Abstract
Background
The emergence of new variants of SARS-CoV-2 has led to the administration of different booster vaccines to mitigate COVID-19. Vaccines with adenoviral vectors have been rarely associated with vaccine-induced immune thrombotic thrombocytopenia (VITT).
Objectives
This study aimed to describe 15 cases of VITT after the third and fourth doses of the COVID-19 vaccine in Brazil.
Methods
Cases were reported after all kinds of anti–SARS-CoV-2 booster vaccinations between October 17, 2021, and September 4, 2022.
Results
Of the 26 suspected cases, 15 cases of VITT were analyzed. Of these, 10 were classified as definite VITT, 2 as probable, 1 as possible, and 2 as unlikely. The estimated frequency of definite, probable, or possible VITT was 0.33 cases per million. Cases were assigned to ChAdOx1 (13 cases), Ad26.COV2.S (1 case), and BNT162b2 (1 case). None of the patients received an adenoviral vaccine as a primary vaccination. The average age of participants was 34 years, and symptoms usually appeared 8 days after vaccination. Headache was the most common symptom, and cerebral veins were the most affected thrombotic site. The overall mortality risk was 53%. Anti-platelet factor 4 enzyme-linked immunosorbent assay serology was positive in 11 out of 15 patients (73.3%), negative in 2 (13.3%), and missing in 2 (13.3%).
Conclusion
The study confirms that VITT is linked to the first exposure to adenoviral vector vaccines. Since January 2023, Brazil has recommended preferably COVID-19 messenger RNA vaccines for individuals aged 18 to 39 years. We suggest that, in the current disease scenario, COVID-19 adenovirus vaccines should not be the first choice for individuals aged <50 years who have not received a previous dose of this type of vaccine.
Keywords: adenoviral-vectored vaccines, booster immunization, Brazil, COVID-19 vaccines, vaccine-induced immune thrombotic thrombocytopenia (VITT)
Essentials
-
•
New variants of SARS-CoV-2 led to the administration of booster vaccines against COVID-19.
-
•
Vaccine-induced immune thrombotic thrombocytopenia (VITT) was recently reported in Brazil.
-
•
We describe 15 cases of suspected VITT after the third and fourth doses of the COVID-19 vaccine.
-
•
The estimated frequency of VITT after the third and fourth ChAdOx1 doses was 0.33 cases per million.
1. Introduction
COVID-19 vaccine rollout began in January 2021 and has since become the primary strategy to mitigate the disease. Newer SARS-CoV-2 variants, such as Omicron, with higher degrees of immune evasion and waning immunity following vaccination have prompted health officials to adopt different booster strategies [1,2]. A homologous booster shot uses the same vaccine previously administered in the primary scheme (first and second doses), while a heterologous booster shot employs a different vaccine [3]. Examples of adenoviral vaccines used in Brazil are ChAdOx1 nCov-19 (Oxford-AstraZeneca) and Ad26.COV2.S (Janssen), while the only messenger RNA (mRNA) vaccine is BNT162b2 (Pfizer–BioNTech).
Homologous and heterologous boosters with ChAdOx1 nCov-19, mRNA-1273 (Moderna), Ad26.COV2.S, BNT162b2, or Sinovac-CoronaVac vaccines presented an acceptable safety profile. All booster vaccines proved immunogenic in adults who had completed the primary COVID-19 vaccine regimen [[3], [4], [5]]. Immunogenicity studies demonstrated an apparent superiority of mRNA booster strategies, irrespective of the primary vaccine series, followed by adenoviral-vectored and inactivated vaccines [3,6].
ChAdOx1, Ad26.COV2.S, and BNT162b2 are available as booster doses in Brazil. Sinovac-CoronaVac, due to its reduced immunogenicity, is offered as a booster only in particular situations, such as the unavailability of other vaccines or when other vaccines are contraindicated. Initially, the preferred option for booster doses in the country was mRNA vaccines. Still, due to availability issues and the need to increase booster dose vaccination coverage, adenoviral-vectored vaccines were also offered for such a strategy, irrespective of the primary vaccination series [7]. Thus, many people were exposed to adenoviral-vectored COVID-19 vaccines during booster shots for the first time.
Despite the significant benefits associated with COVID-19 vaccination (millions of deaths averted worldwide) and a good safety profile, the adenoviral-vectored vaccines, particularly ChAdOx1 and Ad26.COV2.S, have been related to the development of cases of vaccine-induced immune thrombotic thrombocytopenia (VITT) 5 to 30 days after the exposure [8]. Thrombosis with thrombocytopenia syndrome (TTS) is a descriptive term; however, this condition is not necessarily caused by vaccination [9]. VITT is a rare but life-threatening adverse event, with a significantly higher risk among those aged <55 years [10]. Despite mainly being reported after the first vaccine dose, 4 cases of cerebral venous thrombosis due to VITT after a second ChAdOx1 nCoV-19 dose were reported [11].
Bio-Manguinhos/Fiocruz is a public pharmaceutical industry related to the Ministry of Health (MoH), and after the vaccine technology transfer agreement with AstraZeneca to produce ChAdO1x, it has become a substantial supplier of COVID-19 vaccines to Brazil. Bio-Manguinhos/Fiocruz has distributed more than 188 million ChAdOx1 doses, and around 33 million were used as booster doses [12]. Bio-Manguinhos/Fiocruz and the National Immunization Program/MoH developed a collaborative initiative to facilitate early diagnosis of VITT in suspected cases and its treatment [13,14]. Some case series have been reported globally, including one from our group describing VITT cases after Brazil’s first and second doses [13,15,16]. Nevertheless, reporting this rare adverse event is still relevant because it adds information to the scarce evidence about this potentially severe disease. Herein, we describe a series of 15 VITT cases after the third and fourth doses of COVID-19 vaccines in Brazil.
