Abstract
Active packaging is becoming increasingly significant in the food industry. The present study aims to explore the use of Syzygium Cumini Seed Extract (SCSE) as an antioxidant and chitosan as an antibacterial agent to produce active packaging based on polylactic acid (PLA), poly ε-caprolactone (PCL), and polyethylene glycol (PEG) blend. Using advanced characterization techniques, active packaging (PLA/PCL/PEG) incorporating with 0.5 g chitosan-0.5 mL SCSE was evaluated for its mechanical, physical, structural, and antibacterial-antioxidant properties. The addition of chitosan-SCSE caused an 18.57 % increase in tensile strength and decreased the Water Vapor Transmission Rate (WVTR) by up to 52 %, whereas smooth surface microscopy indicated good compatibility between polymers and active agents. Active packaging incorporating chitosan-SCSE reduced 96.66 % of Gram-positive bacteria Staphylococcus aureus and 73.98 % of Gram-negative bacteria, Escherichia coli. During 15 days of storage, the active packaging was able to slow the increase in Total Volatile Basic Nitrogen (TVBN) in beef and prevent the decrease in vitamin C contents in pineapple.
Keywords: Active packaging, Chitosan, Syzygium cumini seed extract (SCSE) extract, PLA, PCL
1. Introduction
Organic acids, enzymes, bacteriocins, fungicides, natural extracts, and ions are incorporated into a matrix (gelatin, starch, and polymers) to form active packaging. Active packaging is one of the novel methods that have been developed, allowing interactions between food ingredients, packaging, and the environment to improve quality, safety, and extend product shelf life. Also known as smart packaging, active packaging is designed to detect and warn of food spoilage [1]. This system utilizes humidity, time, temperature, freshness, spoilage, and biosensors to ensure food safety ([2,3]). Microbiological reactions or developments that occur over time due to the interaction of metabolites produced by microbial growth and chemicals provide visual signals and information about food degradation [4].
Polylactic acid (PLA) is among the most promising biopolymers for active packaging applications among many polymers derived from natural materials. The active packaging production process based on PLA is easier because it employs commercial tools. However, PLA has a drawback for active packaging applications due to its low water vapor barrier properties, rigidity, and heat distortion temperatures ([5,6]). Combining PLA with other biocompatible aliphatic polyesters, such as poly ε-caprolactone (PCL), is expected to compensate for these drawbacks. This combination allows the production of new biomaterials with improved physical, mechanical, and thermal properties ([[7], [8], [9]]). Since the PLA-PCL blend has high rigidity, polyethylene glycol (PEG) is commonly added as a plasticizer to improve flexibility and meet the requirements for active packaging. PEG offers the advantage of having a wide range of molecular weights that effectively increase polymer blend chain mobility, ductility, and durability [10].
The additive used to produce active packaging must be chosen in accordance with established regulations, particularly in terms of toxicological effects [11]. Chitosan is widely used in food packaging because it is non-toxic and decomposes naturally. Chitosan can also form excellent films, has broad antimicrobial activity, is selective against gases (CO2 and O2), and is compatible with other substances such as vitamins and minerals [12]. Table 1 summarizes recent research on various types of polymer blend films with various additives for active packaging applications. Most of the previous works considered PLA in their study; however, they added acetyl tributyl citrate (ATBC), PCL, polybutylene succinate adipate (PBSA), polybutylene adipate terephthalate (PBAT), and PEG to produce the active packaging. Various additives derived from essential oils and plant extracts, as well as their combinations, were used to improve the physical mechanical properties of active packaging. When essential oils, plant extracts, and other polysaccharides were used as natural agents to produce active packaging, variations in tensile strength and WVTR were observed (Table 1). Therefore, the additive used should be compatible to form a homogeneous blend with the biopolymer. It should not be too volatile as it may evaporate with rising process temperature and should not migrate easily to avoid material contamination".
Table 1.
Previous works on different types of polymer blend with additives for active packaging.
| Type of material | Additive | Tensile strength (MPa) | WVTR (g.mm.h−1.m−2.kPa−1) | Ref |
|---|---|---|---|---|
| PLA-ATBC | Carvacrol-thymol | 22.8 | – | [13] |
| PLA-PEG | Chitosan-rosemary | 16.8 | – | [14] |
| PLA | Lemon balm | 45.3 | – | [15] |
| PLA-PBSA | Carvacrol | 38.92 | 06.92 | [16] |
| PLA-PBSA | thymol | 12.32 | 8.261 | [17] |
| PLA-PCL | Thymol-carvacrol | 6.42 | – | [18] |
| PLA | halloysite nanotubes | 37.23 | 0.1183 | [19] |
| PLA | Chitosan-rosemary | 93 | 0.170 | [20] |
| PLA-PBAT | citral | 10.2 MPa | 0.049 | [21] |
| PLA | Mesoporous silica nanoparticles-clove oil | 11.8 MPa | 02.14 | [22] |
| PLA | Tea polyphenol | 2.72 MPa | 5.393 | [23] |
| PLA-PBAT | Carvacrol | 18.90 MPa | 0.68 | [24] |
| PLA | Thymus-mediterranean propolis | 23.2 MPa | – | [25] |
| PLA | Chitosan-AgNPs | 27.8 MPa | 0.120 | [26] |
| PLA-PCL | Green tea | 42.3 MPA | 0.244 | [27] |
A study involved blending PLA/PCL with thymol-carvacrol, and SEM morphology analysis revealed that the resulting film surface became rough, with a large number of pores formed due to the low miscibility between polymers and the additive [28]. Many researchers have extensively used essential oils as antibacterials in the production of active packaging and have concluded that essential oils can increase water vapor permeability [29]. Since essential oils are highly hydrophobic, they influence the hydrophilic/hydrophobic balance of the film by creating a more porous microstructure. Additionally, essential oils improve the oxygen permeability of the film [30]. The addition of essential oil as an additive reduces the compactness of the polymer, resulting in the formation of discontinuities in the film matrix and consequently lowering the tensile strength [31].
Utilizing natural antioxidant and antimicrobial ingredients, such as plant extracts, presents a viable alternative to synthetic active packaging, as indicated by several studies. SCSE has garnered significant attention due to its safe and potent antioxidant and antibacterial properties, effective against a wide range of bacteria ([[32], [33], [34]]). SCSEs belong to a class of natural antioxidant compounds derived from the Syzygium Cumini Linn plant [35]. Its composition is rich in phenolic compounds, including anthocyanins, flavonoids, and tannins, all of which impart functional properties such as antioxidative capabilities, making it suitable for active packaging. The active components within SCSE exhibit robust antioxidant activity, effectively inhibiting the oxidation process in food products [36]. Therefore, the aim of this study is to investigate the impact of the chitosan-SCSE ratio as an antibacterial and antioxidant agent in the production of PLA-PCL-PEG active packaging. To the best of the author's knowledge, no study has reported on the use of hybrid additives, such as chitosan-SCSE, in the formulation of active packaging. The study assesses the mechanical and physical properties, characteristics of the polymer blend, and colony reduction due to the addition of active agents, targeting both Gram-positive bacteria like Staphylococcus aureus and Gram-negative bacteria like Escherichia coli. Subsequently, the application of active packaging was employed in the preservation of food industry materials to investigate their ability to maintain the nutritional quality of perishable foods, including monitoring Total Volatile Basic Nitrogen (TVBN) levels in fresh beef and preserving Vitamin C content in pineapple.
2. Materials and method
2.1. Materials
Reagent grade of PLA (MW: 60,000) procured from NatureWorks Co., Tangerang, Indonesia. Reagent grade of PCL (MW: 80,000) also obtained from NatureWorks Co., Tangerang, Indonesia. Technical grade of PEG-400 supplied by Rudang Jaya, Medan, Indonesia. Technical grade of ethanol 70 % provided by Rudang Jaya, Medan, Indonesia. Pharmaceutical grade of chitosan (MW: 50 kDa, deacetylation: 75–85 %), purchased from PT Fugha Pratama Mandiri, Lhokseumawe, Aceh. Food grade of syzygium cumini seed supply by a local farmer in Lhokseumawe, Aceh. ATCC (American Type Culture Collection) staphylococcus aureus (Gram-positive) and escherichia coli (Gram-negative) obtained from the Laboratory of Pharmaceutical Microbiology, Department of Biology, Universitas Sumatera Utara.
