Abstract
Objective:
In this research we evaluated molecular mechanism of effect of metformin in radio sensitivity of breast cancer cells.
Methods:
This research was done in cellular and molecular research center of Qazvin university of Medical science in 1399 to 1401. Studied samples were two breast cancer cell lines (MCF-7 and MDA-MB-231) they are derived from primary and secondary tumors resected from a single patient. We exposed them to cumulative 50 Gy radiation and constructed radio resistant cell lines. Then resistant cell lines were treated with 50µm of metformin. Our studied groups were resistant cells treated and un treated with metformin. Then, the expression rate of miR-21-5p and SESN1 gene in resistant and control cells was checked by Quantitative Real-time PCR(qRTPCR). After the cell lines were treated with different concentrations of metformin at different intervals, the rate of cell proliferation and cell death was checked by CCK-8 assay and flow cytometry. The Western blot method was also used to confirm the expression of some genes.
Results:
Our results showed that the expression of miR-21-5p was upregulated in radiation-resistant cancer cells (1.8±0.65) (P<0.0001) MCF-7 cell line and (1.6±0.42)(P<0.001) MBA-MD-231 cell line, while the expression of SESN1 was down regulated (0.46±0.12) (P<0.0001) MCF-7 cell line and (0.42±0.22) (P<0.001) MBA-MD-231 cell line. Metformin enhanced the radio sensitivity of breast cancer cells in a dose and time-dependent manner. Also, metformin treatment decreased the expression of miR-21-5p (0.47±0.32) (P<0.0001) MCF-7 Cell line and (0.45±0.21)(P<0.001) MBA-MD-231 cell line and increased the expression of SESN1 (1.65±0.72)(P<0.0001)MCF-7 cell line and (1.73±0.52)(P<0.0001) MBA-MD-231 cell line. The function of metformin was reversed by miR-21-5p inhibitors or the transfection of SESN1 overexpressing plasmids.
Conclusion:
In conclusion, based on this research results, metformin enhanced the radio sensitivity of breast cancer cells via modulating the expression of miR-21-5p and SESN1.
Key Words: miR-21-5p, SESN1 gene, breast cancer, metformin, radio resistant
Introduction
Radiotherapy is one) is the most widely used drug to treat type 2 diabetic patients. Metformin lowers blood glucose levels by suppressing gluconeogenesis in the liver and increasing glucose uptake by skeletal muscle. Metformin decreases blood insulin levels, increases insulin sensitivity, of the treatment methods for breast cancer patients. In this method, the radiation causes damage to the DNA of cancer cells and does not allow the cells to proliferate. Still, in many cases, we see the resistance of cancer cells to radiation, and many factors are involved in this regard (Younget al., 2018). Therefore, we must first understand the molecular mechanisms of this radiation resistance and look for the use of drugs to reduce this resistance. Metformin (1, 1-dimethyl biguanidehydrochlorideincreases free fatty acid utilization, and suppresses the synthesis of proteins, fatty acids, and cholesterol(Witters, 2005).In addition, metformin has been shown in the last several years to possess a strong anti-cancer effect (Dowling et al., 2001; Gonzalez-Angulo et al., 2010; Micic et al., 2011; Alimova et al., 2009). The anti- cancer effects of metformin have been reported in various studies, and its molecular mechanism is the activation of Adenosine monophosphate kinase (AMPK) followed by the inhibition of the mammalian target of rapamycin (mTOR), which prevents the growth and proliferation of cancer cells and leads cancer cells to apoptosis (Rochaet al., 2009). Additionally, it was shown that metformin could enhance the radio sensitivity of tumor cells, such as breast cancer cells, but the exact mechanism of this sensitivity is not fully specified (Chevalieret al., 2020; Samsuriet al., 2017; Zhanget al., 2014).
MicroRNAs (miRNAs/miRs) are a class of non-coding and highly conserved small RNAs containing 19–25 nucleotides that can bind to the 3’untranslated region (3’-UTR) of their target genes to modulate their expression at the post-transcriptional/translation level. Different studies have shown miRNAs play an essential role in regulating the sensitivity of tumor cells to radiotherapy,the miR-15 family and miR-16 are reported to modulate the radio sensitivity of diverse human tumors such as breast cancer, cervical cancer, and non-small cell lung cancer (NSCLC) (Tomasik et al., 2018; Lan et al., 2015; Mei et al., 2015; Wu et al., 2018). Moreover, the role of miR-21-5p in regulating the radio sensitivity of NSCLC cells was shown (Songet al., 2017). Sesterni1(SESN1) is a member of the growth arrest, and DNA damage inducible gene family that are involved in different pathways, such as the AMPK/mTORC1. It is a tumor suppressor and ubiquitously expressed in human tissues, mainly in skeletal muscle, heart, liver, and brain (Pashaet al., 2017). Moreover, it is a common challenge for cells exposed to toxic agents, including ionizing radiation and the overproduction of highly reactive molecules such as reactive oxygen species (ROS). SESN1 and SESN2 can respond to genotoxic stress in a p53-dependent manner (Sunet al., 2020 ). Bioinformatics analysis in this research predicted a potential binding site between miR-21-5p and SESN1 mRNA, suggesting that miR-21-5p may regulate the radio sensitivity of breast cancer cells via modulating SESN1 expression. For the first time, it was proven that metformin enhanced the radio sensitivity of breast cancer cells via modulating the miR-21-5p/SESN1 axis. It is expected that the findings of this study may help to improve the therapeutic effect of radiotherapy in the treatment of breast cancer patients.
Materials and Methods
Cell culture
Human Breast cell lines (MCF-7 and MDA-MB-231) were purchased from the Cell Bank of Institute Pasteur (Tehran, IRAN). Cell culture was done in a 37º incubator containing 5% CO2. Cells were cultured in RPMI-1640 (Gibco, Germany) with 10% heat-inactivated fetal bovine serum (Gibco, Germany), 100 U/ml penicillin, and 100 μg/ml streptomycin (Gibco, Germany), metformin treatment was done with 25, 50 and 100 µm concentration in 12, 24,48,72 and 96 hours interval.
Resistance of cells to radiation
To produce radiation-resistant cell lines, the studied cell lines (MCF-7 and MDA-MB-231) were irradiated consecutively with a low dose of 2 Gy using a 6-MV photon beam produced by a linear accelerator (Clinac 21EX, Varian Medical Systems, Inc., Palo Alto, CA) until the cumulative dose reached 50 Gy. A dose of 50 Gy is used in the radiation of patients with breast cancer. Cells were trypsin zed and subcultured into new flasks when the cells reached ~90% confluence; then, when the cells reached ~70% confluence, they were irradiated again.
Cell transfection
In this regard, the negative control miRNA (miR-NC), miR-21-5p mimics, miR-21-5p inhibitor, SESN1-overexpressing plasmid, empty plasmid, SESN1short interference RNA (siRNA), and control siRNA were purchased (Exiqon, Denmark). Lipofectamine 3000 was used to transfect cells according to the manufacturer’s instructions (Invitrogen; Thermo Fisher Scientific, Inc.). Quantitative real-time PCR (qRT-PCR) was used to evaluate transfection efficiency.
