Abstract
Objective:
Cancer poses a significant challenge in modern medicine, standing as the primary cause of death in many countries, second only to cardiovascular diseases. Among the various treatments available, carboplatin, a chemotherapy drug, is employed for specific cancer types, including brain carcinoma. The main objective of this investigation is to enhance the therapeutic efficacy of carboplatin by utilizing niosomal nanocarriers.
Methods:
We synthesized nanoniosomal carboplatin using the reverse-phase evaporation technique and conducted an assessment of its particle size, zeta potential, and drug-release properties. Subsequently, we evaluated the cytotoxicity of nanoniosomal carboplatin using the C6 rat glioma cell line.
Results:
Our research revealed that these niosomal nanoparticles possessed a particle size of 290.5±5.5 nm and a zeta potential of -21.7±7.4 mV. The amount of encapsulated drug and drug loading level were found to be 60.2±2.3% and 2.5±1.1%, respectively. Importantly, the cytotoxic impact of these nanoniosomes on the C6 rat glioma cell line exhibited a significant increase compared to the free drug (P<0.05).
Conclusion:
Based on our discoveries, it is evident that carboplatin niosomal nanocarriers hold potential as an innovative approach to chemotherapy for brain cancer therapy.
Key Words: Brain cancer, Nanoniosome, Carboplatin, Nanoparticle
Introduction
Cancer is a significant global health concern due to its high mortality rate, particularly among women and men. The most common types of cancer include breast cancer, lung cancer, prostate cancer, brain cancer, sarcoma cancer, and endometrial carcinoma (Fazilat‐Panah, Dehghani et al. 2021, Ahmadi, Sheida et al. 2022, Fayyad, Mohammed Ali et al. 2022, Mohammadinejad, Jafari-Gharabaghlou et al. 2022, Kalantari, Moqadam et al. 2022, khoshravan Azar, Dadashpour et al. 2022, Loghmani, Moqadam et al. 2022, Nayerpour Dizaj, Jafari-Gharabaghlou et al. 2023, Sabzalian, Kharajinezhadian et al. 2023, Zarean Shahraki, Azizmohammad Looha et al. 2023). Brain tumors constitute 85% to 90% of all primary central nervous system (CNS) tumors and rank as the 10th leading cause of death for both men and women (Ostrom, Price et al. 2022). In the year 2020, approximately 180,047 individuals in the United States were estimated to be living with brain and other nervous system cancer (Fan, Zhang et al. 2022). Notably, the incidence of brain tumors is higher among females, accounting for 58.7% of diagnoses, in contrast to males at 41.3% (Miller, Ostrom et al. 2021). Regarding age distribution, adults aged 65 years or older represent 43.5% of all brain tumor cases, followed by adults aged 40 to 64 years at 40.0%, adolescents and young adults aged 15 to 39 years at 14.3%, and children under 15 years at 2.1% (Neff, Price et al. 2023). Cancer, like infectious diseases and Alzheimer’s disease, poses a significant global health challenge. While it shares the spotlight with other infectious diseases, cancer is part of a larger landscape of medical concerns that are influenced by a complex interplay of factors. (Torkaman, Nasiri et al. 2014, Torkaman, Kamachi et al. 2015, Derakhshan, Eslampour et al. 2016, Allahyartorkaman, Mirsaeidi et al. 2019, Farmanfarma, Mohammadian et al. 2019, Vienne-Jumeau, Tafani et al. 2019, Moghimhanjani et al 2020, Hatami et al 2022, Kalani, Zare et al. 2022, Moghimhanjani and Taghavirashidizadeh 2022, Taghavirashidizadeh et al 2022, Khajeh, Ganjavi et al. 2023, Sadrabad, Ghahremanfard et al. 2023, Sadrabad, Pedram et al. 2023). One of the primary treatment options for cancer is chemotherapy, which involves the use of drugs to eradicate cancer cells. Carboplatin, a chemotherapy drug, is commonly used for the treatment of specific cancer types, including brain carcinoma. However, the effectiveness of carboplatin can be enhanced by utilizing innovative drug delivery systems. In the field of cancer research, various studies have explored different aspects of cancer treatment and drug delivery systems. For instance, Alimirzaei and Kieslich (2023) focus on utilizing machine learning models to predict anticancer peptides, potentially contributing to the development of novel cancer therapies. Additionally, the investigation highlights the development of nanocomposites for applications in bone tissue engineering, underscoring the versatility of nanotechnology across various biomedical fields (Askari, Rasouli et al. 2021). Moreover, Rasouli, Darghiasi et al. discuss the application of hydroxyapatite-based nanoparticles for drug delivery and local controlled release, emphasizing the potential of nanomedicine in advancing treatment strategies. These nanoparticles offer multifunctionality and can be customized for specific therapeutic purposes.
