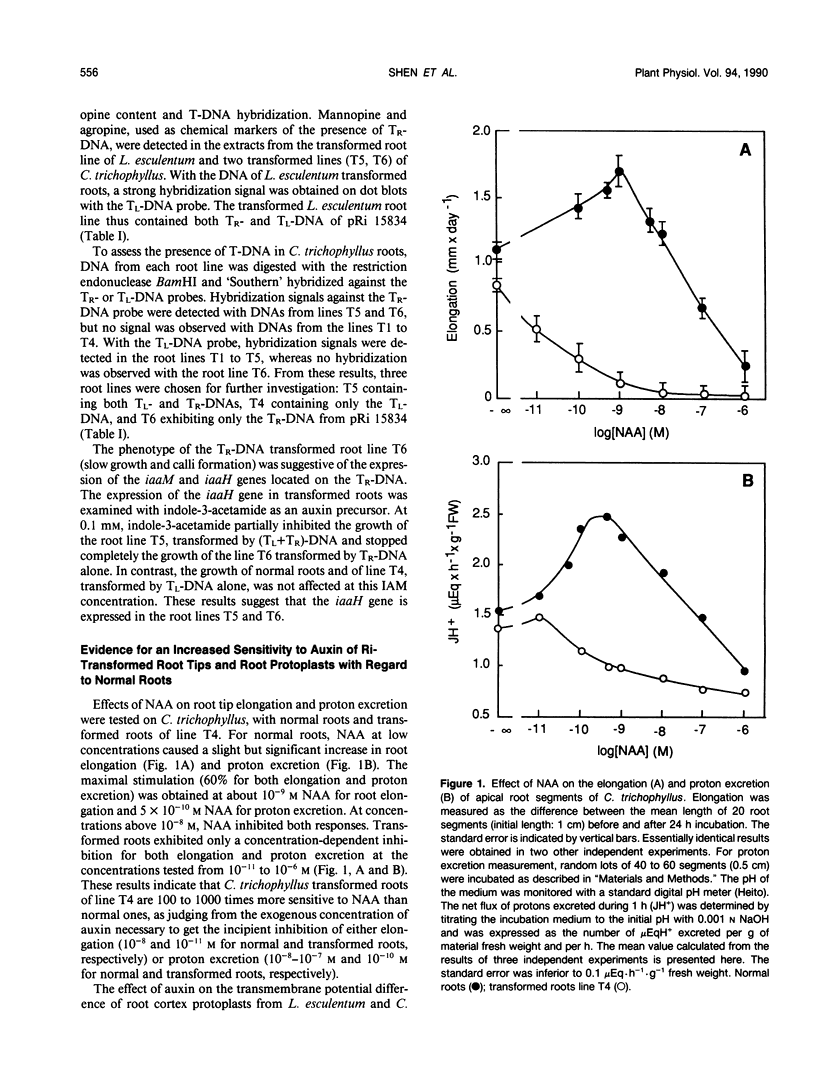
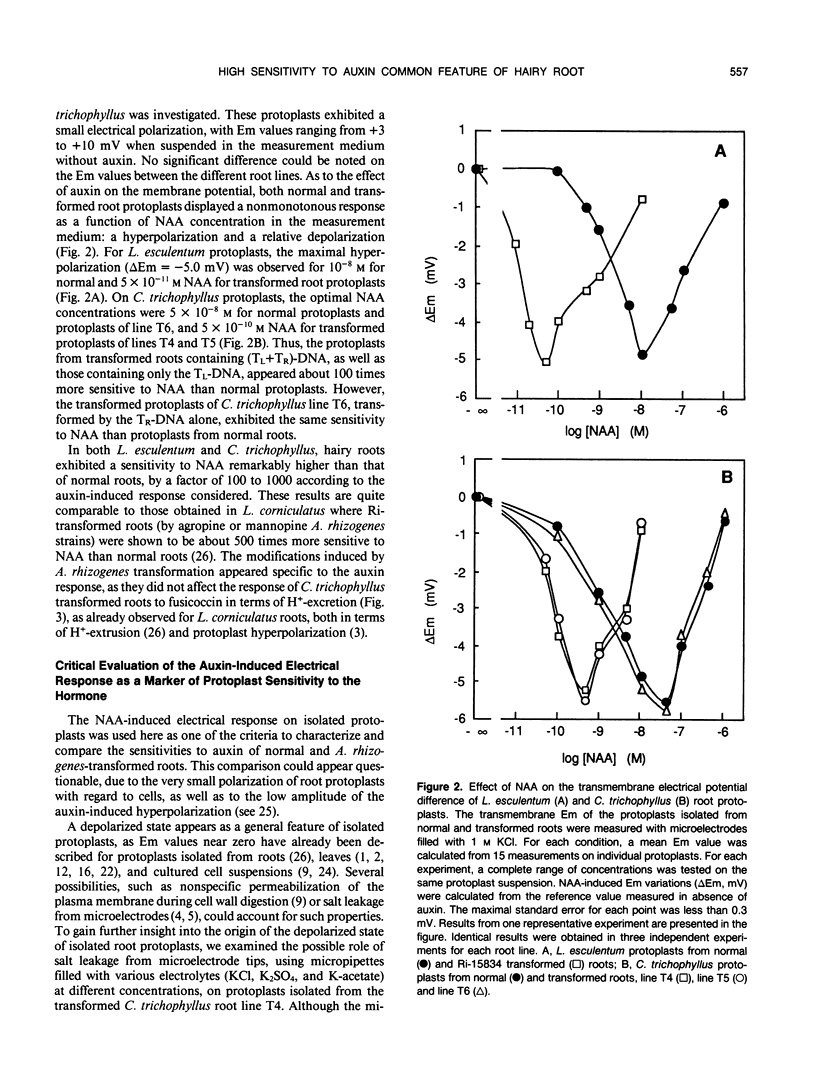
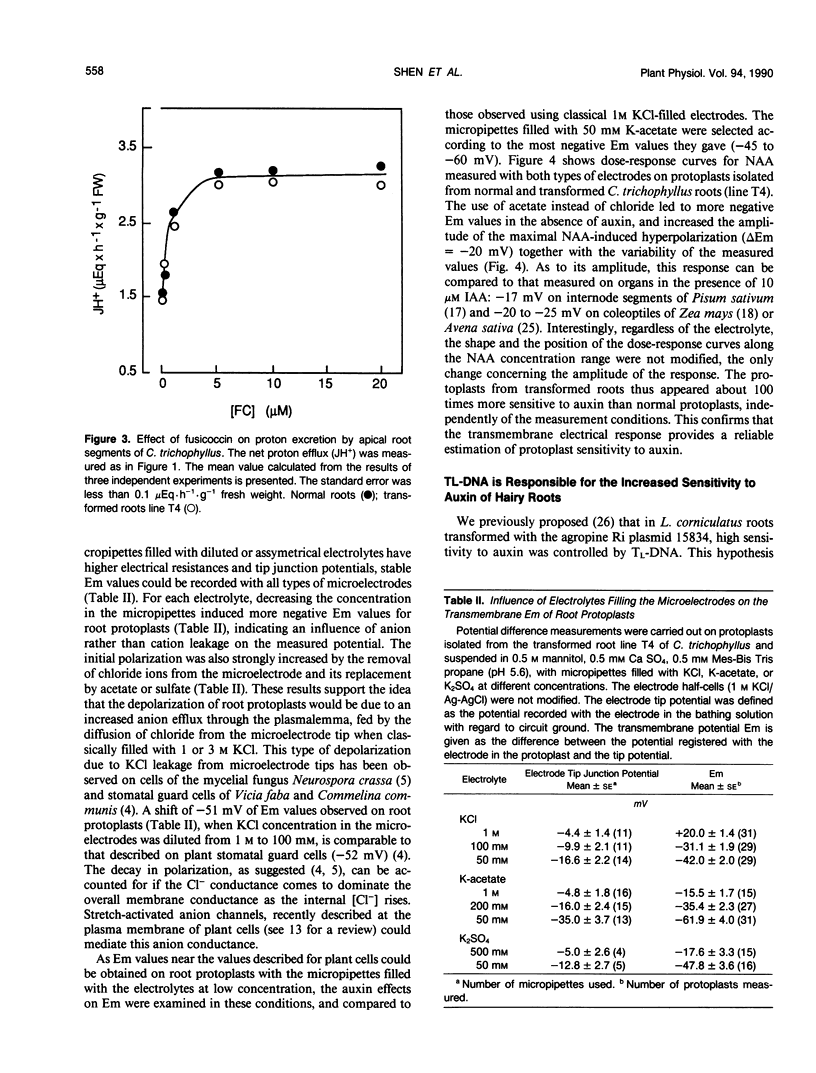
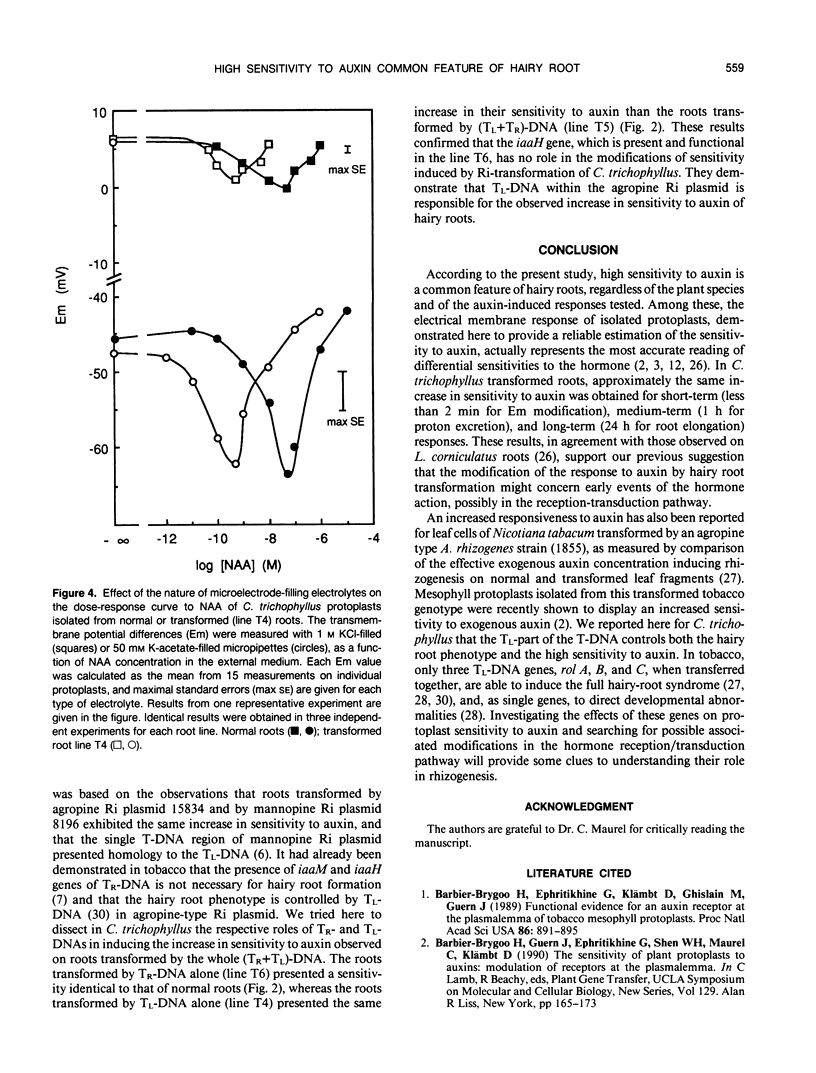
The responses to auxin of Lycopersicon esculentum roots transformed by (Tl+Tr)-DNA of the Ri plasmid of agropine-type Agrobacterium rhizogenes strain 15834 and Catharanthus trichophyllus roots transformed by the (Tl+Tr)-DNA, and by Tl- or Tr- DNA alone of the same bacterial strain were compared to that of their normal counterparts. The transmembrane electrical potential difference of root protoplasts was measured as a function of the concentration of exogenous naphthalene acetic acid. The sensitivity to auxin expressed by this response was shown to be independent of the measurement conditions and of the basal polarization of isolated protoplasts. According to this electrical response, as well as to the modulation by auxin of proton excretion by root tips and root tip elongation, roots transformed by (Tl+Tr) DNA are 100 to 1000 times more sensitive to exogenous auxin than normal roots, as is the case with normal and transformed roots from Lotus corniculatus (WH Shen, A Petit, J Guern, J Tempé [1988] Proc Natl Acad Sci USA 85: 3417-3421). Further-more, transformed roots of C. trichophyllus are not