RNA extraction and qRT-PCR
We used TRIZOL reagent to isolate the total RNA from the cells based on kit protocols (Invitrogen Life Technology Co, USA). Our primer sequences were : SESN1 F:5′-GCGACCAGGACGAGGAACTT-3′, R:5′-TGCATCTGTGCGTCTTCACT-3′; miR21-5p F:5′-GGGGGGTAGCTTATCAGACTG-3′, R:5′-CAGTGCGTGTCGTGGAGT-3′, GAPDH(Internal control)F: 5′-TCAAGAAGGTGGTGAAGCAG-3′, R: 5′-CGCTGTTGAAGTCAGAGGAG-3′,miR30a-5p (Internal control) miR-30a-5p forward, 5′-AACGAGACGACGACAGAC-3′ and reverse, 5′-TGTAAACATCCTCGACTGGAAG-3′.Subsequently, real-time quantification was performed using Rotor gene-Q real-time PCR system (Qiagen, Germany). Each real-time PCR (10 µl) included 1 µl of reverse and forward primers (Exiqon, Denmark), 5 µl of Ampliqon real Q plus 2x master mix green (Ampliqon, Denmark), and 4 µl of diluted cDNA. The reactions were incubated in a 72-well optical strip at 95°Cfor 15 min (enzyme activation), followed by 95°C for 20 s and 60°Cfor 60 s (40 cycles); all reactions were run in triplicate. After the reactions, the mean Ct was determined from the triplicate PCRs. We used Ct values to evaluate the expression levels of the three miRNAs. The expression value of miRNAs relative to internal controls was determined using the 2-ΔCt method.
Analysis of apoptosis
Flow cytometry was used for the evaluation of apoptosis. In this method, two dye was used to distinguish apoptotic from necrotic cells (Annexin V-FITC and propidium iodide (PI)). Apoptotic cells were Annexin V-FITC-positive, PI-positive, and necrotic cells were Annexin V-FITC negative, PI-positive. To do this process, 1.5 × 105 cells/well of MCF-7 and MDA-MB-231 cells were plated in 6-multiwell plates with or without 50µM metformin. After 48 h, cells were detached and analyzed by flow cytometry method. The experiment was carried out according to the Life Technologies Apoptosis Assay protocol, and the samples were analyzed by flow cytometry within 1 hour using the FACS Caliber (Becton Dickinson, CA) in triplicate. Dot plot graphs were used to illustrate the viable cells (the lower left quadrant), early phase apoptotic cells (the lower right quadrant), late phase apoptotic or dead cells (the upper right quadrant), and the necrotic cells (the upper left quadrant).
Western blotting method
Cultured MCF-7 and MDA-MB-231 cells with 50µm metformin and without metformin centrifuged. Total protein was extracted from the HUVECs using lysis buffer (Beyotime Institute of Biotechnology). Total protein was quantified using a bicinchoninic acid kit (Beyotime Institute of Biotechnology). Subsequently, 40 µg of total protein was loaded per lane on a 10% SDS-PAGE gel, electroblotted onto polyvinylidenedifluoride membranes (Thermo Fisher Scientific, Inc.) and blocked using 5% skimmed milk (MilliporeSigma) for 1 h at room temperature. The membranes were then incubated overnight at 4°C with the following primary antibodies: Anti-SESN1 (1:1,000 dilution; cat. no. ab134091), Anti-PAMPK (1:1,000 dilution; cat. No. ab 2533585), Anti-mTOR (1:1,000 dilution; cat. No. ab 325371), Anti-Beta actin(1:1,000 dilution; cat. No. ab 119716)(Abcam,USA).
CCK-8 assay
MCF-7 and MDA-MB-231 cells were harvested in a logarithmic growth phase. The cell density was adjusted to 1 × 103/ml and incubated into 96 well plates (100 μL of cell suspension per well). The 96 well plates were then placed in an incubator to continue the culture. Then, 10 μL of CCK-8 reagent (Dojindo, Japan) were added to the wells at 12, 24, 48, and 72 hr, followed by 2 hr of culture. Subsequently, the absorbance in each well examined at 450 nm using a microplate reader group of experiments was repeated three times.
Dual luciferase reporter gene assay
In this regard we used Luciferase Enhancer Reporter Vector to cloning of wild type or mutant type SESN1 3’ UTR into pGL-3 (E1771,Promega, Madison, WI). The cells were then seeded into 24-well plates at a density of 5,000 cells per well. Then miR-21-5p mimics or negative control miRNA were co transfected in to the cell lines and after 48h cells were collected. Ultimately, luciferase activity was determined using a dual luciferase reporter system (Promega).
Statistical analysis
Data analysis was done with Graph Pad Prism 7 (Graph Pad PRISM V 5.04 analytical software). All data were expressed as mean ± SD. The mean differences between the two groups were analyzed by Student’s t-test and more groups by one-way analysis of variance (ANOVA). The difference was statistically significant at P < 0.05.