2. Methods
This work describes VITT cases reported to Bio-Manguinhos/Fiocruz or the National Immunization Program/MoH following all kinds of anti–SARS-CoV-2 booster vaccinations in Brazil from October 17, 2021, until September 4, 2022. The local ethics committee approved the study (CAAE #52396621.0.0000.5262). Informed consent was waived for this study because notification of cases suspicious for VITT was compulsory in Brazil.
2.1. Eligibility criteria
The inclusion criteria were as follows: complete COVID-19 vaccination data, including date and type/brand of the vaccine, TTS levels 1, 2, or 3 according to the Brighton Collaboration case definition [17], and causality criteria established as definite or indeterminate, according to the Council for International Organizations of Medical Sciences (CIOMS)/World Health Organization (WHO) causality assessment for adverse events following immunization [17,18]. Reports in which it was impossible to retrieve the clinical/laboratory data necessary for evaluation were excluded.
2.2. VITT case definitions
VITT cases were defined according to the Brighton Collaboration criteria and CIOMS/WHO causality assessment [17,19]. The Brighton Collaboration interim definition criteria for VITT focus on providing a standardized framework for identifying and classifying cases. These criteria help in VITT’s accurate and consistent reporting, surveillance, and research. Causality assessment with vaccination refers to the process of determining the likelihood and strength of the relationship between a vaccine and an adverse event. Causality assessment involves evaluating various factors, such as temporal association, biological plausibility, alternative explanations, and available scientific evidence. It aims to determine if the adverse event is a result of the vaccine or if it occurred coincidentally. In summary, the Brighton Collaboration criteria for VITT focus on defining and categorizing cases associated with vaccines, while causality assessment with vaccination involves assessing the causal relationship between a vaccine and an adverse event. Confirmed cases were those that were classified as level 1 VITT and had positive anti-platelet factor 4 (anti-PF4) antibody dosage (enzyme-linked immunosorbent assay [ELISA]) and no other identifiable causes that could explain the event. Then, the included subjects underwent further investigation and were categorized based on the UK Expert Hematology Panel VITT case definition [15], which involved 5 criteria: the timeframe between vaccination and symptom onset (5-30 days or up to 42 days for isolated deep vein thrombosis [DVT] and pulmonary embolism [PE]), presence of thrombocytopenia, evidence of thrombosis, D-dimer value, and anti-PF4 dosage. The case is classified as definite VITT if all 5 criteria are met. The level of diagnostic certainty decreases as the number of criteria met decreases [15].
2.3. Laboratory and image testing
Laboratory and imaging tests were performed during routine care, according to the availability of the healthcare institution responsible for the patient. All collected data were registered in electronic spreadsheets. Regarding anti-PF4 ELISA, tests were performed in private laboratories for 3 patients and at Arthur de Siqueira Cavalcanti State Institute of Hematology (MoH national reference for the diagnosis of VITT) for 10 patients. In 2 patients, this test was not performed. Lifecodes PF4 immunoglobulin G assay and the enhanced ELISA assay (immunoglobulin G, immunoglobulin A, and immunoglobulin M) (Immucor) were performed in Arthur de Siqueira Cavalcanti State Institute of Hematology according to the manufacturer’s instructions. Positivity was defined as an optical density ≥0.40. These data were not available for private laboratory tests. Depending on the affected site, the imaging method used to confirm thrombosis varied, such as computed tomography, computed tomography angiography, Doppler ultrasound, or echocardiography.
2.4. Clinical parameters
The variables analyzed in the study included age, sex, vaccine manufacturer for both primary and booster doses, application date, and vaccine dose (third or fourth). The clinical characteristics were also evaluated, including symptom onset, time to hospital admission, comorbidities, previous use of heparin, and risk factors for thrombosis. Symptoms indicative of thromboses, such as headache, visual blurring, seizure, altered level of consciousness, focal neurological signs, abdominal pain, chest pain, dyspnea, and pain/edema/pallor of lower or upper limbs, were assessed. Additionally, treatment details and outcomes (alive or deceased) were described. Laboratory test results were examined, including platelet count, D-dimer levels, imaging of thrombosis and its location, as well as anti-PF4 ELISA antibody results.
2.5. Statistical analysis
To characterize cases of VITT, descriptive statistics, including frequencies, percentages, medians, and SDs, were employed for variables such as age, sex, vaccine manufacturer, timeframe between vaccination and symptom onset, time between symptom onset and anti-PF4 testing, anti-PF4 positivity, and clinical outcome. The clinical characteristics of suspected VITT cases and vaccination-related information were summarized using frequency and contingency tables, presenting absolute frequencies and percentages. Statistical analysis was performed with International Business Machines SPSS Statistics version 20 (IBM).
