Abstract
A State of the Art lecture titled “Investigating Patients for Bleeding Disorders When Most of the Usual Ones Have Been Ruled Out” was presented at the International Society on Thrombosis and Haemostasis Congress in 2023. Mild to moderate bleeding disorders (MBDs) in patients in whom no diagnosis of an established disorder, such as platelet function defect, von Willebrand disease, or a coagulation factor deficiency, can be identified are classified as bleeding disorders of unknown cause (BDUCs). Prospective data from the Vienna Bleeding Biobank and other studies have revealed a high proportion of BDUCs of up to 70% among patients with MBD who have a similar bleeding phenotype as other MBDs. As BDUC is a diagnosis of exclusion, the accuracy of the diagnostic workup is essential. For example, repeated testing for von Willebrand disease should be considered if von Willebrand factor values are <80 IU/dL. Current evidence does not support the clinical use of global assays such as thromboelastography, platelet function analyzer, or thrombin generation potential. Rare and novel bleeding disorders due to genetic variants in fibrinolytic factors or natural anticoagulants are rare and should only be analyzed in patients with specific phenotypes and a clear family history. In BDUC, blood group O was identified as a risk factor for increased bleeding severity and bleeding risk after hemostatic challenges. Future studies should improve the phenotypical characterization and ideally identify novel risk factors in BDUC, as a multifactorial pathogenesis is suspected. Finally, we summarize relevant new data on this topic presented during the 2023 International Society on Thrombosis and Haemostasis Congress.
Keywords: BDUC, bleeding, natural anticoagulants, platelet function defects (PFD), von Willebrand Diseases (VWD)
1. Introduction
Patients in whom the most usual bleeding disorders have been excluded after extensive investigation of plasmatic coagulation and platelet function are classified as patients with bleeding disorders of unknown cause (BDUCs). This new entity of bleeding disorders has increasingly become a focus of attention among hemostasis experts during the last decade [1,2].
In general, bleeding disorders encompass a broad clinical spectrum. Although severe bleeding disorders often manifest in early childhood and are mostly diagnosed soon, there is a broad variety in mild to moderate bleeding disorders (MBDs). Patients with MBDs present with bleeding symptoms such as easy bruising, epistaxis, heavy menstrual bleeding, and bleeding after hemostatic challenges. The temporal occurrence of these symptoms and the rarity of severe and life-threatening bleeding manifestations result in delayed diagnostic workup and diagnosis in adolescence or adulthood [3,4].
Common MBDs include platelet function defects (PFDs), von Willebrand disease (VWD), or coagulation factor deficiencies (CFDs). Nevertheless, in the last decade, the new entity of BDUC has been acknowledged as the most common MBD [4,5]. Contrary to previous assumptions, patients with BDUC do not differ from those with other MBDs with regard to the bleeding phenotype and bleeding severity [6]. Diagnosis of BDUC as a diagnosis of exclusion is cumbersome as the comprehensive diagnostic workup requires a multistep approach that is often restricted to tertiary centers [3,7].
2. Investigating Patients with MBDs and BDUC: The Vienna Bleeding Biobank
The Vienna Bleeding Biobank (VIBB) was established in 2009 at the Medical University of Vienna with the aim to better characterize patients with MBDs and especially those with BDUC [2]. The VIBB is a single-center cohort study, including patients aged ≥16 years who are referred to our tertiary center with a nontrivial bleeding tendency and without a previous diagnosis of a bleeding disorder [4]. Patients with thrombocytopenia (<100 G/L), infections, malignancy, impaired liver and kidney functions, or 6 weeks after surgery and childbirth are not included. Since 2009, 864 patients have been included in the VIBB. The clinical data registry and biobank provide a valuable source to investigate underlying mechanisms and bleeding outcomes in patients with MBDs and BDUC.
3. Diagnosis of BDUC
BDUC is a diagnosis of exclusion. Consequently, a clear standardization of the diagnostic workup of patients with MBDs is required to justify the classification with BDUC, as previously suggested by Baker and O’Donnell [8]. A broad variety of nonhemostatic disorders or conditions can also result in bleeding manifestations, and it is crucial to rule out these hereditary or acquired nonhemostatic disorders upfront, as reviewed by Thomas et al. [9]. These conditions include hematological disorders such as amyloidosis or hereditary hemorrhagic telangiectasia, congenital disorders such as connective tissue disorders, as well as endocrinological disorders such as hypothyroidism, among others [8,9]. A comprehensive overview of clinical conditions that may resemble a bleeding disorder is provided in Table 1 [[10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23]].
Table 1.
Clinical manifestations of disorders that resemble a bleeding disorder—adapted from Thomas et al. [9].
| Disorder | Clinical manifestation |
|---|---|
| Achenbach’s syndrome [10] | Paroxysmal bruising of the fingers |
| Amyloidosis [11] | Cerebral, cutaneous, intracranial and gastrointestinal bleeding |
| Angina bullosa hemorrhagica | Acute painful blisters in the mount |
| Autoerythrocyte syndrome (also known as psychogenic purpura and Gardner–Diamond syndrome) [12,13] | Recurrent, spontaneous, painful ecchymosis, frequently preceded by a prodrome of pain or itching of the skin |
| Exercise induced purpura [14] | Purpuric patches on the lower limbs after exercise |
| Hereditary hemorrhagic telangiectasia [15] | Recurrent epistaxis, gastrointestinal bleedings, hemorrhage from visceral arteriovenous malformation (eg, cerebral and pulmonary), telangiectasia on skin and mucosal surfaces |
| Hypothyroidism [16] | Often mild mucocutaneous bleeding tendency and rarely severe (eg, hemorrhages after trauma or surgery) |
| Medications | Bruising and bleeding from skin and the gastrointestinal tract |
| Noonan syndrome [17] | Postsurgical bleeding without any coagulation or platelet function abnormality |
| Osteogenesis imperfecta [18] | Variety of bleeding symptoms including bruising, bleeding after interventions and epistaxis |
| Scurvy [19] | Malaise, lethargy, purpura, intracerebral hemorrhage, subperiosteal hemorrhage, perifollicular hemorrhage, corkscrew hairs, poor wound healing and scorbutic gums |
| Senile purpura [20] | Scattered purpuric patches and white pseudoscars with skin atrophy |
| Skin fragility and connective tissue disorders (eg, Ehlers–Danlos syndrome) [21] | Excessive bruising but systemic manifestations of the specific condition |
| Uremia [22] | Bruising and hemorrhage |
| Vasculitis (eg, Henoch–Schönlein purpura) [23] | Purpuric rash and pulmonary hemorrhage |
According to the proposed algorithm, BDUC is diagnosed in patients with MBD, with normal routine and hemostatic test results, including von Willebrand factor (VWF) antigen and activity, assessment of platelet function by light transmission aggregometry and platelet nucleotides, and normal factor XIII antigen and activity and normal levels of FII, FV, FVII, and FX [8].
