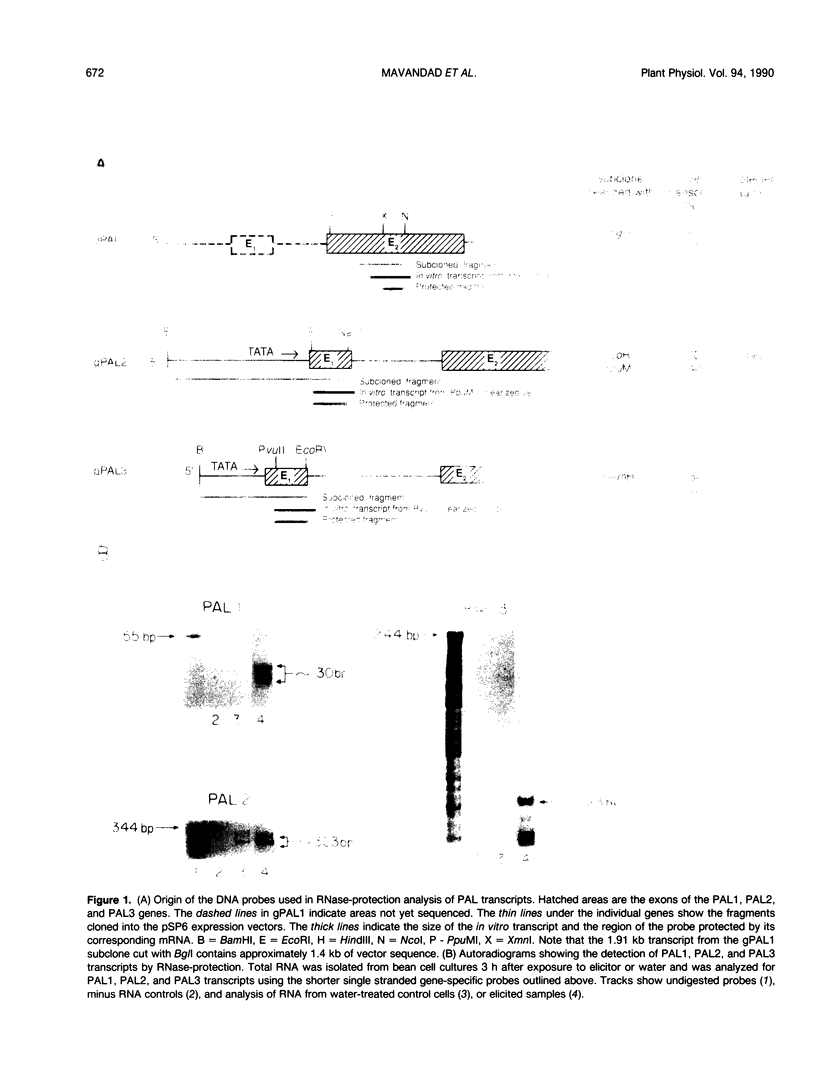
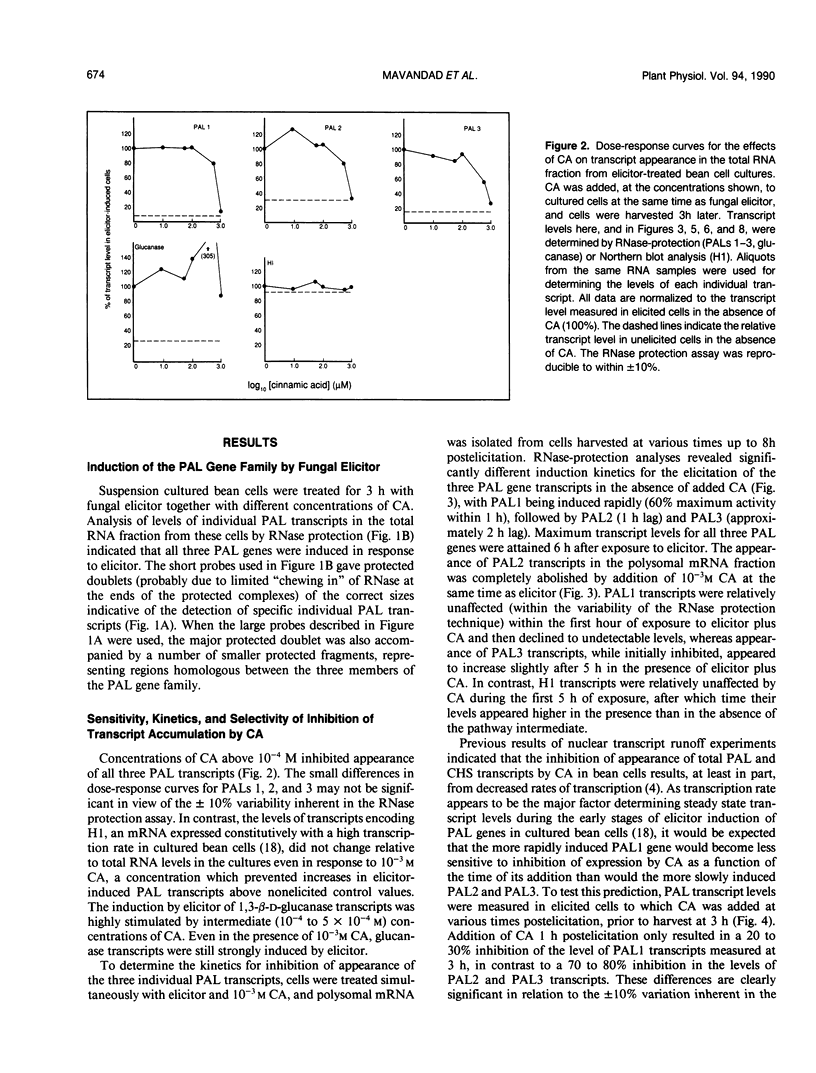
Abstract
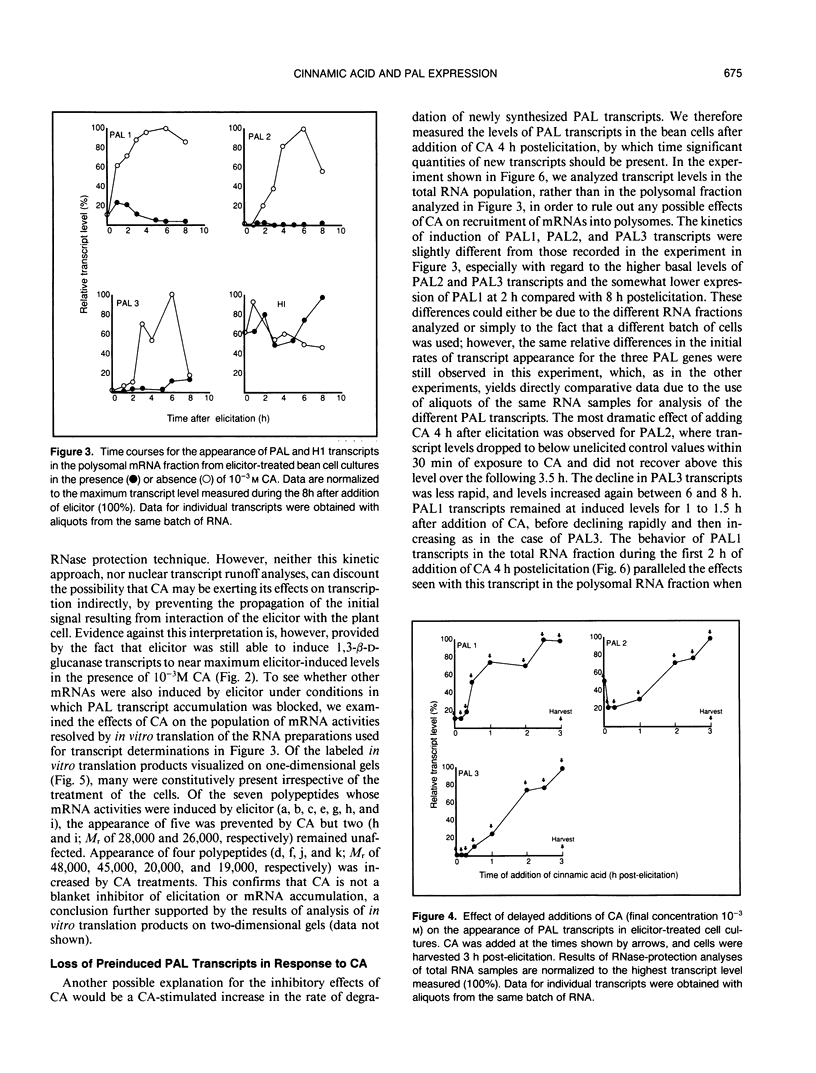
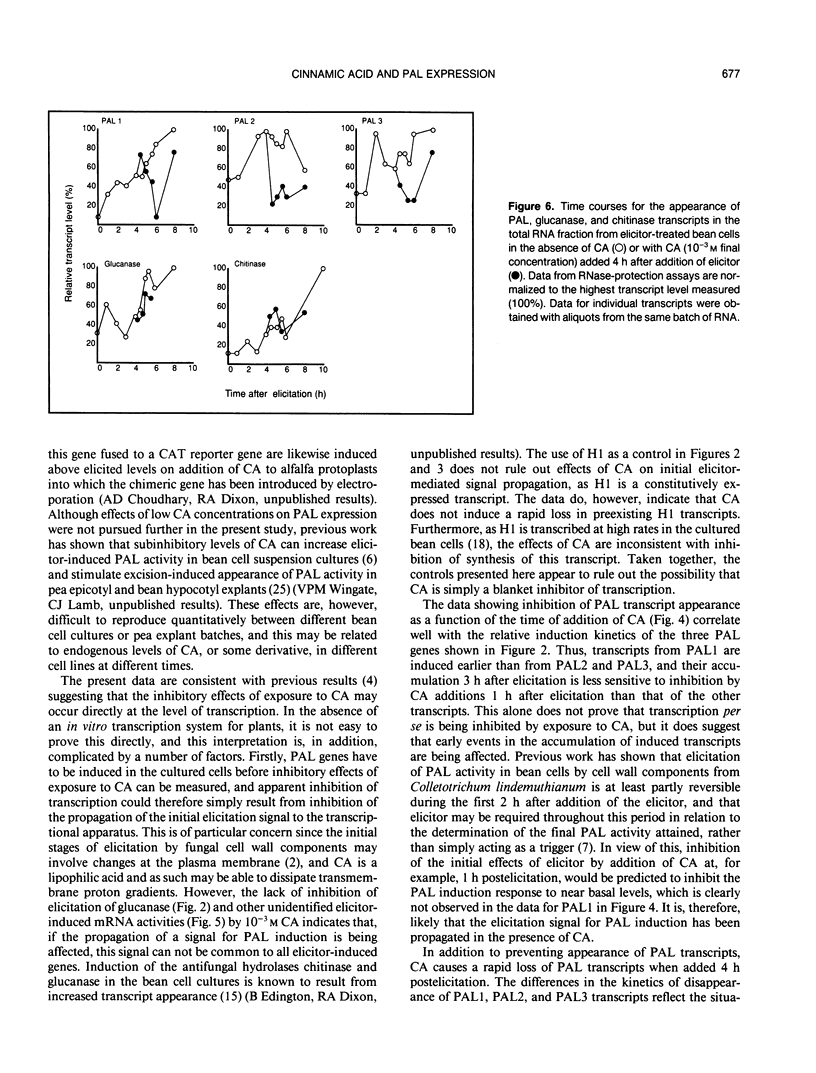
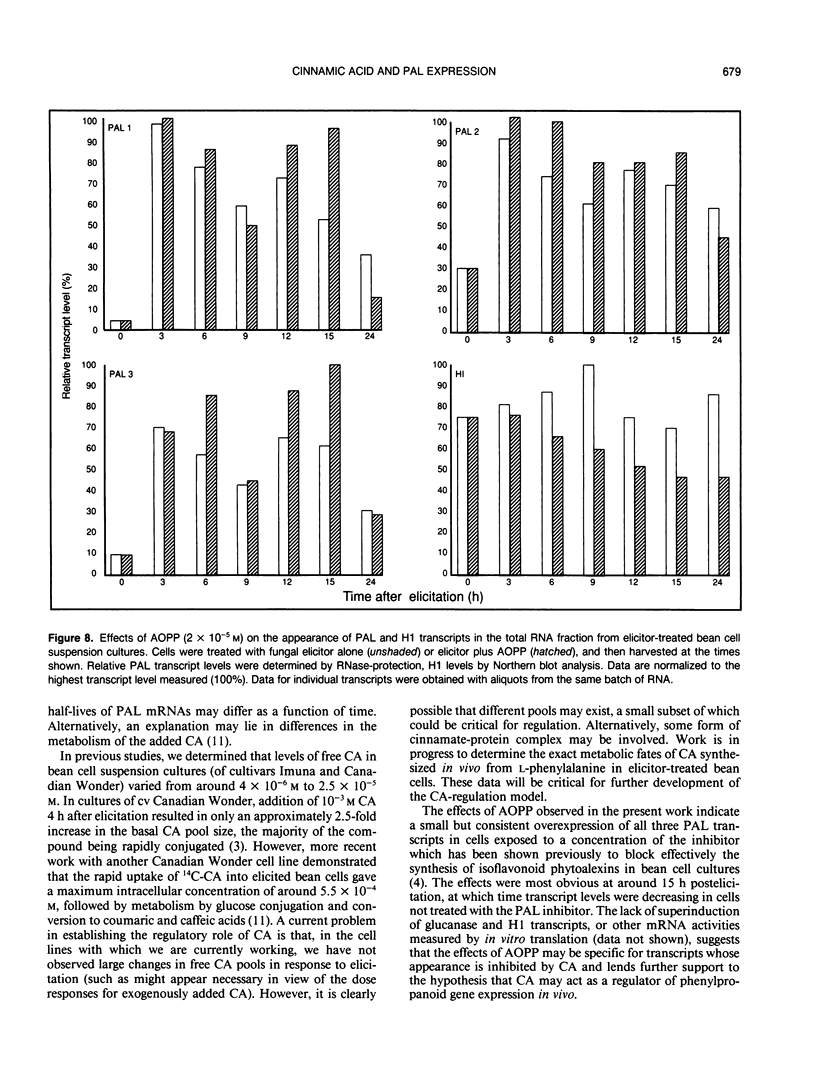
Using DNA probes specific for the three members of the bean (Phaseolus vulgaris L.) phenylalanine ammonia-lyase (PAL) gene family, we have studied the effects of the product of the PAL reaction, trans-cinnamic acid (CA), on the appearance of individual PAL transcripts in suspension cultured bean cells. Concentrations of CA in excess of 10−4 molar inhibited appearance of elicitor-induced transcripts encoding PAL1, PAL2, and PAL3 when added to the cells at the same time as fungal elicitor. Addition of CA 4 hours postelicitation caused a major reduction in the levels of all three PAL transcripts, but with different kinetics and subsequent rates of recovery. The inhibition of accumulation of PAL1, PAL2, or PAL3 transcripts, measured 3 hours after exposure to elicitor, as a function of the time of addition of CA postelicitation reflected the different rates of appearance of the three PAL transcripts in the presence of elicitor alone. The