3. Results
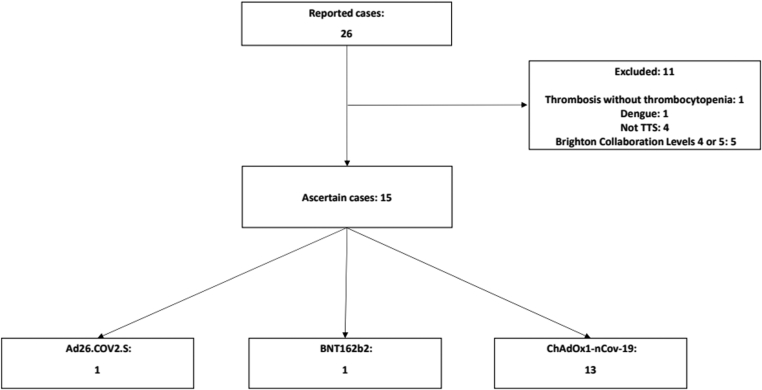
This series of cases evaluated 26 cases that were initially reported as VITT following booster doses of COVID-19 vaccines used in Brazil. After a pharmacovigilance investigation, 11 cases were excluded as they were categorized as level 4 (8 cases) or level 5 (3 cases) according to the Brighton Collaboration classification for TTS [17]. The authors evaluated the remaining 15 cases with the aim of establishing a causal relationship with the vaccine. Thirteen cases were reported after ChAdOx1, 1 was reported after Ad26.COV2.S, and 1 was reported after BNT162b2 (Figure 1). According to the Brighton Collaboration criteria, all cases were classified as level 1 for VITT. As shown in Table 1, 11 cases were classified as having a consistent causal relationship with the vaccine, according to the CIOMS/WHO adverse events following immunization causality assessment criteria (73.3%). Confirmed cases were those that were classified as level 1 and had positive anti-PF4 ELISA and no other identifiable causes that could explain the event. Possible cases were those that were classified as level 1 according to the Brighton classification, had no other possible cause, and lacked the availability of an anti-PF4 test result. Improbable cases were those where, despite a level 1 certainty for VITT, the anti-PF4 test result was negative, and there was a possibility of other diagnoses. According to these criteria, there were 11 confirmed cases, 2 possible cases, and 2 improbable cases. Considering the UK Panel of Hematology Experts criteria, VITT cases were classified as definite (10), probable (2), possible (1), or unlikely (2). For rate calculation, the confirmed, probable, and possible cases were included, totaling 13 cases [13].
Figure 1.
Flowchart illustrating the study design. TTS, thrombosis with thrombocytopenia syndrome.
Table 1.
Distribution of participants according to classifications.
| Classification | Alive | Deceased | Totala |
|---|---|---|---|
| CIOMS/WHO (AEFI) | |||
| A1—consistent | 5/6 (83.3) | 5/8 (62.5) | 11/15 (73.3) |
| B1—indeterminate | 1/6 (16.7) | 3/8 (37.5) | 4/15 (26.7) |
| Total | 6 | 8 | 15 |
| UK Expert Hematology Panel criteria | |||
| Definite | 5/6 (83.3) | 5/8 (62.5) | 10/15 (66.7) |
| Probable | 0/6 (0) | 1/8 (12.5) | 2/15 (13.3) |
| Possible | 0/6 (0) | 1/8 (12.5) | 1/15 (6.7) |
| Unlikely | 1/6 (16.7) | 1/8 (12.5) | 2/15 (13.3) |
| Total | 6 | 8 | 15 |
Data are reported as N or n/N (%).
AEFI, adverse events following immunization; CIOMS, Council for International Organizations of Medical Sciences; WHO, World Health Organization.
One case has no outcome information.
According to Table 2, the median age was 34 years (range, 21-47 years), and 8 patients were women (53.3%). All 10 patients who had their race identified belonged to the White race (66.7%). We did not have data on race/ethnicity in 5 cases (33.3%). Most participants were younger than 40 years of age (11/15, 73.3%). The median number of days between vaccination and symptom onset was 8 (range, 2-16). The median baseline platelet count was 33,000/mm3 (range, 1100-123,000), and the median D-dimer level was 11,618 fibrinogen equivalent units (FEUs) (range, 500-75,000 FEU). Anti-PF4 ELISA serology was positive in 11 out of 15 patients (73.3%), negative in 2 (13.3%), and missing in 2 (13.3%). The median time between symptom onset and hospital admission in deceased and alive groups was 3.5 days and 1.5 days, respectively. The overall median time from symptom onset to anti-PF4 ELISA testing was 27 days (range, 10-86 days); however, this information was missing in 3 cases.
Table 2.
Distribution and descriptive statistics of the clinical and laboratory characteristics of the participants, Brazil, 2021.