In the research by Salehi et al. on 2023, they discusses the challenges of diagnosing and treating cheilitis glandularis, a rare and poorly understood condition. The study emphasizes the importance of medical research in developing new treatments for such medical conditions. Furthermore, Soleymani, Halimehjani et al. 2023 discuss the synthesis of iodine-containing compounds, highlighting efficient methods that involve nanoparticle synthesis for drug delivery applications. The focus is on a chemical synthesis method, indicating a high degree of control over the reaction. The methodology’s advantages include mild reaction conditions, high yields, easy purification, and the use of inexpensive reagents, making the protocol attractive for chemical synthesis. The study also explores the cytotoxicity of the synthesized silver nanoparticles, indicating that their genotoxic effects become more evident at higher concentrations. Furthermore, it sheds light on the challenges associated with treating brain tumors, attributing them to the blood-brain barrier. This barrier impedes the uptake of lipophilic molecules, xenobiotics, potentially toxic metabolites, and drugs. The primary treatment options for brain tumors encompass surgical intervention, radiation therapy, chemotherapy, and targeted drug therapy. Surgery stands out as the most commonly employed treatment for brain tumors, and in many instances, it suffices as the sole therapeutic approach (Rivera, Norman et al. 2021). Radiation therapy employs high-energy beams like X-rays or protons to eliminate cancer cells (Scaringi, Agolli et al. 2018). Chemotherapy drugs are pharmaceuticals designed to eradicate cancer cells. While chemotherapy is rarely employed as a standalone treatment for brain tumors, it is often combined with surgical and/or radiation therapies (Sawyer, Piepmeier et al. 2006, Weiss, Weller et al. 2016). Transporting drugs across the blood-brain barrier presents a significant obstacle in the treatment of brain disorders. To overcome this challenge, researchers are actively exploring innovative strategies, such as utilizing nanoparticles for brain-targeted purposes (Moazzam, Hatamian-Zarmi et al. 2023)Targeted therapy medications are accessible for specific types of brain cancers and benign brain tumors (Wei, Chen et al. 2014). Carboplatin is a versatile anticancer medication currently utilized in chemotherapy to combat ovarian, breast, and brain cancers (Esim, Gedik et al. 2021, Alavi, Raza et al. 2022). To mitigate the adverse effects of carboplatin and enhance its effectiveness in cancer treatment, researchers have developed Carboplatin-Niosomal nanoparticles (Alavi, Raza et al. 2022). Additionally, investigations have explored the use of PEGylated-Niosomes loaded with magnetic nanoparticles for targeted delivery of carboplatin in the fight against tumors (Davarpanah, Khalili Yazdi et al. 2018, Alavi, Raza et al. 2022). Despite previous attempts to employ various carriers for carboplatin delivery, the creation of the ideal nanoniosome formulation for this drug has encountered challenges. This study addresses this issue by refining the formulation of carboplatin nanoniosomes and assessing their impact on the toxicity of brain cancer cell lines.
Materials and Methods
Carboplatin, Span 40, polyethylene glycol (PEG) 400, and cholesterol were procured from Sigma-Aldrich Co., UK. The RPMI 1640 cell culture medium was sourced from Gibco Co., Germany. Furthermore, the C6 cell line was acquired from the Pasteur Institute cell bank in Iran.
Nanoniosomal drug preparation
Niosomal nanoparticles were created through the reverse phase evaporation method. In a concise summary, 6 mg of carboplatin, 65 mg of span40, 40 mg of cholesterol, and 38 mg of PEG 400, with a molar ratio of 60:45:5 per cent, were dissolved in 120 ml of 96% (v/v) ethanol. This mixture was then heated to 37˚C and stirred at 140 rpm for 2 hours until complete dissolution occurred. The solvent was subsequently removed using a rotary evaporator, leaving behind a thin film. This thin film was dissolved in two separate additions of 10 cc of phosphate buffer (pH 7.4). Finally, the formulations underwent sonication for 6 minutes using an ultrasonic bath (Bandelin Sonorex Digitec, Germany). The particles were then stored at 4℃ until the subsequent studies.
A single milligram of the formulation was dissolved in 3 ml of phosphate-buffered saline (PBS). Following this, the nanoniosomes’ absorption at 633 nm was assessed, and their zeta potential and average diameter were measured using a Zetasizer (Nano ZS3600, Malvern Instruments, UK).