In the VIBB, patients with BDUC are diagnosed after exclusion of VWF, CFD, and PFD, as depicted in Figure 1. Following this algorithm, BDUC is diagnosed in 66% of patients in the VIBB, which is in line with other cohorts of patients with MBD from different geographic regions, reporting BDUC in approximately 50% to 70% of all patients with MBDs [5], as summarized in Table 2 [5,[24], [25], [26], [27], [28], [29], [30]].
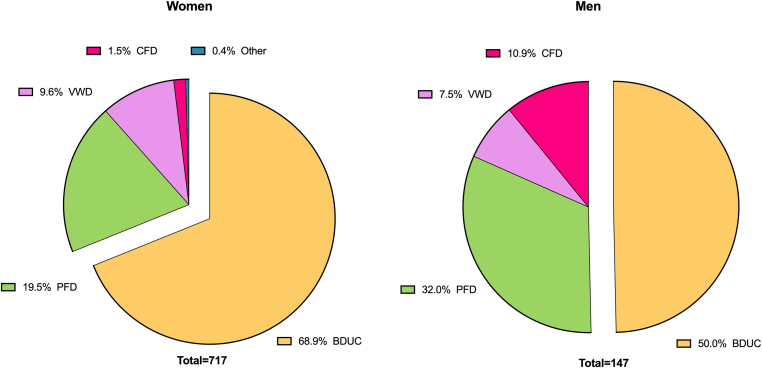
Figure 1.
Diagnoses according to sex in the Vienna Bleeding Biobank. Previously unpublished data from the Vienna Bleeding Biobank. BDUC, bleeding disorder of unknown cause; CFD, coagulation factor deficiency; PFD, platelet function defect; VWD, von Willebrand disease.
Table 2.
Relative frequency of diagnosis in patients with mild to moderate bleeding disorders highlighting the proportion of patients with normal test results (bleeding disorder of unknown cause)—adapted from Quiroga et al. [5].
| Study | Type of study | N | VWD |
PFD |
CFD |
BDUC |
|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | |||
| Parkin et al. [24] | Prospective | 93 | 6 (7) | 17 (18) | NA | 44 (47) |
| Quiroga et al. [25] | Retrospective | 589 | 110 (19) | 23 (21)a | 66 (11) | 324 (55) |
| Quiroga et al. [31] | Prospective | 280b | 50 (18) | 65 (23) | 11 (4) | 167 (60)c |
| Podda et al. [26] | Prospectived | 128 | 7 (6) | 12 (9) | 28 (22) | 63 (49) |
| Gupta et al. [27] | Prospectivee | 2800 | 94 (3) | 312 (11) | 466 (17) | 1928 (69) |
| Agren et al. [32] | Prospective | 586 | 59 (10) | 143 (24) | 25 (4) | 336 (57) |
| Marcus et al. [28] | Prospective | 104 | 30 (29) | 12 (12) | NA | 61 (59) |
| Tosetto et al. [29] | Prospectivef | 105 | 11 (10) | 11 (10) | 5 (5) | 68 (65) |
| Veen et al. [33] | Prospective | 181 | 21 (12)g | 39 (22)g | 5 (3) | 121 (67) |
| Mehic et al. [34] | Prospective | 634h | 59 (9)i | 127 (20)j | 23 (4)k | 422 (67) |
| Zafarani et al. [30] | Retrospective | 397 | 49 (25) | 15 (8) | 88 (45)l | 200 (50) |
BDUC, bleeding disorder of unknown cause; CFD, clotting factor deficiency; NA, not available; PFD, platelet factor deficiency; VWD, von Willebrand disease.
Platelet secretion was performed in only 107 patients.
The sum of all patients is >280 because 13 of them had concomitant diagnosis of VWD, PFD or CFD.
All test results were normal, except for 19% of 160 patients with prolonged bleeding time. Five patients had both low VWF and a PFD.
Fourteen percent of the 128 patients had laboratory abnormalities not associated with bleeding risk (lupus anticoagulant and contact phase defects).
This study included a proportion of patients with acquired disorders of platelet function or with thrombocytopenia.
Ten of the 105 patients had laboratory findings not associated with bleeding risk (senile purpura, Rendu–Weber–Osler disease, and asymptomatic clotting factor deficiency).
Five patients had both low VWF and a PFD.
Three patients with dysfibrinogenemia.
Including 4 patients with additionally possible PFD.
Including 91 with possible (pathological light transmission aggregometry [LTA] with at least 1 agonist at 1 time point) and 36 with definite (pathological LTA with ≥2 agonists at ≥2 time points) PFD.
Including patients with FVIII activity ≤50% (n = 14; 60.9%); FIX activity ≤50% (n = 5; 21.7%), FXI activity ≤60% (n = 3; 13.0%), and FXIII activity ≤10% (n = 1; 4.3%).
Including 54 patients with hemophilia and 34 patients with FVII deficiency.
3. Clinical Characteristics of Patients with BDUC
Unpublished data from the VIBB show a female preponderance in patients with MBD in general, but especially in BDUC, as 69% of females, but only 50% of males are diagnosed with BDUC (Figure 1). The high prevalence (59%) of females among 167 patients with BDUC compared to patients with other MBDs has already been described in 2007 by Quiroga et al. [31] and has consistently been reported in other cohorts, such as by Veen et al. [33] or Agren et al. [32]. Women-specific hemostatic challenges during lifetime including menstrual bleeding and childbirth were made responsible for the sex-difference in MBDs in general and BDUC in specific. Approximately a third of patients with BDUC report a positive family history of bleeding, as shown in the VIBB and other cohorts of patients with MBD [31,33,34,35].
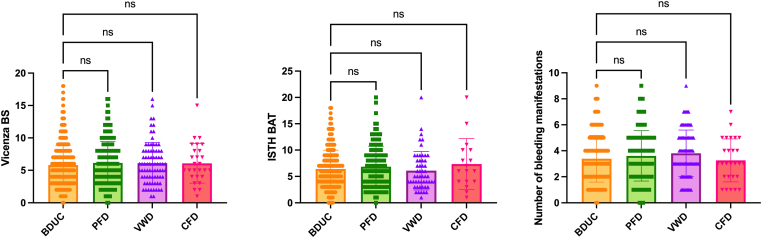
Previously, the bleeding phenotype in patients with BDUC was considered less severe than that in patients with other bleeding disorders [7]. For example, Quiroga et al. [31] (19%) and Veen et al. [33] (54%) found less frequent abnormal bleeding scores in BDUC than in other MBD. Nevertheless, in unpublished data from the VIBB, we found no difference in the bleeding severity compared to other MBDs, as shown in Figure 2. Overall, (abnormal) bleeding scores differed between studies and showed a bad discriminatory power in the VIBB [6].
Figure 2.
Bleeding severity measured by the Vicenza bleeding score, International Society on Thrombosis and Haemostasis bleeding assessment tool, and number of bleeding manifestation according to diagnosis in the Vienna Bleeding Biobank. Previously unpublished data from the Vienna Bleeding Biobank. Bars represent mean and SD. BDUC, bleeding disorder of unknown cause; CFD, coagulation factor deficiency; ns, not significant; PFD, platelet function defect; VWD, von Willebrand disease.