2.2. Extraction of SCSE from jamblang fruit
The extraction procedure was conducted employing a conventional Soxhlet apparatus, in accordance with the methodology outlined by Ref. [36] The process was carried out at a controlled temperature of 55 °C, utilizing ethanol as the solvent. Precisely 20 g of Syzygium cumini seed powder were encased within a filter paper and securely positioned within a thimble holder. Then, the thimble assembly was submerged in the distillation flask containing the solvent. The extraction process was run for 6 h. Following this extraction period, the solute content present in the SCSEs was meticulously separated from the solvent. This separation process was achieved through a distillation technique, which was performed at a controlled temperature of 60 °C, lasting for a duration of 45 min.
2.3. Active packaging production and melt processing
2.3.1. Active packaging production for mechanical properties
The film was produced using a single-screw extrusion process, similar to the technique described by Ref. [37], but with a few modifications. The operating temperature was precisely set at 160 °C, and this condition was maintained for 45 min. In the processing chamber, 9.5 g of PLA and 0.5 g of PCL were carefully put into the processing chamber. The blending process was meticulously carried out at the aforementioned temperature for 45 min at a mixing rate of 20 rpm. The blending of biopolymer material was then extruded and elongated to form fine filaments. Following that, approximately 10 g of the PLA-PCL biopolymer blend was weighed and compressed molded in a heated press. Chitosan, SCSE, and PEG were thoroughly mixed with the PLA-PCL blend using a stirring apparatus at a stirring speed of 40 rpm. The entire mixing and fusion process was carried out at a temperature of 160 °C and a pressure of 100 bar. Table 2 shows the formulations used to develop the active packaging.
Table 2.
Composition of each material in the active packaging production.
| Sample | Matrix |
Plastisizer |
Active Agent (10 % w/v) |
||
|---|---|---|---|---|---|
| PLA (g) | PCL (g) | PEG 30 %(mL) | Chitosan (g) | SCSE (mL) | |
| Xa1 | 9.5 | 0.5 | 3 | 0.1 | 0.9 |
| Xa2 | 9.5 | 0.5 | 3 | 0.2 | 0.8 |
| Xa3 | 9.5 | 0.5 | 3 | 0.3 | 0.7 |
| Xa4 | 9.5 | 0.5 | 3 | 0.4 | 0.6 |
| Xa5 | 9.5 | 0.5 | 3 | 0.5 | 0.5 |
| Xa6 | 9.5 | 0.5 | 3 | 0.6 | 0.4 |
| Xa7 | 9.5 | 0.5 | 3 | 0.7 | 0.3 |
| Xa8 | 9.5 | 0.5 | 3 | 0.8 | 0.2 |
| Xa9 | 9.5 | 0.5 | 3 | 0.9 | 0.1 |
| Xa10 | 9.5 | 0.5 | 3 | 1 | – |
| Xa11 | 9.5 | 0.5 | 3 | – | 1 |
| Xa12 | 9.5 | 0.5 | 3 | – | – |
| Xa13 | 9.5 | 0.5 | – | – | – |
| Xa14 | 10 | – | – | – | – |
2.4. Characterization of syzygium cumini seed extract (SCSE) extract
2.4.1. Characterization of antioxidant compounds from SCSE using the gas Cromatography and mass spectroscopy (GC-MS)
The GC-MS-QP2010 Ultra is connected to a mass spectrometer (Agilent 5975C) using a DB-1MS capillary column (30 × 0.25 mm I.D 0.25 μm layer thickness). The carrier gas was helium, flowing at a rate of 1 mL/min. The temperature starts out at 60 °C (1 min), rises to 240 °C at a rate of 6 °C/min, stays for 6 min, then rises to 250 °C at a rate of 10 °C/min, reaching 250 °C for the final 10 min. Sample SCSE (0.1 μL) were injected with a split less mode.
2.4.2. Antioxidant activity characterization of SCSE using the DPPH method
A total of 100 μL of SCSE with concentrations of 100, 200, 300, 400, and 500 μg/mL, is added with 1.0 mL of 0.4 mM DPPH in a 5 mL tera flask then added ethanol up to the tera limit. Then the mixture is vortexed for 30 s and then put into the incubator for 30 min. The solution is then measured for absorbance using a UV–vis spectrophotometer at a wavelength of 515 nm. Antioxidant activity can be calculated by using Equation (1):
| (1) |
Where I defined as inhibiton, Absw is absorbance from control solution, and Abss is absorbance from initial sample.
2.5. Characteristics of PLA-PCL active packaging with PEG plasticizers and chitosan/SCSE as active agents
2.5.1. Fourier transform Infra red (FTIR)
Fourier transforms infrared (FTIR) spectra are recorded in absorbance mode using a Shimadzu model (IRPrestige 21). FTIR spectroscopy used to analyze the chemical composition and molecular structure of the packaging material. Sampling identified within a wave number range of 400–4000 cm−1, at a resolution of 4 cm−1.
2.5.2. Tensile strength ASTM D-638
The mechanical properties of the films are determined using a universal testing machine (model JIS 2241) according to the standard method of ASTM D-638. The test involved specimens measuring 160 mm × 25 mm, which were evaluated both longitudinally (LD) and transversally (TD). Tensile strength (σmax, MPa), and elongation-at-break (εmax, %), were derived from the stress-strain curves. At a load of 100 kgf and test speed of 2 mm/s, with an average of three repetitions for each sample. The sample is poured into the dumbbell mold as much as 15 mL. All samples are stored at 25 °C and 50 % relative humidity (RH) for 48 h prior to characterization. The obtained tensile strength data were analyzed using standard statistical methods, including analysis of variance (ANOVA), to evaluate significant differences between the various film formulations.
2.5.3. Scanning Electron Microscopy
The morphology of the prepared films was examined using Scanning Electron Microscopy (SEM, JEOL-JSM). SEM images were captured with a high-tension voltage of 1.5 kV and a spot size of 30 μm. Specimens were immersed in liquid nitrogen, gold-coated, and affixed to a glass substrate. The immersion of the sample in liquid nitrogen is primarily aimed at preserving the structural and morphological integrity of the sample. SEM analysis requires the sample to be in a solid-state, and freezing it with liquid nitrogen helps to maintain its physical properties without any distortion or alteration, ensuring that the sample remains stable throughout the analysis. Then, magnified up to 100 times (morphology) then photographed on each surface of the broken specimen.
2.5.4. Water Vapor transmission rate (WVTR)
The water vapor permeability rate (WVPR) of the films was assessed using a gravimetric test following the ASTM standard (2002). Each cup was filled with 10 g of silica gel. A film sample, approximately 0.06 cm in diameter, was positioned on the top of the cup and sealed using a top ring secured in place by tight clamps. The film's surface exposed to vapor transmission measured 10 cm2. The cups were initially weighed and then placed inside desiccators containing a saturated KCl solution. Subsequently, the cups were weighed at certain intervals, and the WVTR calculated based on previous works by Ref. [38]. The water vapor transmission rate is determined by Equation (2):
| (2) |
Where ΔX is Slope of cup after weight increase (g/h), and A is the surface area of the films (m2).
The resulting data were subjected to statistical analysis, including calculation of means and standard deviations, to assess the variations within and between different film formulations. Statistical significance was determined using one-way ANOVA.
2.5.5. Antimicrobial activity
Potato Dextrose Agar (PDA) was employed to assess the antimicrobial activity of the samples against both Gram-negative (E. coli) and Gram-positive (S. aureus) bacteria. Approximately 20 mL of PDA was poured into a sterilized Petri dish using an autoclave of HV and HVE Hirayama type, maintained at 120 °C for 15 min 100 μL of cell culture is coated in solidified PDA at 37 °C for 24 h (Wong et al., 2020). The quantitative inhibitory effect of the films is carried out according to the standard test method for determining the activity of antimicrobial agents under dynamic contact conditions (ASTM E 2149-01). Bacterial growth is visualized after incubation at 37 °C for 24 h (S. aureus) and 48 h (E. coli) using a Colony Counter Scan300. The following formula was used to calculate the percentage inhibition of biofilm formation:
| (3) |
2.6. Application on beef and pineapple
2.6.1. Total Volatile Basic Nitrogen (TVB-N)
Kjeldahl semi-micro distillation was used to determine the TVB-N content of beef. This test is carried out according to Ref. [39]. Beef (10 g) that has been packed in 14 cm × 9 cm film for 0–15 day at 4 °C, vacuum-sealed before analysis to minimize exposure to oxygen as much as possible then unpacked. Grind the beef sample into a fine paste using a mortar and pestle. Transfer the beef paste into a clean beaker. Add distilled water as much as 100 mL to the beef paste in the beaker to create a slurry then, stir the slurry at 100 rpm vigorously with a glass rod to ensure thorough mixing at 25 °C for 45 min. Filter the slurry through a filter paper to obtain a clear beef extract. After filtering, 10 mL of the supernatant obtained was mixed with 10 mL of MgO solution (10 g/L) and then distilled for 5 min using a Kjeldahl distillation unit. The distillate is absorbed by 10 mL of boric acid solution (20 g/L) containing 5–6 drops of indicator. The filtrate was then titrated with a solution of 10 mmol/L HCl, and the results were determined as mg/100 g sample. TVBN measurements were conducted at regular intervals during the storage period. Statistical analysis of the TVBN data was performed using one-way analysis of variance (ANOVA). This analysis enabled us to determine the influence of different active packaging formulations on TVBN content.