inhibitory effects of CA as seen on PAL transcripts were not observed for the constitutively expressed transcript H1, or the elicitor-inducible 1,3-β-D-glucanase. Analysis of in vitro translated polypeptides showed that some elicitor-induced mRNA activities were not down-regulated by CA, and that a number of other mRNA activities were induced by CA, thus providing further evidence for specificity in the action of CA on bean cells. Treatment of elicited cells with L-α-aminooxy-β-phenylpropionic acid, a potent and specific inhibitor of PAL activity, resulted in maintenance of elevated PAL transcript levels beyond 12 hours postelicitation, this effect being greatest for PAL transcripts 2 and 3. Our results are consistent with a model in which CA, or a derivative, could act as a component in a regulatory feedback system operating at the level of phenylpropanoid gene transcription.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amrhein N., Gerhardt J. Superinduction of phenylalanine ammonia-lyase in gherkin hypocotyls caused by the inhibitor, L-alpha-aminooxy-beta-phenylpropionic acid. Biochim Biophys Acta. 1979 Apr 3;583(4):434–442. doi: 10.1016/0304-4165(79)90060-6. [DOI] [PubMed] [Google Scholar]
- Dixon R. A., Harrison M. J. Activation, structure, and organization of genes involved in microbial defense in plants. Adv Genet. 1990;28:165–234. doi: 10.1016/s0065-2660(08)60527-1. [DOI] [PubMed] [Google Scholar]
- Dixon R. A., Lamb C. J. Stimulation of de novo synthesis of L-phenylalanine ammonia-lyase in relation to phytoalexin accumulation in Colletotrichum lindemuthianum elicitor-treated cell suspension cultures of french bean (Phaseolus vulgaris). Biochim Biophys Acta. 1979 Sep 3;586(3):453–463. doi: 10.1016/0304-4165(79)90035-7. [DOI] [PubMed] [Google Scholar]
- Edwards K., Cramer C. L., Bolwell G. P., Dixon R. A., Schuch W., Lamb C. J. Rapid transient induction of phenylalanine ammonia-lyase mRNA in elicitor-treated bean cells. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6731–6735. doi: 10.1073/pnas.82.20.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Hadwiger L. A., Wagoner W. Effect of Heat Shock on the mRNA-Directed Disease Resistance Response of Peas. Plant Physiol. 1983 Jun;72(2):553–556. doi: 10.1104/pp.72.