Abstract
Objective:
This study aimed to evaluate the correlation between maximal standardized uptake value (SUVmax) of primary colon cancer and serum neutrophil-to-lymphocyte ratio (NLR), and to assess the prognostic value of SUVmax and serum NLR in stage I and II colon cancer patients.
Methods:
In this retrospective study a total of 128 patients with pathologically confirmed stage I and II colon cancer diagnosed between January 2014 and December 2017 were included. All patients underwent F-18 Fluorodeoxyglucose (FDG) positron emission tomography (PET)/computed tomography (CT) and differential white blood cell (WBC) counts before surgery. The correlations between SUVmax and NLR were assessed. The prognostic value of SUVmax and NLR for predicting recurrence-free survival (RFS) was investigated.
Results:
The mean NLR was 2.2 ± 1.2, and the mean SUVmax of primary tumor was 15.2 ± 7.9. There was significant correlation between NLR and SUVmax (rho=0.2, p=0.02). Mean follow-up period was 59.8 ± 19.2 months and 12 patients experienced a recurrence. In univariable analysis, NLR (p=0.0084, HR=5.0223, 95% CI=1.5117-16.6853), C-reactive protein (CRP) (p=0.021, HR=4.1115, 95% CI=1.2380-13.6551), carbohydrate antigen 19-9 (CA19-9) (p=0.0134, HR=4.2683, 95% CI=1.3519-13.4766), and Kirsten ras sarcoma viral oncogene (KRAS) mutation (p=0.0338, HR=3.4703, 95% CI=1.0998-10.9499) were significant prognostic factors for the recurrence. In multivariable analysis, NLR (p=0.0256, HR=4.1155, 95% CI=1.1887-14.2490) and CA19-9 (p=0.0257, HR=4.139, 95% CI=1.1880-14.4200) were independent prognostic factors for the recurrence.
Conclusions:
Significant correlation was observed between SUVmax of primary colon cancer and serum NLR. Furthermore, in the multivariable analysis conducted on early colon cancer cases, NLR and CA19-9 were found to be independently associated with RFS. This suggested that NLR could be used as a supplementary tool for identifying patients at high risk of recurrence in early colon cancer. However, SUVmax was not associated with prognosis, suggesting that it cannot be used for predicting prognosis in early colon cancer.
Key Words: Colon cancer, F-18 FDG PET/CT, SUVmax, NLR, prognosis
Introduction
In 2020, approximately 150,000 new cases of colorectal cancer were diagnosed in the United States, resulting in 52,000 deaths from the disease, making it the third leading cause of death (Siegel et al., 2022). Due to increased use of screening colonoscopies, the incidence and mortality rates have been decreasing since 2000. (Siegel et al., 2020) Approximately 40% of colon cancer patients are diagnosed at stage I or II (localized disease), and the 5-year survival rate for these patients is around 89%. Unfortunately, within this subgroup, 11% of patients with early stage colon cancer still experience mortality within 5 years (Siegel et al., 2022).
Chronic inflammation may play a causative role in oncogenesis and can also affect the prognosis of cancer (Greten and Grivennikov, 2019). Among the inflammatory markers, serum neutrophil-to-lymphocyte ratio (NLR) calculated from differential white blood cell (WBC) counts has been reported as a prognostic factor in several solid tumors (Templeton et al., 2014; Ocana et al., 2017).
F-18 Fluorodeoxyglucose (FDG) is a glucose analogue that accumulates in tumor and inflammatory cells via GLUT protein (Mochizuki et al., 2001). F-18 FDG positron emission tomography/computed tomography (PET/CT) is useful for diagnosing tumors, staging, monitoring therapy response, and detecting recurrence in colon cancer cases (Chowdhury et al., 2010; Lee et al., 2017). F-18 FDG PET/CT is also useful for evaluating inflammatory diseases (Hess et al., 2014). The degree of FDG uptake in cancer lesions is reflected by tumor microenvironment, which consists of tumor cells, tumor stromal cells, endothelial cells, immune cells, and non-cellular components (Baghban et al., 2020). Several previous studies have reported the correlation between maximal standardized uptake value (SUVmax) of primary cancer and NLR in head & neck, esophageal, pancreas, cholangiocarcinoma, and lung cancers (Sürücü et al., 2015; Jeong et al., 2017; Guo et al., 2019; Mirili et al., 2019; Seo et al., 2019; Araç et al., 2020; Werner et al., 2021; Bojaxhiu et al., 2022). However, only two studies have investigated the relationship between SUVmax and NLR in colon cancer, (McSorley et al., 2018; Xu et al., 2019) and none have specifically focused on stage I and II colon cancer. Therefore, we intended to evaluate these factors and assess their prognostic value in stage I and II colon cancer. The aim of this study was to evaluate the correlation between SUVmax of primary colon cancer and serum NLR, and to assess the prognostic significance of SUVmax and serum NLR in stage I and II colon cancer patients.