2.6.2. Vitamin C
The content of vitamin C in pineapple was determined by the spectrophotometric method [40]. Quantitative analysis of vitamin C is carried out by titrimetry with 2,6-dichlorophenolindophenol reagents. As much as 50 g of pineapple that has been wrapped in active packaging (15 cm × 15 cm) is stored in a refrigerator at 4 °C for 15 days of storage. The process carried out by homogenizing pineapple piece with a certain amount of water then, the solution is sonicated, centrifuged, and then analyzed with a spectrophotometer (UV–Vis Split 8600U). Distilled water was used as reference. The absorbance was measured at 300 nm and the Vit C content of the sample was expressed as mg/100 mL. The impact of active packaging on the vitamin C content of pineapple samples was assessed using a randomized complete block design (RCBD).
2.7. Limitation of the study
This study has provided valuable insights into the development of active packaging using PLA/PCL/PEG films integrated with chitosan and Syzygium cumini seed extract, therefore it is important to acknowledge certain limitations about real-world testing condition since the research primarily focused on laboratory-scale evaluations and controlled environmental conditions. Futhermore, the influence of various food types, pH levels, and moisture content on the performance of the active packaging materials remains an area for future research also It is essential to assess the long-term stability of these agents to ensure their efficacy throughout the intended shelf life of packaged products.
3. Results and discussion
3.1. Characteristic of syzygium cumini seed extract (SCSE)
3.1.1. Gas Cromatography and mass spectroscopy (GC-MS) analysis
Table 3 presents various antioxidant compounds found in SCSE. SCSE is rich in phenolic compounds, including β-humulene, α-guaiene, methandrostenolone, caryophyllene, β-elemene, α-humulene, γ-gurjenene, and epiglobulol, all of which play a crucial role in free radical scavenging activity.
Table 3.
GC-MS results of the characterization of SCSE.
| No | Retention time | %Area | Components |
|---|---|---|---|
| 1 | 16.065 | 27.06 | β-humulene |
| 2 | 13.46 | 12.48 | α-guaiene |
| 3 | 13.537 | 8.88 | Methandrostenolone |
| 4 | 14.191 | 5.53 | α-guaiene |
| 5 | 13.228 | 3.35 | Caryophyllene |
| 6 | 12.827 | 1.26 | β-elemene |
| 7 | 15.614 | 1.21 | α-guaiene |
| 8 | 13.654 | 1.19 | α-humulene |
| 9 | 13.924 | 0.82 | γ-gurjenene |
| 10 | 14.06 | 0.72 | Epiglobulol |
| 11 | 13.823 | 0.53 | Caryophyllene |
These phenolic compounds within the SCSE group exhibit remarkable antioxidant properties. According to research conducted by Ref. [41], the quantity of colonies of pathogenic microorganisms such as Klebsiella pneumoniae, Escherichia coli, and Pseudomonas aeruginosa can be reduced due to the essential oil compounds humulene and caryophyllene, which possess strong antimicrobial activity. Another study by Ref. [42] has confirmed the antioxidant activity of β-elemene, β-caryophyllene, and α-humulene using the DPPH method, with a value of 113.84 μg/mg. Furthermore, research by Ref. [43] validated the chemical composition and antioxidant activity of essential oils from pine plants, including α-guaiene, methandrostenol, and β-humulene, using GC-MS. Study has identified 30 components in thymus essential oil, with major constituents such as β-caryophyllene (14.5 %), carvacrol (13.4 %), spathulenol (5.3 %), and α-humulene (5.1 %) [44].
SCSE comprises numerous molecules capable of neutralizing free radicals by accepting or donating electrons to eliminate unpaired free radicals. These SCSE molecules react with reactive radicals to create new free radicals that are less active and less harmful than unneutralized free radicals. The free radical scavenging ability of SCSE is attributed to its aromatic ring structure, which can delocalize unpaired electrons through hydroxyl groups. This study aligns with prior work by Ref. [45], wherein β-elemene exhibited antioxidant activity when reacting with oxygen.
3.1.2. DPPH assay (2,2′-diphenyl-1-picrylhydrazyl)
Table 4 presents the antioxidant properties of SCSE measured using the DPPH method. The table demonstrates that SCSE exhibits an impressive antioxidant activity of 53.39 % as determined by the DPPH method. Artificial free radicals, represented by DPPH, react with the antioxidant compounds present in SCSE, resulting in a reduction of free radicals by more than 50 % with an IC50 value of 452.37 μg/mL. The IC50 value is an important metric indicating the amount of SCSE required to neutralize 50 % of the free radicals, according to established standards. This neutralization occurs because diphenylpicrylhydrazyl (DPPH) radicals become neutral and transform into diphenylpicrylhydrazine in the presence of functional groups, including (-OH). This transformation effectively disrupts free radical chains by either accepting or donating electrons, thereby eliminating unpaired free radicals.
Table 4.
Antioxidant activity of SCSE.
| Concentration (μg/mL) | Absorbance | Antioxidant activity (%) | Linear regression equation |
|---|---|---|---|
| 0 | 0.72 | 0 | y = 0.1061x +2.0026 R2 = 0.99 IC50 = 452.37 |
| 100 | 0.62 | 13.89 | |
| 200 | 0.55 | 23.97 | |
| 300 | 0.47 | 34.80 | |
| 400 | 0.39 | 45.02 | |
| 500 | 0.33 | 53.39 |
Notably, the IC50 value obtained in the present study is superior to previous findings using SCSE reported by Ishartati et al. (2021), where they recorded an IC50 value of 492.17 μg/mL. A smaller IC50 value indicates higher antioxidant activity. It's worth mentioning that variations in IC50 values can be attributed to differences in the solvents employed, as demonstrated in another study by Ref. [46]. They investigated various plant extracts using different solvents, resulting in IC50 values ranging from 31.9 to 59.7 μg/mL.
3.2. Characteristics of PLA-PCL active packaging with PEG plasticizers and Chitosan/SCSE as an active agents
3.2.1. Fourier transform Infra red (FTIR) analysis
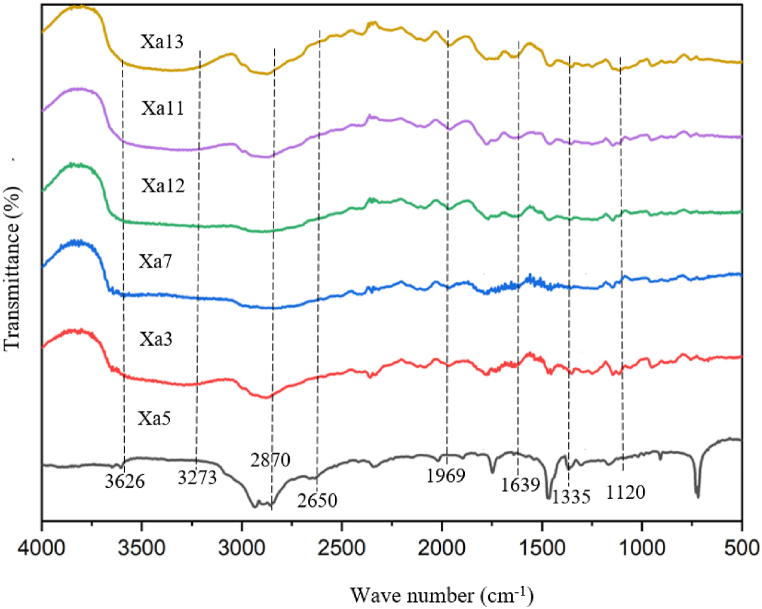
To investigate the interactions among the functional group present in the PLA, PCL, PEG, chitosan, and SCSE films, FTIR spectroscopy was employed to monitor shifts in absorption bands in specific regions. Fig. 1 displays the FTIR spectra of the PLA-PCL-PEG blend incorporating the active agent chitosan and SCSE. Several characteristic absorption bands are observed in the spectra, providing insights into the molecular interactions within the films. Bands at 1969 cm−1, 1639 cm−1, 1335 cm−1, and 1120 cm−1 correspond to C–H chain vibrations, C C stretching, and the C–O peak, respectively (Xa13). In the carbon chain, this group acts as a bond destabilizer. PLA-PCL-PEG films containing chitosan exhibit a broader absorption band at 2870 cm−1, indicating interactions and dispersion between the PLA-PCL-PEG matrix and chitosan (Xa11) [47].