Encapsulation efficiency
Initially, a standard curve for Carboplatin was established by preparing different drug concentrations (0.039, 0.019, 0.009, 0.004, and 0.002 mg/mL) in PBS at a wavelength of 227 nm. Free-drug liposomes were employed as a reference to prevent any interference or overlap from other liposome or nanoparticle components. Subsequently, 2 mL of each formulation was subjected to centrifugation (at 14,000 rpm, 4°C, for 15 minutes), and the absorbance of the supernatant was determined at 227 nm using a spectrophotometer from Hitachi, Japan. EE% and DLE% were then determined using the following equations:
(1)
(2)
Additionally, an assessment was conducted to determine the nanoparticles’ stability. This involved storing a suspension of particles in a refrigerator at 4°C for a duration of 1 months. Subsequently, the nanoparticles underwent a reevaluation, considering factors such as size, zeta potential, encapsulation, and drug loading efficiency.
Drug release study
To assess the release pattern of nanoniosomal carboplatin, we took 1 ml of the niosomal drug suspension and its control, placing them both in a dialysis bag with a molecular weight cut-off of 10,000 Daltons. This bag was then immersed in 80 ml of PBS buffer with a pH of 7.4 and stirred at room temperature for a duration of 40 hours, at a stirring speed of 140 rpm. After this period, 2 ml of the old PBS buffer was removed and replaced with 2 ml of fresh PBS buffer. We measured the optical absorbance of the samples at 227 nm and calculated the concentration of the free drug using a standard curve. The cumulative percentage of drug release was determined using the following formula:
Drug release (%) = Released drug from particles (mg) /Total drug in particles (mg) × 100
Cell culture
The C6 cell line was cultivated in RPMI 1640 medium, to which 10% fetal bovine serum, 100 μg/ml of streptomycin, and 100 U/ml of penicillin were added as supplements. The cell culture was consistently maintained at conditions of 37°C temperature, 5% CO2, and 90% humidity. In each cell culture, 100 µl of a cell suspension, containing 10,000 cells, was introduced into the wells of a 96-well plate. The plate was then kept in an incubator at 37°C with 5% CO2. After a period of 24 hours, the supernatant was gathered from the cells. Subsequently, varying concentrations of the nanoniosomal carboplatin formulation, the control, and the standard carboplatin drug were introduced to the cells and incubated for an additional 24 hours. This procedure was repeated following another 24-hour interval (Amiri, Ebrahimi-Far et al. 2016, Ebrahimifar, Nili-Ahmadabadi et al. 2017).
Cytotoxicity assay
Cytotoxicity assessment was conducted using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. After a 48-hour exposure period, the supernatant was aspirated, and a 100 µl solution of MTT (0.5 mg/ml) was introduced to the cells. The cells were then kept in incubation for 3 hours, followed by the addition of a 100 µl solution of isopropanol to the culture medium. After a 30-minute interval, the optical absorbance of the formazan product was quantified at 570 nm using a plate reader (Synergy Multi-Mode Elisa Reader, Bio-Tek, USA). Lastly, the IC50 value was computed using the pharm program.
Statistical analysis
Statistical analyses for this study were conducted with GraphPad Prism software version 8.00, and a Student’s t-test was employed to assess statistical variances. The outcomes were presented as mean values along with their corresponding standard deviations (SD) based on a sample size of three (n = 3). Statistical significance was established when the p-value was below 0.05.
Results
Particle size and zeta potential analysis
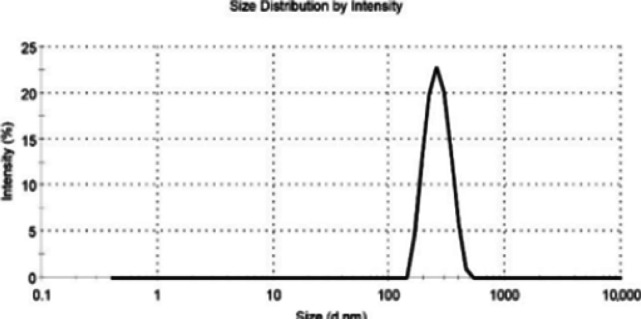
As depicted in Figure 1, the average size of nanoniosomal carboplatin was measured at 290.5±5.5 nm, and the zeta potential was found to be -21.7±7.4 mV. Furthermore, the findings from measurements of encapsulation, drug loading efficiency, size, and zeta potential after a one-month period affirmed the nanoparticles’ reliable stability (refer to Table 1).
Figure 1.
The Mean Size of Nanoniosomal Carboplatin
Table 1.