| Variable | Alive | Deceased | Totala |
|---|---|---|---|
| Age (y) | |||
| Median (IQR) | 31.5 (26.25-35.75) | 37.5 (29.50-39.75) | 34 (28-40) |
| Range | 21-41 | 22-47 | 21-47 |
| Total | 6 | 8 | 15 |
| Sex | |||
| Female | 0/6 (0) | 7/8 (87.5) | 8/15 (53.3) |
| Male | 6/6 (100) | 1/8 (12.5) | 7/15 (46.7) |
| Total | 6 | 8 | 15 |
| Race/ethnicity | |||
| White | 5/6 (83.3) | 4/8 (50) | 10/15 (66.7) |
| Non-White | 0 | 0 | 0 |
| Not informed | 1/6 (16.7) | 4/8 (50) | 5/15 (33.3) |
| Total | 6 | 8 | 15 |
| Days since vaccination | |||
| Median (IQR) | 7 (8) | 9 (4) | 8 (6) |
| Range | 2-16 | 6-16 | 2-16 |
| Total | 6 | 8 | 15 |
| Days between symptoms and admissionb | |||
| Median (IQR) | 3.5 (8.25) | 1.5 (2) | 2.5 (4) |
| Range | 0-12 | 0-9 | 0-12 |
| Total | 6 | 8 | 14 |
| Platelets per cubic millimeter (lower value) | |||
| Median (IQR) | 38,500 (89,065.75) | 21,500 (31,862.5) | 33,000 (39,000) |
| Range | 1337-123,000 | 1100-64,000 | 1100-123,000 |
| Total | 6 | 8 | 15 |
| D-dimer (FEU) (upper value)c | |||
| Median (IQR) | 15,318 (25,949.5) | 11,235 (11,855) | 11,617.5 (13,724.5) |
| Range | 500-61,800 | 690-20,000 | 500-75,000 |
| Total | 6 | 5 | 12 |
| Anti-PF4 ELISAd | |||
| Positive | 5/6 (83.3) | 6/6 (100) | 11/13 (84.6) |
| Negative | 1/6 (16.7) | 0/6 (0) | 2/13 (15.4) |
| Total | 6 | 6 | 13 |
| Vaccine received | |||
| ChAdOx1 nCov-19 | 5/6 (83.3) | 8/8 (100) | 13/15 (86.7) |
| Ad26.COV2.S | 1/6 (16.7) | 0/8 (0) | 1/15 (6.7) |
| BNT162b2 | 0/6 (0) | 0/8 (0) | 1/15 (6.7) |
| Total | 6 | 8 | 15 |
| Dose received | |||
| Third | 4/6 (66.7) | 2/8 (25) | 7/15 (46.7) |
| Fourth | 2/6 (33.3) | 6/8 (75) | 8/15 (53.3) |
| Total | 6 | 8 | 15 |
Data are reported as N or n/N (%), unless otherwise specified.
Anti-PF4, anti-platelet factor 4; ELISA, enzyme-linked immunosorbent assay; FEU, fibrinogen equivalent units.
One case has no outcome information.
Information not available for 1 case.
Information not available for 3 cases.
Information not available for 2 cases.
Seven patients received a third dose (46.7%), and 8 received a fourth (53.3%) dose of the vaccine against SARS-CoV-2. None of the patients received an adenoviral vector vaccine as the primary or first homologous booster dose (previous COVID-19 vaccines were CoronaVac or BNT162b2). Although not statistically tested, there seems to be a trend toward older age, female sex, and lower platelet count in the deceased group, according to Table 2. The median age of the deceased and alive groups was 37.5 and 31.5 years, respectively. Also, among the deceased patients, 7 were women (87.5%) and 1 (12.5%) was a man. The median platelet count in the deceased group was 21,500/mm3, as opposed to 38,500/mm3 in the alive group. A detailed case description is disclosed in the Supplementary Table.
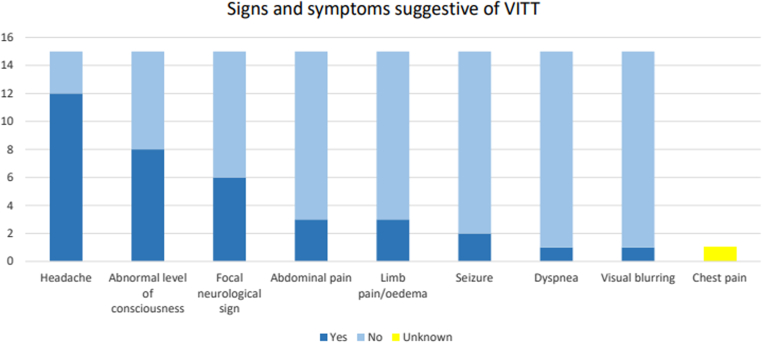
The most frequent signs and symptoms of VITT were headache in 12 cases (80%). Eight patients had abnormal consciousness levels (53.3%); 6 had focal neurological deficits (40%); 3 had abdominal pain (20%); 3 had pain, swelling, or limb pallor (20%); and 2 had seizures (13.3%), as shown in Figure 2.
Figure 2.
Distribution of signs and symptoms suggestive of vaccine-induced immune thrombosis with thrombocytopenia (VITT) observed in our cases.
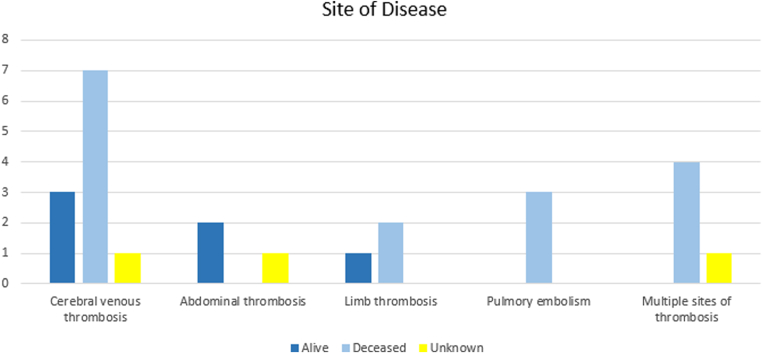
All 15 cases exhibited venous thrombosis. Two patients presented thrombosis at arterial cerebral beds as well: one with subarachnoid hemorrhage and right subclavian vein thrombosis and the other with cerebral venous sinus thrombosis (CVST), intracranial hemorrhage, DVT, and PE. Microvascular thrombosis, such as thrombotic microangiopathic anemia, was not observed. Cerebral veins were the affected thrombotic site in 11 individuals (73.3%), as shown in Figure 3. In 8 patients, CVST was complicated by secondary intracranial hemorrhage (53.3%). There were 3 cases of abdominal vein thrombosis (20%), 3 cases of thrombosis in the upper or lower limb veins (20%), 3 cases of PE (20%), and 5 cases of multiple thrombotic sites (33.3%). Nine patients (60%) exhibited thrombotic risk factors: smoking (2), 1 of whom also presented sickle cell disease (1); previous DVT (2); oral contraceptive medication use (3); and obesity (2).