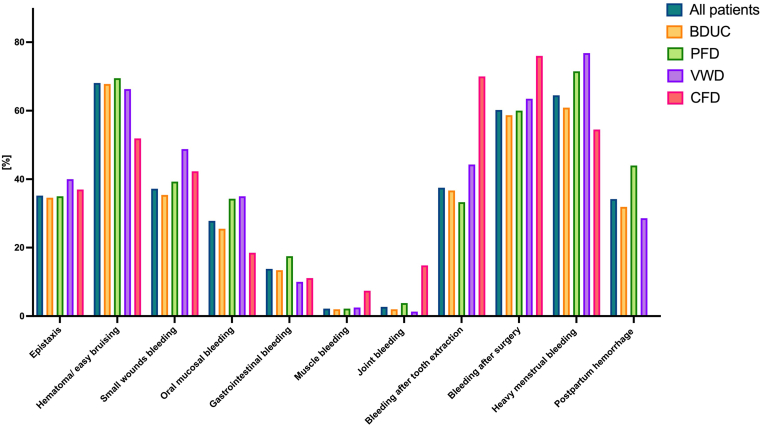
With regards to bleeding phenotype, Quiroga et al. [31] showed a similar distribution of bleeding symptoms between patients with BDUC and patients with another MBD diagnosis, apart from a higher prevalence of hematomas and major bleeding events requiring transfusion in those with a definite MBD diagnosis. Veen et al. [33] retrospectively analyzed patients who were referred to hemostatic investigations due to hematomas and postsurgical bleeding and found no major difference in these manifestations between patients with BDUC and patients with other MBDs. In the VIBB, minor differences with higher rates of postinvasive bleeding in CFD, heavy menstrual bleeding in VWD, and postpartum hemorrhage in patients with PFD compared to BDUC were observed, whereas most of the bleeding manifestations occurred with a similar frequency (unpublished data, Figure 3).
Figure 3.
Presence of bleeding symptoms according to diagnosis in the Vienna Bleeding Biobank. Previously unpublished data from the Vienna Bleeding Biobank. BDUC, bleeding disorder of unknown cause; CFD, coagulation factor deficiency; PFD, platelet function defect; VWD, von Willebrand disease.
4. Investigating Patients with BDUC
As based on our data, BDUC cannot be distinguished by clinical presentation and is a diagnosis of exclusion; the diagnosis is dependent on the diagnostic accuracy of excluded diagnoses.
The following paragraphs will shed light on some important aspects in the diagnostic workup of patients with MBDs and BDUC. The following clinically relevant questions will be covered in detail:
-
1)
Do we need repeated testing to exclude bleeding disorders?
-
2)
Do we need global tests of primary and secondary hemostasis?
-
3)
Which rare known bleeding disorders should be investigated in patients with BDUC?
-
4)
Are there known risk factors for (re)bleeding in patients with BDUC?
4.1. Do we need repeated testing to exclude bleeding disorders?
The accurate diagnosis of MBDs such as VWD or PFD poses considerable challenges, even for specialized centers [3,7,8]. These challenges arise from fluctuations in relevant diagnostic parameters and error-prone laboratory methods [3,7]. In general, a confirmation of pathological test results is required, for example, to diagnose CFD, PFD, and VWD [8]. Nevertheless, there might be false normal test results, which might require repeated testing to consequently exclude a certain bleeding disorder. This especially holds true for VWD as levels of VWF can increase due to various endogenous and exogenous factors [36].
In patients with bleeding, VWD is diagnosed at VWF levels ≤50 IU/L [37]. Consequently, patients with VWF levels >50 IU/dL will be categorized as patients with BDUC upon exclusion of other bleeding disorders [8]. These diagnostic criteria rely on single measurements of VWF levels, as, according to guidelines, repeated VWF measurements are not mandatory for VWD diagnosis [37]. This concept of single VWF measurements to diagnose or exclude VWD has recently been challenged by us and others [[38], [39], [40], [41]]. Our data on 277 patients with MBD from the VIBB showed that 13% of patients had changing VWF levels below and above the cutoff of 50 IU/dL in repeated VWF measurements [42]. Forty-five percent of the patients who were initially diagnosed with VWD had levels above the diagnostic cutoff in subsequent measurements, and 11% of patients with baseline VWF levels between 51 and 80 IU/dL fulfilled the VWD diagnostic criteria in subsequent measurements. We have identified VWF levels of 80 IU/dL yielding a low probability of 1.2% (95% CI, 0.3-4.9) of crossing the diagnostic threshold, resulting in negative predictive values of 98.1% for the changing status.
Our data suggest repeated testing for VWD in patients with VWF levels below 80 IU/dL to minimize an overlap between patients with low VWF levels and BDUC [43]. Our data showed that of the 63 patients who fulfilled the diagnostic VWD criteria at least once during the study period, only 49 (77.7%) were diagnosed at the baseline visit, whereas the other 14 patients (23.3%) were primarily diagnosed with BDUC. Similar results were obtained in pediatric patients, as in only 70% of pediatric patients with a bleeding disorder VWD was diagnosed at initial testing [39]. In alignment with our results, a retrospective analysis of 39 adolescents with heavy menstrual bleeding revealed that 5 out of 16 (31%) VWD patients remained undetected in initial VWF testing, also underlining the necessity of repeated VWD testing [3].
4.2. Do we need global tests of primary and secondary hemostasis?
The role of global tests for primary and secondary hemostasis as a diagnostic tool in MBD has been debated [3,7]. To date, global assays, including thromboelastography on whole blood or platelet rich plasma, platelet function analyzer (PFA-100, Dade Behring Inc) from whole blood, and skin bleeding time as well as measurement of thrombin generation potential both from whole blood or plasma, are not established in clinical routine for the diagnostic workup of MBDs [3], which is mostly based on their low sensitivity and specificity and the paucity of scientific data in MBDs and especially BDUC [44]. Furthermore, alterations in global hemostatic tests in most cases do not provide information on the underlying hemostatic defect and, thus, are of limited use in the diagnostic workup of patients with BDUC.
Most data on global assays in patients with BDUC are available for the measurement of thrombin generation, as recently summarized by Thomas et al. [45]. Nevertheless, those assays are poorly standardized and results are conflicting [33,[46], [47], [48], [49], [50], [51]], which limits the general validity of the assay in patients with BDUC. In the VIBB, reduced thrombin potential reflected by a prolonged lag time, time to peak, and lower peak thrombin velocity index and the endogenous thrombin potential in BDUC has been described [47], which was partly in line with altered thrombin generation in other BDUC patient cohorts [48,50,51]. On the other side, in 3 studies based on 2 different thrombin generation assays, no differences were found when comparing patients with BDUC with healthy controls [33,46,49].
Based on current evidence, the clinical use of global hemostatic assays to guide diagnostic steps or treatment cannot be recommended in patients with MBDs or BDUC [3]. Nevertheless, better standardized, and reproducible global assays might be interesting research tools and potentially help to subcategorize patients with BDUC and guide the scientific and clinical approach in the future.