Materials and Methods
Patients
This retrospective study included a total of 128 patients diagnosed with stage I or II colon cancer between January 2014 and December 2017. All patients underwent F-18 FDG PET/CT scans prior to surgery. Differential WBC counts, serum carcinoembryonic antigen (CEA), serum carbohydrate antigen 19-9 (CA19-9), serum C-reactive protein (CRP), and serum albumin tests were conducted within 30 days before surgery. Patients with a history of cancer were excluded from the study. The pathologic stages of the colon cancer patients were determined according to the American Joint Committee on Cancer Manual, 8th edition. (Amin MB, 2017) After curative surgery, adjuvant chemotherapy was performed in stage II patients with high-risk features (Baxter et al., 2022). All patients underwent routine follow-up evaluation including laboratory tests, colonoscopy, CT, or PET/CT every 3-12 months. Patients who did not experience recurrence were censored at the date of their last follow-up.
FDG PET/CT imaging
For all patients included in the study, oral intake and intravenous glucose injection were prohibited for at least 6 h before the PET/CT scan. Blood glucose levels of the patients were measured before administering F-18 FDG, and PET/CT scans were only conducted if the blood glucose level was below 200 mg/dl. A whole-body scan, covering the area from head to thigh (torso) was performed 60 minutes after intravenous injection of approximately 370 MBq of F-18 FDG. The PET/CT examinations were performed using PET/CT scanners (Discovery STE or Discovery 690; GE Healthcare, Milwaukee, WI, USA). The CT images were acquired using a multidetector CT equipment with a standard protocol that consisted of 140 kV, 60–80mA, and a section thickness of 3.75 mm. Emission PET data were acquired for 2 minutes per bed position. PET images were reconstructed using an ordered-subset expectation maximization iterative reconstruction algorithm with three iterations, 18 subsets, a matrix size of 256x256, and a transaxial field-of-view of 50 cm. Subsequently, the PET images were fused with the CT images.
Image evaluation
Semi-quantitative analysis was performed independently by 2 experienced nuclear medicine physicians who were blinded to the clinicopathologic results. For semi-quantitative analysis, a three-dimensional volume of interest (3D VOI) was drawn on the primary tumor, and SUVmax was calculated for the pixels within the 3D VOI.
Statistical analysis
Statistical analyses were conducted using MedCalc for Windows, version 20.210 (MedCalc Software, Ostend, Belgium). Receiver operating characteristic (ROC) curve analysis was performed to evaluate the diagnostic performance of NLR for differentiating recurrence group and non-recurrence group. Based on the optimal cutoff for NLR, patients were assigned to either low NLR group or high NLR group. The recurrence-free survival (RFS) was defined as the time from colon cancer surgery to recurrence. The Kaplan-Meier survival curve was used to assess RFS rates, and RFS curves were compared using the log-rank test. Univariate and multivariate analyses were performed, and hazard ratios (HR) with 95% confidence interval (CI) were obtained. For all statistical comparisons, P-value less than 0.05 was considered statistically significant.
Results
Characteristics of the study population
The characteristics of the patients included in the study are shown in Table 1. A total of 128 patients were included in this study (68 male and 60 female patients; mean age, 66.5 ± 11.5 years). The pathologic staging of colon cancer was stage I in 46 patients (36%), and stage II in 82 patients (64%). None of the stage I patients received adjuvant chemotherapy. While 46 patients (56%) of stage II received adjuvant chemotherapy (FOLFOX or capecitabine). Kirsten ras sarcoma viral oncogene (KRAS) mutation was found in 39 patients (30%). High microsatellite instability (MSI-H) was found in 22 patients (17%). The mean number of harvested lymph nodes was 30.0 ± 11.5. The mean levels of CEA were 4.8 ± 9.3, and the mean levels of CA19-9 were 15.6 ± 42.8.
Table 1.
Patient Characteristics
| Characteristics | Number (%) | Value (mean±SD) |
|---|---|---|
| All | 128 | |
| Age (years) mean±SD | 66.5±11.5 | |
| Sex | ||
| Male | 68 (53) | |
| Female | 60 (47) | |
| Histologic grade | ||
| Low | 23 (18) | |
| Intermediate | 101 (79) | |
| High | 4 (3) | |
| Stage | ||
| I | 46 (36) | |
| II | 82 (64) | |
| KRAS status | ||
| Wild-type | 89 (70) | |
| Mutation | 39 (30) | |
| Microsatellite status | ||
| MSS | 106 (83) | |
| MSI-H | 22 (17) | |
| Lymphovascular invasion | ||
| Negative | 115 (90) | |
| Positive | 13 (10) | |
| Perineural invasion | ||
| Negative | 112 (88) | |
| Positive | 16 (12) | |
| Harvested lymph nodes | 30.0±11.5 | |
| CEA (ng/mL) | 4.8±9.3 | |
| <2.8 | 66 (52) | 1.5±0.7 |
| ≥2.8 | 62 (48) | 8.4±12.4 |
| CA19-9 (U/mL) | 15.6±42.8 | |
| <14.7 | 94 (73) | 6.9±3.9 |
| ≥14.7 | 34 (27) | 39.5±78.8 |
| CRP (mg/dL) | 1.2±3.2 | |
| <0.44 | 85 (66) | 0.2±0.1 |
| ≥0.44 | 43 (34) | 3.2±5.1 |
| NLR | 2.2±1.2 | |
| <2.5 | 39 (31) | 1.6±0.4 |
| ≥2.5 | 89 (69) | 3.6±1.2 |
| SUVmax | 15.2±7.9 | |
| <16.7 | 84 (66) | 10.8±3.6 |
| ≥16.7 | 44 (34) | 23.7±6.7 |
SD, standard deviation; KRAS, Kirsten ras sarcoma viral oncogene; MSS, microsatellite stable; MSI-H, high microsatellite instability; CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen 19-9; CRP, c-reactive protein; NLR, neutrophil-lymphocyte ratio; SUVmax, maximum standardized uptake value.
