Although accurate estimates of the rate of occurrence of venous thromboembolism (VTE) in patients with COVID-19 are unknown, results from observational studies show that it is considerably higher than that of critically ill hospitalized patients [1]. This situation raises the question as to whether any of the known coagulation biomarkers could be useful to stratify for the VTE risk and help in adopting tailored antithrombotic prophylaxis. In this respect, D-dimer was considered as the candidate parameter and has been massively used since the outbreak of the pandemic. Retrospective studies tried to determine the risk of VTE according to a predefined threshold of D-dimer at admission [2]. This situation generated reassurance that the predictive value of D-dimer estimated in reported studies could be generalized to the population of patients with COVID-19 when D-dimer is measured with different platforms. As a matter of fact, there are many platforms available, but D-dimer measurement has not yet been standardized [3] and there are no between-platform comparisons for D-dimer levels in patients with COVID-19. Hence, we aimed to compare D-dimer levels measured with 3 widely used platforms in a population of COVID-19.
Ninety-one Caucasian patients hospitalized because of swab-proven COVID-19 (33 women; median age, 73 years; range, 28-78 years), for whom samples were collected at baseline from November to December 2020, were eligible. Eighty-seven had D-dimer results available and were included in this analysis, which was approved by the institutional review board. Blood was collected into 1/10 volumes of trisodium citrate 0.109 M and subjected to single-spin centrifugation for 15 minutes at 2000 g. D-dimer was measured by the latex-based turbidimetric immunoassay STA-DD on a STA coagulometer (Stago). Leftover plasma was frozen within 2 hours and stored at −70 °C until shipping (dry-ice) to the other hospital (Milano), where D-dimer was measured no later than 2 months, using 2 additional immunoturbidimetric platforms (HSD-Dimer on ACLTOP [Werfen] and Innovance D-dimer on Sysmex coagulometer [Siemens]). The 3 platforms report D-dimer as fibrinogen equivalent units and were validated for VTE exclusion with cutoffs of 500 μg/L. Data are reported as medians and IQRs. To assess for equivalence of platforms, Cohen’s κ coefficient was taken as a measure of agreement, and Bland-Altman plots were constructed for paired D-dimer. Cohen’s κ statistic was calculated for samples that were independently classified by the 3 platforms in a priori–defined D-dimer categories (see below and Figure 1).
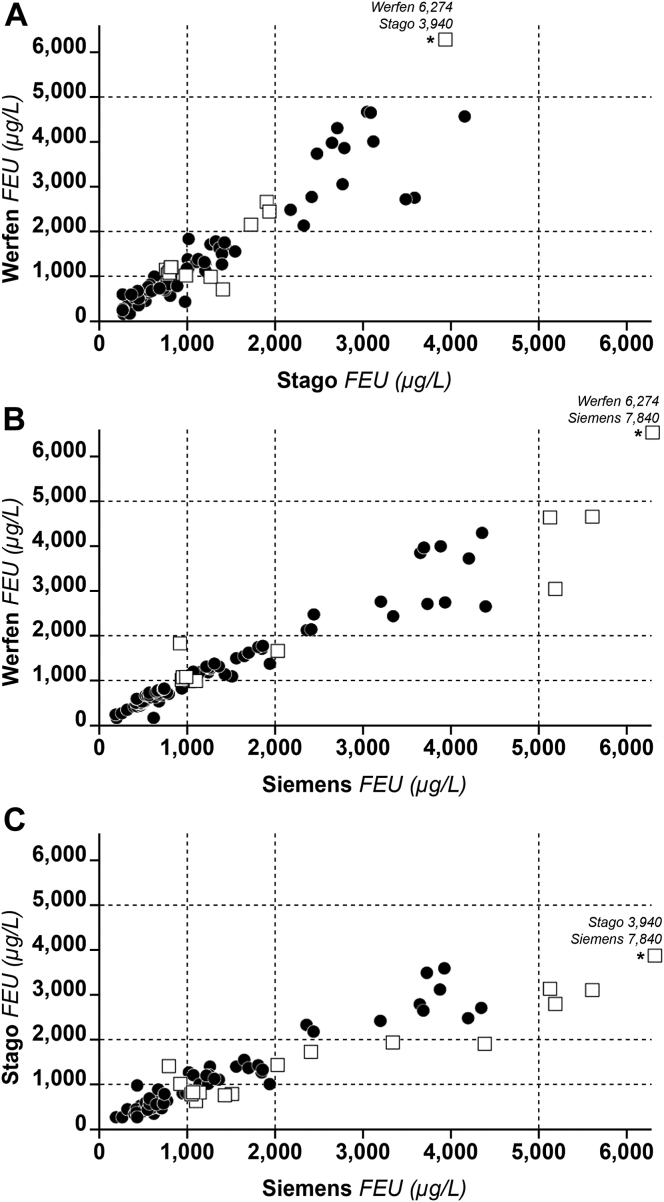
Figure 1.