The Physicochemical Properties of Carboplatin Nanoparticles During Production and after a 1-month Period
| Time | Zeta potential (mV) | Size (nm) | EE (%) | DLE (%) |
|---|---|---|---|---|
| Production time | -21.1 | 290.4 | 60.1 | 2.5 |
| 1 months after production | -23.9 | 314.5 | 55.2 | 2.1 |
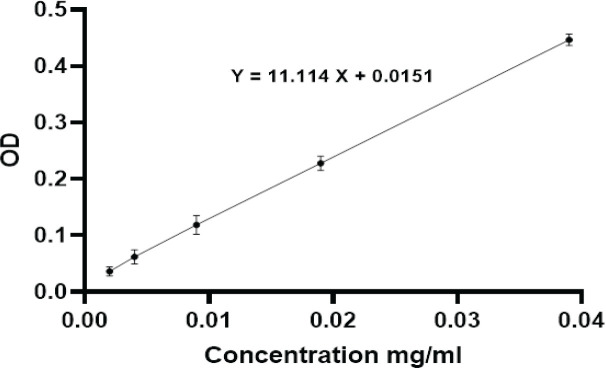
Furthermore, EE% and DLC% were calculated using the standard curve (Figure 2) and the following formula.
Figure 2.
A Standard Carboplatin Curve was Generated by Measuring the Absorbance (optical density; OD) of Various Dilutions of Carboplatin Concentrations
Optical density OD= 11.114X + 0.0151
The calculations revealed an encapsulation efficiency of 60.2±2.3% and a drug loading content of 2.5±1.1%.
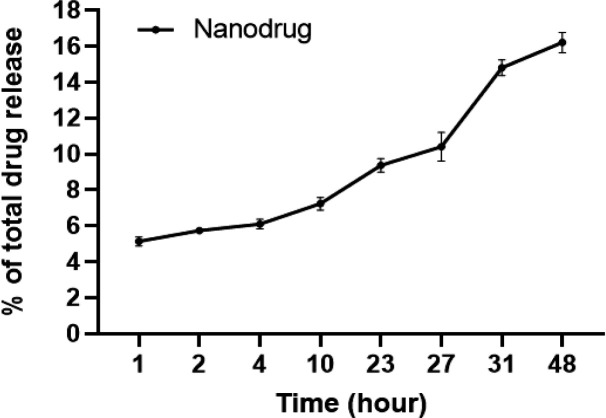
In vitro drug release studies
As illustrated in Figure 3, we monitored the release of carboplatin from the nanoniosomes into the PBS buffer at specific time points: 1, 2, 4, 10, 23, 27, 31, and 48 hours. To ascertain the amount released, we employed a standard carboplatin curve. The findings indicated that, within the 48-hour duration, the nanoniosomes released approximately 74.6±4.8% of the carboplatin.
Figure 3.
The Graph Illustrates How Carboplatin is Released in Vitro from the Carboplatin Nanoniosomes into a PBS Buffer. The information is depicted as the mean value along with the standard deviation (SD), and it originates from three distinct experiments, with each experiment conducted twice
Cytotoxicity assay
As illustrated in Figure 4, the IC50 values exhibited a noteworthy decrease in a dose-dependent fashion after exposing brain cell lines to both free carboplatin and its nanoniosomal formulation. This determination was made through the MTT assay (Figure 4). The cytotoxic effects exhibited a direct relationship with drug concentrations, with the nanodrug having an IC50 of 90.5±2.1 µM, demonstrating superior cytotoxicity compared to the standard carboplatin with an IC50 of 160.7±4.4 µM.
Figure 4.
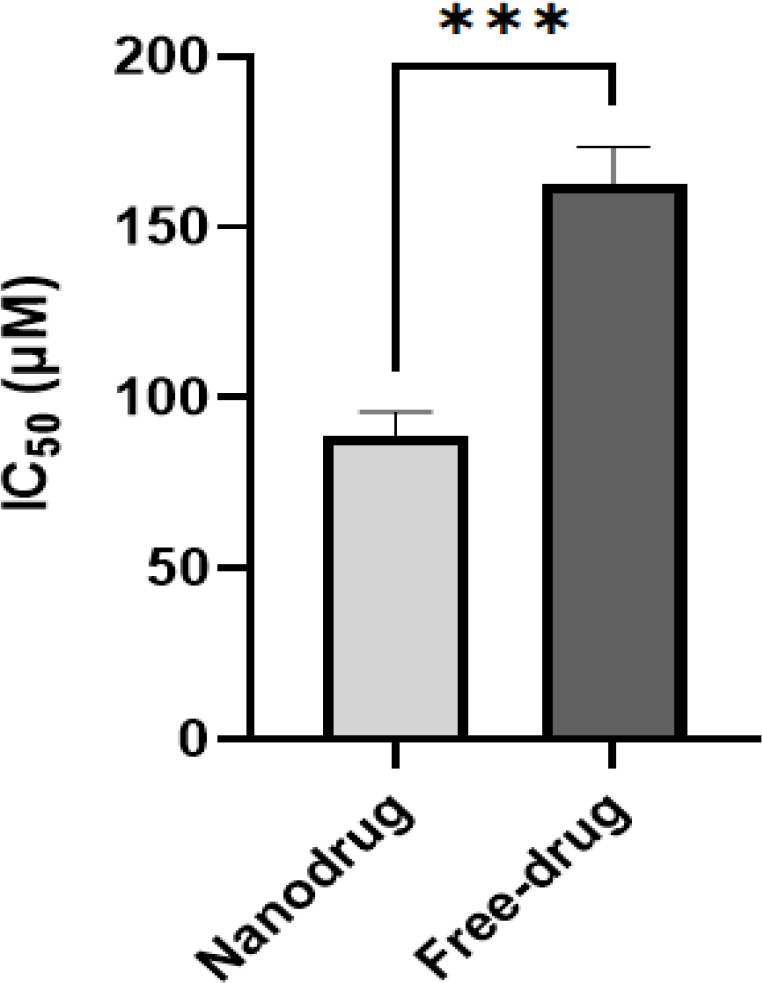
Viability Effects of Carboplatin-loaded Nanoparticles Compared to the Standard Drug (carboplatin) on C6 Rat Glioma Cell Line after 48-h Incubation. (Graph represents mean ± SD; n= 3 independent experiments, ***p = 0.0002, two-way ANOVA with Šidák test)
Discussion
Cancer is a condition arising from the uncontrolled growth and division of cells. These cells proliferate without regulation, infiltrate healthy tissues and organs, and eventually disseminate throughout the body. Moreover, cancer cells do not undergo programmed cell death, known as apoptosis, when subjected to conditions that would typically trigger it in normal cells, such as DNA damage. The initiation of apoptosis is a crucial approach for eliminating cancer cells with minimal side effects, and the majority of anti-cancer treatments function by inducing apoptosis (Mohamadi, Kazemi et al. 2017). In this study, carboplatin-loaded niosomal nanoparticles were prepared using the reverse phase evaporation technique. This method was selected due to its suitability for producing