modified in their sensitivity to fusicoccin, illustrating the specificity of the modification of the auxin sensitivity. Roots transformed by the Tr-DNA alone showed the same sensitivity to auxin as normal roots, whereas the roots transformed by the Tl-DNA alone exhibited an auxin sensitivity as high as the roots transformed by (Tl+Tr)-DNA. It was concluded that the high sensitivity to auxin is controlled by the Tl-DNA in agropine type Ri plasmids.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbier-Brygoo H., Ephritikhine G., Klämbt D., Ghislain M., Guern J. Functional evidence for an auxin receptor at the plasmalemma of tobacco mesophyll protoplasts. Proc Natl Acad Sci U S A. 1989 Feb;86(3):891–895. doi: 10.1073/pnas.86.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt M. R., Slayman C. L. KCl leakage from microelectrodes and its impact on the membrane parameters of a nonexcitable cell. J Membr Biol. 1983;72(3):223–234. doi: 10.1007/BF01870589. [DOI] [PubMed] [Google Scholar]
- Brevet J., Tempé J. Homology mapping of T-DNA regions on three Agrobacterium rhizogenes Ri plasmids by electron microscope heteroduplex studies. Plasmid. 1988 Mar;19(2):75–83. doi: 10.1016/0147-619x(88)90046-7. [DOI] [PubMed] [Google Scholar]