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick S. A., Bell J. N., Boller T., Lamb C. J. Chitinase cDNA cloning and mRNA induction by fungal elicitor, wounding, and infection. Plant Physiol. 1988 Jan;86(1):182–186. doi: 10.1104/pp.86.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb C. J. Regulation of enzyme levels in phenylpropanoid biosynthesis: characterization of the modulation by light and pathway intermediates. Arch Biochem Biophys. 1979 Jan;192(1):311–317. doi: 10.1016/0003-9861(79)90097-3. [DOI] [PubMed] [Google Scholar]
- Lamb C. J. trans-Cinnamic acid as a mediator of the light-stimulated increase in hydroxycinnamoyl-CoA: quinate hydroxycinnamoyl transferase. FEBS Lett. 1977 Mar 15;75(1):37–40. doi: 10.1016/0014-5793(77)80047-1. [DOI] [PubMed] [Google Scholar]
- Liang X. W., Dron M., Cramer C. L., Dixon R. A., Lamb C. J. Differential regulation of phenylalanine ammonia-lyase genes during plant development and by environmental cues. J Biol Chem. 1989 Aug 25;264(24):14486–14492. [PubMed] [Google Scholar]
- Lois R., Dietrich A., Hahlbrock K., Schulz W. A phenylalanine ammonia-lyase gene from parsley: structure, regulation and identification of elicitor and light responsive