Fig. 1.
The interaction between the fuctional group of the PLA-PCL-PEG and the active agent chitosan/SCSE based on FTIR analysis.
The addition of chitosan is also marked by the appearance of an amine group (N–H) peak at 1631 cm−1. Further interactions are evident when the PLA-PCL-PEG blend interacts with SCSE (Xa12), influencing the stretching of the O–H group at 2870 cm−1 and the C–H group at 1969 cm−1. These shifts suggest interfacial adhesion between the PLA-PCL-PEG functional groups and SCSE. Another noteworthy spectrum, Xa7, displays a broad absorption band at 3626 cm−1, corresponding to the stretching vibration (O–H) of hydroxyl groups found in alcohols and phenols. Additionally, absorbance peaks at 2870 cm−1 correspond to the stretching vibrations of N–H groups in chitosan, while peaks at 1969 cm−1 indicate the presence of O–H and C–H aromatic compounds. Furthermore, sample Xa3 exhibits carbonyl group (C O) stretching vibrations. The absorption band shift from 1639 cm−1 to 1969 cm−1 is attributed to the presence of phenolic compounds, specifically flavonoids, in SCSE, which induce stretching of the aromatic rings (C C). Stretching of the N–H group also occurs again in the absorption band 3273 cm−1. Another significant presence of hydroxyl groups (O–H) in 1355 cm−1 and 3626 cm−1 absorption bands within the bioplastic film may ascribed to the potential movement of hydroxyl groups associated with SCSE during interactions between the bioplastic matrix and the active substance.
3.2.2. Tensile strength
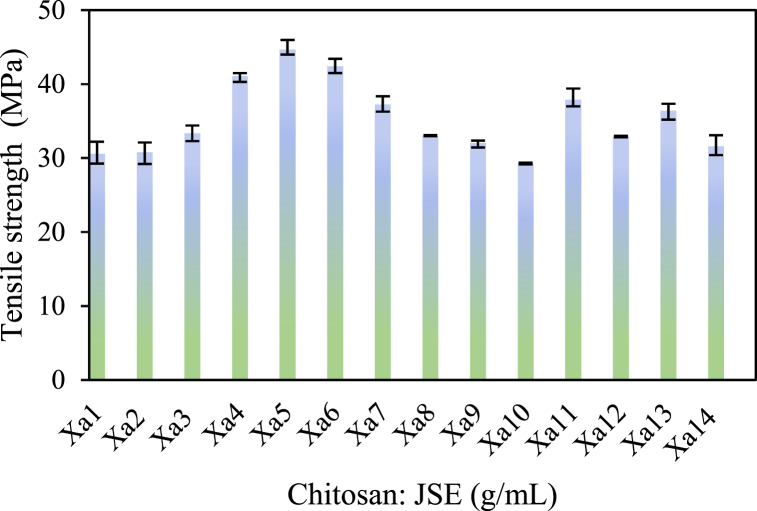
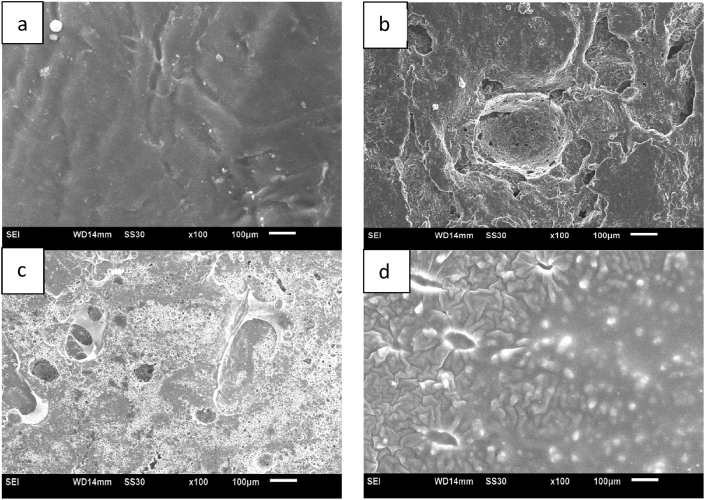
Fig. 2 illustrates the tensile strength of active packaging films, tested using the Universal Tensile Machine (UTM) following ASTM D-638 standards. The matrix with PEG plasticizer (Xa12) exhibited a tensile strength of 32.91 MPa. Since PEG is non-polar, it leads to limited interaction and compatibility between the matrix and plasticizer. The use of non-polar plasticizers can reduce mechanical properties by up to 15 %. Sample Xa5 displayed the highest tensile strength, approximately 44.69 MPa. This superior performance can be attributed to the presence of fewer voids in the blends, as observed in Fig. 3(b), which result from better film-forming properties and material compatibility, thus enhancing their mechanical properties. Since chitosan molecules contain amino and hydroxyl groups that can form hydrogen bonds with the polymer chains in the blend, these interactions strengthen the intermolecular forces within the material, enhancing its mechanical properties. On the other hand, SCSE containing antioxidant compounds, such as phenolic groups have the ability to scavenge free radicals. When incorporated into the packaging material, these antioxidants can protect the polymer chains from oxidative degradation. This protection can prevent the breakdown of polymer bonds and maintain the structural integrity of the material over time. Therefore, the compatibility of SCSE compounds with chitosan can disrupt the formation of a uniform crystal structure in the polymer matrix. This disruption can weaken intermolecular hydrogen bonds and inhibit interactions between polymer chains, resulting in a material with a denser structure. This improved structure contributes to higher tensile strength.
Fig. 2.
The effect of the active agent chitosan/SCSE on the tensile strength value for each sample.
Fig. 3.
Morphology of films (a)Xa12, (b)Xa5, (c)Xa10, and (d)Xa11.
Chitosan, when added at 3%–5% by weight, acted as a filler and restricted the movement of the matrix segmental chains, consequently strengthening the bioplastic films. The tensile strength increased by 5 % with the addition of chitosan. This increase is associated with the effective dispersion of chitosan and favorable interactions with other components [48].
In contrast, a 34.50 % reduction in tensile strength was observed in samples Xa6-Xa10. This decrease was caused by the formation of chitosan aggregates surrounded by weak interfacial interactions, resulting in a gradual decrease in tensile strength as depicted in Fig. 3. (a), and (c). The combination of phenolic compounds, such as SCSE, with chitosan can disrupt the formation of a uniform crystal structure within the matrix, weaken intermolecular hydrogen bonds, and inhibit interactions between polymer chains. High tensile strength values were also observed in samples containing only SCSE additives. Sample Xa11, for instance, exhibited a tensile strength of 37.90 MPa. This increase can be attributed to the compatibility between polymers and SCSE, as depicted in Fig. 3(d). This compatibility results in a denser surface due to the interaction of polymer and SCSE interfaces. Since SCSE contains hydroxyl groups, it is highly reactive and forms bonds with polymeric chains, contributing to the increased tensile strength of the film.
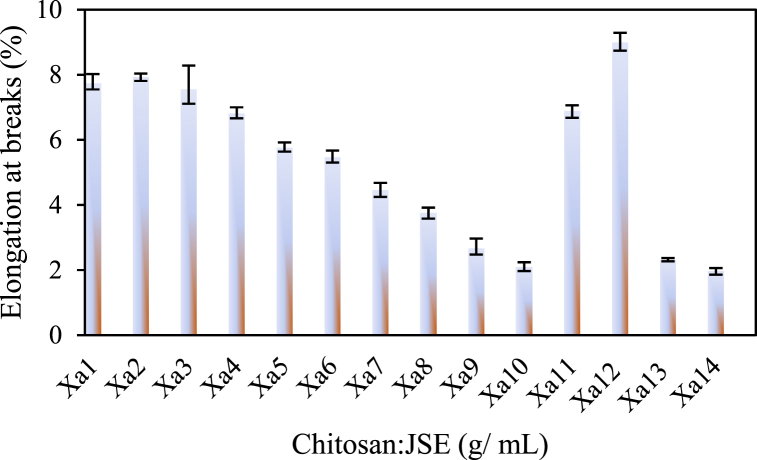
3.2.3. Elongation at breaks
Fig. 4 illustrates the impact of the active agent chitosan/SCSE on the elongation at break for each sample of active packaging films. The introduction of 30 wt% PEG to the PLA-PCL blend without active agents (Xa12) resulted in an increase in elongation at break. As indicated in Fig. 4, sample Xa12 exhibits a higher elongation at break, reaching up to 8.9 %. This enhancement is attributed to the smaller molecular size of PEG with a low molecular weight, facilitating its incorporation into the matrix chain and thus promoting a more effective plasticizing effect. PEG plasticization enhances the mobility of PLA and PCL chains, increasing their ductility and compatibility (PLA/PCL/PEG), thereby broadening the range of potential applications. Using PEG as a plasticizer improved flexibility and processability in PLA/PCL blends [49].