Results
Comparison of the expression ratio of miR-21-5pand SESN1 in resistant and nonresistant cell lines
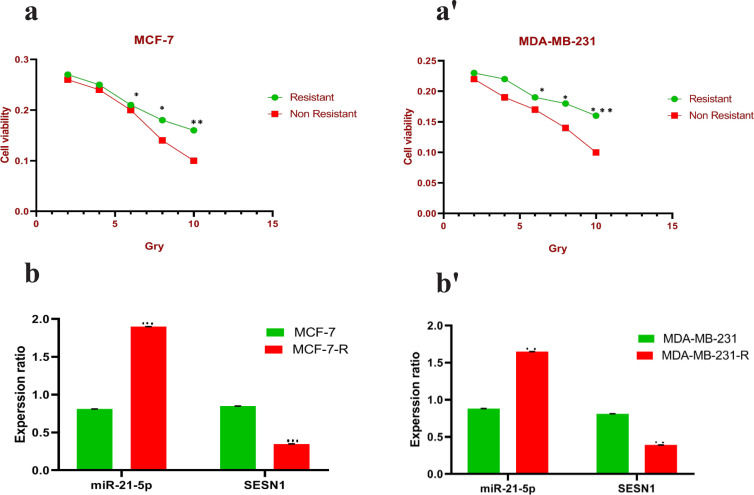
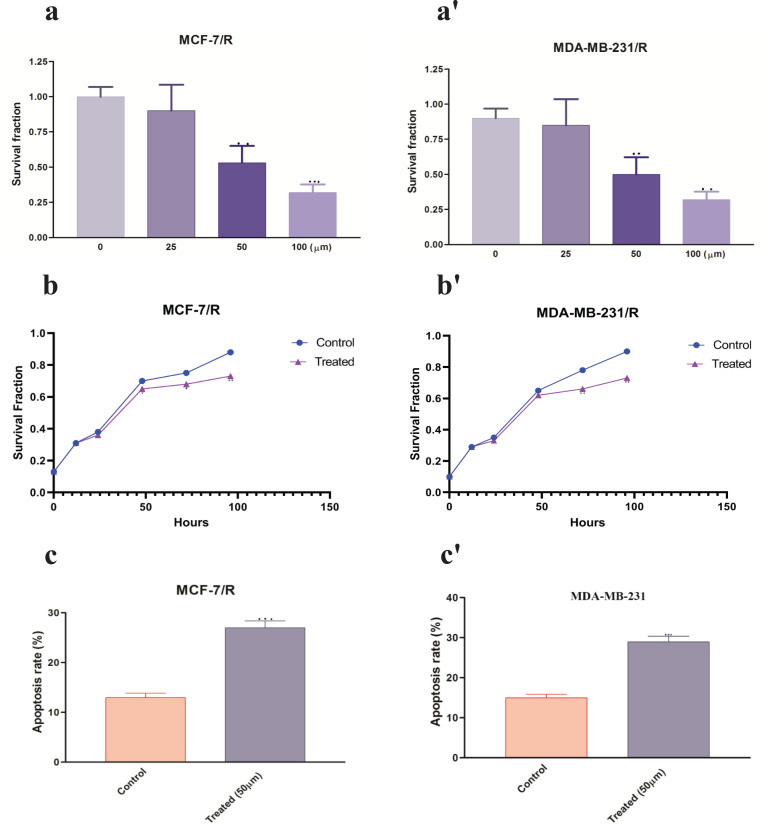
After the resistance of cell lines (MCF-7 and MDA-MB-231) against radiation, the survival rates of resistant and control cells against different doses of radiation were investigated. The results showed that the survival rate against different doses of radiation in resistant cells is higher than in control cells (Figure 1a-a’). The expression ratio of miR-21-5p and SESN1 was evaluated in resistant and control cell lines. Our results showed significant up regulation of miR-21-5p(1.8±0.65)(P<0.0001) in MCF-7 cell line , (1.6±0.42)(P<0.001) in MBA-MD-231 cell line, and down regulation of the SESN1 gene in resistant cell lines in comparison to controls (0.46±0.12)(P<0.0001)in MCF-7 cell line and (0.42±0.22)(P<0.0001) in MBA-MD-231 cell line (Figure 1b-b’).
Figure 1.
After Radiosensitive Cell Lines were Constructed (a-a'): CCK-8 assay was used to confirm the successful construction of radio-resistant cell lines exposed to different doses of radiation (b-b'): Shows significant differential expression of miR-21-5p and SESN1 gene between resistant and sensitive cell lines. *p<0.05 **p < 0.01 and ***p < 0.001
MiR-21-5p directly targeted SESN1 gene
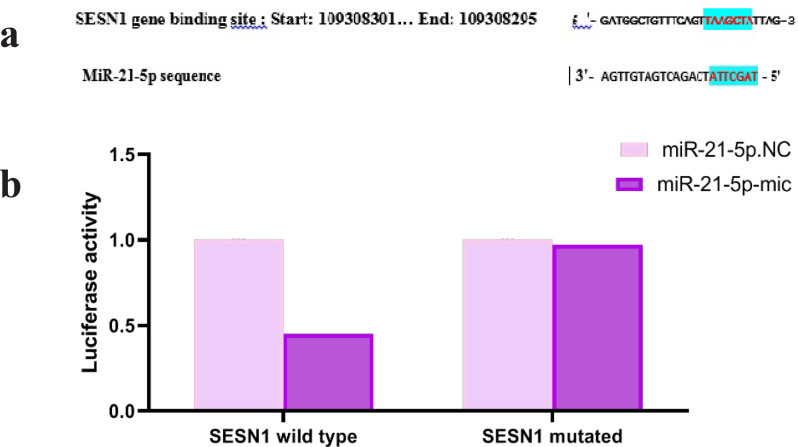
Target scan analysis results showed SESN1 gene has one binding site for miR-21-5p (Figure 2a). In this regard we used a luciferase reporter assay to verify this prediction.MiR-21-5p mimics notably repressed the luciferase activity of 293T cells transfected with SESN1-WT vectors, but miR-21-5p mimics had not significant effect on the luciferase activity of SESN1-MUT vectors (Figure 2b).
Figure 2.
MiR-21-5p Directly Targeted SESN1. (a) Shows potential binding site for miR-21-5p and SESN1 (Target Scan Release 7.2) . (b) Luciferase activity was analyzed in 293T cells co transfected with SESN1-WT or SESN1-MUT in conjunction with miR-21-5p or miR-NC. **p < 0.01, *** p < 0.001
miR-21-5p/SESN1 axis involve in resistance of cell lines
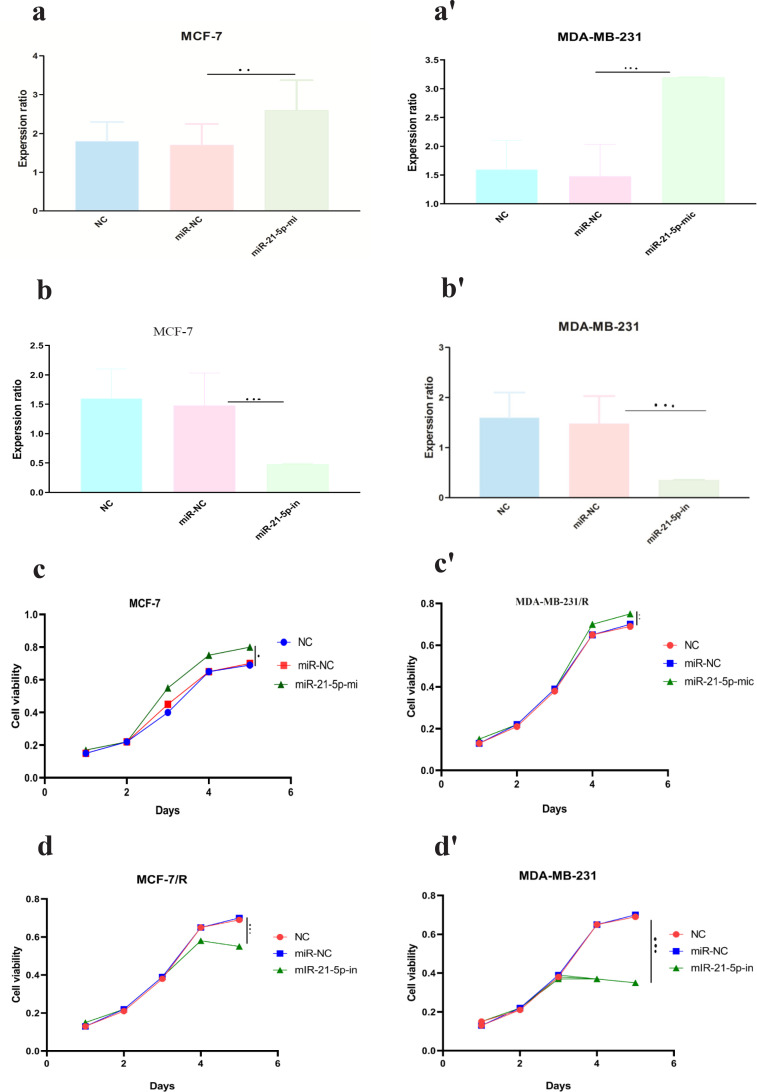
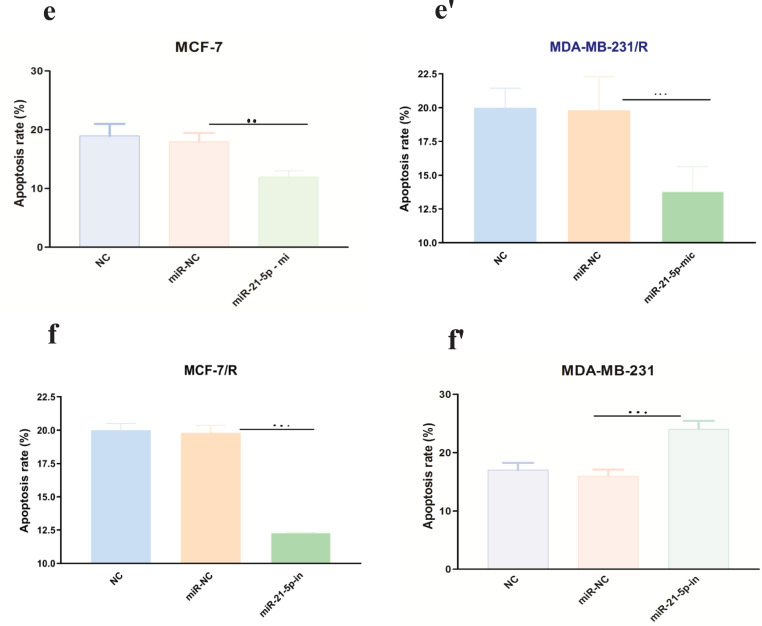
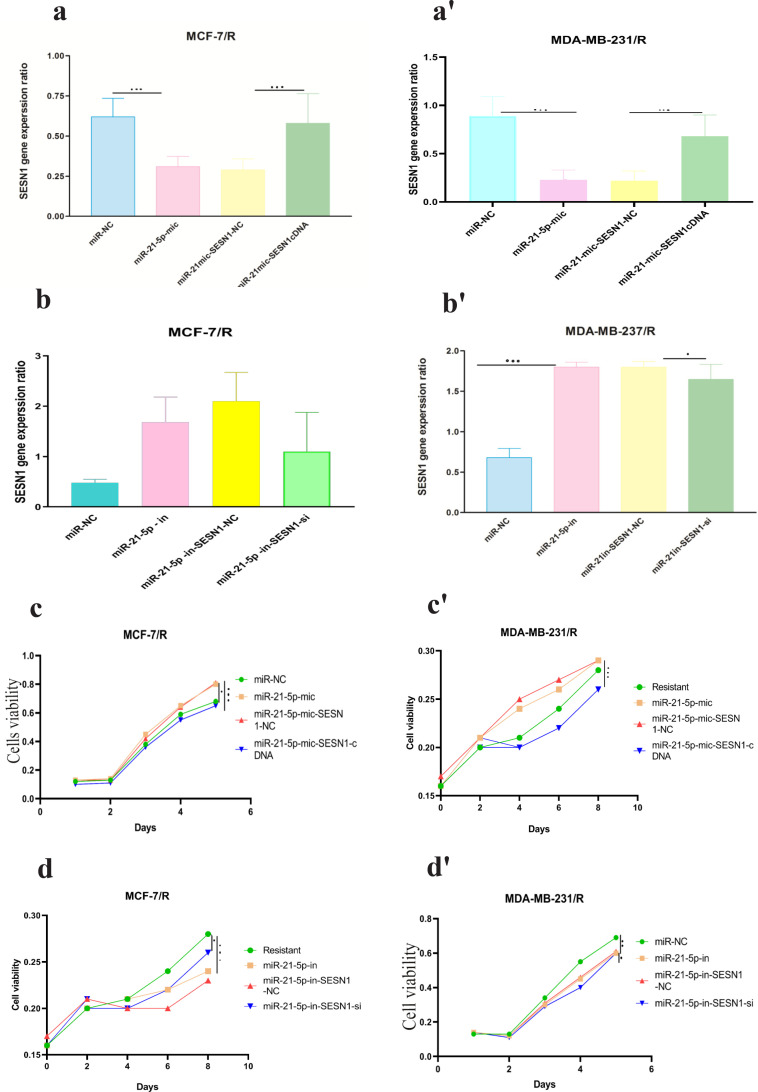
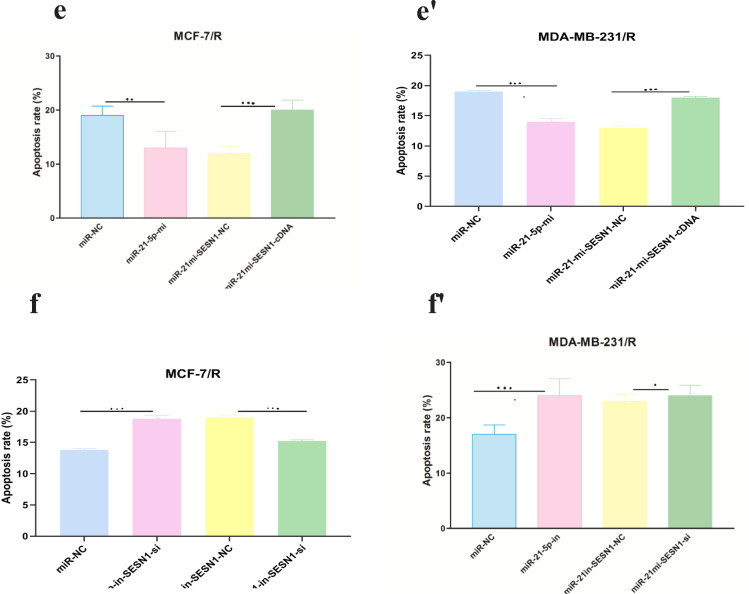
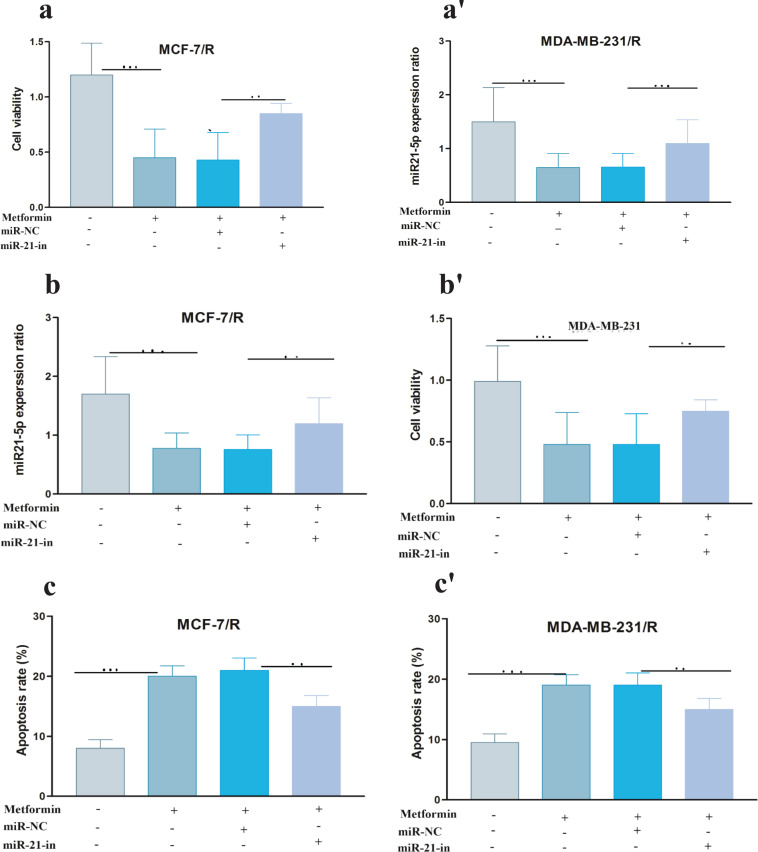