- De Paolis A., Mauro M. L., Pomponi M., Cardarelli M., Spanò L., Costantino P. Localization of agropine-synthesizing functions in the TR region of the root-inducing plasmid of Agrobacterium rhizogenes 1855. Plasmid. 1985 Jan;13(1):1–7. doi: 10.1016/0147-619x(85)90050-2. [DOI] [PubMed] [Google Scholar]
- Ephritikhine G., Barbier-Brygoo H., Muller J. F., Guern J. Auxin effect on the transmembrane potential difference of wild-type and mutant tobacco protoplasts exhibiting a differential sensitiity to auxin. Plant Physiol. 1987 Apr;83(4):801–804. doi: 10.1104/pp.83.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman G. A., White F. F., Gordon M. P., Nester E. W. Hairy-root-inducing plasmid: physical map and homology to tumor-inducing plasmids. J Bacteriol. 1984 Jan;157(1):269–276. doi: 10.1128/jb.157.1.269-276.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouanin L. Restriction map of an agropine-type Ri plasmid and its homologies with Ti plasmids. Plasmid. 1984 Sep;12(2):91–102. doi: 10.1016/0147-619x(84)90055-6. [DOI] [PubMed] [Google Scholar]
- Pomponi M., Spanò L., Sabbadini M. G., Costantino P. Restriction endonuclease mapping of the root-inducing plasmid of Agrobacterium rhizogenes 1855. Plasmid. 1983 Sep;10(2):119–129. doi: 10.1016/0147-619x(83)90064-1. [DOI] [PubMed] [Google Scholar]