cis-acting elements. EMBO J. 1989 Jun;8(6):1641–1648. doi: 10.1002/j.1460-2075.1989.tb03554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami E., Ozeki Y., Matsuoka M., Koizuka N., Tanaka Y. Structure and some characterization of the gene for phenylalanine ammonia-lyase from rice plants. Eur J Biochem. 1989 Oct 20;185(1):19–25. doi: 10.1111/j.1432-1033.1989.tb15075.x. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D. Magnesium precipitation of ribonucleoprotein complexes. Expedient techniques for the isolation of undergraded polysomes and messenger ribonucleic acid. Biochemistry. 1974 Aug 13;13(17):3606–3615. doi: 10.1021/bi00714a032. [DOI] [PubMed] [Google Scholar]
- Schröder J., Kreuzaler F., Schäfer E., Hahlbrock K. Concomitant induction of phenylalanine ammonia-lyase and flavanone synthase mRNAs in irradiated plant cells. J Biol Chem. 1979 Jan 10;254(1):57–65. [PubMed] [Google Scholar]
- Shields S. E., Wingate V. P., Lamb C. J. Dual control of phenylalanine ammonia-lyase production and removal by its product cinnamic acid. Eur J Biochem. 1982 Apr 1;123(2):389–395. doi: 10.1111/j.1432-1033.1982.tb19781.x. [DOI] [PubMed] [Google Scholar]