Figure 3.
Sites of disease in the 15 ascertain patients.
Treatment information was available for all the patients. Intravenous immunoglobulin (IVIG), systemic glucocorticoids, and nonheparin anticoagulation were used in 6 (40%), 4 (26.7%), and 8 (53.3%) of the 15 patients, respectively. Six patients underwent a surgical procedure: decompressive craniectomy (4), emergency limb fasciotomy (1), and inferior vena cava filter implant (1). No patient received plasma exchange therapy or rituximab. Five patients received blood transfusions (53.3%): platelets (3), plasma (1), red blood cells (1), and an unreported blood cell component (1). Information about previous heparin use was unavailable for 11 patients (73.3%). Two patients reported previous heparin use, and 2 did not. Despite the current guidelines, 3 individuals (20%) received heparin as anticoagulant treatment—1 patient died and 2 survived. Overall, 8 patients died, 6 survived, and 1 had no outcome information. Thus, the overall mortality risk was 53%. Considering only patients with CVST and those with CVST complicated by intracranial hemorrhage, the mortality rates were 64% and 88%, respectively.
Considering the number of ChadOx1 vaccine doses administered in the period in Brazil, the rate of VITT, using the data of our case series, was 0.39 cases per million doses administered (or 35 cases in 89,773,509 for first and second doses) [12,13]. For booster doses, the rate of VITT was 0.33 cases per million doses administered (or 13 cases in 39,305,270 for the third or fourth dose).
4. Discussion
Our study presented VITT data consistent with those reported in the international literature. VITT is far more common after the first dose of an adenoviral vector vaccine rather than after subsequent doses [20]. This case series described individuals who developed VITT after the third or fourth anti–SARS-CoV-2 vaccine dose but received adenoviral vaccines for the first time. The rate of VITT after booster doses was similar to the first- and second-dose VITT rates. The estimated frequency of VITT after the third and fourth ChAdOx1 doses was 0.33 cases per million.
The median age at diagnosis of the cohort in this study was comparable to that of the British cohort of 220 individuals (34 and 48 years old, respectively) [15]. The incidence of VITT among persons aged <50 years in the United Kingdom was 1:50,000, which was compatible with other reports [15]. The worst-case scenario was observed in Norway, where the incidence of VITT was considerably higher in the <55-years age group, with medium and high estimates ranging from 1 in 60,000 persons to 1 in 20,000 persons [10].
The first case of VITT after COVID-19 booster vaccination was described in a 34-year-old man with right testicular edema and an excruciating headache [21] and is included in this case series. Our previous study described 39 VITT cases that developed during primary vaccination (first and second doses) [13]. Similar to our previous study’s findings, here we noticed that cental nervous system veins were the most common site of thrombosis. The median number of days between vaccination and symptom onset was the same, and the median platelet count and D-dimer values resembled those in the case series. The median time between symptom onset and hospital admission was lower among survivors compared to the deceased group in both case series, which emphasizes the urgency to treat this disease. We did not observe age and sex differences in mortality in our previous study, where 10 (41.6%) of 24 women and 10 (66.6%) of 15 men died, and the median ages in the deceased and alive groups were 37 and 45.5 years, respectively [13]. However, we did notice a lower platelet count trend in our previous case series (23,000/mm3 and 46,000/mm3 for the deceased and alive groups, respectively). This finding could be related to a higher risk of cerebral bleeding in patients with cerebral thrombosis. Pavord et al. [15] identified the baseline platelet count and the presence of intracranial hemorrhage as being independently associated with death, with 73% mortality among patients with platelet counts below 30,000/mm3 and intracranial hemorrhage. The apparent age, sex, and platelet count differences in mortality in this work could have occurred by chance due to a small number of cases. Future meta-analysis should gather information from local case series and test these hypotheses.
Of note, 2 cases here were classified as unlikely VITT using the UK Expert Hematology Panel criteria. Despite not fulfilling all 5 diagnostic criteria, these 2 cases were nonetheless suggestive of VITT due to the presence of other typical findings: the time frame between vaccination and symptoms in these cases was 2 and 16 days; both presented limb pain and swelling—one of them also presented with altered conscious level and dyspnea—and thrombocytopenia (123,000/mm3 and 40,000/mm3). One patient showed external iliac vein thrombosis, and the other had intracardiac thrombosis and suspected PE. However, the D-dimer level was not high in these cases (500 and 690 FEU), and 1 patient presented a negative anti-PF4 ELISA, while it was not available in the other one. Because of the lack of clinically relevant details, these cases were described in this work but were excluded from the VITT rate calculation.
By comparing both case series, we found that the median age in the booster vaccination group was lower than that in the primary scheme group (34 vs 41 years) [13]. This might be explained by younger people or healthcare workers who received BNT162b2 and CoronaVac as the primary scheme (due to availability) and an adenoviral vaccine as a booster. In the United Kingdom and Australia, educational programs have been developed to reduce mortality risk due to VITT, which was reduced to 22% and 5.6%, respectively [15,22]. Despite all efforts, we identified that 28 of 54 patients died (51.8% mortality rate), which was more significant than in these countries. Few of our cases were treated with IVIG, the standard VITT treatment. This finding is likely attributable to diagnostic and therapeutic challenges and IVIG shortage.
