Abstract
Objective
The 24-h urine protein remains the gold standard to diagnose proteinuria in suspected preeclamptic patients. However, this test is time consuming and sometimes inaccurate. In this study, we aimed to analyse the correlation between the random urine protein/creatinine ratio (UPCR) and 24-h urine protein and to explore the clinical value of UPCR in the diagnosis of preeclampsia.
Method
We retrospectively evaluated 109 pregnant women from our hospital who had hypertensive diseases. They were grouped according to time of urine collection and disease severity to compare differences in random urine protein, urine creatinine, and UPCR. The correlation between the UPCR and 24-h urine protein was determined by Pearson's linear correlation.
Results
We found no statistically significant differences in random urine protein, urine creatinine, or UPCR among the four time of sampling groups. Further, random urine protein, UPCR, and 24-h urine protein between the gestational hypertension and preeclampsia groups differed significantly (P < 0.001). Correlation analysis showed significant positive correlation between random urine protein, and 24-h urine protein, and UPCR and 24-h urine protein, with r values of 0.789 and 0.810, respectively. According to the receiver operating characteristic (ROC) curve, the optimal threshold, sensitivity, specificity, and area under the curve of UPCR for the diagnosis of preeclampsia were 0.456 g/mmol, 67.8 %, 78.3 %, and 0.747, respectively (95 % confidence interval [CI], 0.65–0.844).
Conclusion
This study indicated that UPCR is significantly correlated with 24-h urine protein and is expected to replace the 24-h urine protein test as a diagnostic indicator of preeclampsia.
Keywords: Preeclampsia, Random urine protein, Random urine protein/creatinine ratio, 24-h urine protein
1. Introduction
Preeclampsia is defined as new-onset hypertension after 20 weeks of gestation combined with proteinuria (>0.3 g/day), or signs and symptoms of end-organ dysfunction. At present, the incidence of preeclampsia in China is as high as 5–7% [1], which seriously endangers the health and safety of mothers and infants. As an important clinical indicator, urine protein can reflect renal involvement and is key to identifying disease development. It is also associated with severe adverse pregnancy outcomes [2,3]. A 24-h urine protein test is considered the “gold standard” for the quantitative diagnosis of proteinuria. However, the 24-h urine protein test is time-consuming and may be inaccurate [4]. Patient compliance is often poor, especially in patients with pregnancy-induced hypertension (PIH). Therefore, it is of practical clinical significance to seek a simple and accurate method to replace 24-h urine protein quantification. Among them, the random urine protein/creatinine ratio (UPCR) and random urine protein test have attracted much attention. However, owing to different detection methods and different time points of specimen collection, the results of the UPCR random urine protein test, and 24-h urine protein test reported in the literature are not consistent [5]. This study aimed to analyse the correlation between random urine albumin/creatinine ratio, random urine protein, and 24-h urine protein. Additionally, we aimed to find a random urine test index that can replace tests requiring 24-h urine collection and to explore the best threshold of this index in the diagnosis of preeclampsia.
2. Methods
2.1. Study design
This retrospective cohort study included all pregnant women undergoing regular check-ups at the Second Affiliated Hospital of Wenzhou Medical University in China between January and December 2022. A total of 109 hospitalised pregnant women with hypertensive disorders complicating pregnancy were enrolled: 60 in the gestational hypertension group and 49 in the preeclampsia group. The diagnostic criteria for hypertensive disorders of pregnancy matched the criteria of the 2015 Chinese Society of Obstetrics and Gynaecology guidelines [6], which are consistent with the diagnostic criteria of the International Society for the Study of Hypertension in Pregnancy published in 2018 [7]. Exclusion criteria were pregnancy complicated with chronic hypertension, kidney disease, cardiovascular disease, immune disease, metabolic diseases (gestational diabetes mellitus, thyroid disease, etc.), intrahepatic cholestasis of pregnancy, multiple pregnancies, and foetal dysplasia. None of the patients took antihypertensive or cardiovascular drugs before diagnosis.
The urine collection method and precautions are described here in detail. A random urine sample was collected from pregnant women on admission; midstream urine was collected after cleaning the vulvar secretions. The 24-h urine collection was performed as follows: patients were instructed to empty the bladder at 7 a.m., and subsequently urine was collected from 7 a.m. that day until 7 a.m. the next day. All urine samples were collected in large clean containers, and 10 g boric acid for embalming was added immediately after the first urine sample was left in the container. The 24-h urine was pooled together, 5 mL was taken into a special container, and the 24-h total urine volume was recorded. Collection of specimens was approved by the ethics committee of our hospital, and informed consent was obtained from all the patients.
