Abstract
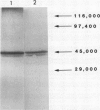
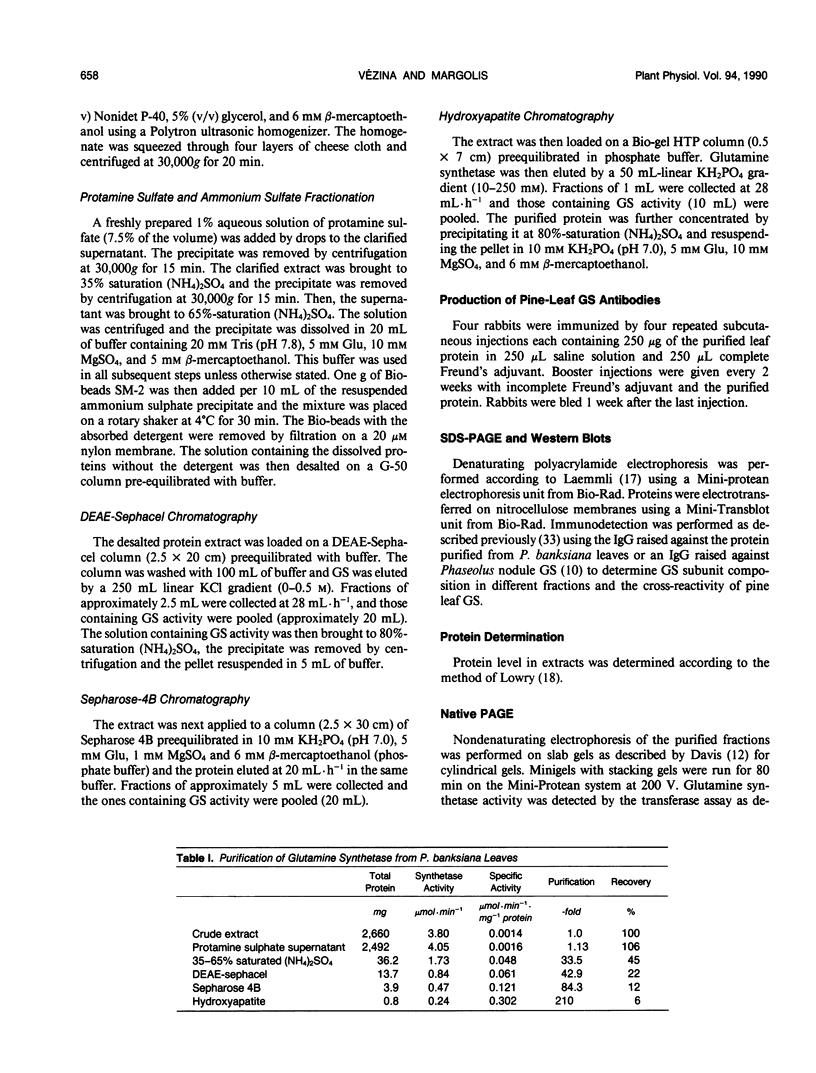
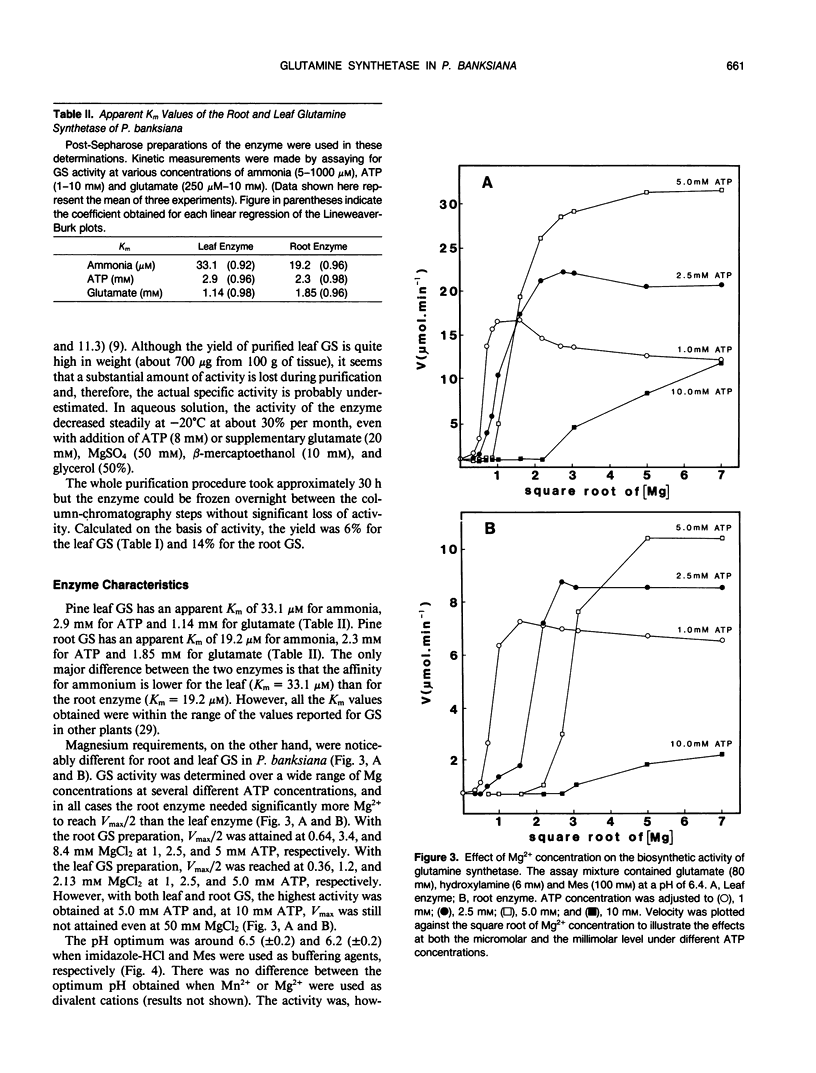
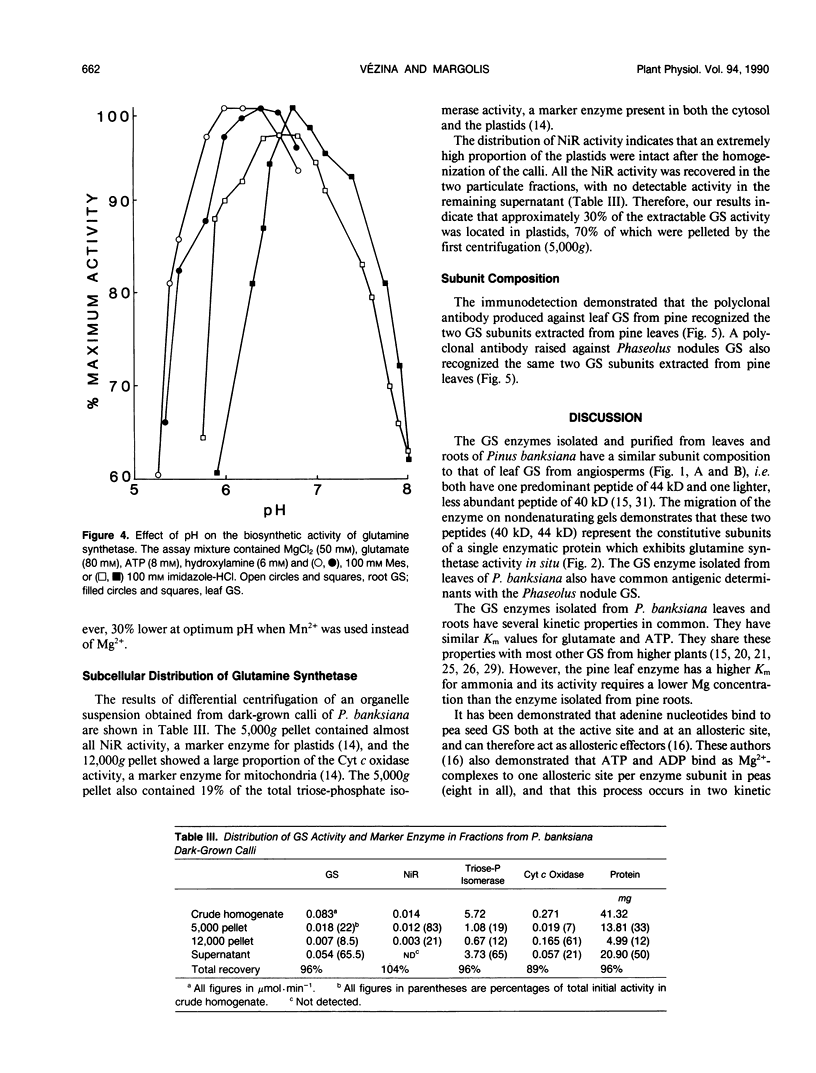
A method is described for the purification of glutamine synthetase (GS; EC. 6.3.1.2) from the leaves and roots of Pinus banksiana Lamb., a conifer which utilizes ammonium as its primary nitrogen source. The enzyme was purified to apparent homogeneity by a procedure involving salt fractionation as well as ion-exchange, size exclusion, and affinity chromatography. Since the final preparation produced two bands on SDS polyacryamide gels but only one band on a nondenaturating gel, it is concluded that the two subunits (44 and 40 kilodaltons, respectively) are part of a single enzymatic protein which shows GS activity. The pH optimum for leaf GS ranged between 6.2 and 6.5, one pH unit lower than the values reported for higher plants which utilize primarily nitrate nitrogen. Magnesium requirements for GS in P. banksiana were different for leaves and roots, showing Vmax/2 values of 2.5 and 8 millimolar, respectively at 5 millimolar ATP. Furthermore, Km values for ammonium were higher for the enzyme in leaves (33.1 micromolar) than in roots (19.2 micromolar). Km values for ATP and for glutamate, on the other hand, were similar for the two tissues. A polyclonal antibody was produced against the purified leaf GS. Western blots of leaf homogenates produced two bands, the lighter one being more abundant. The same pattern was found when immunodetection was performed using an anti GS IgG produced against purified GS from Phaseolus nodules thus indicating common antigenic determinants. At least 30% of total GS was recovered in a plastid-fraction of dark-grown calli produced from the basal part of P. banksiana hypocotyls.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes R. L. Glutamine Synthesis & Translocation in Pine. Plant Physiol. 