Mimics and inhibitors of miR-21-5p were transfected into MCF-7/R and MDA-MB-231/R cells, to further confirm whether the miR-21-5p / SESN1 axis plays a role in the resistance of cells to radiation and expression of miR-21-5p was evaluated by qRT-PCR. Our findings showed up regulation of this miRNA in transfected cells in comparison to un transfected cells (2.4±0.98) (P<0. 001) in MCF-7 cell line, (3.2±0.85) (P<0.0001) in MBA-MD-231 cell line (Figure 3a-a’, b-b’). Also, the viability and apoptosis of the cells were evaluated. The results showed that miR-21-5p mimic increased the survival rate of resistant cells in comparison to un transfected cells (0.85±0.24vs 0.6±0.12)( P<0.05) in MCF-7/R cell line and (0.79±0.24 vs 0.6±0.12)( P<0.001) in MDA-MB-231/R cell line. Apoptosis rate was decreased in transfected cells in comparison to non transfected cells (13% vs 19%)(P<0.001) in MCF-7/R cell line and (13.2% vs 18%) (P<0.0001) in MDA-MB-231/R cell line. In the cells with miR-21-5p inhibitor the results showed down regulation of miR-21-5p in transfected cells in comparison to un transfected cells (0.39±0.12)(P<0. 0001) MCF-7 cell line, (0.31±0.11) (P<0.0001) MBA-MD-231 cell line. Also we observed viability decreasing in transfected cells in comparison to un transfected cells (0.48±0.12 vs 0.69±0.31) (P<0.0001) in MCF-7/R cell line and (0.39±0.22 vs 0.68±0.42) (P<0.0001) MBA-MD-231 cell line and increasing of apoptosis(19.3% vs 13.2%)(P<0.0001) in MCF-7/R cell line and (24% vs 13.8%) MBA-MD-231 cell line (P<0.0001) (Figure 3c-c’, d-d’ , e-e’,f-f’). In the next step, the studied cell lines were transfected with pcDNASESN1,miR-21-5p-mi and SESN1-siRNA , miR-21-5p-in. Our results showed co-transfection of miR-21-5p-mi and pcDNA-SESN1 significantly down regulated the expression level of SESN1 (0.39±0.12) (P<0.0001) in MCF/R cell line and (0.29±0.13) (P<0.0001)in MDA-MB-231 cell line, but not as much as when we used only miR-21-5p-mi (0.65±0.31) in MCF/R cell line and (0.71±0.34) in MDA-MB-231 cell line. Similarly, co-transfection of miR-21-5p-in and pcDNA-SESN1-si significantly upregulated the expression level of SESN1 (1.87±0.87) (P<0.0001) but not as much as when we used only miR-21-5p-in (Figure 4 a-a’, b-b’, c-c’, d-d’ , e-e’, f-f’). Based on the results, we conclude that miR-21-5p has an essential role in SESN1 expression regulation, and miR-21-5p/SESN1 is involved in the regulation of radio-resistance of breast cancer cells.