nanoparticles with the desired characteristics. Additionally, PEG (polyethylene glycol) was incorporated into the study for multiple reasons. PEG serves to enhance stability in the bloodstream, is soluble in water, exhibits minimal immunogenicity and antigenicity, and prolongs the duration of drug release (Kanaani, Ebrahimifar et al. 2017). The zeta potential of -21 mV verified the stability of the particles, highlighting the suitability of niosome nanoparticles as a promising drug delivery system. Niosomes can serve as drug reservoirs and regulate drug release by altering their compositions (Kanaani, Tabrizi et al. 2017). The literature contains various formulations of niosomes incorporating chemotherapeutics (Ahmadi, Seraj et al. 2022, Honarvari, Karimifard et al. 2022). As an example, Ahmadi, Seraj et al. in 2022 created controlled-release nanoniosomes to enhance the delivery and effectiveness of Letrozole in breast cancer treatment. Their research showed the formulation’s capacity for efficient delivery. Additionally, Honarvari, Karimifard et al. 2022 explored Folate-Targeted Curcumin-Loaded niosomes for precise site-specific delivery in breast cancer treatment. In a distinct study, niosomes loaded with melittin were investigated for the treatment of breast cancer. These nanoparticles possessed a size of 197 nm and an encapsulation efficiency of 60%. A pharmacokinetic study conducted in vivo revealed that the targeted nanoparticles displayed extended circulation duration and improved bioavailability. This ultimately led to a noteworthy reduction in clearance and distribution volume when compared to both doxorubicin solution and non-targeted niosomes (Dabbagh Moghaddam, Akbarzadeh et al. 2021). Pharmaceutical biotechnology played a crucial role in promoting the adoption of innovative drug delivery strategies. In this context, nanocarriers, including niosomal nanoparticles, demonstrated great promise as viable options (Moghtaderi, Sedaghatnia et al. 2022).
This research assessed how various substances, including a non-ionic surfactant and cholesterol, impact encapsulation efficiency, nanoparticle dimensions, and drug delivery. The release of drugs plays a pivotal role in drug delivery systems (Porta-i-Batalla, Eckstein et al. 2016). In our current investigation, we successfully achieved a sustained release of carboplatin from the nanoparticles, characterized by an initial rapid release, which signifies the release of the drug that was initially adsorbed onto the nanoparticles. After 48 hours, approximately 16% of the drug had been released. The inclusion of PEG in the nanoparticle composition has the potential to improve stability and enhance the prospects of delivering the drug to tumor sites (Ebrahimifar, Nili-Ahmadabadi et al. 2017). This could be attributed to the nanoparticles’ ability to retain the drug, a feature likely influenced by the presence of PEG in the formulation. Furthermore, enhancing particle stability can contribute to the drug’s effectiveness by facilitating its delivery to tumor sites (Poy, Akbarzadeh et al. 2016).