1962 May;37(3):323–326. doi: 10.1104/pp.37.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton P. M., Waley S. G. The active centre of triose phosphate isomerase. Biochem J. 1966 Sep;100(3):702–710. doi: 10.1042/bj1000702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dalling M. J., Tolbert N. E., Hageman R. H. Intracellular location of nitrate reductase and nitrite reductase. I. Spinach and tobacco leaves. Biochim Biophys Acta. 1972 Dec 14;283(3):505–512. doi: 10.1016/0005-2728(72)90266-6. [DOI] [PubMed] [Google Scholar]
- Hirel B., Gadal P. Glutamine Synthetase in Rice: A COMPARATIVE STUDY OF THE ENZYMES FROM ROOTS AND LEAVES. Plant Physiol. 1980 Oct;66(4):619–623. doi: 10.1104/pp.66.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight T. J., Langston-Unkefer P. J. Adenine nucleotides as allosteric effectors of pea seed glutamine synthetase. J Biol Chem. 1988 Aug 15;263(23):11084–11089. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McNally S. F., Hirel B., Gadal P., Mann A. F., Stewart G. R. Glutamine Synthetases of Higher Plants : Evidence for a Specific Isoform Content Related to Their Possible Physiological Role and Their Compartmentation within the Leaf. Plant Physiol. 1983 May;72(1):22–25. doi: 10.1104/pp.72.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McParland R. H., Guevara J. G., Becker R. R., Evans H. J. The purification and properties of the glutamine synthetase from the cytosol of Soya-bean root nodules. Biochem J. 1976 Mar 1;153(3):597–606. doi: 10.1042/bj1530597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momoi T. The presence of lipophilic glycoprotein interacting with insulin. Biochem Biophys Res Commun. 1979 Mar 30;87(2):541–549. doi: 10.1016/0006-291x(79)91829-1. [DOI] [PubMed] [Google Scholar]