At least 5 countries have instituted restrictions on the ChAdOx1 nCoV-19 vaccine based on age [23], and in May 2022, the US Food and Drug Administration issued restrictions on the administration of Ad26.COV2.S in the United States [24]. In Brazil, since January 2023, persons aged 18 to 39 should preferentially receive mRNA-based COVID-19 vaccines; however, viral vector vaccines can be used in remote places or places with unavailable mRNA-based vaccines [25]. Before this period, there were no age-related restrictions to adenoviral vaccines. Contraindications to adenoviral vector vaccines remain for pregnant women after the first VITT case report in pregnant women in 2021 [26]; individuals with severe side effects of previous doses, such as hypersensitivity to components, anaphylactic reactions, VITT or capillary-leak syndrome, and individuals aged <18 years [27]. Apart from being aged <50 years and having the first dose of the adenoviral vaccine, there are no known risk factors for VITT development [8]. Genetic predisposition is suggested based on worldwide incidences and recent anti-PF4 oligoclonality data [28]. Bio-Manguinhos/Fiocruz is conducting an ongoing study investigating genetic susceptibility to rare and severe ChadOx1 adverse effects, including VITT (ClinicalTrials.gov identifier: NCT05630313). The first Brazilian case report of VITT with genetic analysis was recently published by our group, describing a patient who developed VITT after the first dose of ChadOx1 [29]. Which vaccine constituent triggers the immune response to PF4 and why the most affected thrombosis sites are cerebral and abdominal in patients with VITT remain as knowledge gaps. Despite not being so frequently reported as in Europe, Australia, and the United States, VITT remains relevant in low- and middle-income countries where adenoviral vector-based vaccines are the cornerstone of COVID-19 vaccination.
Our study has limitations regarding selection bias, underreporting, surveillance failure, challenges in clinical investigation, and missing important examinations of few suspected cases. The strengths include a nationwide well-documented report of VITT during the heterologous booster COVID-19 vaccination campaign. Herein, we added information about demography and clinical laboratory case characterization.
5. Conclusions
Despite the educational initiative, there are concerns about underrecognition and appropriate treatment. Our data reinforce that VITT is linked to the first exposure to an adenoviral vector vaccine. Policymakers should consider the COVID-19 disease burden and the vaccine's side effects, costs, and availability. In Brazil, adenoviral vaccines were critical during primary vaccination to help control the spread of COVID-19 and reduce the mortality risk. Nevertheless, we should consider the changes in COVID-19 epidemiology, the availability of alternative vaccines, and the potential severity of VITT in younger patients. Based on our experience, we suggest that, in the current disease scenario, adenoviral-vectored COVID-19 vaccines should not be administered to individuals aged <50 years who have never received a previous dose of an adenoviral COVID-19 vaccine.
Appendix
Following are the members of the Brazilian VITT Investigative Collaboration Group:
-
•
Anna Paula Bise Viegas and Liliam Cristiana Júlio: Florianópolis, Santa Catarina, Brazil;
-
•
Ana Paula Pietrowski Bertuol: Brasília, Distrito Federal, Brazil;
-
•
Eder Gatti Fernandes: São Paulo, São Paulo, Brazil;
-
•
Risoleide Marques de Figueiredo and Rosane Ferreira: Rio de Janeiro, Rio de Janeiro, Brazil;
-
•
Cláudia Weingaertner Palm and Marion Burger: Curitiba, Paraná, Brazil;
-
•
Diogenes Seraphim Ferreira, Georgia Karina Morgenstern, and Tsukiyo Obu Kamoi: Curitiba, Paraná, Brazil;
-
•
Isis Mattos de Carvalho, Juliana Jenifer da Silva Araújo Cunha, Nadja Greffe, Nathalya Macedo Nascimento Costa, and Thaina Genuino de Souza: Rio de Janeiro, Rio de Janeiro, Brazil;
-
•
Renata Loss Lima Frizzera: Vitória, Espírito Santo, Brazil;
-
•
Barbara Emoingt Furtado: Cotia, São Paulo, Brazil;
-
•
Isis Mattos de Carvalho, Juliana Jenifer da Silva Araújo Cunha, Gabriele Tantos Nunes, and Tatiana Garcez: Rio de Janeiro, Rio de Janeiro, Brazil;
-
•
Rubens Costa Filho: Rio de Janeiro, Rio de Janeiro, Brazil;
-
•
Juliana Vassalo Rodrigues: Niterói, Rio de Janeiro, Brazil;
-
•
Luzia Zago: Uberaba, Minas Gerais, Brazil;
-
•
Claudio Renato Freire: Piracicaba, São Paulo, Brazil.
Acknowledgments
We thank the entire Department of Medical Affairs, Clinical Studies, and Post-Registration Surveillance (DEAME) of Bio-Manguinhos/Fiocruz, the Ministry of Health and local surveillance teams, the Ministry of Health and local immunization teams, and healthcare professionals that made the vaccination campaign possible and successful in Brazil. We are grateful to the Brazilian VITT Investigative Collaboration Group for engaging in case investigation and helpful discussions.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. Bio-Manguinhos/Fiocruz paid the article processing charges.
Ethics statement
The local ethics committee approved the study (CAAE #52396621.0.0000.5262). Informed consent was waived for this study because notification of cases suspicious for vaccine-induced immune thrombotic thrombocytopenia was compulsory in Brazil.
Author contributions
D.P.M.-d.-A. and V.B.G.P. conceptualized the study, performed clinical analysis, and wrote the original draft of the manuscript. L.M.d.A.F. wrote the manuscript and performed the anti-platelet factor 4 serology. R.S.P., P.M.N.d.O., P.R.G.T., L.K.L., G.V.T., T.d.S.P., and D.L.A. curated the data, wrote the manuscript, investigated the clinical and diagnostic data, and developed the methodology. J.R.X. and T.d.M.d.C. performed statistical analysis. M.d.L.d.S.M. conceptualized the study; acquired funding; and wrote, reviewed, and edited the manuscript.