All samples were analysed using a Siemens ADVIA 2400 automatic biochemical analyser. Urine protein was quantified by colorimetry, and the 24-h urine protein was calculated as urine protein quantity multiplied by the 24-h urine volume. Urine creatinine was measured using the creatinine enzymatic method, and the UPCR was calculated from random urine protein quantification and urine creatinine test results. All reagents were obtained from Siemens Medical Diagnostic Products.
2.2. Statistical analysis
Data analysis was performed using the SPSS version 18.0 statistical software for the differences in random urine protein, UPCR, and 24-h urine protein between the two groups using the rank sum test, and the data were expressed as (P25, P75). Pearson's linear correlation was used to analyse the correlation of random urine protein, UPCR, and 24-h urine protein. Random urine protein or UPCR was set as the independent variable, and 24-h urine protein quantification was set as the dependent variable. Bivariate scatter plots were used to analyse the correlation between the two variables. According to the receiver operating characteristic (ROC) curve, the maximum Youden index (sensitivity + specificity −1) was used to determine the cut-off value.
3. Results
3.1. Comparison of random urine protein, random urine creatinine, and UPCR according to time grouping
According to the time at which random urine samples was collected after admission, they were divided into four time of sampling groups as following: 00:00–06:00 (4 cases), 06:00–12:00 (42 cases), 12:00–18:00 (53 cases), and 18:00–24:00 (10 cases). Random urine protein, random urine creatinine, and UPCR levels did not differ significantly among these four groups (Table 1). Therefore, urine samples can be randomly collected according to the time pregnant women arrive at the hospital. This procedure was simple and convenient, and patients’ compliance was good.
Table 1.
Comparison of random urine protein, random urine creatinine, and UPCR among the time of sampling groups.
| Test items | P25–P75 | Z value | P value |
|---|---|---|---|
| Random urine protein | (0.155–1.97) | 1.415 | 0.702 |
| Random urine creatinine | (5.45–14.005) | 0.458 | 0.928 |
| UPCR | (0.018527–0.1659) | 1.680 | 0.641 |
UPCR: random urine protein/creatinine ratio.
3.2. Comparison of random urine protein, urine creatinine, UPCR and 24-h urine protein in groups according to disease severity
The random urine protein, UPCR, and 24-h urine protein levels were compared between the gestational hypertension (60 cases) and preeclampsia (49 cases) groups, and the difference was statistically significant (P < 0.001). However, there was no significant difference between the two groups in terms of random urine creatinine levels (Table 2). Therefore, random urine protein, UPCR, and 24-h urine protein levels increased with disease severity.
Table 2.
Comparison of random urine protein, random urine creatinine, UPCR, and 24-h urine protein according to disease severity.
| Random urine protein (P25–P75) | Random urine creatinine (P25–P75) | UPCR (P25–P75) | 24-h urine protein (P25–P75) | |
|---|---|---|---|---|
| Gestational hypertension | (0.122, 0.56) | (5.38, 13.76) | (0.016, 0.052) | (0.16, 0.44) |
| Preeclampsia | (0.225, 7.75) | (5.56, 15.195) | (0.03, 0.908) | (0.345, 5.44) |
| Z | −4.353 | −0.615 | −4.429 | −5.045 |
| p | <0.001 | 0.538 | <0.001 | <0.001 |
3.3. Correlation of random urine protein, UPCR, and 24-h urine protein quantification
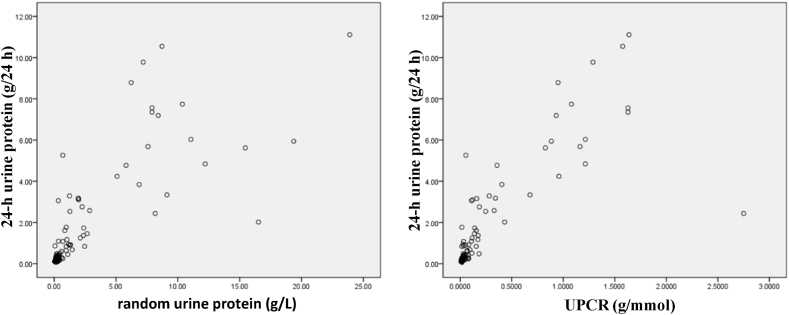
Correlation analysis of random urine protein and 24-h urine protein showed a significant positive linear correlation (r = 0.789, P < 0.001). There was also a significant positive linear correlation between UPCR and 24-h urine protein levels (r = 0.810, P < 0.001). However, the correlation between UPCR and 24-h urine protein quantification was better (Fig. 1).