Scatterplots of D-dimer levels (micrograms per liter) obtained from patients COVID-19 using 3 commercial platforms. Open squares identify samples with discrepant results (see text for more details). Asterisks identify values that were higher than the scale of the graph. Dashed horizontal and vertical lines identify the D-dimer categories corresponding to >1000 to <2000 μg/L, >2000 to <5000 μg/L, or >5000 μg/L. To assess for equivalence of platforms, Cohen’s κ coefficient was taken as a measure of agreement. Cohen’s κ statistic was calculated for samples that were independently classified by the 3 platforms in the above a priori–defined D-dimer categories. The interagreement κ statistics showed good agreement (Stago vs Werfen: κ = 0.76; 95% CI, 0.64-0.88; P < .001; Siemens vs Werfen: κ = 0.81; 95% CI, 0.70-0.90; P < .001; Siemens vs Stago: κ = 0.70; 95% CI, 0.56-0.82; P < .001). It is common practice to consider between-method agreement as insufficient when κ statistic is <0.4, discrete when κ is 0.4 to 0.6, good when κ is 0.6 to 0.8, and excellent when κ is 0.8 to 1.0. FEU, fibrinogen equivalent unit.
The Stago platform measured a slightly lower median D-dimer level (1005 μg/L; IQR, 570-2180) than that measured by the Werfen (1139 μg/L; IQR, 666-2468) or Siemens (1120 μg/L; IQR, 630-2820) platform. The interagreement κ statistics showed good agreement (Stago vs Werfen: κ = 0.76; 95% CI, 0.64-0.88; P < .001; Siemens vs Werfen: κ = 0.81; 95% CI, 0.70-0.90; P < .001; and Siemens vs Stago: κ = 0.70; 95% CI, 0.56-0.82; P < .001). Between-platform agreement was also evaluated for other D-dimer categories that could be of clinical interest. κ values for the category of D-dimer <1000 vs >1000 μg/L ranged from 0.75 (95% CI, 0.60-0.88) for Stago vs Werfen to 0.81 (95% CI, 0.66-0.93) for Werfen vs Siemens. κ values for the category <2000 vs >2000 μg/L ranged from 0.81 (95% CI, 0.66-0.94) for Stago vs Siemens to 0.92 (95% CI, 0.81-1.00) for Werfen vs Siemens. Overall, the above between-platform agreement can be considered acceptable over the entire range of D-dimer values. Scatterplots showed that D-dimer levels measured with the platforms were concordant for most patients (Figure 1). The greatest number (percentage) of discrepant results were recorded for Stago vs Siemens (n = 17, 20%) and the smallest for Werfen vs Siemens (n = 11, 13%). Most discrepancies were recorded for the category 1000 to 2000 μg/L. Between-platform differences were smaller for values <3000 μg/L than for values >3000 μg/L (Figure 2). The estimated median bias was −30 μg/L and −180 μg/L for the comparison of Werfen vs Siemens and Stago vs Siemens, respectively. Concerning the comparison of Werfen vs Stago, an overestimation of the former compared to the latter was observed (median bias Werfen vs Stago, 198 μg/L).