Association between NLR and SUVmax
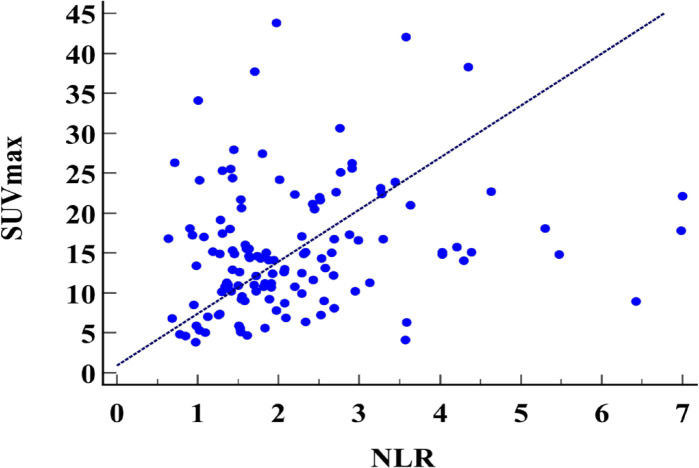
The mean NLR was 2.2 ± 1.2, and the mean SUVmax of primary tumor was 15.2 ± 7.9. There was significant correlation between NLR and SUVmax (rho=0.2, p=0.02; Figure 1a).
Figure 1a.
Scatter Plots between the NLR and SUVmax. NLR, neutrophil to lymphocyte ratio; SUVmax, maximum standardized uptake value
Association between NLR and CRP
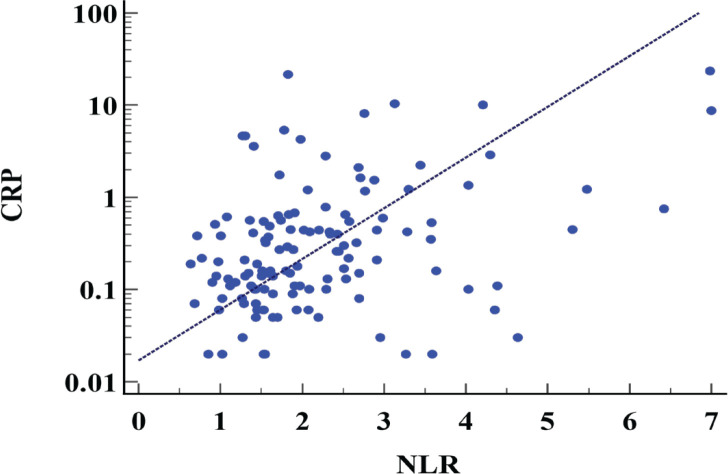
The mean NLR was 2.2 ± 1.2, and the mean serum CRP was 1.2 ± 3.2 (mg/dl). There was significant correlation between NLR and CRP (rho=0.37, p<0.0001; Figure 1b).
Figure 1b.
Scatter Plots between the NLR and CRP. NLR, neutrophil to lymphocyte ratio; CRP, c-reactive protein
ROC curve analysis
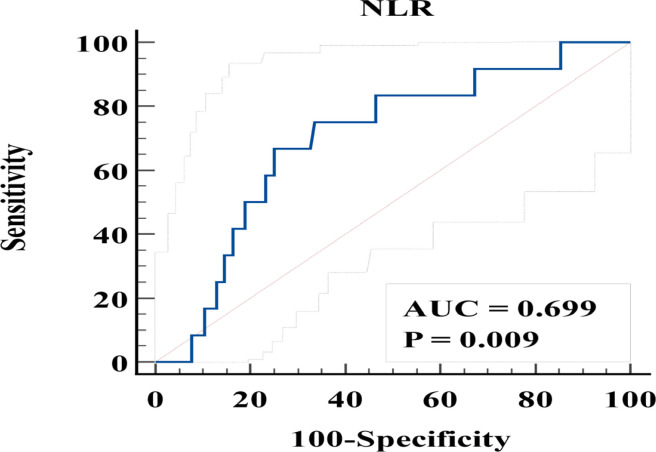
In the ROC curve analysis for differentiation of the recurrence group from the non-recurrence group, a cutoff of NLR of 2.5 yielded the highest accuracy (sensitivity, 66%, specificity, 75%; and area under curve [AUC], 0.699; Figure 2). The optimal cutoff for SUVmax, CRP, CEA, and CA19-9 was 16.7, 0.44, 2.8, and 14.7, respectively.
Figure 2.