4.3. Which rare bleeding disorders should be investigated?
The diagnostic workup for the diagnosis of BDUC encompasses common bleeding disorders such as VWD, PFD, or CFD. Nevertheless, rare bleeding disorders, which are not detected by routine global coagulation tests, might not be identified if not directly investigated. These rare bleeding disorders, among others, include disorders with increased function of natural anticoagulants as well as hyperfibrinolytic disorders and cause a similar bleeding phenotype as BDUC [52,53].
Pathological variants in 2 natural anticoagulant pathways, thrombomodulin (TM) [[54], [55], [56]] and tissue factor pathway inhibitor (TFPI) [[57], [58], [59]], were identified in single patients or families as the underlying cause for a bleeding tendency [53].
TM-associated coagulopathy is caused by heterozygous variants in the THBD gene (c.1611C > A and c.1487delCM), leading to elevated soluble(s) TM levels [[54], [55], [56]]. Due to loss of the C-terminal cytoplasmatic domain, shedding of TM from the endothelium into the plasma is induced. Patients had mostly postsurgical and/or posttraumatic bleeding, but in some cases also spontaneous abdominal bleeding was reported. Of note, coagulation screening tests such as activated partial thromboplastin time (aPTT) and prothrombin time, levels of clotting factors, and platelet function tests were within normal limits, only reduced thrombin generation was found in these patients [53]. However, investigating soluble (s) TM levels in a large cohort from the VIBB did not reveal increased levels in BDUC or other patients with MBD, and no association of sTM with the bleeding phenotype or thrombin generation was identified [60].
With regards to TFPI, novel B-domain variants in exon 13 of the FV-encoding gene (F5) resulting in FV splice variants have been identified to cause the East Texas, FV-Amsterdam, and FV-Atlanta bleeding disorders [[57], [58], [59]]. These variants lead to the activation of the rarely used splice donor leading to the truncated form of FV (FV-short), which prolongs the half-life of free TFPIα by better binding to it and protecting it from degradation and cleavage. Besides their clinical bleeding manifestations, including menorrhagia, bruising, epistaxis, and massive bleedings after trauma or surgery, patients also usually have a prolonged aPTT and prothrombin time as well as reduced thrombin generation [53,57,61,62]. We investigated levels of free TFPIα in more than 600 patients from the VIBB and found increased levels in patients with BDUC or PFD that were associated with abnormal thrombin generation, including a prolonged lag time and time to peak [63]. Nevertheless, no relevant variants in the F5 gene causing an increase of FV-short were identified [64], and patients did not reach as high plasma levels of TFPI as patients with disease-causing variants [53].
Another rare bleeding disorder caused by a natural anticoagulant is α1-antitrypsin Pittsburgh (α1-AT-P), characterized by a Met 358 to Arg substitution in α1-AT. α1-Antitrypsin Pittsburgh functions as a potent thrombin inhibitor as well as an inhibitor of the contact pathway, resulting in a severe bleeding disorder [65,66].
Hyperfibrinolytic disorders are characterized by mucocutaneous bleeding and prolonged bleeding after interventions and trauma [52,67], as seen in MBDs. Genetic variants in fibrinolytic genes are rare and have only been described in single patients and families, including patients with α2-antiplasmin deficiency (14 homozygous and 104 heterozygous), PAI-1 deficiency (n = 26), Quebec platelet disorder (n = 23), and tissue plasminogen activator excess (n = 4) [52].
Genetic analysis by high-throughput whole exome sequencing of 96 genes related to coagulation and platelet function within the Thrombogenomics project has revealed a pathological genetic variant only in approximately 3% of more than 600 patients with BDUC [64]. Also, no pathological variant of a hyperfibrinolytic disorder was identified in 370 patients with BDUC from the VIBB [64] in the analysis of SERPINE1 (PAI-1 deficiency), SERPINF2 (α2-antiplasmin deficiency), PLAT (tissue plasminogen activator), or PLAU (Quebec platelet disorder).
Also, recently, a novel bleeding disorder associated with tissue factor (TF) deficiency was discovered in a woman with unexplained bleeding. This variant in the TF encoding gene (F3) leads to an early termination of protein translation, resulting in a shortened TF protein. In mice experiments, this variant led to decreased TF production resulting in reduced hemostatic capacity [68].
As these above-described bleeding disorders due to alterations of natural anticoagulants, TF, or fibrinolytic factors are very rare, their investigation in our opinion might be limited to selected patients with an impressive family history and a specific bleeding phenotype.
Identifying which patients with BDUC should undergo extensive testing remains a challenging issue, and currently, no clear guidelines exist for selecting patients and laboratory tests. While recognizing the practical and financial challenges surrounding extensive testing for rare bleeding disorders in BDUC, it is essential to emphasize the current limitations in the management of patients with BDUC, who have a substantial risk of recurrent bleeding episodes and no personalized treatment options. In our opinion, these gaps in clinical patient care encourage further investigations of patients with BDUC, which might be limited to specialized tertiary care centers, and underscore the need for ongoing research and collaborative efforts in this field.
4.4. What are the risk factors for (recurrent) bleeding?
Considering the varying bleeding phenotype in MBDs, a multifactorial combination of mild bleeding risk factors resulting in the clinical bleeding tendency has been hypothesized.
ABO blood type has been acknowledged to play a role in both thrombosis and hemostasis [69,70]. Blood type non-O has been identified as an independent risk factor for venous thromboembolism in large epidemiological studies [69,71]. On the other side, blood type O was found to be overrepresented in bleeding patients [70] and is associated with lower VWF levels [72]. Also, in 422 patients with BDUC from the VIBB, blood group O was overrepresented compared to 23,145 healthy blood donors from the same geographic region (47% vs 38%; odds ratio, 1.48; 95% CI, 1.22-1.79) [34]. We also found that blood group O was associated with higher bleeding scores and number of bleeding manifestations in BDUC, even after adjustment for VWF levels, FVIII levels, and sex. Also, oral cavity bleeding was more prevalent in patients with blood group O than in those with other blood types, which was again independent of VWF levels, FVIII levels, and sex, whereas other bleeding symptoms did not differ. In the same study, we found that blood group O was associated with increased clot density measured by rotational thromboelastometry (ROTEM) and turbidimetric measurement of plasma clot formation [34], which might reflect an altered clot composition but the exact underlying mechanisms of ABO blood type as hemostatic modifier remains unclear.
Patients with MBDs and BDUC have a high risk for recurrent bleeding for most bleeding symptoms, as recently identified by our group [35]. In a retrospective Dutch study, an increased risk of bleeding after surgery and childbirth was also identified in both patients with MBD and those with BDUC [73], which is in line with data from the VIBB [35]. Also, Castle et al. [74] reported a high risk of postpartum hemorrhage in patients with BDUC, which was even higher than that in the study from our group [35].
In multivariable analysis, we identified a higher bleeding score at baseline and longer follow-up time as independent risk factors for bleeding during follow-up. For bleeding after hemostatic challenges, blood group O, previous postsurgical bleeding, and having an established diagnosis of an MBD, including VWD, PFD, and CFD, were associated with increased bleeding risk [35].