In our current study, we employed niosomal nanoparticles to enhance the anticancer effectiveness of carboplatin against the C6 rat glioma cell line. The cytotoxic effects exhibited a direct relationship with drug concentrations, with the nanodrug having an IC50 of 90 µM, demonstrating superior cytotoxicity compared to the standard carboplatin with an IC50 of 160 µM. This enhanced efficacy was attributed to the sustained release of the drug from the nanocarrier. To our knowledge, this is the first investigation to explore the effectiveness of carboplatin-loaded niosomal nanoparticles on the C6 rat glioma cell line. The nanoparticles generated in this study exhibited favorable characteristics in both size and zeta potential. Additionally, the resulting nanodrug demonstrated a lower IC50 value in terms of cytotoxicity compared to the free drug. These findings indicate that the niosomal formulation of carboplatin is more effective at targeting and destroying brain carcinoma cells. The improved performance can be attributed to the niosomal nanocarrier’s ability to prolong the drug’s half-life in the bloodstream and provide protective benefits. Despite the low loading capacity in this study, the presence of PEG during nanoparticle preparation allows them to potentially remain concealed. Consequently, they can circulate in the bloodstream for extended periods, leading to enhanced drug efficacy (Kanaani, Ebrahimifar et al. 2017). To investigate the drug release behavior, the dialysis tubing technique was employed. The release profile of carboplatin-loaded nanoparticles exhibited an initial rapid diffusion phase, followed by a slower diffusion phase. Based on the findings, it can be inferred that the majority of drug releases occurred within the first four hours. Nonetheless, additional research is required to develop an improved carboplatin-loaded nanoparticle with enhanced loading capacity and efficiency. Such advancements would make it a more promising option for treating brain cancer and other types of cancers. In this context, it is advisable to consider the utilization of folate ligands, dextran, or monoclonal antibodies.
Author Contribution Statement
Reza Reihanisaransari, Mohadeseh Abbasi and Fatemeh poustchi performed the experimental tests. Cell culture was carried out by Mohammadreza Allahyartorkaman. Parizad Ghanbarikondori and Ali hheidari performed the statistical analysis. Hanifeh Salehi, Vahid Salehi, Farimah Moazzam and Mohaddeseh Izadkhah wrote the article.
Acknowledgements
None.
Data availability
Not applicable as we used information from previously published articles.
Approved by any scientific Body
Not applicable as the manuscript is not a part of any student thesis or study.
Ethical issue and approval
Not applicable as we used information from previously published articles.
Consent for publication
All authors have given consent for publication.
Conflict of interest
The authors declare no potential conflict of interest.
References
- Ahmadi MS, Sheida F, Ameri A, et al. Ewing’s Sarcoma of Mandible: Practical Approach to a Challenging Case. Case Rep Oncol. 2022;15:927–35. doi: 10.1159/000525608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadi S, Seraj M, Chiani M, et al. In vitro development of controlled-release nanoniosomes for improved delivery and anticancer activity of letrozole for breast cancer treatment. Int J Nanomedicine. 2022:6233–55. doi: 10.2147/IJN.S384085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alavi SE, Raza A, Esfahani MKM, et al. Carboplatin niosomal nanoplatform for potentiated chemotherapy. J Pharm Sci. 2022;111:3029–37. doi: 10.1016/j.xphs.2022.06.002. [DOI] [PubMed] [Google Scholar]
- Alimirzaei F, Kieslich CA. Computer Aided Chemical Engineering . Vol. 52. Elsevier; 2023. Machine learning models for predicting membranolytic anticancer peptides; pp. 2691–6. [Google Scholar]
- Allahyartorkaman M, Mirsaeidi M, Hamzehloo G, et al. Low diagnostic accuracy of Xpert MTB/RIF assay for extrapulmonary tuberculosis: A multicenter surveillance. Sci Rep. 2019;9:18515. doi: 10.1038/s41598-019-55112-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiri B, Ebrahimi-Far M, Saffari Z, et al. Preparation, characterization and cytotoxicity of silibinin-containing nanoniosomes in T47D human breast carcinoma cells. Asian Pac J Cancer Prev. 2016;17:3835–8. [PubMed] [Google Scholar]