ROC Curve of NLR for Predicting Recurrence in Stage I, II Colon Cancer Patients. AUC, area under curve; NLR, neutrophil to lymphocyte ratio; ROC, receiver operating characteristic curve
Recurrence-free survival
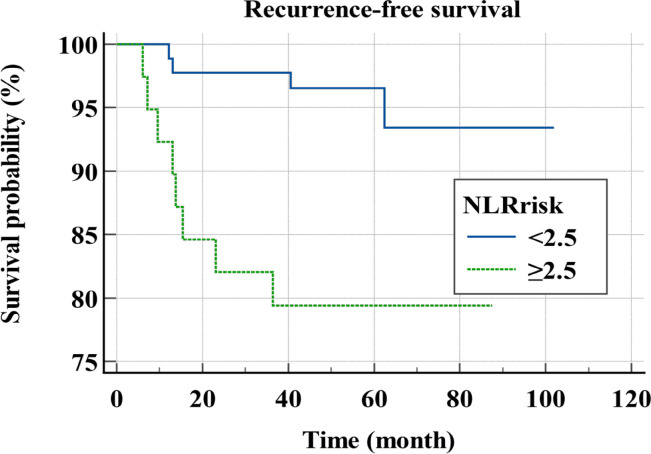
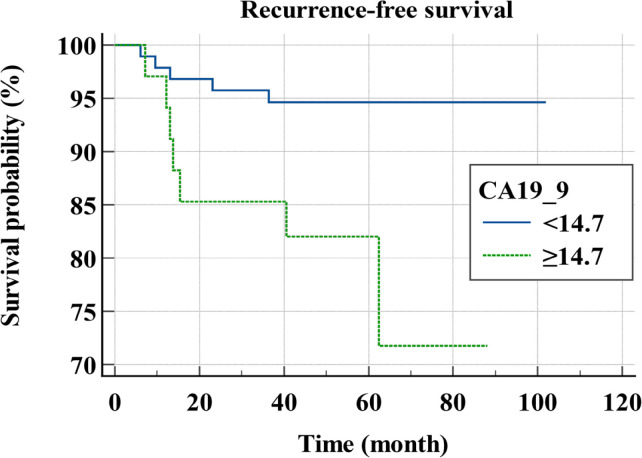
Mean follow-up period was 59.8 ± 19.2 months and 12 patients experienced a recurrence. Mean RFS was 94 months. In univariable analysis, NLR (p=0.0084, HR=5.0223, 95% CI=1.5117-16.6853), CRP (p=0.021, HR=4.1115, 95% CI=1.2380-13.6551), CA19-9 (p=0.0134, HR=4.2683, 95% CI=1.3519-13.4766), and KRAS mutation (p=0.0338, HR=3.4703, 95% CI=1.0998-10.9499) were significant prognostic factors for the recurrence (Table 2). In multivariable analysis, NLR (p=0.0256, HR=4.1155, 95% CI=1.1887-14.2490) and CA19-9 (p=0.0257, HR=4.139, 95% CI=1.1880-14.4200) were independent prognostic factors for the recurrence (Table 3). The survival curves according to NLR and CA19-9 are shown in Figure 3.
Table 2.
Univariable Analyses of RFS
| Variable | Hazard ratio | P value | 95% CI |
|---|---|---|---|
| Age | 0.6889 | 0.2554-2.4644 | |
| <65 | 1 | ||
| ≥65 | 0.7933 | ||
| Sex | 0.1723 | 0.1305-1.4396 | |
| Male | 1 | ||
| Female | 0.4335 | ||
| Histologic grade | 0.8799 | 0.2460-5.1369 | |
| Low | 1 | ||
| Intermediate, high | 1.1242 | ||
| Stage | 0.4434 | 0.4512-6.1584 | |
| I | 1 | ||
| II | 1.667 | ||
| KRAS status | 0.0338 | 1.0998-10.9499 | |
| Wild-type | 1 | ||
| Mutation | 3.4703 | ||
| Microsatellite status | 0.4572 | 0.4441-6.0723 | |
| MSS | 1 | ||
| MSI | 1.6422 | ||
| Lymphovascular invasion | 0.819 | 0.1016-6.1010 | |
| Negative | 1 | ||
| Positive | 0.7874 | ||
| Perineural invasion | 0.6477 | 0.0800-4.8086 | |
| Negative | 1 | ||
| Positive | 0.6204 | ||
| CEA (ng/mL) | 0.059 | 0.9531-13.0272 | |
| <2.8 | 1 | ||
| ≥2.8 | 3.5237 | ||
| CA19-9 (U/mL) | 0.0134 | 1.3519-13.4766 | |
| <14.7 | 1 | ||
| ≥14.7 | 4.2683 | ||
| CRP (mg/dL) | 0.021 | 1.2380-13.6551 | |
| <0.44 | 1 | ||
| ≥0.44 | 4.1115 | ||
| NLR | 0.0084 | 1.5117-16.6853 | |
| <2.5 | 1 | ||
| ≥2.5 | 5.0223 | ||
| SUVmax | 0.45 | 0.1634-2.2334 | |
| <16.7 | 1 | ||
| ≥16.7 | 0.6041 | ||
SD, standard deviation; KRAS, Kirsten ras sarcoma viral oncogene; MSS, microsatellite stable; MSI, microsatellite instability; CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen 19-9; CRP, c-reactive protein; NLR, neutrophil-lymphocyte ratio; SUVmax, maximum standardized uptake value.
Table 3.