Figure 3.
miR-21-5p was Involved in Regulating Radioresistance miR-21-5p Mimics and Inhibitorswere Transfected into MCF-7/R and MDA-MB-231/R Cells, Successful Transfection was Confirmed by qRT-PCR (a-a' and b-b'). CCK-8 assay and flow cytometry were used to detect the proliferation and apoptosis of breast cancer cells, respectively(c-c', d-d' , e-e', f-f'). *p<0.05 **p < 0.01 and ***p < 0.001
Figure 3.
miR-21-5p was Involved in Regulating Radioresistance miR-21-5p Mimics and Inhibitorswere Transfected into MCF-7/R and MDA-MB-231/R Cells, Successful Transfection was Confirmed by qRT-PCR (a-a' and b-b'). CCK-8 assay and flow cytometry were used to detect the proliferation and apoptosis of breast cancer cells, respectively(c-c', d-d' , e-e', f-f'). *p<0.05 **p < 0.01 and ***p < 0.001
Figure 4.
miR-21-5p/SESN1 are Involved in Radio Sensitivity of Breast Cancer Cells .miR-21-5p mimics and pcDNA-SESN1 were co-transfected into MCF-7/R cells, while miR-21-5p inhibitors and si-SESN1 was transfected into MDA-MB-231/R cells, and successful transfection was confirmed by qRT-PCR. (a-a', b-b') CCK-8 assay and flow cytometry were used to detect the proliferation and apoptosis of breast cancer cells, respectively(c-c', d-d' , e-e', f-f'). *p<0.05, **p < 0.01 and ***p < 0.001
Figure 4.
miR-21-5p/SESN1 are Involved in Radio Sensitivity of Breast Cancer Cells .miR-21-5p mimics and pcDNA-SESN1 were co-transfected into MCF-7/R cells, while miR-21-5p inhibitors and si-SESN1 was transfected into MDA-MB-231/R cells, and successful transfection was confirmed by qRT-PCR. (a-a', b-b') CCK-8 assay and flow cytometry were used to detect the proliferation and apoptosis of breast cancer cells, respectively(c-c', d-d' , e-e', f-f'). *p<0.05, **p < 0.01 and ***p < 0.001
Metformin involved in radio sensitivity of breast cancer cell lines by underexpression of miR-21-5pand upregulation of SESN1
In the next step, the studied cell lines (MCF-7/R and MDA-MB-231/R) were treated with different doses of metformin at different times intervals. The results showed that this drug decreases cells viability and increase apoptosis rate of resistant cells in a dose and time-dependent manner (Figure 5 a-a´, b-b´, c-c´).