Fig. 4.
Effect of the active agent chitosan/SCSE on elongation at break for each bioplastic film sample.
Fig. 4 further demonstrates that the inclusion of chitosan and SCSE reduces the elongation at break from 8.9 % to 2.67 %. This reduction occurs because chitosan acts as a reinforcing agent, increasing the rigidity of the films. Additionally, films with a higher SCSE content (10 % by weight) exhibit reduced elongation at break, primarily due to the abundance of phenolic compounds present.
3.2.4. Water vapor transmission rate (WVTR)
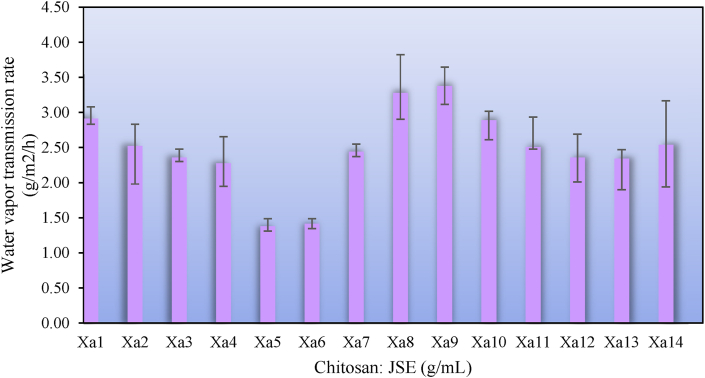
Fig. 5 shows the addition of active agent to the matrix reduced the WVTR by 52 %, from 2.91 g/m2/hour (Xa1) to 1.38 g/m2/hour (Xa5). One of the carboxylic groups from chitosan (-COOH) interacts with the hydroxyl group (-OH) of the SCSE group, resulting in the release of one molecule of water and the formation of a hydrophobic carbonyl group (-C O) for each (-COOH). As water molecules are removed, the crosslink resulting in a more compact film. The less hydrophilic due to the formation of the (-C O) group, can explain the lower WVTR value. The decrease is also influenced by the PLA-PCL blend which has a high moisture barrier due to its hydrophobic properties.
Fig. 5.
Effect of chitosan/SCSE on water vapor transmission rate.
The increase in SCSE content resulted in much more hydrogen bonding through the adsorbed water and resulted in a surface with high hydrophilicity. Another factor that causes the WVTR higher is the roughness of the surface. The film matrix has more pores for transporting gas and vapor permeations, causing a higher WVTR. The addition of PEG also increasing WVTR value in this study. PEG, as a hydrophilic plasticizer, changes the functional and increases the WVTR value. The PEG functional group increases the free volume for water adsorption on the matrix chain, resulting in a looser matrix polymer network.
3.2.5. Antimicrobial activity
All samples exhibited antibacterial activity against both S. aureus and E. coli, though there were significant differences in their antibacterial effects. Additionally, chitosan-SCSE showed stronger inhibitory activity against Gram-positive bacteria (S. aureus) than Gram-negative bacteria (E. coli). This difference is due to structural differences in bacterial cell walls. Gram-negative bacteria have an outer lipopolysaccharide membrane, whereas Gram-positive bacteria have a peptidoglycan structure with a single layer ([50,51]). Furthermore, when the proportion of SCSE exceeded that of chitosan, a higher percentage of colony reduction was observed for both types of bacteria. The absence of additional solvents, resulting from the acid group, during the mechanical production of active packaging films did not lead to an increase in the degree of deacetylation of chitosan. It is worth noting that chitosan has been found to inhibit the formation of bacterial and fungal biofilms ([52,53]). The degree of deacetylation and molecular weight play pivotal roles in influencing antibacterial activity. Bacterial biofilms represent microbial populations attached to surfaces and enclosed within a matrix of self-produced extracellular polymeric substances. The interaction between chitosan, SCSE, and microbial cell membranes, related to both positive and negative charges, can lead to the disruption of bacterial biofilms, resulting in the leakage of proteins and other cellular components, ultimately leading to bacterial death.
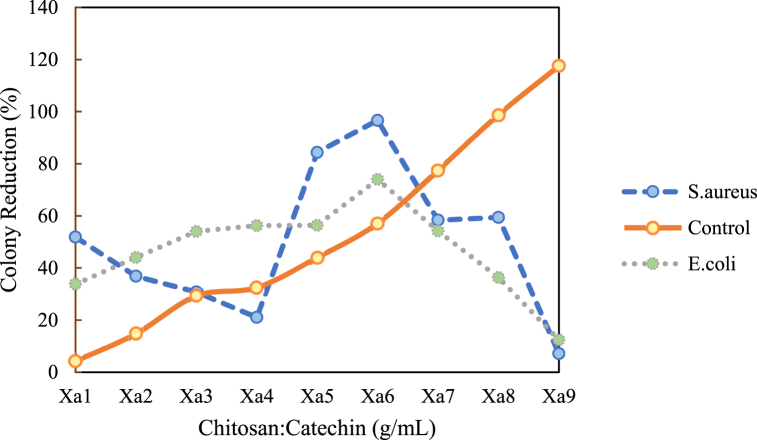
The addition of a small quantity of positively charged chitosan may inadvertently lead to bacterial accumulation since its slight positive charge is insufficient to neutralize the negative charge on bacteria, ultimately promoting bacterial growth over time. Furthermore, the concentration of SCSE also exerts a notable influence on its inhibitory effect against bacterial growth. As illustrated in Fig. 6, the impact of chitosan/SCSE as an active agent is depicted. It is observed that all samples effectively inhibit biofilm formation. Specifically, the percentages of inhibition for biofilm formation by S. aureus and E. coli at the same concentration (Xa6) are 96.66 % and 73.98 %, respectively.
Fig. 6.
Reduction of bacterial colonies on each bioplastic film with the addition of chitosan/SCSE as an active agent.
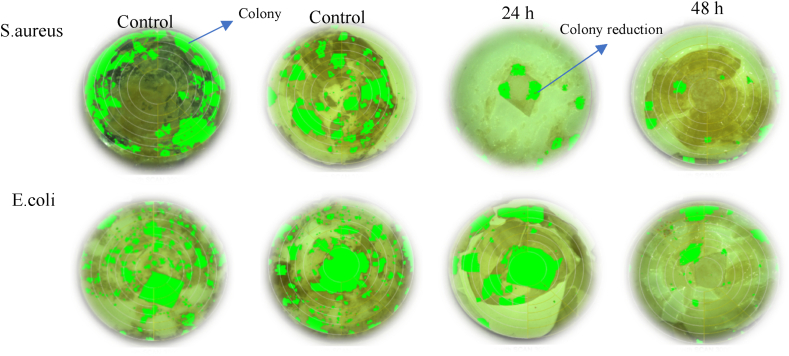
The lipophilic properties of phenolic compounds present in SCSE significantly contribute to their antibacterial activity. The active agent chitosan/SCSE disrupts the bacterial cell membrane, leading to direct binding with genomic DNA and subsequently reducing the formation of colonies, as illustrated in Fig. 7. A decrease in the green coloration of bacterial colonies is observed after 24 and 48 h of storage. The concentrations of SCSE impact cell membrane permeability, which can result in the leakage of cellular materials and alterations in cell morphology. Additionally, SCSE induces minor changes in the secondary structure of DNA and modifies DNA morphology due to the interaction between the active agent chitosan/SCSE and genomic DNA.
Fig. 7.
Colony counter assesment for S.aureus and E.coli bacteria for Xa6.
3.2.6. Application
3.2.6.1. Total Volatile Basic Nitrogen (TVB-N) in beef
Films containing active agents such as chitosan and SCSE, which are phenolic compound-rich products, have been shown to positively delay microbial proliferation and growth in beef products. Fig. 8 shows the effect of the active agent chitosan/SCSE concentration on the increase of TVB-N after 15 days of storage at 4 °C. Based on Fig. 9, the amount of TVB-N in all samples has increased. This demonstrates that even the low temperature does not kill microorganisms, but rather slows their growth and reproduction. The increase of TVB-N in all samples is caused by primary or secondary hydrolysis (extensive hydrolysis).