Multivariable Analyses of RFS
| Variable | Hazard ratio | P value | 95% CI |
|---|---|---|---|
| KRAS status | 0.0587 | 0.9587-10.2469 | |
| Wild-type | 1 | ||
| Mutation | 3.1344 | ||
| CA19-9 (U/mL) | 0.0331 | 1.1105-12.2760 | |
| <14.7 | 1 | ||
| ≥14.7 | 3.6923 | ||
| CRP (mg/dL) | 0.0712 | 0.9071-10.5676 | |
| <0.44 | 1 | ||
| ≥0.44 | 3.096 | ||
| NLR | 0.0246 | 1.1969-13.7780 | |
| <2.5 | 1 | ||
| ≥2.5 | 4.0609 | ||
SD, standard deviation; KRAS, Kirsten ras sarcoma viral oncogene; MSS, microsatellite stable; MSI, microsatellite instability; CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen 19-9; CRP, c-reactive protein; NLR, neutrophil-lymphocyte ratio; SUVmax, maximum standardized uptake value.
Figure 3a.
Recurrence-Free Survival Curves for Stage I, II Colon Cancer Patients According to NLR. NLR, neutrophil to lymphocyte ratio
In stage II colon cancer, NLR (p=0.0406), and CRP (p=0.253) were significant prognostic factors for the recurrence in univariate analysis. However, in multivariate analysis, NLR (p=0.1700, HR=2.7147, 95% CI=0.6520-11.3024) and CRP (p=0.1038, HR=3.8448, 95% CI=0.7590-19.4756) were not associated with the recurrence.
Figure 3b.
Recurrence-Free Survival Curves for Stage I, II Colon Cancer Patients According to CA19_9. CA19-9, Carbohydrate Antigen 19-9
Discussion
In the patients with stage I and II colon cancer, the overall 5-year survival rate is relatively high at approximately 89%. However, 11% of patients experience mortality within 5 years (Siegel et al., 2022). It is important to identify patients with a low risk of relapse who may suffer a recurrence, for proper patient management. In this study, SUVmax showed a weak association with NLR. Both NLR and CA19-9 were significantly associated with the RFS of stage I and II colon cancer.
Chronic inflammation has been recognized as significant factor in the development of cancer (Greten and Grivennikov, 2019). The inflammatory process associated with cancer can be viewed from two perspectives. Firstly, in terms of anti-tumorigenic immune response, lymphocytes play an important role in adaptive immune responses. Cytotoxic T lymphocytes produce tumor related antigens and cytokines, that plays important role in antitumor immunity. Tumor infiltrating lymphocytes are related to improved clinical outcome in many cancers. (Dunn et al., 2004) Secondly, in the aspect of pro-tumorigenic immune response, neutrophils, specifically N1 tumor associated neutrophils, play a crucial role in cancer growth and progression by releasing various chemokines, cytokines, and enzymes. They promote tumor angiogenesis by secreting proangiogenic factors such as vascular endothelial growth factors (Galdiero et al., 2013; Shaul and Fridlender, 2018).
In this study, a significant but very weak correlation between NLR and SUVmax was observed (rho=0.2, p=0.02). The correlation between SUVmax of primary cancer and NLR has been reported in various cancers. In head and neck cancers, esophageal cancers, pancreas cancers, and cholangiocarcinoma, no significant correlation was found between SUVmax and NLR (Sürücü et al., 2015; Seo et al., 2019; Araç et al., 2020; Werner et al., 2021; Bojaxhiu et al., 2022). In lung cancers, there were significant but very weak or weak correlations between SUVmax and NLR (r=0.237, p=0.0005; r=0.004, p=0.004; r=0.36, p=0.011) (Jeong et al., 2017; Guo et al., 2019; Mirili et al., 2019). In colon cancer, two previous studies reported the association between SUVmax and NLR. In one study, there was significant differences in median SUVmax between patients with high NLR and those with low NLR who experienced recurrent colon cancer (McSorley et al., 2018). In the other study, SUVmax was very weakly correlated with NLR (r=0.164, p=0.013) (Xu et al., 2019). Overall, there was no significant correlation between SUVmax and NLR in various types of cancers (Sürücü et al., 2015; Seo et al., 2019; Araç et al., 2020; Werner et al., 2021; Bojaxhiu et al., 2022). In some cases, there was significant correlation between SUVmax and NLR, however those correlations were either weak or very weak (Jeong et al., 2017; Guo et al., 2019; Mirili et al., 2019; Xu et al., 2019). These findings suggest that inflammation may have minimal effect on the SUVmax of primary tumor (Cottone et al., 2011).
In this study, both NLR (p=0.0256, HR=4.1155, 95% CI=1.1887-14.2490) and CA19-9 (p=0.0257, HR=4.139, 95% CI=1.1880-14.4200) were identified as independent prognostic factors for recurrence. A previous study demonstrated that increased NLR (>2.36) was significantly related to poor outcomes in early colon cancer patients (p=0.0182, HR=2.33, 95% CI=1.15-4.70)(Galizia et al., 2015). In addition to colon cancer, increased NLR was associated with poor outcomes in various other solid tumors (Templeton et al., 2014; Ocana et al., 2017). In patients with stage II colon cancer, adjuvant chemotherapy is typically recommended for those with high risk factors such as pT4, bowel obstruction, bowel perforation, lymphovascular invasion, perineural invasion, inadequate lymph node sampling (<12 nodes), and poorly differentiated histology (G3) (Rebuzzi et al., 2020). Although NLR is not considered a risk factor for adjuvant chemotherapy in these patients, patients with high NLR have a significantly higher rate of recurrence compared to those with low NLR. Therefore, NLR could help identify patients with high risk of recurrence who require more careful follow-up.