- Askari E, Rasouli M, Darghiasi SF, et al. Reduced graphene oxide-grafted bovine serum albumin/bredigite nanocomposites with high mechanical properties and excellent osteogenic bioactivity for bone tissue engineering. Bio-Des Manuf. 2021;4:243–57. [Google Scholar]
- Dabbagh Moghaddam F, Akbarzadeh I, Marzbankia E, et al. Delivery of melittin-loaded niosomes for breast cancer treatment: an in vitro and in vivo evaluation of anti-cancer effect. Cancer Nanotechnol. 2021;12:14. [Google Scholar]
- Davarpanah F, Khalili Yazdi A, Barani M, Mirzaei M, Torkzadeh-Mahani M. Magnetic delivery of antitumor carboplatin by using PEGylated-Niosomes. DARU J Pharm Sci. 2018;26:57–64. doi: 10.1007/s40199-018-0215-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derakhshan A, Eslampour A, Safinezhad E, Hasanzadeh S. The effect of intravitreal bevacizumab injection on the corneal endothelial cells. Rev Clin Med. 2016;3:78–83. [Google Scholar]
- Ebrahimifar M, Nili-Ahmadabadi A, Akbarzadeh A, et al. Preparation, characterization and cytotoxic effects of pegylated nanoliposomal containing carboplatin on ovarian cancer cell lines. Indian J Clin Biochem. 2017;32:230–4. doi: 10.1007/s12291-016-0596-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esim O, Gedik ME, Dogan AL, Gunaydin G, Hascicek C. Development of carboplatin loaded bovine serum albumin nanoparticles and evaluation of its effect on an ovarian cancer cell line. J Drug Deliv Sci Technol. 2021;64:102655. [Google Scholar]
- Fan Y, Zhang X, Gao C, et al. Burden and trends of brain and central nervous system cancer from 1990 to 2019 at the global, regional, and country levels. Arch Public Health. 2022;80:1–14. doi: 10.1186/s13690-022-00965-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmanfarma KK, Mohammadian M, Shahabinia Z, Hassanipour S, Salehiniya H. Brain cancer in the world: an epidemiological review. World Cancer Res J. 2019;6:1–5. [Google Scholar]
- Honarvari B, Karimifard S, Akhtari N, et al. Folate-targeted curcumin-loaded niosomes for site-specific delivery in breast cancer treatment: In silico and In vitro study. Molecules. 2022;27:4634. doi: 10.3390/molecules27144634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalani N, Zare MR, Safniyat S, Hatami N, Razavizadegan SA. The First Case of the Subretinal Abscess After Sars-CoV2 Infection. Acta Med Iran. 2022:132–4. [Google Scholar]
- Kanaani L, Ebrahimifar M, Khiyavi AA, Mehrdiba T. Effects of cisplatin-loaded niosomal nanoparticleson BT-20 human breast carcinoma cells. Asian Pac J Cancer Prev. 2017;18:365. doi: 10.22034/APJCP.2017.18.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaani L, Tabrizi MM, Khiyavi AA, Javadi I. Improving the efficacy of cisplatin using niosome nanoparticles against human breast cancer cell line BT-20: An in vitro study. Asian Pac J Cancer Biol. 2017;2:27–9. [Google Scholar]
- Khajeh S, Ganjavi M, Panahi G, et al. D-allose: Molecular Pathways and Therapeutic Capacity in Cancer. Curr Mol Pharmacol. 2023;16:801–10. doi: 10.2174/1874467216666221227105011. [DOI] [PubMed] [Google Scholar]
- Miller KD, Ostrom QT, Kruchko C, et al. Brain and other central nervous system tumor statistics, 2021. CA Cancer J Clin. 2021;71:381–406. doi: 10.3322/caac.21693. [DOI] [PubMed] [Google Scholar]
- Moazzam F, Hatamian-Zarmi A, Hosseinzadeh BE, et al. Preparation and characterization of brain-targeted polymeric nanocarriers (Frankincense-PMBN-lactoferrin) and in-vivo evaluation on an Alzheimer’s disease-like rat model induced by scopolamine. Brain Res. 2023:148622. doi: 10.1016/j.brainres.2023.148622. [DOI] [PubMed] [Google Scholar]
- Moghimhanjani M, Taghavirashidizadeh A. Analysis of liver cancer detection based on image processing. 2022 arXiv preprint arXiv:2207.08032. [Google Scholar]
- Moghtaderi M, Sedaghatnia K, Bourbour M, et al. Niosomes: a novel targeted drug delivery system for cancer. Med Oncol. 2022;39:240. doi: 10.1007/s12032-022-01836-3. [DOI] [PubMed] [Google Scholar]