Fig. 1.
Scatter plot of random urine protein, UPCR, and 24-h urine protein.
3.4. Optimal threshold of UPCR for the diagnosis of preeclampsia
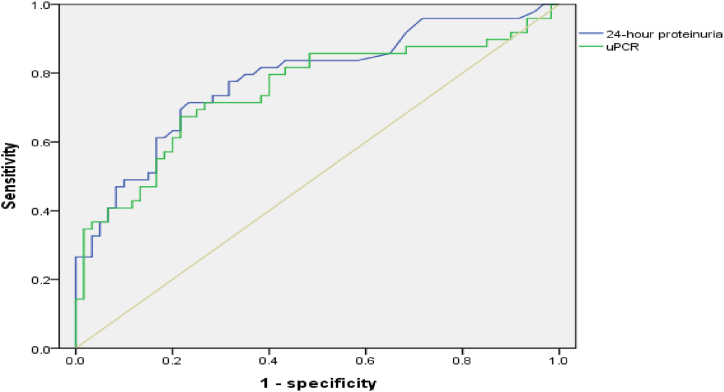
According to the ROC curve, the optimal threshold of UPCR for the diagnosis of preeclampsia was 0.456 g/mmol, sensitivity was 67.8 %, specificity was 78.3 %, and area under the curve was 0.747 (95 % CI, 0.65–0.844) (Fig. 2).
Fig. 2.
Areas under ROC curve were 0.747 (95 % CI, 0.65–0.844).
4. Discussion
Preeclampsia is an idiopathic and common complication of pregnancy, with an incidence of 5–7%, and it is often accompanied by acute kidney injury [1,8,9]. Urine protein, an important urine biochemical indicator, can reflect renal involvement and is key to identifying disease development. Studies have shown that urine protein levels are associated with serious adverse pregnancy outcomes [2,10]. Therefore, the timely and accurate detection of urine protein levels has important clinical significance in the diagnosis of preeclampsia. The 24-h urine protein assay is considered the “gold standard” for the quantitative determination of urine protein. However, the 24-h urine collection process is complicated [4], and patient compliance is poor, especially for patients in the third trimester. Because of the increased frequency of nocturia in the third trimester, a bedpan was placed on the toilet for each collection, the collected urine was poured into a large container, and the bedpan was cleaned. If pregnant women have high blood pressure, urination at night may lead to a risk of fainting. Therefore, assistance from family members is required in most cases. Additionally, there is no preservative or mixing in the collection process, which will lead to inaccuracies in the detection of 24-h urine protein, and sometimes it needs to be repeated many times to obtain accurate results. Therefore, it is of practical clinical significance to seek a simple and accurate method that can replace 24-h urine protein quantification.
To understand whether urine protein production fluctuated throughout the day and whether the biochemical indicators related to random urine were affected by time point, the random urine samples were divided into four groups according to the time of urine collection after admission. The results showed no statistically significant differences in the random urine protein, random urine creatinine, or UPCR among the four time of sampling groups. Therefore, random urine does not need to be collected at a specific time point; and it can be collected in pregnant women with PIH at any time after admission to the hospital. This is not only very convenient for patients to comply with but also greatly shortens the length of time required for urine protein detection; therefore providing the necessary diagnostic basis for timely clinical diagnosis of preeclampsia. In this study, the comparison between the gestational hypertension group and preeclampsia group showed that there were significant differences in the random urine protein, UPCR, and 24-h urine protein (P < 0.001), but there was no significant difference in the random urine creatinine between the two groups. Therefore, the random urine protein and UPCR in patients with preeclampsia are significantly higher than those in patients with gestational hypertension, thereby suggesting that random urine protein and UPCR have a good correlation with the severity of the disease and can be further used as diagnostic indicators to predict preeclampsia.