- Racusen R. H., Kinnersley A. M., Galston A. W. Osmotically induced changes in electrical properties of plant protoplast membranes. Science. 1977 Oct 28;198(4315):405–407. doi: 10.1126/science.198.4315.405. [DOI] [PubMed] [Google Scholar]
- Senn A. P., Goldsmith M. H. Regulation of electrogenic proton pumping by auxin and fusicoccin as related to the growth of Avena coleoptiles. Plant Physiol. 1988 Sep;88(1):131–138. doi: 10.1104/pp.88.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W. H., Petit A., Guern J., Tempé J. Hairy roots are more sensitive to auxin than normal roots. Proc Natl Acad Sci U S A. 1988 May;85(10):3417–3421. doi: 10.1073/pnas.85.10.3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanò L., Mariotti D., Cardarelli M., Branca C., Costantino P. Morphogenesis and Auxin Sensitivity of Transgenic Tobacco with Different Complements of Ri T-DNA. Plant Physiol. 1988 Jun;87(2):479–483. doi: 10.1104/pp.87.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spena A., Schmülling T., Koncz C., Schell J. S. Independent and synergistic activity of rol A, B and C loci in stimulating abnormal growth in plants. EMBO J. 1987 Dec 20;6(13):3891–3899. doi: 10.1002/j.1460-2075.1987.tb02729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