5. Conclusion
BDUC is a diagnosis of exclusion after extensive laboratory workup, and patients cannot be distinguished from those with MBD by the clinical phenotype. Repeated testing might be necessary for some parameters such as VWF in order not to omit an established MBD such as VWD. Global hemostatic tests in MBD or patients with BDUC are currently not included in the clinical workup and their role needs to be further elucidated. Rare known coagulation disorders are indeed very rare and thus targeted investigations might be limited to selected patients with BDUC. Patients with BDUC are at risk for recurrent bleeding, especially after hemostatic challenges, and some risk factors, such as blood group O, previous postsurgical bleeding, or a high bleeding score, might help in predicting bleeding risk at future interventions.
6. International Society on Thrombosis and Haemostasis 2023 Congress Report
Besides the State of the Art lecture by Prof Johanna Gebhart, also novel data regarding patients with BDUC were also presented at the International Society on Thrombosis and Haemostasis 2023 Congress. Our group (Mehic et al., PB 1206 [75]) investigated fluorogenic plasmin generation (PG) in 367 patients with BDUC and compared it to that in 100 healthy controls. We found an impaired PG potential reflected by lower plasmin peak in patients with BDUC, while there was no robust association with bleeding severity or symptoms. Overall, the PG assay showed a good ability to distinguish BDUC from healthy controls, but the underlying mechanism for a counterintuitive reduced PG potential remained unclear.
Baby et al. (PB0553) [76] investigated RICOF, ROTEM, and aPTT clot wave form analysis (CWA) in 72 patients with BDUC. There were no differences for RICOF and ROTEM parameters, while CWA showed significant prolongation of the maximum peak of the second derivative in patients with BDUC compared to healthy controls, though all CWA parameters were within the normal range.
Berkowitz et al. (PB1376) [77] retrospectively investigated periprocedural hemostatic prophylaxis (with antifibrinolytics, desmopressin, platelets, and/or cryoprecipitate) and bleeding outcomes in patients with BDUC. Overall, 77 surgical procedures (51 minor and 26 major) in a total of 70 patients with BDUC with a mean age of 44 and mean bleeding score of 9.4 were analyzed. The authors identified that in 25% of all procedures, no hemostatic prophylaxis was applied and that major or clinically relevant minor bleeding occurred in 5.4% (3/56) of procedures with prophylaxis and 10.5% (2/19) of procedures without prophylaxis. Albeit all deliveries received hemostatic treatment, postpartum hemorrhage still occurred in 40% (2/5) of deliveries.
7. Future Directions
The awareness of BDUC as a nontrivial bleeding disorder has increased over the last decade [1] due to the relevant bleeding risk and high burden of disease in patients with BDUC [35,[73], [74], [78]]. Nevertheless, a standardized approach and consensus for the diagnostic workup and (prophylactic) treatment of patients with BDUC is urgently needed. International and collaborative initiatives on patients with BDUC from distinct regions and ethnic/race backgrounds are required to characterize these patients more comprehensively and ideally cluster them according to their phenotype. Research endeavors should focus on revealing novel pathophysiologically relevant hemostatic mechanisms as causes for BDUC. As in a proportion of patients with BDUC, a multifactorial cause for the bleeding phenotype is suspected, and novel risk factors and their effects on the hemostatic balance, such as the role of blood group O, should be addressed.
Acknowledgments
Funding
The Vienna Bleeding Biobank is supported by an unrestricted grant from CSL Behring, the Medical-Scientific Fund of the Mayor of the federal capital Vienna (grant number 20023), and the Anniversary Fund of the Austrian National Bank (grant number 18500). D.M. received the Physician Pathway Scholarship of the Medical University of Vienna for protected research time.
Author contributions
D.M., I.P., and J.G. performed the literature review and wrote and revised the manuscript.
Relationship Disclosures
D.M. received honoraria for advisory board meetings and lectures from CSL Behring. I.P. has received honoraria from Bayer, CSL Behring, Novo-Nordisk, Pfizer, Roche, Sobi, and Takeda for lectures and advisory board meetings. J.G. received honoraria for lectures and advisory board meetings and research funding for the Medical University of Vienna from CSL Behring, Novartis, Amgen, Sobi, and Takeda.
Footnotes
Handling Editor: Prof. Michael Makris
References
- 1.Thomas W., Downes K., Evans G., Gidley G., Lowe G., MacDonald S., et al. Current practice and registration patterns among United Kingdom Haemophilia Centre Doctors’ Organisation centers for patients with unclassified bleeding disorders. J Thromb Haemost. 2021;19:2738–2743. doi: 10.1111/jth.15492. [DOI] [PubMed] [Google Scholar]
- 2.Thomas W. The natural history of bleeding disorder of unknown cause. J Thromb Haemost. 2023;21:1747–1749. doi: 10.1016/j.jtha.2023.03.025. [DOI] [PubMed] [Google Scholar]
- 3.Rodeghiero F., Pabinger I., Ragni M., Abdul-Kadir R., Berntorp E., Blanchette V., et al. Fundamentals for a systematic approach to mild and moderate inherited bleeding disorders: an EHA consensus report. Hemasphere. 2019;3:e286. doi: 10.1097/HS9.0000000000000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gebhart J., Hofer S., Panzer S., Quehenberger P., Sunder-Plassmann R., Hoermann G., et al. High proportion of patients with bleeding of unknown cause in persons with a mild-to-moderate bleeding tendency: results from the Vienna Bleeding Biobank (VIBB) Haemophilia. 2018;24:405–413. doi: 10.1111/hae.13422. [DOI] [PubMed] [Google Scholar]
- 5.Quiroga T., Mezzano D. Is my patient a bleeder? A diagnostic framework for mild bleeding disorders. Hematology Am Soc Hematol Educ Program. 2012;2012:466–474. doi: 10.1182/asheducation-2012.1.466. [DOI] [PubMed] [Google Scholar]
- 6.Gebhart J., Hofer S., Kaider A., Rejtö J., Ay C., Pabinger I. The discriminatory power of bleeding assessment tools in adult patients with a mild to moderate bleeding tendency. Eur J Intern Med. 2020;78:34–40. doi: 10.1016/j.ejim.2020.04.023. [DOI] [PubMed] [Google Scholar]
