Abstract
The photosynthetic bacterium Rhodobacter capsulatus has been shown to carry out nitrogenase “switch-off,” a rapid, reversible inhibition of in vivo activity. Here, we demonstrate that highly nitrogen-limited cultures of both the wild-type strain and a draT draG mutant are capable of nitrogenase switch-off while moderately nitrogen-limited cultures show instead a “magnitude” response, with a decrease in in vivo nitrogenase activity that is proportional to the amount of added NH4+.
Nitrogenase is an enzymatic complex consisting of two proteins, MoFe protein (dinitrogenase) and Fe protein (dinitrogenase reductase), which catalyze the reduction of dinitrogen to ammonia. Since nitrogen fixation presents significant demands on cellular energy supplies, many diazotrophs have developed complex regulatory systems for tight control of nitrogenase synthesis and/or activity. Several different types of diazotrophs have been shown to be capable of nitrogenase “switch-off/switch-on,” a short-term (<10-min), reversible modulation of their in vivo activity in response to various environmental stimuli. In some cases, as amply demonstrated for the photosynthetic bacterium Rhodospirillum rubrum (13, 15), this response appears to be uniquely mediated by a two-enzyme system (dinitrogenase reductase ADP-ribosyltransferase [DRAT] and dinitrogenase reductase-activating glycohydrolase [DRAG]), which causes a covalent modification/demodification of the Fe protein via ADP-ribosylation.
Previous studies have shown that the photosynthetic bacterium Rhodobacter capsulatus is capable of nitrogenase switch-off in response to ammonium additions or darkness (3, 8, 19) and that the Fe protein from this organism is subject to in vivo (3, 8, 10, 17) and in vitro (4) ADP-ribosylation. Similarly to R. rubrum, R. capsulatus strains mutated in draT and draG have been reported to be unable to regulate in vivo nitrogenase activity in response to NH4+ and darkness (16). However, in apparent contradiction of these results, it has been reported that this organism was capable of nitrogenase switch-off in response to ammonium addition even though the Fe protein had been genetically altered so that it no longer contained the arginine residue that is the target for ADP-ribosylation (19). These differences might be explained by there being another cellular target for DRAT (15). Alternatively, the previously noted modification-independent regulation might be subtly altered by growth conditions which have been shown to affect the switch-off response of some photosynthetic bacteria (1, 24). A full understanding of the differences and commonalities of these two processes will require extensive biochemical and genetic analysis. As a prerequisite, we undertook a study of how the switch-off of nitrogenase activity, involving both ADP-ribosylation and ADP-ribosylation-independent processes, is affected by nitrogen limitation.
The effects of ammonium additions on nitrogenase activity and Fe protein modification are different depending upon the degree of nitrogen limitation.
We used cultures of strain SB1003, grown with different limiting amounts of initial NH4+ (0 to 7.6 mM) in completely filled 1.6- by 12.5-cm screw-capped tubes in a Biotronette mark III environmental chamber (Labline Instruments) equipped with three 150-W incandescent bulbs (∼3 klx at the tube surface), for the study of the relationship between in vivo nitrogenase regulation and Fe protein ADP-ribosylation.
The severity of nitrogen limitation varied with the initial NH4+ used for growth. Highly nitrogen-limited (HNL) cultures were grown on RCV medium without an added nitrogen source. Moderately nitrogen-limited (MNL) cultures were grown on RCV medium supplemented with 7.6 mM NH4+. HNL cultures had higher whole-cell glutamine synthetase (GS) activity (6, 9, 22) and a lower GS adenylylation state (6, 9, 22) than MNL cultures (Table 1). In the enteric bacteria Salmonella typhimurium and Klebsiella pneumoniae, the size of the intracellular glutamine pool appears to reflect the cellular nitrogen status, with external nitrogen limitation causing a drop in this pool (5). In agreement with this, quantitation of the levels of glutamine in R. capsulatus HNL and MNL cultures clearly showed great differences in the pools of this central nitrogen metabolite (Table 1).