In many types of cancers, SUVmax of primary cancer has been identified as a significant factor associated with prognosis (Paesmans et al., 2010; Ghooshkhanei et al., 2014; Zhu et al., 2017; Wang et al., 2019; Lee et al., 2021b). However, majority of the studies on colon cancer have reported no significant association between SUVmax of primary colorectal cancer and prognosis. These studies often included a small number of patients with poorly differentiated tumors (Ogawa et al., 2015; Lee et al., 2017; Baik et al., 2018; Yin et al., 2021). In the present study, there was also no significant association between SUVmax and RFS in stage I and II colon cancer patients (p=0.4500). Generally, SUVmax correlates with tumor size and differentiation (Na et al., 2009). In several cancers, poorly differentiated tumors tend to have higher SUVmax than well or moderately differentiated tumors (Vesselle et al., 2008; Kidd et al., 2009; Ekmekcioglu et al., 2013). Previous studies reported that patients with a higher number of poorly differentiated clusters tend to have a poorer prognosis compared to those with a lower number of poorly differentiated clusters in early colon cancer (Ueno et al., 2012; Kinoshita et al., 2015). The small number of patients with poorly differentiated tumors in the present study may explain the lack of a significant relationship between SUVmax and prognosis (Table 1). Future studies with a larger sample size, specifically including more patients with poorly differentiated tumors are needed.
In the context of F-18 FDG PET/CT, the SUV of bone marrow and spleen may be a better indicator of systemic inflammation than the SUV of primary cancer. Previous studies have reported that bone marrow to liver ratio and spleen to liver ratio were significantly associated with prognosis of colon cancer (Lee et al., 2018; Lee et al., 2021a). However, in early colon cancer, no studies have investigated the association between prognosis and glucose metabolism of the bone marrow or spleen on F-18 FDG PET/CT. Therefore, further evaluation is necessary.
Our study had some limitations. Firstly, the number of patients with early stage colon cancer who had recurrence was small, and we did not perform an overall survival analysis. Thus, further studies with larger sample size are required. Secondly, we did not evaluate volumetric parameters such as metabolic tumor volume (MTV) and total lesion glycolysis (TLG). Although SUVmax was easy to calculate, it only represented the highest SUV within tumors and therefore does not provide volumetric information. MTV and TLG, which represented the metabolically active volume, would be more relevant as markers of systemic inflammation, and further evaluation using these parameters is warranted.
In conclusion, there was weak correlation between SUVmax of primary colon cancer and serum NLR. NLR and CA19-9 were independent factors associated with RFS in multivariable analysis in early colon cancer. NLR could be used supplementarily for identifying patients who have high risk of recurrence in early colon cancer. However, SUVmax was not associated with the prognosis, and thus it cannot be used for predicting prognosis in early colon cancer.
Author Contribution Statement
SJ contributed to the study design, data analysis, and writing of the manuscript. JS, HJ, MS, and KB contributed to data collection and analysis. SS contributed to data analysis and interpretation and supervised the finding of this work. All authors discussed the results and contributed to final manuscript.
Acknowledgements
This study was not approved by any scientific Body and is not part of an approved student thesis.
Ethics statement
The study was approved by the Institutional Review Board of Inje University Busan Paik Hospital (IRB No. 2022-11-046) and was performed in accordance with the ethical standards proposed in the 1964 Declaration of Helsinki and its later amendments.
Availability of data
Participants of this study did not agree for their data to be shared publicly, sharing the data is not available.
Conflict of Interest
The authors declare no other potential conflicts of interest relevant to this article.