Relationship disclosure
D.P.M.-d.-A., P.M.N.d.O., R.S.P., P.R.G.T., L.K.L., G.V.T., T.d.S.P., D.L.A., J.R.X., T.d.M.d.C., and M.d.L.d.S.M. work for Bio-Manguinhos/Fiocruz, the producer of ChadOx1 nCoV-19 in Brazil.
Footnotes
Handling Editor: Dr Vânia Morelli
The online version contains supplementary material available at https://doi.org/10.1016/j.rpth.2023.102243
Contributor Information
Daniela P. Mendes-de-Almeida, Email: daniela.mendes@bio.fiocruz.br.
The Brazilian VITT Investigative Collaboration Group:
Anna Paula Bise Viegas, Liliam Cristiana Júlio, Ana Paula Pietrowski Bertuol, Eder Gatti Fernandes, Risoleide Marques de Figueiredo, Rosane Ferreira, Cláudia Weingaertner Palm, Marion Burger, Diogenes Seraphim Ferreira, Georgia Karina Morgenstern, Tsukiyo Obu Kamoi, Isis Mattos de Carvalho, Juliana Jenifer da Silva Araújo Cunha, Nadja Greffe, Nathalya Macedo Nascimento Costa, Thaina Genuino de Souza, Renata Loss Lima Frizzera, Barbara Emoingt Furtado, Gabriele Tantos Nunes, Tatiana Garcez, Rubens Costa Filho, Juliana Vassalo Rodrigues, Luzia Zago, and Claudio Renato Freire
Supplementary material
Demographic and clinical parameters of the cases
References
- 1.Mbaeyi S., Oliver S.E., Collins J.P., Godfrey M., Goswami N.D., Hadler S.C., et al. The Advisory Committee on Immunization Practices’ Interim Recommendations for additional primary and booster doses of COVID-19 vaccines—United States, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1545–1552. doi: 10.15585/mmwr.mm7044e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burki T.K. Omicron variant and booster COVID-19 vaccines. Lancet Respir Med. 2022;10:e17. doi: 10.1016/S2213-2600(21)00559-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atmar R.L., Lyke K.E., Deming M.E., Jackson L.A., Branche A.R., El Sahly H.M., et al. Homologous and heterologous Covid-19 booster vaccinations. N Engl J Med. 2022;386:1046–1057. doi: 10.1056/NEJMoa2116414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fadlyana E., Setiabudi D., Kartasasmita C.B., Putri N.D., Rezeki Hadinegoro S., Mulholland K., et al. Immunogenicity and safety in healthy adults of full dose versus half doses of COVID-19 vaccine (ChAdOx1-S or BNT162b2) or full-dose CoronaVac administered as a booster dose after priming with CoronaVac: a randomised, observer-masked, controlled trial in Indonesia. Lancet Infect Dis. 2023;23:545–555. doi: 10.1016/S1473-3099(22)00800-3. [DOI] [PubMed] [Google Scholar]
- 5.Munro A.P.S., Janani L., Cornelius V., Aley P.K., Babbage G., Baxter D., et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. Lancet. 2021;398:2258–2276. doi: 10.1016/S0140-6736(21)02717-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costa Clemens S.A., Weckx L., Clemens R., Almeida Mendes A.V., Ramos Souza A., Silveira M.B.V., et al. Heterologous versus homologous COVID-19 booster vaccination in previous recipients of two doses of CoronaVac COVID-19 vaccine in Brazil (RHH-001): a phase 4, non-inferiority, single blind, randomised study. Lancet. 2022;399:521–529. doi: 10.1016/S0140-6736(22)00094-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Secretaria de Vigilância em Saúde, Departamento de Imunizações e Doenças Transmissíveis, Ministério da Saúde Plano nacional de operacionalização da vacinação contra a COVID-19—2a edição. 2022. https://sbim.org.br/images/files/notas-tecnicas/pno-2a-edicao-isbn-equivalente-14.pdf [accessed April 8, 2023].
- 8.Greinacher A., Thiele T., Warkentin T.E., Weisser K., Kyrle P.A., Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384:2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Makris M., Pavord S. Most cases of thrombosis and thrombocytopenia syndrome (TTS) post ChAdOx-1 nCov-19 are vaccine-induced immune thrombotic thrombocytopenia (VITT) Lancet Reg Health Eur. 2022;12 doi: 10.1016/j.lanepe.2021.100274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan BTB, Bobos P, Odutayo A, Pai M. Meta-analysis of risk of vaccine-induced immune thrombotic thrombocytopenia following ChAdOx1-S recombinant vaccine. Preprint. Posted online May 8, 2021. medRxiv 2021.05.04.21256613. https://doi.org/10.1101/2021.05.04.21256613
- 11.Krzywicka K., van de Munckhof A., Zimmermann J., Bode F.J., Frisullo G., Karapanayiotides T., et al. Cerebral venous thrombosis due to vaccine-induced immune thrombotic thrombocytopenia after a second ChAdOx1 nCoV-19 dose. Blood. 2022;139:2720–2724. doi: 10.1182/blood.2021015329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rede Nacional de Dados de Saúde, Subsistema de Atenção à Saúde Indígena do SUS Vacinômetro COVID-19. 2023. https://infoms.saude.gov.br/extensions/SEIDIGI_DEMAS_Vacina_C19/SEIDIGI_DEMAS_Vacina_C19.html# [accessed April 8, 2023].