TABLE 1.
Nitrogen status and NH4+ transport activities in HNL and MNL cultures of R. capsulatusa
| Culture type | GS activity (μmol min−1 mg of protein−1) | GS adenylylation stateb | Intracellular glutamine (nmol mg of protein−1) | NH4+ uptake (nmol min−1 mg of protein−1) | [14C]methylammonium uptake (nmol min−1 mg of protein−1) |
|---|---|---|---|---|---|
| HNL | 3.0 ± 0.3 | 0.70 ± 0.04 | 3.75 ± 1.39 | 46.8 ± 10.6 | 4.87 ± 2.37 |
| MNL | 1.9 ± 0.16 | 0.57 ± 0.02 | 138.0 ± 38 | 37.8 ± 8.5 | 0.63 ± 0.28 |
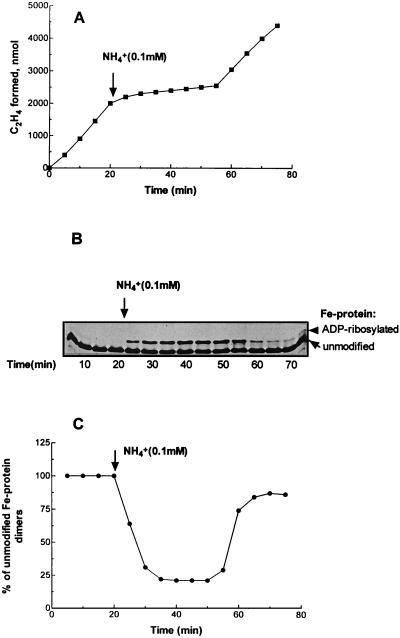
HNL cultures demonstrated a classical in vivo nitrogenase switch-off response to added NH4+. Complete inhibition of acetylene reduction (measured anaerobically at 30°C with 5-ml samples in 25-ml vials under high light intensity [4]) was evident within 2 to 3 min of NH4+ addition, and full recovery of the initial rate of nitrogenase activity was obtained within a relatively short period of time, which depended upon the amount of ammonium added (Fig. 1A). To monitor the modification state of Fe protein in R. capsulatus cells under different conditions, a rapid method of extraction with sodium dodecyl sulfate (SDS), which avoids artifactual changes in the modification state of Fe protein, was used to directly lyse cells without prior treatment and to solubilize protein for SDS-polyacrylamide gel electrophoresis and subsequent immunoblotting (26). The percentage of unmodified/modified Fe protein dimers was calculated as ADP-ribosylated Fe protein/total Fe protein (14). Since only one of the two Fe protein subunits in the Fe protein dimer becomes modified, 100% modification of Fe protein dimers corresponds to two equal-intensity bands. Immunoblot analysis (Fig. 1B and C) showed that the levels of unmodified Fe protein dimers coincided approximately both temporally and quantitatively with changes in in vivo nitrogenase activity.
FIG. 1.
Effects of ammonium on in vivo nitrogenase activity (C2H2 reduction) and the Fe protein modification state in HNL cultures of R. capsulatus. Cultures were grown under photoheterotrophic anaerobic conditions on RCV medium without an added nitrogen source to early stationary phase (∼12 h; extracellular ammonium, ∼10 μM; optical density at 660 nm, 1.1; nitrogenase activity, 196 nmol of C2H4 · min−1 · mg of protein−1), and 5-ml aliquots were transferred by syringe to 25-ml argon-filled vials for simultaneous analyses of nitrogenase activity by acetylene reduction and Fe protein modification state by SDS-polyacrylamide gel electrophoresis and immunoblotting. At the times indicated by the arrows, NH4Cl was added to a final concentration of 0.1 mM. (A) In vivo nitrogenase activity (C2H2 reduction). (B) Immunoblot of Fe protein in culture samples withdrawn during the C2H2 reduction assay. The lower bands correspond to the monomer form of unmodified Fe protein, and the upper bands correspond to the ADP-ribosylated monomer. It should be noted that only one of the two Fe protein subunits in the Fe protein dimer becomes modified; thus, 100% modification of Fe protein dimers corresponds to two equal-intensity bands consisting of an ADP-ribosylated subunit and an unmodified subunit. (C) Results of scanning densitometry of the immunoblot from panel B, calculated as percent unmodified Fe protein dimers. Each point represents an average of at least three replicate assays.