- 7.Mezzano D., Quiroga T. Diagnostic challenges of inherited mild bleeding disorders: a bait for poorly explored clinical and basic research. J Thromb Haemost. 2019;17:257–270. doi: 10.1111/jth.14363. [DOI] [PubMed] [Google Scholar]
- 8.Baker R.I., O’Donnell J.S. How I treat bleeding disorder of unknown cause. Blood. 2021;138:1795–1804. doi: 10.1182/blood.2020010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas W., Downes K., Desborough M.J.R. Bleeding of unknown cause and unclassified bleeding disorders; diagnosis, pathophysiology and management. Haemophilia. 2020;26:946–957. doi: 10.1111/hae.14174. [DOI] [PubMed] [Google Scholar]
- 10.Jiménez P.R., Ocampo M.I., Castañeda-Cardona C., Rosselli D. Achenbach’s syndrome: case report and systematic review of the literature. Rev Colomb Reumatol (Engl Ed) 2017;24:230–236. [Google Scholar]
- 11.Sucker C., Hetzel G.R., Grabensee B., Stockschlaeder M., Scharf R.E. Amyloidosis and bleeding: pathophysiology, diagnosis, and therapy. Am J Kidney Dis. 2006;47:947–955. doi: 10.1053/j.ajkd.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 12.Gardner F.H., Diamond L.K. Autoerythrocyte sensitization; a form of purpura producing painful bruising following autosensitization to red blood cells in certain women. Blood. 1955;10:675–690. [PubMed] [Google Scholar]
- 13.Zhao H., Luo F., Li H. Autoerythrocyte sensitization syndrome presenting with general neurodermatitis: factitious purpura or psychophysiological entity? Dermatol Ther (Heidelb) 2012;2:5. doi: 10.1007/s13555-012-0005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramelet A.A. Exercise-induced purpura. Dermatology. 2004;208:293–296. doi: 10.1159/000077837. [DOI] [PubMed] [Google Scholar]
- 15.Kritharis A., Al-Samkari H., Kuter D.J. Hereditary hemorrhagic telangiectasia: diagnosis and management from the hematologist’s perspective. Haematologica. 2018;103:1433–1443. doi: 10.3324/haematol.2018.193003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franchini M. Hemostatic changes in thyroid diseases: haemostasis and thrombosis. Hematology. 2006;11:203–208. doi: 10.1080/10245330600667591. [DOI] [PubMed] [Google Scholar]
- 17.Nugent D.J., Romano A.A., Sabharwal S., Cooper D.L. Evaluation of bleeding disorders in patients with Noonan syndrome: a systematic review. J Blood Med. 2018;9:185–192. doi: 10.2147/JBM.S164474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gooijer K., Rondeel J.M.M., van Dijk F.S., Harsevoort A.G.J., Janus G.J.M., Franken A.A.M. Bleeding and bruising in Osteogenesis Imperfecta: International Society on Thrombosis and Haemostasis bleeding assessment tool and haemostasis laboratory assessment in 22 individuals. Br J Haematol. 2019;187:509–517. doi: 10.1111/bjh.16097. [DOI] [PubMed] [Google Scholar]
- 19.Fleming J.D., Martin B., Card D.J., Mellerio J.E. Pain, purpura and curly hairs. Clin Exp Dermatol. 2013;38:940–942. doi: 10.1111/ced.12118. [DOI] [PubMed] [Google Scholar]
- 20.Dyer J.M., Miller R.A. Chronic skin fragility of aging: current concepts in the pathogenesis, recognition, and management of dermatoporosis. J Clin Aesthet Dermatol. 2018;11:13–18. [PMC free article] [PubMed] [Google Scholar]
- 21.Kumskova M., Flora G.D., Staber J., Lentz S.R., Chauhan A.K. Characterization of bleeding symptoms in Ehlers-Danlos syndrome. J Thromb Haemost. 2023;21:1824–1830. doi: 10.1016/j.jtha.2023.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Remuzzi G. Bleeding disorders in uremia: pathophysiology and treatment. Adv Nephrol Necker Hosp. 1989;18:171–186. [PubMed] [Google Scholar]
- 23.Du L., Wang P., Liu C., Li S., Yue S., Yang Y. Multisystemic manifestations of IgA vasculitis. Clin Rheumatol. 2021;40:43–52. doi: 10.1007/s10067-020-05166-5. [DOI] [PubMed] [Google Scholar]
- 24.Parkin J.D., Smith I.L., O’Neill A.I., Ibrahim K.M., Butcher L.A. Mild bleeding disorders. A clinical and laboratory study. Med J Aust. 1992;156:614–617. [PubMed] [Google Scholar]
- 25.Quiroga T., Pérez M., Rodríguez S., Muñoz B., Aranda E., Morales M., et al. Skin and mucous membrane hemorrhages: clinical assessment, study sequence and relative frequency of hereditary diseases of the hemostasis in a Chilean population. Article in Spanish. Rev Med Chil. 1997;125:409–418. [PubMed] [Google Scholar]
- 26.Podda G.M., Bucciarelli P., Lussana F., Lecchi A., Cattaneo M. Usefulness of PFA-100 testing in the diagnostic screening of patients with suspected abnormalities of hemostasis: comparison with the bleeding time. J Thromb Haemost. 2007;5:2393–2398. doi: 10.1111/j.1538-7836.2007.02752.x. [DOI] [PubMed] [Google Scholar]
- 27.Gupta P.K., Charan V.D., Saxena R. Spectrum of von Willebrand disease and inherited platelet function disorders amongst Indian bleeders. Ann Hematol. 2007;86:403–407. doi: 10.1007/s00277-006-0244-8. [DOI] [PubMed] [Google Scholar]
- 28.Marcus P.D., Nire K.G., Grooms L., Klima J., O’Brien S.H. The power of a standardized bleeding score in diagnosing paediatric type 1 von Willebrand’s disease and platelet function defects. Haemophilia. 2011;17:223–227. doi: 10.1111/j.1365-2516.2010.02390.x. [DOI] [PubMed] [Google Scholar]
- 29.Tosetto A., Castaman G., Plug I., Rodeghiero F., Eikenboom J. Prospective evaluation of the clinical utility of quantitative bleeding severity assessment in patients referred for hemostatic evaluation. J Thromb Haemost. 2011;9:1143–1148. doi: 10.1111/j.1538-7836.2011.04265.x. [DOI] [PubMed] [Google Scholar]
- 30.Zafarani A., Ghodratnia E., Amirzargar M.R., Mahmoudi M., Taghavi-Farahabadi M., Tavangar F., et al. Bleeding disorder of unknown cause: results from Iranian study. Transfus Apher Sci. 2023;62:103730. doi: 10.1016/j.transci.2023.103730. [DOI] [PubMed] [Google Scholar]
- 31.Quiroga T., Goycoolea M., Panes O., Aranda E., Martínez C., Belmont S., et al. High prevalence of bleeders of unknown cause among patients with inherited mucocutaneous bleeding. A prospective study of 280 patients and 299 controls. Haematologica. 2007;92:357–365. doi: 10.3324/haematol.10816. [DOI] [PubMed] [Google Scholar]
- 32.Agren A., Wiman B., Stiller V., Lindmarker P., Sten-Linder M., Carlsson A., et al. Evaluation of low PAI-1 activity as a risk factor for hemorrhagic diathesis. J Thromb Haemost. 2006;4:201–208. doi: 10.1111/j.1538-7836.2005.01709.x. [DOI] [PubMed] [Google Scholar]