Owing to different detection methods, the results of quantitative correlation between UPCR, random urine protein, and 24-h urine protein reported in foreign literature are inconsistent, and the correlation coefficient ranges from 0.600 to 0.099 [5,11,12]. As early as 2002, the Clinical Practice Guidelines for Chronic Kidney Disease and Dialysis of the American Nephrology Association recommended UPCR instead of 24-h urine protein quantification [13,14]. However, previous studies suggested that 24-h urine creatinine excretion varies greatly and is affected by individual, diet, muscle volume, exercise status, renal function level, and other factors. In some diseases, such as hyperthyroidism and acromegaly, loss of muscle volume can reduce urine creatinine excretion. Diabetic nephropathy and hypothyroidism increase urine creatinine excretion [15]. The instability of urine creatinine excretion can lead to inaccuracy in the measurement of UPCR during the monitoring and evaluation of urine protein. Therefore, in this study, random urine protein and UPCR were introduced, and a correlation analysis was carried out between random urine protein and 24-h urine protein. The results showed a significant positive linear correlation between random urine protein and 24-h urine protein as well as between UPCR and 24-h urine protein, with r values of 0.789 and 0.810, respectively. Notably, the correlation between UPCR and 24-h urine protein quantification was stronger than that between random urine protein and 24-h urine protein. In this study, owing to the small number of cases with 24-h urine protein >3 g/24 h, the correlation between random urine protein, UPCR, and 24-h urine protein quantification began to fluctuate. In future studies, the sample size should be increased, and especially include more patients with high 24-h urine protein quantification.
The correlation between UPCR and 24-h urine protein quantification was stronger than that between random urine protein and 24-h urine protein; therefore, we further constructed the ROC curve of UPCR and 24-h urine protein quantification to predict preeclampsia. This study found that the optimal threshold of UPCR for the diagnosis of preeclampsia was 0.456 g/mmol, sensitivity was 67.8 %, specificity was 78.3 %, and area under the curve was 0.747 (95 % CI, 0.65–0.844). There was no statistically significant difference between the ROC curves for UPCR and 24-h urine protein quantification. Therefore, UPCR was found to have good predictive value in the diagnosis of preeclampsia. Early assessment of target organ involvement and prediction of preeclampsia complications are particularly important for improving maternal and infant outcomes. Therefore, it is necessary to obtain timely and accurate urine protein secretion data in clinical practice. UPCR is not only simple to perform but also greatly reduces the time required for preeclampsia specimen collection, which increases the possibility of timely diagnosis and evaluation of preeclampsia. Some studies have found that UPCR may have good application value in the risk prediction of preeclampsia. Therefore, UPCR can be considered as a predictive method for the clinical severity of preeclampsia and adverse maternal and infant outcomes [16,17].
5. Conclusions
The results of this study suggest that random UPCR in patients with preeclampsia has a significant correlation with 24-h urine protein quantification and has the advantages of being fast, simple, and accurate. Simultaneously, UPCR is expected to replace 24-h urine protein quantification as a diagnostic index for preeclampsia. The best UPCR cut-off value for the diagnosis of preeclampsia was 0.456.
Research funding
This work was supported by grant from the Wenzhou Science and Technology Bureau (grant numbers 2023Y0253).
CRediT authorship contribution statement
Jingjing Guo: Writing – original draft. Lianlian Zhou: Formal analysis. Suzhen Pan: Data curation. Baoqing Li: Writing – review & editing.
Declaration of competing interest
The authors declare no potential conflicts of interest.
Acknowledgements
We would like to thank Editage (www.editage.cn) for English language editing.
Data availability
Data will be made available on request.