Fig. 8.
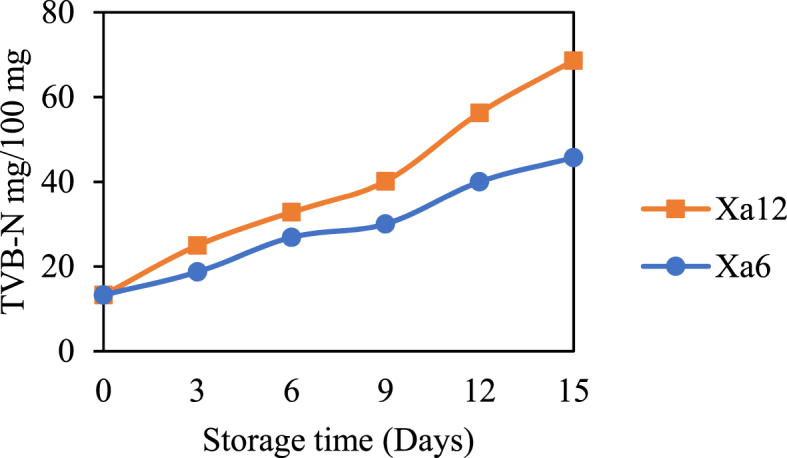
The effect of storage time on the increase of TVB-N in beef.
Fig. 9.
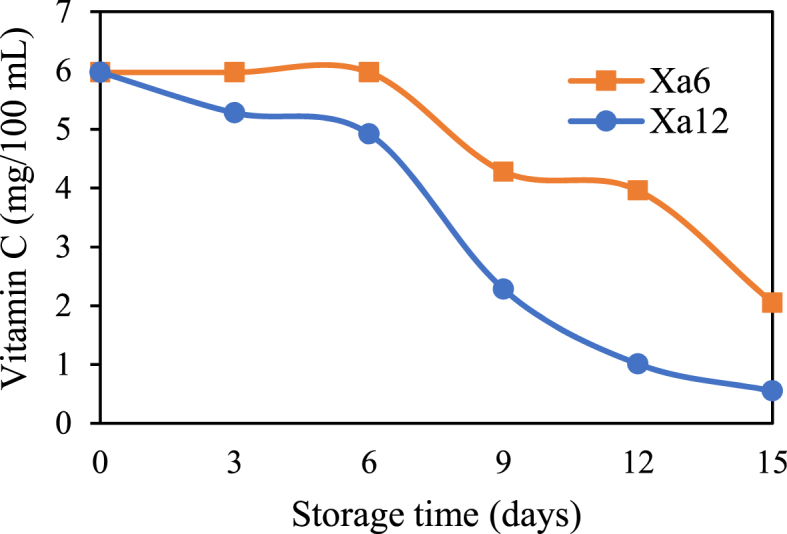
The effect of storage time on the decrease in vitamin C in pineapple.
When wrapped using PLA/PCL/PEG film that does not contain an active substance (Xa12), the oxidation process becomes faster. The amount of TVB-N in beef increased from 32.84 mg/100 mg (6th day) to 68.65 mg/100 mg (15th day). This increase reached 52.16 % comparing to the samples wrapped with packaging contains active agent chitosan/SCSE (Xa6). On the same day, the increase in the TVB-N from the packaged sample (Xa6) is 26.90 mg/100 mg–45.71 mg/100 mg or around 41.11 %. Chitosan as an active agent is bacteriocidal which kills bacteria present in beef and inhibits its growth. SCSE is a group of polyphenols, which work by denaturing proteins, changing cell wall permeability, and causing cell leakage [54]. Similar findings have been reported in previous studies on essential oils containing antibacterial and antioxidant effectively inhibit food degradation during storage [55].
3.2.6.2. Vitamin C in pineapple
Fig. 9 shows a reduction in vitamin C contents in pineapples cut packed with bioplastic films containing the active agent chitosan/SCSE (Xa6). The vitamin C content of all samples decreased linearly with storage time. This could be due to Vitamin C oxidation and consumption by physiological metabolism. Overall, the contens of vitamin C in pineapple packed in active packaging film containing the active agent chitosan/SCSE (Xa6) are higher than pineapple packed in a PLA/PCL/PEG film (Xa12).
The vitamin C content in all samples decreased linearly with storage time. This could be due to Vitamin C oxidation and consumption by physiological metabolism. Fig. 9 shows that samples containing the active agent chitosan/SCSE could keep vitamin C content at 1.43 mg/100 mL until the sixth day of storage. Chitosan's hydrophobic properties helps to keep vitamin C contents in pineapple. These properties create an environment with adequate CO2 and low O2 concentrations, which slows respiration and deteriorative reactions. The right concentration of CO2 can prevent vitamin C depletion due to enzymatic reactions.
Samples wrapped PLA/PCL/PEG film decreased the vitamin C content from 1.43 mg/100 mL on day 0–0.52 mg/mL after sixth day storage. Enzymatic reactions caused a 63.63 % reduction in vitamin C contents. The polyphenol oxidase (PPO) enzyme found in pineapple, which is high in antioxidants, is involved in the enzymatic reaction. Phenols begin to oxidize into quinones when phenolic and PPO compounds from food are exposed to oxygen. Then, in a number of reactions, these quinones and their derivatives are polymerized to create melanin, a brown pigment that is relatively insoluble. The melanin serves as an indication of vitamin deterioration.
4. Conclusion
The active packaging containing 0.5 g chitosan/0.5 mL SCSE (Xa5) had the best mechanical and physical properties. Morphology observation using SEM shows a relatively smooth surface with small amounts of voids resulting in a tensile strength of 44.69 MPa and a WVTR of 1.38 g/m2/hour. Colony decreased for each Gram-positive bacteria S.aureus (96.66 %) and Gram-negative bacteria E.coli (73.98 %). The active packaging films also able to maintain beef quality according to their TVB-N up to 22.94 % (storage for 15 days) and vitamin C content in pineapple 1.43 mg/100 mL after 6 days of storage.
5. Data availability statement
For the readers who asking for data available please directly contact corresponding author via e-mail (halimatuddahliana@usu.ac.id).
CRediT authorship contribution statement
Halimatuddahliana Nasution: Conceptualization, Data curation, Formal analysis. Hamidah Harahap: Funding acquisition, Investigation, Methodology. Elisa Julianti: Project administration, Resources, Software. Aida Safitri: Supervision, Validation, Visualization. Mariatti Jaafar: Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The author wishes to express his gratitude and appreciation to the Republic of Indonesia's Ministry of Research, Technology, and Higher Education, as well as Universitas Sumatera Utara, for funding through grant number 7/UN5.2.3.1/PPM/KP-WCU/2022.