- Mohamadi N, Kazemi SM, Mohammadian M, et al. Toxicity of cisplatin-loaded poly butyl cyanoacrylate nanoparticles in a brain cancer cell line: Anionic polymerization results. Asian Pac J Cancer Prev. 2017;18:629. doi: 10.22034/APJCP.2017.18.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff C, Price M, Cioffi G, et al. Complete prevalence of primary malignant and non‐malignant brain tumors in comparison to other cancers in the United States. Cancer. 2023;129:2514–21. doi: 10.1002/cncr.34837. [DOI] [PubMed] [Google Scholar]
- Ostrom QT, Price M, Neff C, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2015–2019. Neurooncol. 2022;24:v1–v95. doi: 10.1093/neuonc/noac202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porta-i-Batalla M, Eckstein C, Xifré-Pérez E, et al. Sustained, controlled and stimuli-responsive drug release systems based on nanoporous anodic alumina with layer-by-layer polyelectrolyte. Nanoscale Res Lett. 2016;11:1–9. doi: 10.1186/s11671-016-1585-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poy D, Akbarzadeh A, Ebrahimi Shahmabadi H, et al. Preparation, characterization, and cytotoxic effects of liposomal nanoparticles containing cisplatin: an in vitro study. Chem Biol Drug Des. 2016;88:568–73. doi: 10.1111/cbdd.12786. [DOI] [PubMed] [Google Scholar]
- Rasouli M, Darghiasi S, Naghib S, Rahmanian M. Multifunctional Hydroxyapatite-based Nanoparticles for Biomedicine: Recent Progress in Drug Delivery and Local Controlled Release. Curr Mech Adv Mater. 2020 [Google Scholar]
- Rivera M, Norman S, Sehgal R, Juthani R. Updates on surgical management and advances for brain tumors. Curr Oncol Rep. 2021;23:1–9. doi: 10.1007/s11912-020-01005-7. [DOI] [PubMed] [Google Scholar]
- Sadrabad MJ, Ghahremanfard F, Sohanian S, et al. Knowledge and Attitude of Cancer Patients’ Companions towards Chemotherapy and Radiotherapy-induced Oral Complications and Dental Considerations. Iran Red Crescent Med J. 2023:25. [Google Scholar]
- Sadrabad MJ, Pedram A, Saberian E, Emami R. Clinical efficacy of LLLT in treatment of trigeminal neuralgia–Case report. J Oral Maxillofac Surg Med Pathol. 2023;35:568–71. [Google Scholar]
- Salehi N, Salehi A, Kalantari M. Unusual upper lip swelling: A review and a case report of cheilitis landularis. World J Adv Res Rev. 2023;19:181–7. [Google Scholar]
- Sawyer AJ, Piepmeier JM, Saltzman WM. Cancer issue: new methods for direct delivery of chemotherapy for treating brain tumors. Yale J Biol Med. 2006;79:141. [PMC free article] [PubMed] [Google Scholar]
- Scaringi C, Agolli L, Minniti G. Technical advances in radiation therapy for brain tumors. Anticancer Res. 2018;38:6041–5. doi: 10.21873/anticanres.12954. [DOI] [PubMed] [Google Scholar]
- Soleymani F, Halimehjani AZ, Asar FJ, Thatcher GR. Iodocyclization of S–(homo) propargyl dithiocarbamates: Regiospecific synthesis of 2-imino (iminium)-1, 3-dithiolanes/dithianes/dithiepanes. Tetrahedron Lett. 2023;128:154702. [Google Scholar]
- Torkaman MR, Nasiri MJ, Farnia P, et al. Estimation of Recent Transmission of Mycobacterium Tuberculosis Strains among Iranian and Afghan Immigrants: A Cluster-Based Study. J Clin Diagn Res. 2014;8:Dc05–8. doi: 10.7860/JCDR/2014/8886.4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torkaman MRA, Kamachi K, Nikbin VS, Lotfi MN, Shahcheraghi F. Comparison of loop-mediated isothermal amplification and real-time PCR for detecting Bordetella pertussis. J Med Microbiol. 2015;64:463–5. doi: 10.1099/jmm.0.000021. [DOI] [PubMed] [Google Scholar]
- Vienne-Jumeau A, Tafani C, Ricard D. Environmental risk factors of primary brain tumors: A review. Rev Neurol. 2019;175:664–78. doi: 10.1016/j.neurol.2019.08.004. [DOI] [PubMed] [Google Scholar]
- Wei X, Chen X, Ying M, Lu W. Brain tumor-targeted drug delivery strategies. Acta Pharm Sin B. 2014;4:193–201. doi: 10.1016/j.apsb.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss T, Weller M, Roth P. Immunological effects of chemotherapy and radiotherapy against brain tumors. Expert Rev Anticancer Ther. 2016;16:1087–94. doi: 10.1080/14737140.2016.1229600. [DOI] [PubMed] [Google Scholar]
- Zarean Shahraki S, Azizmohammad Looha M, Mohammadi Kazaj P, et al. Time-related survival prediction in molecular subtypes of breast cancer using time-to-event deep-learning-based models. Front Oncol. 2023;13:1147604. doi: 10.3389/fonc.2023.1147604. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable as we used information from previously published articles.