- 33.Veen C.S.B., Huisman E.J., Cnossen M.H., Kom-Gortat R., Rijken D.C., Leebeek F.W.G., et al. Evaluation of thromboelastometry, thrombin generation and plasma clot lysis time in patients with bleeding of unknown cause: a prospective cohort study. Haemophilia. 2020;26:e106–e115. doi: 10.1111/hae.13991. [DOI] [PubMed] [Google Scholar]
- 34.Mehic D., Hofer S., Jungbauer C., Kaider A., Haslacher H., Eigenbauer E., et al. Association of ABO blood group with bleeding severity in patients with bleeding of unknown cause. Blood Adv. 2020;4:5157–5164. doi: 10.1182/bloodadvances.2020002452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehic D., Neubauer G., Janig F., Kaider A., Ay C., Pabinger I., et al. Risk factors for future bleeding in patients with mild bleeding disorders: longitudinal data from the Vienna Bleeding Biobank. J Thromb Haemost. 2023;21:1757–1768. doi: 10.1016/j.jtha.2023.03.006. [DOI] [PubMed] [Google Scholar]
- 36.Abou-Ismail M.Y., James P.D., Flood V.H., Connell N.T. Beyond the guidelines: how we approach challenging scenarios in the diagnosis and management of von Willebrand disease. J Thromb Haemost. 2023;21:204–214. doi: 10.1016/j.jtha.2022.11.042. [DOI] [PubMed] [Google Scholar]
- 37.James P.D., Connell N.T., Ameer B., Di Paola J., Eikenboom J., Giraud N., et al. ASH ISTH NHF WFH 2021 guidelines on the diagnosis of von Willebrand disease. Blood Adv. 2021;5:280–300. doi: 10.1182/bloodadvances.2020003265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mehic D., Kraemmer D., Tolios A., Bücheler J., Quehenberger P., Ay C., et al. Repeated testing unfolds the challenge of diagnosing von Willebrand disease: longitudinal data from the Vienna Bleeding Biobank. Blood. 2022;140:8433–8434. [Google Scholar]
- 39.Doshi B.S., Rogers R.S., Whitworth H.B., Stabnick E.A., Britton J., Butler R.B., et al. Utility of repeat testing in the evaluation for von Willebrand disease in pediatric patients. J Thromb Haemost. 2019;17:1838–1847. doi: 10.1111/jth.14591. [DOI] [PubMed] [Google Scholar]
- 40.Weyand A.C., Kouides P., Malvar J., Jaffray J. Is ≥ 100% the magic number to rule out the laboratory diagnosis of von Willebrand disease based on initial testing? Am J Hematol. 2021;96:E439–E441. doi: 10.1002/ajh.26343. [DOI] [PubMed] [Google Scholar]
- 41.Brown M.C., White M.H., Friedberg R., Woods K., Childress K., Kulkarni M., et al. Elevated von Willebrand factor levels during heavy menstrual bleeding episodes limit the diagnostic utility for von Willebrand disease. Res Pract Thromb Haemost. 2021;5 doi: 10.1002/rth2.12513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mehic D, Kraemmer D, Tolios A, Bücheler J, Quehenberger P, Haslacher H, et al. The necessity of repeat testing for von Willebrand disease in adult patients with mild to moderate bleeding disorders. J Thromb Haemost. Published online September 21, 2023. https://doi.org/10.1016/j.jtha.2023.09.010 [DOI] [PubMed]
- 43.O’Donnell J.S., Baker R.I. Low von Willebrand disease: a bleeding disorder of unknown cause? Hamostaseologie. 2023;43:44–51. doi: 10.1055/a-1980-8198. [DOI] [PubMed] [Google Scholar]
- 44.Baccolo A., Falcinelli E., Mezzasoma A.M., Guglielmini G., Borghi M., Bury L., et al. Usefulness of global tests of primary hemostasis in the initial screening of mild/moderate bleeding disorders for orienting towards von Willebrand disease or inherited platelet functions disorders. Thromb Res. 2023;221:79–82. doi: 10.1016/j.thromres.2022.11.021. [DOI] [PubMed] [Google Scholar]
- 45.Thomas W., White D., MacDonald S. Thrombin generation measured by two platforms in patients with a bleeding tendency: comment. J Thromb Haemost. 2021;19:2896–2899. doi: 10.1111/jth.15524. [DOI] [PubMed] [Google Scholar]
- 46.Ay C., Haselböck J., Laczkovics C., Koder S., Pabinger I. Thrombin generation in patients with a bleeding tendency of unknown origin. Ann Hematol. 2011;90:1099–1104. doi: 10.1007/s00277-011-1201-8. [DOI] [PubMed] [Google Scholar]
- 47.Hofer S., Ay C., Rejtö J., Wolberg A.S., Haslacher H., Koder S., et al. Thrombin-generating potential, plasma clot formation, and clot lysis are impaired in patients with bleeding of unknown cause. J Thromb Haemost. 2019;17:1478–1488. doi: 10.1111/jth.14529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holm E., Zetterberg E., Lövdahl S., Berntorp E. Patients referred for bleeding symptoms of unknown cause: does evaluation of thrombin generation contribute to diagnosis? Mediterr J Hematol Infect Dis. 2016;8 doi: 10.4084/MJHID.2016.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alves G.S.A., Orsi F.A., Santiago-Bassora F.D., Quaino S.K.P., Montalvão S.A.L., de Paula E.V., et al. Laboratory evaluation of patients with undiagnosed bleeding disorders. Blood Coagul Fibrinolysis. 2016;27:500–505. doi: 10.1097/MBC.0000000000000444. [DOI] [PubMed] [Google Scholar]
- 50.MacDonald S., White D., Langdown J., Downes K., Thomas W. Investigation of patients with unclassified bleeding disorder and abnormal thrombin generation for physiological coagulation inhibitors reveals multiple abnormalities and a subset of patients with increased tissue factor pathway inhibitor activity. Int J Lab Hematol. 2020;42:246–255. doi: 10.1111/ijlh.13155. [DOI] [PubMed] [Google Scholar]
- 51.Cornette M., Monteyne T., De Kesel P.M., Devreese K.M.J. Thrombin generation measured by two platforms in patients with a bleeding tendency. J Thromb Haemost. 2021;19:1460–1471. doi: 10.1111/jth.15292. [DOI] [PubMed] [Google Scholar]
- 52.Saes J.L., Schols S.E.M., van Heerde W.L., Nijziel M.R. Hemorrhagic disorders of fibrinolysis: a clinical review. J Thromb Haemost. 2018;16:1498–1509. doi: 10.1111/jth.14160. [DOI] [PubMed] [Google Scholar]
- 53.Mehic D., Colling M., Pabinger I., Gebhart J. Natural anticoagulants: a missing link in mild to moderate bleeding tendencies. Haemophilia. 2021;27:701–709. doi: 10.1111/hae.14356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dargaud Y., Scoazec J.Y., Wielders S.J.H., Trzeciak C., Hackeng T.M., Négrier C., et al. Characterization of an autosomal dominant bleeding disorder caused by a thrombomodulin mutation. Blood. 2015;125:1497–1501. doi: 10.1182/blood-2014-10-604553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Langdown J., Luddington R.J., Huntington J.A., Baglin T.P. A hereditary bleeding disorder resulting from a premature stop codon in thrombomodulin (p.Cys537Stop) Blood. 2014;124:1951–1956. doi: 10.1182/blood-2014-02-557538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Westbury S.K., Whyte C.S., Stephens J., Downes K., Turro E., Claesen K., et al. A new pedigree with thrombomodulin-associated coagulopathy in which delayed fibrinolysis is partially attenuated by co-inherited TAFI deficiency. J Thromb Haemost. 2020;18:2209–2214. doi: 10.1111/jth.14990. [DOI] [PubMed] [Google Scholar]