References
- 1.Rana S., Lemoine E., Granger J.P., Karumanchi S.A. Preeclampsia: pathophysiology, challenges, and perspectives. Circ. Res. 2019;124(7):1094–1112. doi: 10.1161/CIRCRESAHA.118.313276. [DOI] [PubMed] [Google Scholar]
- 2.Brown M.A., Magee L.A., Kenny L.C., Karumanchi S.A., McCarthy F.P., Saito S., et al. Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension. 2018;72(1):24–43. doi: 10.1161/HYPERTENSIONAHA.117.10803. [DOI] [PubMed] [Google Scholar]
- 3.Morikawa M., Mayama M., Saito Y., Nakagawa-Akabane K., Umazume T., Chiba K., et al. Severe urine protein as a parameter of worse perinatal/neonatal outcomes in women with preeclampsia. Pregnancy Hypertens. 2020;19:119–126. doi: 10.1016/j.preghy.2019.12.013. [DOI] [PubMed] [Google Scholar]
- 4.Cote A.M., Firoz T., Mattman A., Lam E.M., von Dadelszen P., Magee L.A. The 24-hour urine collection: gold standard or historical practice? Am. J. Obstet. Gynecol. 2008;199(6):625. doi: 10.1016/j.ajog.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 5.Kyle P.M., Fielder J.N., Pullar B., Horwood L.J., Moore M.P. Comparison of methods to identify significant urine protein in pregnancy in the outpatient setting. BJOG. 2008;115(4):523–527. doi: 10.1111/j.1471-0528.2007.01621.x. [DOI] [PubMed] [Google Scholar]
- 6.Hypertensive disorders in pregnancy subgroup CSoO, gynecology CMA, hypertensive disorders in pregnancy subgroup Chinese society of O, gynecology Chinese medical A. [Diagnosis and treatment guideline of hypertensive disorders in pregnancy (2015)] Zhonghua Fu Chan Ke Za Zhi. 2015;50(10):721–728. [PubMed] [Google Scholar]
- 7.Brown M.A., Magee L.A., Kenny L.C., Karumanchi S.A., McCarthy F.P., Saito S., et al. The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2018;13:291–310. doi: 10.1016/j.preghy.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Cornelis T., Odutayo A., Keunen J., Hladunewich M. The kidney in normal pregnancy and preeclampsia. Semin. Nephrol. 2011;31(1):4–14. doi: 10.1016/j.semnephrol.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Penning M.E., Bloemenkamp K.W., van der Zon T., Zandbergen M., Schutte J.M., Bruijn J.A., et al. Association of preeclampsia with podocyte turnover. Clin. J. Am. Soc. Nephrol. 2014;9(8):1377–1385. doi: 10.2215/CJN.12811213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu X., Wang Y., Xu H., Kang Y., Zhu Q. Association between urine protein and maternal and neonatal outcomes in pre-eclampsia pregnancy: a retrospective observational study. J. Int. Med. Res. 2020;48(4) doi: 10.1177/0300060520908114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aggarwal N., Suri V., Soni S., Chopra V., Kohli H.S. A prospective comparison of random urine protein-creatinine ratio vs 24-hour urine protein in women with preeclampsia. Medscape J. Med. 2008;10(4):98. [PMC free article] [PubMed] [Google Scholar]
- 12.Nisell H., Trygg M., Back R. Urine albumin/creatinine ratio for the assessment of albuminuria in pregnancy hypertension. Acta Obstet. Gynecol. Scand. 2006;85(11):1327–1330. doi: 10.1080/00016340600808747. [DOI] [PubMed] [Google Scholar]
- 13.Goolsby M.J. National Kidney Foundation Guidelines for chronic kidney disease: evaluation, classification, and stratification. J. Am. Acad. Nurse Pract. 2002;14(6):238–242. doi: 10.1111/j.1745-7599.2002.tb00119.x. [DOI] [PubMed] [Google Scholar]
- 14.National Kidney F. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am. J. Kidney Dis. 2002;39(2 Suppl 1):S1–S266. [PubMed] [Google Scholar]
- 15.Newman D.J., Pugia M.J., Lott J.A., Wallace J.F., Hiar A.M. Urinary protein and albumin excretion corrected by creatinine and specific gravity. Clin. Chim. Acta. 2000;294(1–2):139–155. doi: 10.1016/s0009-8981(00)00181-9. [DOI] [PubMed] [Google Scholar]
- 16.Elia E.G., Robb A.O., Hemming K., Price M.J., Riley R.D., French-Constant A., et al. Is the first urinary albumin/creatinine ratio (ACR) in women with suspected preeclampsia a prognostic factor for maternal and neonatal adverse outcome? A retrospective cohort study. Acta Obstet. Gynecol. Scand. 2017;96(5):580–588. doi: 10.1111/aogs.13123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guida J.P., Parpinelli M.A., Surita F.G., Costa M.L. The impact of urine protein on maternal and perinatal outcomes among women with pre-eclampsia. Int. J. Gynaecol. Obstet. 2018;143(1):101–107. doi: 10.1002/ijgo.12487. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.