References
- Amin MB ES, Greene FL, Byrd DR. AJCC Cancer Staging Manual. 8th ed. Springer; 2017. [Google Scholar]
- Araç E, Can C, Kömek H. Associations of volumetric whole-body (18)F-FDG PET/CT parameters with the CA 19-9 level and haemogram parameters in pancreatic adenocarcinoma. Hell J Nucl Med. 2020;23:40–7. doi: 10.1967/s002449912015. [DOI] [PubMed] [Google Scholar]
- Baghban R, Roshangar L, Jahanban-Esfahlan R, et al. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun Signaling. 2020;18:59. doi: 10.1186/s12964-020-0530-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baik H, Lee SM, Seo SH, et al. Prognostic value of positron emission tomography/computed tomography for adjuvant chemotherapy of colon cancer. ANZ J Surg. 2018;88:587–91. doi: 10.1111/ans.14098. [DOI] [PubMed] [Google Scholar]
- Baxter NN, Kennedy EB, Bergsland E, et al. Adjuvant Therapy for Stage II Colon Cancer: ASCO Guideline Update. J Clin Oncol. 2022;40:892–910. doi: 10.1200/JCO.21.02538. [DOI] [PubMed] [Google Scholar]
- Bojaxhiu B, Sinovcic D, Elicin O, et al. Correlation between hematological parameters and PET/CT metabolic parameters in patients with head and neck cancer. Radiat Oncol. 2022;17:141. doi: 10.1186/s13014-022-02112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury FU, Shah N, Scarsbrook AF, et al. [18F]FDG PET/CT imaging of colorectal cancer: a pictorial review. Postgrad Med J. 2010;86:174–82. doi: 10.1136/pgmj.2009.079087. [DOI] [PubMed] [Google Scholar]
- Cottone L, Valtorta S, Capobianco A, et al. Evaluation of the Role of Tumor-Associated Macrophages in an Experimental Model of Peritoneal Carcinomatosis Using <sup>18</sup>F-FDG PET. J Nucl Med. 2011;52:1770–7. doi: 10.2967/jnumed.111.089177. [DOI] [PubMed] [Google Scholar]
- Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–60. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- Ekmekcioglu O, Aliyev A, Yilmaz S, et al. Correlation of 18F-fluorodeoxyglucose uptake with histopathological prognostic factors in breast carcinoma. Nucl Med Commun. 2013;34:1055–67. doi: 10.1097/MNM.0b013e3283658369. [DOI] [PubMed] [Google Scholar]
- Galdiero MR, Bonavita E, Barajon I, et al. Tumor associated macrophages and neutrophils in cancer. Immunobiology. 2013;218:1402–10. doi: 10.1016/j.imbio.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Galizia G, Lieto E, Zamboli A, et al. Neutrophil to lymphocyte ratio is a strong predictor of tumor recurrence in early colon cancers: A propensity score-matched analysis. Surgery. 2015;158:112–20. doi: 10.1016/j.surg.2015.02.006. [DOI] [PubMed] [Google Scholar]
- Ghooshkhanei H, Treglia G, Sabouri G, et al. Risk stratification and prognosis determination using 18F-FDG PET imaging in endometrial cancer patients: a systematic review and meta-analysis. Gynecol Oncol. 2014;132:669–76. doi: 10.1016/j.ygyno.2014.01.039. [DOI] [PubMed] [Google Scholar]
- Greten FR, Grivennikov SI. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity. 2019;51:27–41. doi: 10.1016/j.immuni.2019.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D, Jin F, Jing W, et al. Incorporation of the SUVmax Measured From FDG PET and Neutrophil-to-lymphocyte Ratio Improves Prediction of Clinical Outcomes in Patients With Locally Advanced Non-small-cell Lung Cancer. Clin Lung Cancer. 2019;20:412–9. doi: 10.1016/j.cllc.2019.06.008. [DOI] [PubMed] [Google Scholar]
- Hess S, Hansson SH, Pedersen KT, et al. FDG-PET/CT in Infectious and Inflammatory Diseases. PET Clin. 2014;9:497–519. doi: 10.1016/j.cpet.2014.07.002. [DOI] [PubMed] [Google Scholar]
- Jeong E, Hyun SH, Moon SH, et al. Relation between tumor FDG uptake and hematologic prognostic indicators in stage I lung cancer patients following curative resection. Medicine (Baltimore) 2017;96:e5935. doi: 10.1097/MD.0000000000005935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd EA, Spencer CR, Huettner PC, et al. Cervical cancer histology and tumor differentiation affect 18F-fluorodeoxyglucose uptake. Cancer. 2009;115:3548–54. doi: 10.1002/cncr.24400. [DOI] [PubMed] [Google Scholar]
- Kinoshita O, Kishimoto M, Murayama Y, et al. Poorly differentiated clusters with larger extents have a greater impact on survival: a semi-quantitative pathological evaluation for 239 patients with non-mucinous pT2-3 colorectal carcinoma. World J Surg Oncol. 2015;13:140. doi: 10.1186/s12957-015-0550-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J-H, Lee HS, Kim S, et al. Prognostic significance of bone marrow and spleen 18F-FDG uptake in patients with colorectal cancer. Sci Rep. 2021a;11:12137. doi: 10.1038/s41598-021-91608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JW, Baek MJ, Ahn TS, et al. Fluorine-18-fluorodeoxyglucose uptake of bone marrow on PET/CT can predict prognosis in patients with colorectal cancer after curative surgical resection. Eur J Gastroenterol Hepatol. 2018;30:187–94. doi: 10.1097/MEG.0000000000001018. [DOI] [PubMed] [Google Scholar]