- 57.Vincent L.M., Tran S., Livaja R., Bensend T.A., Milewicz D.M., Dahlbäck B. Coagulation factor VA2440G causes east Texas bleeding disorder via TFPIα. J Clin Invest. 2013;123:3777–3787. doi: 10.1172/JCI69091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cunha M.L.R., Bakhtiari K., Peter J., Marquart J.A., Meijers J.C.M., Middeldorp S. A novel mutation in the F5 gene (factor V Amsterdam) associated with bleeding independent of factor V procoagulant function. Blood. 2015;125:1822–1825. doi: 10.1182/blood-2014-08-592733. [DOI] [PubMed] [Google Scholar]
- 59.Zimowski K.L., Ho M.D., Shields J.E., Denning G., Petrillo T., Jhita N., et al. Factor V Atlanta: a novel mutation in the F5 gene reveals potential new cis-acting elements involved in regulating FV-short and TFPI levels. Blood American Society of Hematology. 2017;130:366. [Google Scholar]
- 60.Mehic D., Tolios A., Hofer S., Ay C., Haslacher H., Downes K., et al. Thrombomodulin in patients with mild to moderate bleeding tendency. Haemophilia. 2021;27:1028–1036. doi: 10.1111/hae.14433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dahlbäck B. Novel insights into the regulation of coagulation by factor V isoforms, tissue factor pathway inhibitorα, and protein S. J Thromb Haemost. 2017;15:1241–1250. doi: 10.1111/jth.13665. [DOI] [PubMed] [Google Scholar]
- 62.Dahlbäck B. Pro- and anticoagulant properties of factor V in pathogenesis of thrombosis and bleeding disorders. Int J Lab Hematol. 2016;38:4–11. doi: 10.1111/ijlh.12508. [DOI] [PubMed] [Google Scholar]
- 63.Mehic D., Tolios A., Hofer S., Ay C., Haslacher H., Rejtö J., et al. Elevated levels of tissue factor pathway inhibitor in patients with mild to moderate bleeding tendency. Blood Adv. 2021;5:391–398. doi: 10.1182/bloodadvances.2020003464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Downes K., Megy K., Duarte D., Vries M., Gebhart J., Hofer S., et al. Diagnostic high-throughput sequencing of 2396 patients with bleeding, thrombotic, and platelet disorders. Blood. 2019;134:2082–2091. doi: 10.1182/blood.2018891192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scott C.F., Carrell R.W., Glaser C.B., Kueppers F., Lewis J.H., Colman R.W. Alpha-1-antitrypsin-Pittsburgh. A potent inhibitor of human plasma factor XIa, kallikrein, and factor XIIf. J Clin Invest. 1986;77:631–634. doi: 10.1172/JCI112346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Owen M.C., Brennan S.O., Lewis J.H., Carrell R.W. Mutation of antitrypsin to antithrombin. Alpha 1-antitrypsin Pittsburgh (358 Met leads to Arg), a fatal bleeding disorder. N Engl J Med. 1983;309:694–698. doi: 10.1056/NEJM198309223091203. [DOI] [PubMed] [Google Scholar]
- 67.Mehic D., Pabinger I., Ay C., Gebhart J. Fibrinolysis and bleeding of unknown cause. Res Pract Thromb Haemost. 2021;5 doi: 10.1002/rth2.12511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schulman S., El-Darzi E., Florido M.H.C., Friesen M., Merrill-Skoloff G., Brake M.A., et al. A coagulation defect arising from heterozygous premature termination of tissue factor. J Clin Invest. 2020;130:5302–5312. doi: 10.1172/JCI133780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morelli V.M., De Visser M.C.H., Vos H.L., Bertina R.M., Rosendaal F.R. ABO blood group genotypes and the risk of venous thrombosis: effect of factor V Leiden. J Thromb Haemost. 2005;3:183–185. doi: 10.1111/j.1538-7836.2004.01071.x. [DOI] [PubMed] [Google Scholar]
- 70.Dentali F., Sironi A.P., Ageno W., Bonfanti C., Crestani S., Frattini F., et al. Relationship between ABO blood group and hemorrhage: a systematic literature review and meta-analysis. Semin Thromb Hemost. 2013;39:72–82. doi: 10.1055/s-0032-1329550. [DOI] [PubMed] [Google Scholar]
- 71.Englisch C., Moik F., Nopp S., Raderer M., Pabinger I., Ay C. ABO blood group type and risk of venous thromboembolism in patients with cancer. Blood Adv. 2022;6:6274–6281. doi: 10.1182/bloodadvances.2021006283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Desch K.C. Regulation of plasma von Willebrand factor. F1000Res. 2018;7:96. doi: 10.12688/f1000research.13056.1. –6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Veen C.S.B., Huisman E.J., Romano L.G.R., Schipaanboord C.W.A., Cnossen M.H., de Maat M.P.M., et al. Outcome of surgical interventions and deliveries in patients with bleeding of unknown cause: an observational study. Thromb Haemost. 2021;121:1409–1416. doi: 10.1055/s-0041-1726344. [DOI] [PubMed] [Google Scholar]
- 74.Castle D., Desborough M.J.R., Kemp M., Lowe G., Thomas W., Obaji S. Outcomes and management of pregnancy in women with bleeding disorder of unknown cause. J Thromb Haemost. 2022;20:2519–2525. doi: 10.1111/jth.15871. [DOI] [PubMed] [Google Scholar]
- 75.Mehic D., Reitsma S., Haslacher H., Köller M., De Laat B., Ay C., et al. PB1206 Plasmin generation is impaired in patients with bleeding disorders of unknown cause. Res Practice Thrombosis Haemostasis. 2023;7:101037. [Google Scholar]
- 76.Baby S., Thomas L., Dave R., Geevar T., Vijayan R., Singh S., et al. PB0553 Role of international society of thrombosis and hemostasis-bleeding assessment tool, RICOF, ROTEM, and APTT-clot wave form analysis (CWA) in evaluation of patients with bleeding of unknown cause (BUC) Res Pract Thrombosis Haemostasis. 2023;7:100761. [Google Scholar]
- 77.Berkowitz C., Ma A., Miller V., Kirkland K., Key N. PB1376 Periprocedural hemostatic outcomes in bleeding of unknown cause. Res Pract Thrombosis Haemostasis. 2023;7:101530. [Google Scholar]
- 78.Mehic D., Schwarz S., Shulym I., Ay C., Pabinger I., Gebhart J. Health-related quality of life is impaired in bleeding disorders of unknown cause: results from the Vienna Bleeding Biobank. Res Pract Thromb Haemost. 2023;7 doi: 10.1016/j.rpth.2023.102176. [DOI] [PMC free article] [PubMed] [Google Scholar]