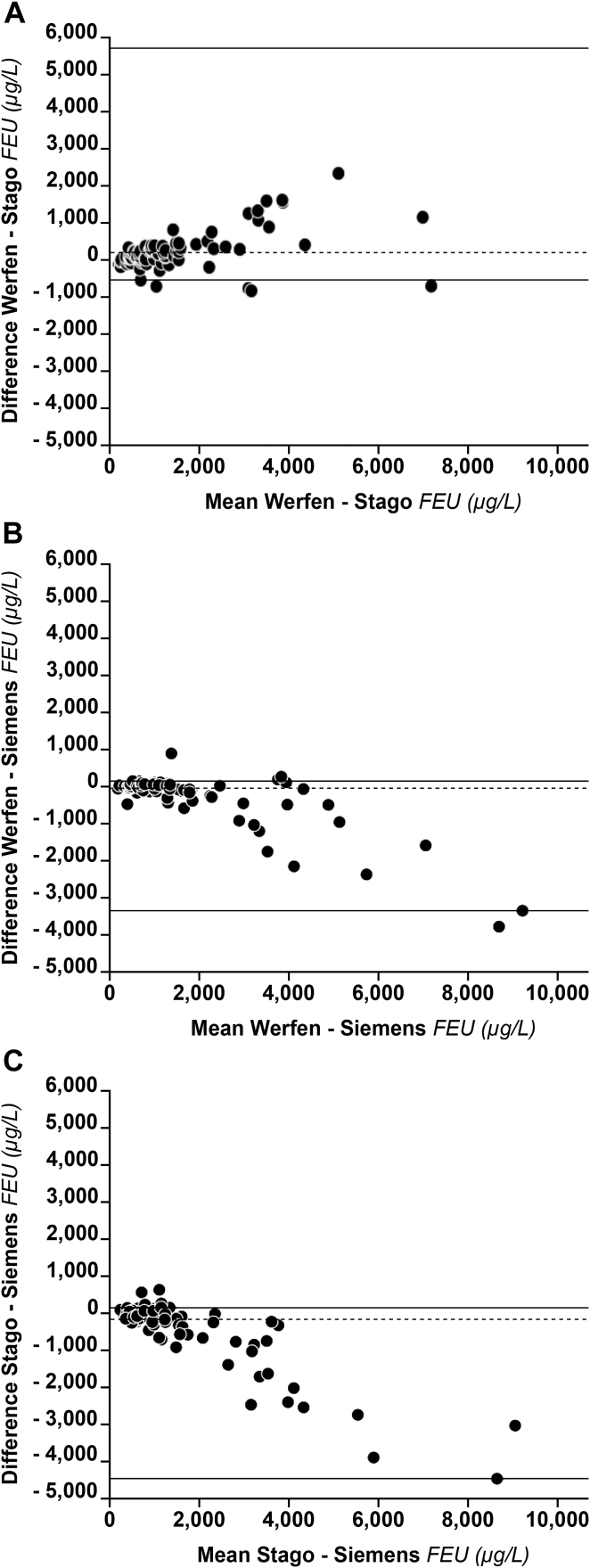
Figure 2.
Bland-Altman plots for D-dimer levels measured with 3 commercial platforms in patients with COVID-19. A nonparametric estimation was used because of the observed highly skewed distribution of differences. Since the between-method differences do not always have a normal distribution as required by a standard Bland-Altman plot, we felt that an approach describing such data without any distribution assumption of differences was preferable. Dashed lines represent the median systematic bias. Solid lines represent the limits of agreement defined as the 5th and the 95th percentile from the distribution of differences. FEU, fibrinogen equivalent unit.
Cumulatively, the observed bias, although measurable, especially at values >3000 μg/L can be hardly considered clinically relevant.
Generally, elevated D-dimer level is considered as the hallmark of coagulation activation, intravascular fibrin deposition, and thrombus formation. However, fibrin deposition may occur in some conditions (especially sepsis, massive infections, and acute respiratory distress syndrome) even in the extravascular compartment [4], where it may undergo digestion by fibrinolysis, thus producing D-dimer. The pool of extravascular D-dimer may eventually enter the vascular system and be measured at the time of blood sampling. This pool, however, should not be considered a biomarker of intravascular fibrin deposition and thrombus formation. It can be speculated that this might be one of the reasons why D-dimer is a useful biomarker in healthy ambulatory subjects suspected of VTE [5] and is of little value in those who are hospitalized and/or critically ill [6]. In the latter, such comorbidities as pneumonia, malignancy, congestive heart failure, etc., may increase D-dimer even in the absence of VTE.
Since the beginning of the COVID-19 pandemic, D-dimer has been extensively used as a biomarker for 3 reasons: (i) to assess prognosis [7,8], (ii) to assess for VTE risk [2], and (iii) to make a decision on the intensity of antithrombotic prophylaxis [9]. The latter received great attention as there was no guidance based on randomized trials to make a decision on the intensity of prophylaxis. Nauka et al. [2] attempted to evaluate the VTE risk in a population of patients with COVID-19 for whom D-dimer levels at admission and imaging-confirmed VTE were available. The authors calculated the odds of developing VTE during admission or within 14 days from discharge based on a priori specified D-dimer categories (the same used in this study). The results showed that the odds of developing VTE increased from the reference (ie, D-dimer of <1000 μg/L) to 2.3 (95% CI, 0.8-73) in the category of >1000 to <2000μg/L, to 2.9 (95% CI, 1.0-8.9) in the category of >2000 to <5000 μg /L, and to 10.7 (95% CI, 3.7-30.3) in the category of >5000 μg/L. The plausible conclusion would be that an appropriate cutoff D-dimer level could be used to assess for the VTE risk and possibly to make a decision on the intensity of prophylaxis. That conclusion and (most importantly) the practical application should, however, be tempered by considering that D-dimer results, when measured with different platforms, may not be comparable and that cutoffs used to make a decision when using one platform are not necessarily applicable to others.