Investigating the function of metformin in increasing the radiation sensitivity of cells through modulation of the expression of miR-21-5p/SESN1
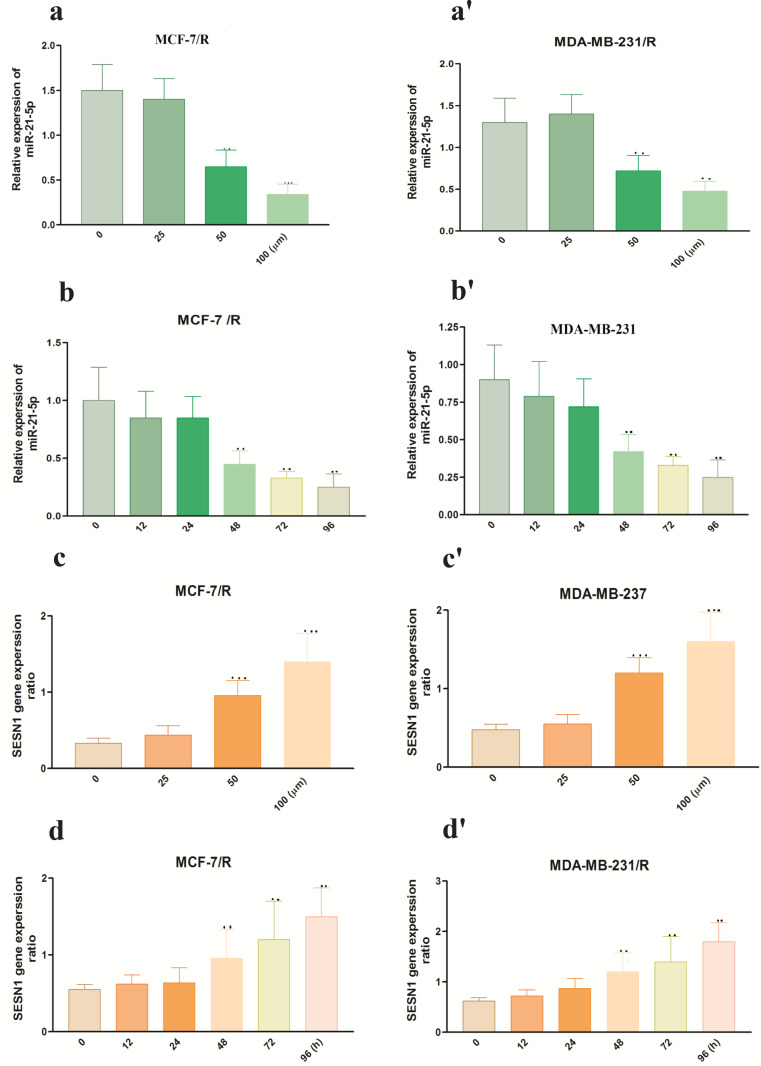
In order to investigate the modulation mechanism of metformin in radio sensitivity of breast cancer cells, we evaluated miR-21-5p and SESN1 expression in MCF-7/R and MDA-MB-231/R cells in different concentrations of metformin and different durations of metformin treatment. Results of qRT-PCR showed that 50 µm metformin after 48h caused a noticeable decrease in miR-21-5p (0.62±0.22) (P<0.001) MCF-7/R cell line, (0.68±0.13)(P<0.001) MBA-MD-231 cell line and increased SESN1 expression (1.52±0.22) (P<0.0001) MCF-7/R cell line, (1.68±0.33) (P<0.0001) MBA-MD-231 cell line (Figures 6, 7. a-a´, b-b´, c-c´, d-d´).
Figure 5.
Metformin Radio Sensitized, Radioresistant Breast Cancer Cells (a-a') After treatment with different concentrations of metformin, CCK-8 assay was used to detect breast cells viability after 48 hr. (b-b') CCK-8 assay was used to detect the cells viability of MCF-7/R and MDA-MB-231/R cells in different rate of miR-21-5p in MCF-7/R and MDA-MB-231/R cells were detected by qRT-PCR after treatment times after treatment with 50 μm metformin. (c-c') Apoptosis rate was detected 48 h after treatment with 50 µm metformin. *p<0.05, **p < 0.01 and ***p < 0.001
Evaluation function of metformin on SESN1 overexpression
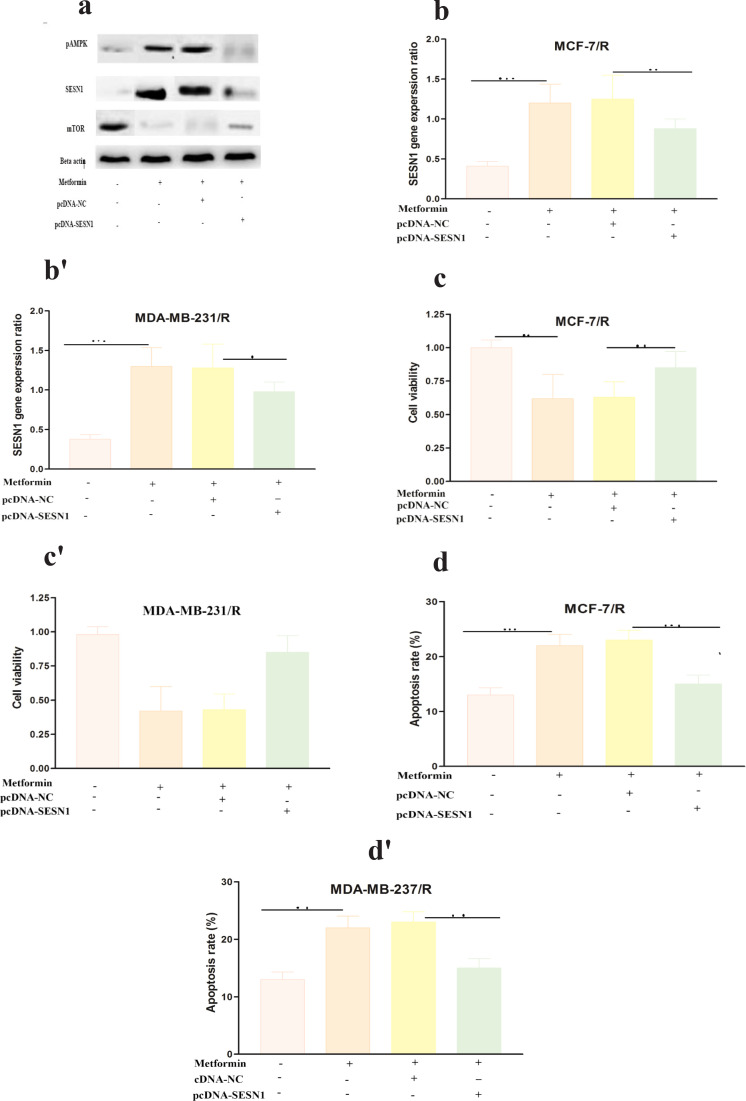
To detect whether SESN1 was involved in the mechanism of action of metformin, qRT-PCR and western blot results implied that 50 µm metformin after 48h significantly upregulated SESN1 expression in breast cancer cells (Figure 8 a , b-b´). Additionally, our western blot results showed that 50 µm metformin after 48h upregulated activated pAMPK and downregulated mTOR expression in the studied breast cancer cell lines (Figure 8. a). Accordingly, SESN1 overexpression affects cell viability and apoptosis (Figure 8c-c´, d-d´). These data indicated that the function of metformin in promoting breast cancer radio sensitivity was mediated by regulating the miR-21- 5p/SESN1 axis.