References
- 1.Salem A., Jridi M., Abdelhedi O., Fakhfakh N., Nasri M., Debeaufort F., Zouari N. Development and characterization of fish gelatin-based biodegradable film enriched with Lepidium sativum extract as active packaging for cheese preservation. Heliyon. 2021;7(10) doi: 10.1016/j.heliyon.2021.e08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nasution H., Harahap H., Julianti E., Safitri A., Jaafar M. Smart packaging based on polylactic acid: the effects of antibacterial and antioxidant agents from natural extracts on physical–mechanical properties, colony reduction, perishable food shelf life, and future prospective. Polymers. 2023;15(20):4103. doi: 10.3390/polym15204103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bandara R., Indunil G.M. Food packaging from recycled papers: chemical, physical, optical properties and heavy metal migration. Heliyon. 2022;8(10) doi: 10.1016/j.heliyon.2022.e10959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chowdhury M.A., Hossain N., Badrudduza M.D., Rana M.M. Development and characterization of natural sourced bioplastic for food packaging applications. Heliyon. 2023;9(2) doi: 10.1016/j.heliyon.2023.e13538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ezati P., Rhim J.W., Molaei R., Rezaei Z. Carbon quantum dots-based antifungal coating film for active packaging application of avocado. Food Packag. Shelf Life. 2022;33 doi: 10.1016/j.fpsl.2022.100878. [DOI] [Google Scholar]
- 6.Safitri A., Sinaga P.S.D., Nasution H., Harahap H., Masyithah Z., Hasibuan R. The role of various plastisizers and fillers additions in improving tensile strength of starch-based bioplastics: a mini review. In IOP Conference Series: earth and Environmental Science. IOP Publishing. 2022, December;1115(No. 1) doi: 10.1088/1755-1315/1115/1/012076. [DOI] [Google Scholar]
- 7.Nashchekina Y., Chabina A., Nashchekin A., Mikhailova N. Different conditions for the modification of polycaprolactone films with L-arginine. Int. J. Mol. Sci. 2020;21(19):6989. doi: 10.3390/ijms21196989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sirisinha K., Kamphunthong W., Srisawat K. A comparison of natural rubber latex and polyethylene glycol as fiber carriers in melt-compounded polylactic acid/cellulose microfibril composites. J. Elastomers Plast. 2018;50(8):697–709. doi: 10.1177/0095244318758156. [DOI] [Google Scholar]
- 9.Teixeira-Costa B.E., Andrade C.T. Natural polymers used in edible food packaging—history, function and application trends as a sustainable alternative to synthetic plastic. Polysaccharides. 2022;3(1):32–58. doi: 10.3390/polysaccharides3010002. [DOI] [Google Scholar]
- 10.Flores Z., San-Martin D., Beldarraín-Iznaga T., Leiva-Vega J., Villalobos-Carvajal R. Effect of homogenization method and carvacrol content on microstructural and physical properties of chitosan-based films. Foods. 2021;10(1):141. doi: 10.3390/foods10010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Celebi H., Gunes E. Combined effect of a plasticizer and carvacrol and thymol on the mechanical, thermal, morphological properties of poly (lactic acid) J. Appl. Polym. Sci. 2018;135(8) doi: 10.1002/app.45895. [DOI] [Google Scholar]
- 12.Vasile C., Stoleru E., Darie-Niţa R.N., Dumitriu R.P., Pamfil D., Tarţau L. Biocompatible materials based on plasticized poly (lactic acid), chitosan and rosemary ethanolic extract I. effect of chitosan on the properties of plasticized poly (lactic acid) materials. Polymers. 2019;11(6):941. doi: 10.3390/polym11060941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gavril G.L., Wrona M., Bertella A., Świeca M., Râpă M., Salafranca J., Nerín C. Influence of medicinal and aromatic plants into risk assessment of a new bioactive packaging based on polylactic acid (PLA) Food Chem. Toxicol. 2019;132 doi: 10.1016/j.fct.2019.110662. [DOI] [PubMed] [Google Scholar]
- 14.Yang C., Tang H., Wang Y., Liu Y., Wang J., Shi W., Li L. Development of PLA-PBSA based biodegradable active film and its application to salmon slices. Food Packag. Shelf Life. 2019;22 doi: 10.1016/j.fpsl.2019.100393. [DOI] [Google Scholar]
- 15.Suwanamornlert P., Kerddonfag N., Sane A., Chinsirikul W., Zhou W., Chonhenchob V. Poly (lactic acid)/poly (butylene-succinate-co-adipate)(PLA/PBSA) blend films containing thymol as alternative to synthetic preservatives for active packaging of bread. Food Packag. Shelf Life. 2020;25 doi: 10.1016/j.fpsl.2020.100515. [DOI] [Google Scholar]
- 16.Risyon N.P., Othman S.H., Basha R.K., Talib R.A. Characterization of polylactic acid/halloysite nanotubes bionanocomposite films for food packaging. Food Packag. Shelf Life. 2020;23 doi: 10.1016/j.fpsl.2019.100450. [DOI] [Google Scholar]
- 17.Fiore A., Park S., Volpe S., Torrieri E., Masi P. Active packaging based on PLA and chitosan-caseinate enriched rosemary essential oil coating for fresh minced chicken breast application. Food Packag. Shelf Life. 2021;29 doi: 10.1016/j.fpsl.2021.100708. [DOI] [Google Scholar]
- 18.Laorenza Y., Harnkarnsujarit N. Carvacrol, citral and α-terpineol essential oil incorporated biodegradable films for functional active packaging of Pacific white shrimp. Food Chem. 2021;363 doi: 10.1016/j.foodchem.2021.130252. [DOI] [PubMed] [Google Scholar]
- 19.Lu W., Cui R., Zhu B., Qin Y., Cheng G., Li L., Yuan M. Influence of clove essential oil immobilized in mesoporous silica nanoparticles on the functional properties of poly (lactic acid) biocomposite food packaging film. J. Mater. Res. Technol. 2021;11:1152–1161. doi: 10.1016/j.jmrt.2021.01.098. [DOI] [Google Scholar]
- 20.Wang X., Huang X., Zhang F., Hou F., Yi F., Sun X.…Liu Z. Characterization of chitosan/zein composite film combined with tea polyphenol and its application on postharvest quality improvement of mushroom (Lyophyllum decastes Sing.) Food Packag. Shelf Life. 2022;33 doi: 10.1016/j.fpsl.2022.100869. [DOI] [Google Scholar]
- 21.Klinmalai P., Srisa A., Laorenza Y., Katekhong W., Harnkarnsujarit N. Antifungal and plasticization effects of carvacrol in biodegradable poly (lactic acid) and poly (butylene adipate terephthalate) blend films for bakery packaging. Lwt. 2021;152 doi: 10.1016/j.lwt.2021.112356. [DOI] [Google Scholar]
- 22.Ardjoum N., Chibani N., Shankar S., Fadhel Y.B., Djidjelli H., Lacroix M. Development of antimicrobial films based on poly (lactic acid) incorporated with Thymus vulgaris essential oil and ethanolic extract of Mediterranean propolis. Int. J. Biol. Macromol. 2021;185:535–542. doi: 10.1016/j.ijbiomac.2021.06.194. [DOI] [PubMed] [Google Scholar]
- 23.Mohammadalinejhad S., Almasi H., Esmaiili M. Physical and release properties of poly (lactic acid)/nanosilver-decorated cellulose, chitosan and lignocellulose nanofiber composite films. Mater. Chem. Phys. 2021;268 doi: 10.1016/j.matchemphys.2021.124719. [DOI] [Google Scholar]
- 24.Sadeghi A., Razavi S.M.A., Shahrampour D. Fabrication and characterization of biodegradable active films with modified morphology based on polycaprolactone-polylactic acid-green tea extract. Int. J. Biol. Macromol. 2022;205:341–356. doi: 10.1016/j.ijbiomac.2022.02.070. [DOI] [PubMed] [Google Scholar]
- 25.Lukic I., Vulic J., Ivanovic J. Antioxidant activity of PLA/PCL films loaded with thymol and/or carvacrol using scCO2 for active food packaging. Food Packag. Shelf Life. 2020;26 doi: 10.1016/j.fpsl.2020.100578. [DOI] [Google Scholar]
- 26.Abdollahi M., Damirchi S., Shafafi M., Rezaei M., Ariaii P. Carboxymethyl cellulose-agar biocomposite film activated with summer savory essential oil as an antimicrobial agent. Int. J. Biol. Macromol. 2019;126:561–568. doi: 10.1016/j.ijbiomac.2018.12.115. [DOI] [PubMed] [Google Scholar]
- 27.Atarés L., Chiralt A. Essential oils as additives in biodegradable films and coatings for active food packaging. Trends Food Sci. Technol. 2016;48:51–62. doi: 10.1016/j.tifs.2015.12.001. [DOI] [Google Scholar]
- 28.Chakravarty A., Ahmad I., Singh P., Sheikh M.U.D., Aalam G., Sagadevan S., Ikram S. Green synthesis of silver nanoparticles using fruits extracts of Syzygium Cumini and their bioactivity. Chem. Phys. Lett. 2022;795 doi: 10.1016/j.cplett.2022.139493. [DOI] [Google Scholar]