The results of our study, comparing directly for the first time D-dimer levels of patients with COVID-19, show that the 3 platforms presented with good agreement when analyzed cumulatively for their ability to allocate the respective results within the same a priori specified D-dimer categories. When results for individual patients were compared, the highest frequency of discrepancy was recorded for D-dimer between 1000 and 2000 μg/L, and the absolute value of difference increased further when the D-dimer was >3000 μg/L. Another study with a different design showed retrospectively that D-dimer measured with different platforms upon harmonization of results is valuable to assess in-hospital mortality in COVID-19 [10].
In conclusion, the study shows that D-dimer in patients with COVID-19 varies according to the platform used when values are >3000 μg/L, even though the above differences can be hardly considered clinically relevant. The reasons for discrepancies are not completely understood. They are most likely due to different sensitivities/specificities of monoclonal antibodies employed to capture D-dimer from plasma and different standards to express results. Finally, it cannot be excluded that there are other yet unknown causes explaining the observed discrepancies. It is, however, reassuring that when D-dimer levels obtained with the 3 most used platforms were cumulatively compared to assess whether their results are allocated within the same range of values, the between-platform agreement was acceptable. This implies that cutoff values, when used to stratify the VTE risk or prognosis in COVID-19, although not generalizable in absolute terms, may be of value for a broad assessment regardless of the D-dimer platform used. The results and conclusion are valid only for the investigated platforms and should not be generalized to other commercial brands.
Acknowledgments
Funding
The study was partially supported by the Italian Ministry of Health—Bando Ricerca Corrente 2022.
Author contributions
A.T. conceived the study and wrote the manuscript. E.S. and M.C. performed testing and data analyses and reviewed results. M.C., C.D.N., and S.T. recruited patients, collected plasma samples, and reviewed results. S.S. (biostatistician) supervised data analyses. F.P. reviewed results. All the authors reviewed and approved the final manuscript.
Relationship Disclosure
There are no competing interests to disclose.
Footnotes
Handling Editor: Dr Michelle Sholzberg
References
- 1.Porfidia A., Valeriani E., Pola R., Porreca E., Rutjes A.W.S., Di Nisio M. Venous thromboembolism in patients with COVID-19: systematic review and meta-analysis. Thromb Res. 2020;196:67–74. doi: 10.1016/j.thromres.2020.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nauka P.C., Baron S.W., Assa A., Mohrmann L., Jindal S., Oran E., et al. Utility of D-dimer in predicting venous thromboembolism in non-mechanically ventilated COVID-19 survivors. Thromb Res. 2021;199:82–84. doi: 10.1016/j.thromres.2020.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Linkins L.A., Takach Lapner S. Review of D-dimer testing: good, bad, and ugly. Int J Lab Hematol. 2017;39:98–103. doi: 10.1111/ijlh.12665. [DOI] [PubMed] [Google Scholar]
- 4.Idell S. Extravascular coagulation and fibrin deposition in acute lung injury. New Horiz. 1994;2:566–574. [PubMed] [Google Scholar]
- 5.Perrier A., Desmarais S., Miron M.J., de Moerloose P., Lepage R., Slosman D., et al. Non-invasive diagnosis of venous thromboembolism in outpatients. Lancet. 1999;353:190–195. doi: 10.1016/S0140-6736(98)05248-9. [DOI] [PubMed] [Google Scholar]
- 6.Aksamit T.R. Thromboembolism occurrence and diagnosis in the medical intensive care unit. Semin Thromb Hemost. 2001;27:47–57. doi: 10.1055/s-2001-12847. [DOI] [PubMed] [Google Scholar]
- 7.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. Erratum in: Lancet 2020;395:1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stawiarski K., Loutoo A., Velardi L., Zarich S. D-dimer driven deep vein thrombosis prophylaxis strategy for hospitalized patients with COVID-19. Thromb Res. 2021;201:151–153. doi: 10.1016/j.thromres.2021.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.García de Guadiana-Romualdo L., Morell-García D., Favaloro E.J., Vílchez J.A., Bauça J.M., Alcaide Martín M.J., et al. Harmonized D-dimer levels upon admission for prognosis of COVID-19 severity: results from a Spanish multicenter registry (BIOCOVID-Spain study) J Thromb Thrombolysis. 2022;53:103–112. doi: 10.1007/s11239-021-02527-y. [DOI] [PMC free article] [PubMed] [Google Scholar]