Figure 6.
Metformin Decreased the Expression of miR-21-5p and Increased the Expression of SESN1 in a Dose and Time-Dependent Manner. (a-a´) The expression with different concentrations of metformin for 48 h. (b-b') Shows the expression rate of miR-21-5p after MCF-7/R and MDA-MB-231/R cells were treated with 50 μm of metformin at different times (c-c') which shows the expression rate of SESN1 in MCF-7/R and MDA-MB-231/R cells after treatment with different concentrations of metformin for 48 hr. (d-d') Shows expression rate of SESN1 after MCF-7/R and MDA-MB-231/R cells were treated with 50 μm of metformin for different times. *p<0.05, **p < 0.01 and ***p < 0.001
Figure 7.
Inhibition of miR-21-5p Reversed the Function of Metformin. (a-a') shows the results of qRT-PCR after transfection of miR-21-5p inhibitor in studied cell lines (b-b') shows cells viability after transfection of miR-21-5p inhibitor in studied cell lines. (c-c') Shows apoptosis rate after transfection of miR-21-5p inhibitor in studied cell lines. **p < 0.01 and ****p < 0.001
Figure 8.
SESN1 Expression Reversed the Function of Metformin (a-a') Shows results of successful transfection in MCF-7 cells by western blot method. (b-b') Shows results of successful transfection in MCF-7 and MDA-MB-231 cells by QRT PCR method (c-c') Shows results of cell viability after transfection (d-d') Results of apoptosis rate after transfection **p < 0.01 and ****p < 0.001
Discussion
The anti-cancer effect of metformin in different cancers was shown (Linet al., 2014). In this research, we studied the effect of metformin on the radio sensitivity of breast cancer cell lines, and for the first time, we showed the radio sensitivity effect of metformin via modulating miR-21-5p and SESN1 expressions. Metformin is the most widely used drug by type 2 diabetic patients. Metformin reduces blood glucose levels by suppressing gluconeogenesis in the liver and increasing glucose uptake by skeletal muscle; furthermore, metformin increases the radio sensitivity of cancer cells (Safai et al., 2018). Based on other and this research western blot results, activation of AMPK and suppression of mTOR appeared to play an essential role in the cytotoxic and radio-sensitizing effect of metformin in vitro as well as in vivo (Rocha et al., 2011). But the exact molecular mechanism of AMPK activation and mTOR suppuration was unknown.Mounting evidence has revealed that miRNAs are also engaged in regulating cancer progression, including enhancing or reducing the radio sensitivity of cancer cells (Mei et al., 2015). The miRNA-21 is located at 17q23.2, vacuole membrane protein-1 (VMP1) coding region, the tenth intron of the VMP1 gene; studies have shown that some tumors have genomic 17q23.2 amplification mutations, such as breast and prostate cancer, and Hodgkin’s lymphoma. Overexpression of miRNA-21-5p has been confirmed in various cancers, such as breast, prostate, and gastric cancer(Li et al., 2009; Kumar et al., 2013; Li et al., 2012). Liu et al., (2021) showed a significant correlation of this miRNA expression between the breast tissue and plasma; they introduced miR-21-5p as a diagnostic biomarker in breast cancer(Liuet al. 2021). In another study, Li et al., (201) showed the effect of downregulation of miR-21-5p in radio sensitivity of esophageal squamous cell carcinoma (Liet al., 2018). Various studies have been shown that metformin and radiation, via the activation of AMPK and inhibition of mTOR, decrease the proliferation and viability of cancer cells. The combination of metformin and irradiation was more efficient than radiationonly (Rocha et al., 2011; Sanli et al., 2010). However, the precise molecular mechanism of this radio sensitivity and activation of AMPK is unknown. As mentioned in this research, the miR-21-5p/ SESN1 axis in radio sensitivity of metformin was studied, and we showed the significant effect of this axis on the activation of AMPK. SESN1 gene encodes a member of the sestrin family. Sestrins are induced by the p53 tumor suppressor protein and play a role in the cellular response to DNA damage and oxidative stress. The encoded protein mediates p53 inhibition of cell growth by activating AMPK protein kinase, which results in the inhibition of the mammalian target of mtTOR (Donaldson et al., 2017). Regarding the effect of metformin on miR-21-5p/ SESN1 in line with our results, Pulito et al., (2017) showed metformin causes downregulation of miR-21-5p and upregulation of SESN1 in breast cancer cells. Still, about the effect of metformin on radio sensitivity of breast cancer cells via this axis, there has been no report so far. In conclusion, for the first time, we showed that metformin could cause radio sensitivity, increase apoptosis, decrease cancer cell viability, activate AMPK, and suppurate mTOR in breast cancer cells via modulating the SESN1/miR-21-5p axis. This work provides new potential strategies to improve the efficacy of radiotherapy in breast cancer patients.
Author Contribution Statement
Participated in research design: Dr Sahar Moghbelinejad, Dr Fatemeh Saffari. Conducted experiments: Abdolmabood Momeni, Mehrzad Ramezani. Contributed new reagents or analytic tools: Dr Fatemeh Saffari, Abdolmabood Momeni. Performed data analysis: Zohreh Estaki, Yasamin Ansari. Wrote or contributed to the writing of the manuscript: Dr Sahar Moghbelinejad, Zohreh Estaki.
Acknowledgements
We appreciate all of the participants in current study. Also authors would like to thank Fonateam translation and localization company for the English languish edition.
Found
This research was supported by cellular and molecular research center Qazvin university of medical science no. 83411.
Data Availability statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author.
Ethical approve
This research was approved by Ethical committee of Qazvin university of medical science IR.QUMS.REC.1400.410.
Disclosure statement
The authors declare no conflicts of interest for this work.