- Lee JY, Yoon SM, Kim JT, et al. Diagnostic and prognostic value of preoperative (18)F-fluorodeoxyglucose positron emission tomography/computed tomography for colorectal cancer: comparison with conventional computed tomography. Intest Res. 2017;15:208–14. doi: 10.5217/ir.2017.15.2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MI, Jung YJ, Kim DI, et al. Prognostic value of SUVmax in breast cancer and comparative analyses of molecular subtypes: A systematic review and meta-analysis. Medicine (Baltimore) 2021b;100:e26745. doi: 10.1097/MD.0000000000026745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSorley ST, Khor BY, Tsang K, et al. The relationship between (18) F-FDG-PETCT-derived markers of tumour metabolism and systemic inflammation in patients with recurrent disease following surgery for colorectal cancer. Colorectal Dis. 2018;20:407–15. doi: 10.1111/codi.13973. [DOI] [PubMed] [Google Scholar]
- Mirili C, Guney IB, Paydas S, et al. Prognostic significance of neutrophil/lymphocyte ratio (NLR) and correlation with PET-CT metabolic parameters in small cell lung cancer (SCLC) Int J Clin Oncol. 2019;24:168–78. doi: 10.1007/s10147-018-1338-8. [DOI] [PubMed] [Google Scholar]
- Mochizuki T, Tsukamoto E, Kuge Y, et al. FDG Uptake and Glucose Transporter Subtype Expressions in Experimental Tumor and Inflammation Models. J Nucl Med. 2001;42:1551–5. [PubMed] [Google Scholar]
- Na SJ, Chung Y-A, Maeng L-s, et al. Comparison between FDG Uptake and Pathologic or Immunohistochemical Parametersin Pre-operative PET/CT Scan of Patient with Primary Colorectal Cancer. Nucl Med Mol Imaging. 2009;43:557–64. [Google Scholar]
- Ocana A, Nieto-Jiménez C, Pandiella A, et al. Neutrophils in cancer: prognostic role and therapeutic strategies. Mol Cancer. 2017;16:137. doi: 10.1186/s12943-017-0707-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Itabashi M, Kondo C, et al. Prognostic Value of Total Lesion Glycolysis Measured by 18F-FDG-PET/CT in Patients with Colorectal Cancer. Anticancer Res. 2015;35:3495–500. [PubMed] [Google Scholar]
- Paesmans M, Berghmans T, Dusart M, et al. Primary Tumor Standardized Uptake Value Measured on Fluorodeoxyglucose Positron Emission Tomography Is of Prognostic Value for Survival in Non-small Cell Lung Cancer: Update of a Systematic Review and Meta-Analysis by the European Lung Cancer Working Party for the International Association for the Study of Lung Cancer Staging Project. J Thor Oncol. 2010;5:612–9. doi: 10.1097/JTO.0b013e3181d0a4f5. [DOI] [PubMed] [Google Scholar]
- Rebuzzi SE, Pesola G, Martelli V, et al. Adjuvant Chemotherapy for Stage II Colon Cancer. Cancers (Basel) 2020;12:2584. doi: 10.3390/cancers12092584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S, Yoh T, Morino K, et al. The Relationship Between (18)F-FDG Uptake on PET/CT and Markers of Systemic Inflammatory Response in Patients Undergoing Surgery for Intrahepatic Cholangiocarcinoma. Anticancer Res. 2019;39:341–6. doi: 10.21873/anticanres.13117. [DOI] [PubMed] [Google Scholar]
- Shaul ME, Fridlender ZG. Cancer-related circulating and tumor-associated neutrophils – subtypes, sources and function. FEBS J. 2018;285:4316–42. doi: 10.1111/febs.14524. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Goding Sauer A, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145–64. doi: 10.3322/caac.21601. [DOI] [PubMed] [Google Scholar]
- Sürücü E, Demir Y, Şengöz T. The correlation between the metabolic tumor volume and hematological parameters in patients with esophageal cancer. Ann Nucl Med. 2015;29:906–10. doi: 10.1007/s12149-015-1020-4. [DOI] [PubMed] [Google Scholar]
- Templeton AJ, McNamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- Ueno H, Kajiwara Y, Shimazaki H, et al. New Criteria for Histologic Grading of Colorectal Cancer. Am J Surg Pathol. 2012;36:193–201. doi: 10.1097/PAS.0b013e318235edee. [DOI] [PubMed] [Google Scholar]
- Wang L, Bai J, Duan P. Prognostic value of 18F-FDG PET/CT functional parameters in patients with head and neck cancer: a meta-analysis. Nucl Med Commun. 2019;40:361–9. doi: 10.1097/MNM.0000000000000974. [DOI] [PubMed] [Google Scholar]
- Werner J, Strobel K, Lehnick D, et al. Overall Neutrophil-to-Lymphocyte Ratio and SUV(max) of Nodal Metastases Predict Outcome in Head and Neck Cancer Before Chemoradiation. Front Oncol. 2021;11:679287. doi: 10.3389/fonc.2021.679287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Li Y, Hu S, et al. The significant value of predicting prognosis in patients with colorectal cancer using (18)F-FDG PET metabolic parameters of primary tumors and hematological parameters. Ann Nucl Med. 2019;33:32–8. doi: 10.1007/s12149-018-1299-z. [DOI] [PubMed] [Google Scholar]
- Yin YX, Xie MZ, Liang XQ, et al. Clinical Significance and Prognostic Value of the Maximum Standardized Uptake Value of (18)F-Flurodeoxyglucose Positron Emission Tomography-Computed Tomography in Colorectal Cancer. Front Oncol. 2021;11:741612. doi: 10.3389/fonc.2021.741612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D, Wang L, Zhang H, et al. Prognostic value of 18F-FDG-PET/CT parameters in patients with pancreatic carcinoma: A systematic review and meta-analysis. Medicine. 2017;96:e7813. doi: 10.1097/MD.0000000000007813. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Participants of this study did not agree for their data to be shared publicly, sharing the data is not available.