- 13.Mouta Nunes de Oliveira P., Mendes-de-Almeida D.P., Bertollo Gomes Porto V., Crespo Cordeiro C., Vitiello Teixeira G., Saraiva Pedro R., et al. Vaccine-induced immune thrombotic thrombocytopenia after COVID-19 vaccination: description of a series of 39 cases in Brazil. Vaccine. 2022;40:4788–4795. doi: 10.1016/j.vaccine.2022.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.da Saúde Ministério. Nota técnica no 933/2021 - CGPNI/DEIDT/SVS/MS—Atualização das orientações para a investigação da Síndrome de Trombose com Trombocitopenia no contexto da vacinação contra a covid-19 no Brasil. 2021. https://www.gov.br/saude/pt-br/coronavirus/vacinas/plano-nacional-de-operacionalizacao-da-vacina-contra-a-covid-19/notas-tecnicas/nota-tecnica-no-933-2021-cgpni-deidt-svs-ms.pdf/view [accessed December 14, 2021].
- 15.Pavord S., Scully M., Hunt B.J., Lester W., Bagot C., Craven B., et al. Clinical features of vaccine-induced immune thrombocytopenia and thrombosis. N Engl J Med. 2021;385:1680–1689. doi: 10.1056/NEJMoa2109908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.See I., Lale A., Marquez P., Streiff M.B., Wheeler A.P., Tepper N.K., et al. Case series of thrombosis with thrombocytopenia syndrome after COVID-19 vaccination-United States, December 2020 to August 2021. Ann Intern Med. 2022;175:513–522. doi: 10.7326/M21-4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen R. Updated Brighton Collaboration case definition for thrombosis with thrombocytopenia syndrome (TTS) 2021. https://brightoncollaboration.org/wp-content/uploads/2023/08/TTS-Interim-Case-Definition-v10.16.3-May-23-2021.pdf [accessed June 22, 2023].
- 18.World Health Organization Causality assessment of an adverse event following immunization (AEFI): user manual for the revised WHO classification, 2nd ed., 2019 update. 2021. https://www.who.int/publications/i/item/9789241516990 [accessed April 23, 2023].
- 19.World Health Organization Guidance for clinical case management of thrombosis with thrombocytopenia syndrome (TTS) following vaccination to prevent coronavirus disease (COVID-19) 2023. https://www.who.int/publications/i/item/9789240061989 [accessed April 23, 2023]. [PubMed]
- 20.Pavord S., Makris M. Second-dose VITT: rare but real. Blood. 2022;139:2581–2583. doi: 10.1182/blood.2022016118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Costa-Filho R.C., Pena Luz C., Monteiro de Souza A.T.A., Tantos Nunes G., Melo Amorim Filho L., Saddy F., et al. Vaccine-induced immune thrombotic thrombocytopenia (VITT) by a third dose of Chadox1 Ncov-19 (Astrazeneca) after BNT162b2 (Pfizer–BioNTech) Virol Immunol J. 2022;6:1–5. [Google Scholar]
- 22.Therapeutic Goods Administration, Department of Health and Age Care, Australian Government COVID-19 vaccine weekly safety report - 23-09-2021. 2021. https://www.tga.gov.au/news/covid-19-vaccine-safety-reports/covid-19-vaccine-weekly-safety-report-23-09-2021 [accessed September 23, 2021].
- 23.Cines D.B., Bussel J.B. SARS-CoV-2 vaccine-induced immune thrombotic thrombocytopenia. N Engl J Med. 2021;384:2254–2256. doi: 10.1056/NEJMe2106315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.US Food and Drug Administration Coronavirus (COVID-19) update: FDA limits use of Janssen COVID-19 vaccine to certain individuals. 2022. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-limits-use-janssen-covid-19-vaccine-certain-individuals [accessed May 5, 2022].
- 25.Ministério da Saúde Nota Técnica No 393/2022-CGPNI/DEIDT/SVS/MS. 2023. https://www.gov.br/saude/pt-br/coronavirus/notas-tecnicas/2022 [accessed April 15, 2023].
- 26.Mendes-de-Almeida D.P., Martins-Gonçalves R., Morato-Santos R., De Carvalho G.A.C., Martins S.A., Palhinha L., et al. Intracerebral hemorrhage associated with vaccine-induced thrombotic thrombocytopenia following ChAdOx1 nCOVID-19 vaccine in a pregnant woman. Haematologica. 2021;106:3025–3028. doi: 10.3324/haematol.2021.279407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ministério da Saúde Informe técnico operacional de vacinação contra a Covid-19. 2023. https://www.gov.br/saude/pt-br/coronavirus/informes-tecnicos/2023/informe-tecnico-operacional-de-vacinacao-contra-a-covid-19/view [accessed April 13, 2023].
- 28.Kanack A.J., Bayas A., George G., Abou-Ismail M.Y., Singh B., Kohlhagen M.C., et al. Monoclonal and oligoclonal anti-platelet factor 4 antibodies mediate VITT. Blood. 2022;140:73–77. doi: 10.1182/blood.2021014588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mendes-de-Almeida D.P., Kehdy F.S.G., Martins-Gonçalves R., Bokel J., Grinsztejn E., Mouta Nunes De Oliveira P., et al. A case report of vaccine-induced immune thrombotic thrombocytopenia (VITT) with genetic analysis. Front Cardiovasc Med. 2023;10:1189320. doi: 10.3389/fcvm.2023.1189320. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Demographic and clinical parameters of the cases