References
- Alimova IN. Metformin inhibits breast cancer cell growth, colony formation and induces cell cycle arrest in vitro. Cell Cycle. 2009;8:909–15. doi: 10.4161/cc.8.6.7933. [DOI] [PubMed] [Google Scholar]
- Chevalier B, Pasquier D, Lartigau EF, et al. Metformin: (future) best friend of the radiation oncologist? Radio Ther Oncol. 2020;151:95–105. doi: 10.1016/j.radonc.2020.06.030. [DOI] [PubMed] [Google Scholar]
- Dowling R JO, Goodwin RJ, Stambolic V. Understanding the benefit of metformin use in cancer treatment. BMC Med. 2001;9:33. doi: 10.1186/1741-7015-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson MC, Katanayeva N, Oricchio ES. A tumor suppressor that can be rescued. Mol Cell Oncol. 2017;21:1365107. doi: 10.1080/23723556.2017.1365107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Angulo AM, Meric-Bernstam F. Metformin: A therapeutic opportunity in breast cancer. Clin Cancer Res. 2010;16:1695–1700. doi: 10.1158/1078-0432.CCR-09-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Keerthana R, Pazhanimuthu A, Perumal P. Over expression of circulating miRNA-21 and miRNA-146a in plasma samples of breast cancer patients. Indian J Biochem Biophys. 2013;50:210–4. [PubMed] [Google Scholar]
- LanF, Yue X, RenG, et al. miR-15a/16 enhances radiation sensitivity of non-small cell lung cancer cells by targeting theTLR1/NF-κ B signaling pathway. Int J Radiat Oncol Biol Phys. 2015;91:73–81. doi: 10.1016/j.ijrobp.2014.09.021. [DOI] [PubMed] [Google Scholar]
- Lin HC, Kachingwe BH, Lin HL, et al. Effects of metformin dose on cancer risk reduction in patients with type 2 diabetes mellitus: a 6-year follow-up study. Pharmacotherapy. 2014;34:36–45. doi: 10.1002/phar.1334. [DOI] [PubMed] [Google Scholar]
- Li T, Li D, Sha J, Sun P, Huang Y. MicroRNA-21 directly targets MARCKS and promotes apoptosis resistance and invasion in prostate cancer cells. Biochem Biophys Res Commun. 2009;383:280–5. doi: 10.1016/j.bbrc.2009.03.077. [DOI] [PubMed] [Google Scholar]
- Li BS, Zhao YL, Guo G, Li W, Zhu ED, Luo X, et al. Plasma micro-RNAs, miR-223, miR-21 and miR-218, as novel potential biomarkersfor gastric cancer detection. PLoS One. 2012;7:e41629. doi: 10.1371/journal.pone.0041629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Mo F, Song X, He Y, Yuan Y, Yan J, Yang Y, Huang J, Zhang S. Exosomal hsa-miR-21-5p is a biomarker for breast cancer diagnosis. PeerJ. 2021;17:e12147. doi: 10.7717/peerj.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei Z, Su T, Ye J, Yang C, Zhang S, Xie C. The miR-15 family enhances the radio sensitivity of breast cancer cells by targeting G2 checkpoints. Radiat Res. 2015;183:196–207. doi: 10.1667/RR13784.1. [DOI] [PubMed] [Google Scholar]
- Micic D, CvijovicG, Trajkovic V, Duntas L H, Polovina S. Metformin: Its emerging role in oncology. Hormones. 2011;10:5–15. doi: 10.14310/horm.2002.1288. [DOI] [PubMed] [Google Scholar]
- Mei Z, Su T, Ye J, Yang C, Zhang S, Xie C. The miR-15 family enhances the radio sensitivity of breast cancer cells by targeting G2 checkpoints. Radiat Res . 2015;183:196–207. doi: 10.1667/RR13784.1. [DOI] [PubMed] [Google Scholar]
- Pasha M, Eid AH, Eid AA, Gorin Y, Munusamy S. Sestrin2 as a novel biomarker and therapeutic target for various diseases. Oxid Med Cell Longev. 2017;2017:3296294. doi: 10.1155/2017/3296294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulito C, Mori F, Sacconi A, Goeman F, Ferraiuolo M, Pasanisi P. Metformin-induced ablation of microRNA 21-5p releases Sestrin-1 and CAB39L antitumoral activities. Cell Discov. 2017;4:17022. doi: 10.1038/celldisc.2017.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha, G. et al. Metformin amplifies chemotherapy-induced AMPK activation and anti tumoral growth. Clin C ancer Res. 2011;17:3993–4005. doi: 10.1158/1078-0432.CCR-10-2243. [DOI] [PubMed] [Google Scholar]
- Safai N, Suvitaival T, Ali A, Spégel P, Al-Majdoub M. Effect of metformin on plasma metabolite profile in the Copenhagen Insulin and Metformin Therapy (CIMT) trial. Diabet Med. 2018;35:944–953. doi: 10.1111/dme.13636. [DOI] [PubMed] [Google Scholar]
- Sanli, T. et al. Ionizing radiation activates AMP-activated kinase (AMPK): a target forradiosensitization of human cancer cells. Int J Radiat Oncol BiolPhys. 2010;78:221–229. doi: 10.1016/j.ijrobp.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Samsuri NAB, Leech M, Marignol L. Metformin and improved treatment outcomes in radiation therapy - A review. Cancer Treat Rev. 2017;55:150–162. doi: 10.1016/j.ctrv.2017.03.005. [DOI] [PubMed] [Google Scholar]
- Song Y, Zuo Y, Qian XL, Chen ZP, Wang SK, Song L, Peng LP. Inhibition of MicroRNA-21-5p Promotes the Radiation Sensitivity of Non-Small Cell Lung Cancer Through HMSH2. Cell Physiol Biochem. 2017;43:1258–1272. doi: 10.1159/000481839. [DOI] [PubMed] [Google Scholar]
- Sun W, Wang Y, Zheng Y, Quan N. The emerging role of Sestrin2 in cell metabolism, and cardiovascular and agerelated diseases. Aging Dis. 2020;11:154–63. doi: 10.14336/AD.2019.0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasik B, Chałubinska-Fendler J, Chowdhury D, Fendler W. Potential of serum microRNAs as biomarkers of radiation injury and tools for individualization of radiotherapy. Transl Res. 2018;201:71–83. doi: 10.1016/j.trsl.2018.06.001. [DOI] [PubMed] [Google Scholar]
- Wu Y, Huang J, Xu H, Gong Z. Over-expression of miR-15a-3p enhances the radiosensitivity of cervical cancer by targeting tumorproteinD52. Biomed Pharmaco ther . 2018;105:1325–1334. doi: 10.1016/j.biopha.2018.06.033. [DOI] [PubMed] [Google Scholar]
- X, Chen D, Li M, Gao X, Shi G, Zhao H. The CADM2/Akt pathway is involved in the inhibitory effect of miR-21-5p down regulation on proliferation and apoptosis in esophageal squamous cell carcinoma cells. ChemBiol Interact. 2018;25:76–82. doi: 10.1016/j.cbi.2018.04.021. [DOI] [PubMed] [Google Scholar]
- Young Shin Ko, Hana Jin, Jong Sil Lee, Sang Won Park, Ki Churl Chang. Radio resistant breast cancer cells exhibit increased resistance to chemotherapy and enhanced invasive properties due to cancer stem cells. Oncol Rep. 2018;40:3752–62. doi: 10.3892/or.2018.6714. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Storr SJ, Johnson K, Green AR, Rakha EA, Ellis IO, Morgan DA, Martin SG. Involvement of metformin and AMPK in the radioresponse and prognosis of luminal versus basal-like breast cancer treated with radiotherapy. Oncotarget. 2014;30:12936–49. doi: 10.18632/oncotarget.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author.