- 29.Kumar M., Hasan M., Lorenzo J.M., Dhumal S., Nishad J., Rais N.…Zhang B. Jamun (Syzygium Cumini (L.) Skeels) seed bioactives and its biological activities: a review. Food Biosci. 2022 doi: 10.1016/j.fbio.2022.102109. [DOI] [Google Scholar]
- 30.Filipini G., Romani V.P., Guimarães Martins V. Biodegradable and active-intelligent films based on methylcellulose and jambolão (Syzygium Cumini) skins extract for food packaging. Food Hydrocolloids. 2020;109 doi: 10.1016/j.foodhyd.2020.106139. [DOI] [Google Scholar]
- 31.Mahindrakar K.V., Rathod V.K. Ultrasonic assisted aqueous extraction of SCSE seed extract and gallic acid from Syzygium Cumini seed kernel and evaluation of total phenolic, flavonoid contents and antioxidant activity. Chemical Engineering and Processing - Process Intensification. 2020;149 doi: 10.1016/j.cep.2020.107841. [DOI] [Google Scholar]
- 32.Lopez de Dicastillo C., Bruna J., Torres A., Alvarado N., Guarda A., Galotto M.J. A traditional aboriginal condiment as an antioxidant agent in the development of biodegradable active packaging. J. Appl. Polym. Sci. 2017;134(15) doi: 10.1002/app.44692. [DOI] [Google Scholar]
- 33.Hasanin M.S., Youssef A.M. Ecofriendly bioactive film doped CuO nanoparticles based biopolymers and reinforced by enzymatically modified nanocellulose fibers for active packaging applications. Food Packag. Shelf Life. 2022;34 doi: 10.1016/j.fpsl.2022.100979. [DOI] [Google Scholar]
- 34.Wong L.W., Hou C.Y., Hsieh C.C., Chang C.K., Wu Y.S., Hsieh C.W. Preparation of antimicrobial active packaging film by capacitively coupled plasma treatment. LWT. 2020;117 doi: 10.1016/j.lwt.2019.108612. [DOI] [Google Scholar]
- 35.Wang J., Euring M., Ostendorf K., Zhang K. Biobased materials for food packaging. Journal of Bioresources and Bioproducts. 2022;7(1):1–13. doi: 10.1016/j.jobab.2021.11.004. [DOI] [Google Scholar]
- 36.Zhang C., Li W., Zhu B., Chen H., Chi H., Li L.…Xue J. The quality evaluation of postharvest strawberries stored in nano-Ag packages at refrigeration temperature. Polymers. 2018;10(8):894. doi: 10.3390/polym10080894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fkiri S., Mezni F., Ouarghi A., Ghazghazi H., Khouja M.L., Khaldi A., Nasr A. Variability of phenolic compounds and antioxidant efficacy in needles extracts of Pinus nigra Arn. Journal of new sciences, Agriculture and Biotechnology. 2018;53(1):3528–3535. [Google Scholar]
- 38.Boga M., Ozkan E.E., Ersoy E., Tuncay E., Canturk Y.Y., Cinar E.…Zengin G. Identification and quantification of phenolic and volatile constituents in five different Anatolian Thyme species using LC–MS/MS and GC-MS, with biological activities. Food Biosci. 2021;43 doi: 10.1016/j.fbio.2021.101141. [DOI] [Google Scholar]
- 39.Zhai B., Zhang N., Han X., Li Q., Zhang M., Chen X.…Sui X. Molecular targets of β-elemene, a herbal extract used in traditional Chinese medicine, and its potential role in cancer therapy: a review. Biomed. Pharmacother. 2019;114 doi: 10.1016/j.biopha.2019.108812. [DOI] [PubMed] [Google Scholar]
- 40.Ishartati E., Roeswitawati D., Rohman S. 3rd KOBI Congress, International and National Conferences (KOBICINC 2020) Atlantis Press; 2021, June. α-Glucosidase and α-amylase inhibitory activities of jambolan (Syzygium Cumini (L.) SKEELS) fruit and seed; pp. 256–260. [Google Scholar]
- 41.Alami-Milani M., Zakeri-Milani P., Valizadeh H., Fathi M., Salatin S., Salehi R., Jelvehgari M. PLA–PCL–PEG–PCL–PLA based micelles for improving the ocular permeability of dexamethasone: development, characterization, and in vitro evaluation. Pharmaceut. Dev. Technol. 2020;25(6):704–719. doi: 10.1080/10837450.2020.1733606. [DOI] [PubMed] [Google Scholar]
- 42.Boyacioglu S., Kodal M., Ozkoc G. A comprehensive study on shape memory behavior of PEG plasticized PLA/TPU bio-blends. Eur. Polym. J. 2020;122 doi: 10.1016/j.eurpolymj.2019.109372. [DOI] [Google Scholar]
- 43.Pereira M.A., Faustino M.A.F., Tomé J.P.C., Neves M.G.P.M.S., Tomé A.C., Cavaleiro J.A.S.…Almeida A. Influence of external bacterial structures on the efficiency of photodynamic inactivation by a cationic porphyrin. Photochem. Photobiol. Sci. 2014;13(4):680–690. doi: 10.1039/c3pp50408e. [DOI] [PubMed] [Google Scholar]
- 44.Megrian D., Taib N., Witwinowski J., Beloin C., Gribaldo S. One or two membranes Diderm Firmicutes challenge the Gram‐positive/Gram‐negative divide. Mol. Microbiol. 2020;113(3):659–671. doi: 10.1111/mmi.14469. [DOI] [PubMed] [Google Scholar]
- 45.Tan Y., Ma S., Leonhard M., Moser D., Ludwig R., Schneider-Stickler B. Co-immobilization of cellobiose dehydrogenase and deoxyribonuclease I on chitosan nanoparticles against fungal/bacterial polymicrobial biofilms targeting both biofilm matrix and microorganisms. Mater. Sci. Eng. C. 2020;108 doi: 10.1016/j.msec.2019.110499. [DOI] [PubMed] [Google Scholar]
- 46.Refate A., Mohamed Y., Mohamed M., Sobhy M., Samhy K., Khaled O.…Mehanny S. 2023. Influence of Electrospinning Parameters on Biopolymers Nanofibers, with Emphasis on Cellulose & Chitosan. Heliyon. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Acsa I., Lilly Caroline B., Philip Njeru N., Lucy Wanjiru N. Preliminary study on disinfectant susceptibility/resistance profiles of bacteria isolated from slaughtered village free-range chickens in Nairobi, Kenya. International Journal of Microbiology. 2021;2021:1–7. doi: 10.1155/2021/8877675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Njoku I.S., Rahman N.U., Khan A.M., Otunomo I., Asekun O.T., Familoni O.B., Ngozi C. Chemical composition, antioxidant, and antibacterial activity of the essential oil from the leaves of Pinus sylvestris. Pac J Sci Technol. 2022;23(1):85–93. https://www.akamai.university/uploads/1/2/7/7/127725089/pjst23_1_85 [Google Scholar]
- 49.Da Silva Dannenberg G., Funck G.D., dos Santos Cruxen C.E., de Lima Marques J., da Silva W.P., Fiorentini Â.M. Essential oil from pink pepper as an antimicrobial component in cellulose acetate film: potential for application as active packaging for sliced cheese. LWT--Food Sci. Technol. 2017;81:314–318. doi: 10.1016/j.lwt.2017.04.002. [DOI] [Google Scholar]
- 50.Fu D., Ding Y., Guo R., Zhang J., Wang H., Niu B., Yan H. Polylactic acid/polyvinyl alcohol-quaternary ammonium chitosan double-layer films doped with novel antimicrobial agent CuO@ ZIF-8 NPs for fruit preservation. Int. J. Biol. Macromol. 2022;195:538–546. doi: 10.1016/j.ijbiomac.2021.12.022. [DOI] [PubMed] [Google Scholar]
- 51.Le Anh Dao N., Phu T.M., Douny C., Quetin-Leclercq J., Hue B.T.B., Bach L.T.…Scippo M.L. Screening and comparative study of in vitro antioxidant and antimicrobial activities of ethanolic extracts of selected Vietnamese plants. Int. J. Food Prop. 2020;23(1):481–496. doi: 10.1080/10942912.2020.1737541. [DOI] [Google Scholar]
- 52.Thinh B.B., Khoi N.T., Doudkin R.V., Thin D.B., Ogunwande I.A. Chemical composition of essential oil and antioxidant activity of the essential oil and methanol extracts of Knema globularia (Lam.) Warb. from Vietnam. Nat. Prod. Res. 2023;37(10):1625–1631. doi: 10.1080/14786419.2022.2103698. [DOI] [PubMed] [Google Scholar]
- 53.Zhang X., Li Y., Guo M., Jin T.Z., Arabi S.A., He Q.…Liu D. Antimicrobial and UV blocking properties of composite chitosan films with curcumin grafted cellulose nanofiber. Food Hydrocolloids. 2021;112 doi: 10.1016/j.foodhyd.2020.106337. [DOI] [Google Scholar]
- 54.Costa S.M., Ferreira D.P., Teixeira P., Ballesteros L.F., Teixeira J.A., Fangueiro R. Active natural-based films for food packaging applications: the combined effect of chitosan and nanocellulose. Int. J. Biol. Macromol. 2021;177:241–251. doi: 10.1016/j.ijbiomac.2021.02.105. 10.101fmah6/j.ijbiomac.2021.02.105. [DOI] [PubMed] [Google Scholar]
- 55.Yuvaraj D., Iyyappan J., Gnanasekaran R., Ishwarya G., Harshini R.P., Dhithya V.…Gomathi K. Advances in bio food packaging–An overview. Heliyon. 2021;7(9) doi: 10.1016/j.heliyon.2021.e07998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
For the readers who asking for data available please directly contact corresponding author via e-mail (halimatuddahliana@usu.ac.id).