- O'Neal D., Joy K. W. Glutamine synthetase of pea leaves. I. Purification, stabilization, and pH optima. Arch Biochem Biophys. 1973 Nov;159(1):113–122. doi: 10.1016/0003-9861(73)90435-9. [DOI] [PubMed] [Google Scholar]
- O'neal D., Joy K. W. Glutamine synthetase of pea leaves: divalent cation effects, substrate specificity, and other properties. Plant Physiol. 1974 Nov;54(5):773–779. doi: 10.1104/pp.54.5.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze E. D. Air Pollution and Forest Decline in a Spruce (Picea abies) Forest. Science. 1989 May 19;244(4906):776–783. doi: 10.1126/science.244.4906.776. [DOI] [PubMed] [Google Scholar]
- Tingey S. V., Walker E. L., Coruzzi G. M. Glutamine synthetase genes of pea encode distinct polypeptides which are differentially expressed in leaves, roots and nodules. EMBO J. 1987 Jan;6(1):1–9. doi: 10.1002/j.1460-2075.1987.tb04710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vézina L. P., Langlois J. R. Tissue and Cellular Distribution of Glutamine Synthetase in Roots of Pea (Pisum sativum) Seedlings. Plant Physiol. 1989 Jul;90(3):1129–1133. doi: 10.1104/pp.90.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vézina L. P., Margolis H. A., Ouimet R. The activity, characterization and distribution of the nitrogen assimilation enzyme, glutamine synthetase, in jack pine seedlings. Tree Physiol. 1988 Jun;4(2):109–118. doi: 10.1093/treephys/4.2.109. [DOI] [PubMed] [Google Scholar]
- Wallsgrove R. M., Lea P. J., Miflin B. J. Distribution of the Enzymes of Nitrogen Assimilation within the Pea Leaf Cell. Plant Physiol. 1979 Feb;63(2):232–236. doi: 10.1104/pp.63.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallsgrove R. M., Miflin B. J. Ferredoxin-Sepharose as an affinity absorbent for the purification of glutamate synthase and other ferredoxin-dependent enzymes. Biochem Soc Trans. 1977;5(1):269–271. doi: 10.1042/bst0050269. [DOI] [PubMed] [Google Scholar]