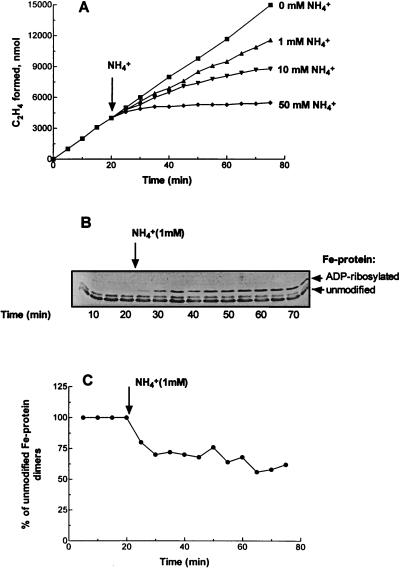
R. capsulatus cultures grown on RCV medium supplemented with increasing amounts of ammonium (1.9, 3.8, and 5.7 mM) showed a progressive decrease in the typical switch-off effect. At an even higher initial ammonium concentration, 7.6 mM (MNL culture), the typical switch-off effect was completely lost. At relatively low concentrations of added ammonium (Fig. 2), only partial inhibition of nitrogenase activity was observed and there appeared to be no recovery. Unlike the typical switch-off effects previously observed, where the quantity of added ammonium affects the duration of the response but not its magnitude (3, 27), with MNL cultures the quantity of added ammonium affects the magnitude of the inhibition. These effects were not due to differences in cell density or light intensity, since concentration of HNL cultures or dilution of MNL cultures immediately prior to assay gave essentially the same results as found with untreated cultures. A similar response has also been observed in the cyanobacterium Anabaena variabilis (21, 25) and in the methane-oxidizing bacterium Methylococcus capsulatus (18). This different response, which we term the nitrogenase “magnitude” response, was accompanied by Fe protein ADP-ribosylation, but the decrease in activity was 1.7-fold greater than the decrease in unmodified Fe protein (Fig. 2). In these cells, the modification of Fe protein was not proportional to the added NH4+ concentration, and similar levels of Fe protein ADP-ribosylation (25 to 40% modified dimers) were induced by the addition of 1 or 50 mM NH4+. Thus, depending on the severity of nitrogen limitation, NH4+-limited cultures of R. capsulatus demonstrate two different in vivo nitrogenase responses to NH4+ addition, switch-off and magnitude responses.
FIG. 2.
Effects of ammonium on in vivo nitrogenase activity (C2H2 reduction) and the Fe protein modification state in MNL cultures of R. capsulatus. Cultures were grown under photoheterotrophic anaerobic conditions on RCV medium containing 7.6 mM NH4+ as a limiting nitrogen source to early stationary phase (∼16 h; extracellular ammonium, ∼10 μM; optical density at 660 nm, 4.6; nitrogenase activity, 62 nmol of C2H4 · min−1 · mg of protein−1). Experimental details are as described in the legend to Fig. 1. (A) In vivo nitrogenase activity (C2H2 reduction). (B) Corresponding immunoblot analysis of Fe protein. (C) Content of unmodified Fe protein dimers calculated from the scan of the immunoblot presented in panel B. Each point represents an average of at least three replicate assays.
MNL cultures have a decreased level of high-affinity ammonium transport.
Externally added NH4+ must be transported into the cell to affect nitrogenase activity, suggesting that the observed differences might lie at the level of NH4+ uptake. R. capsulatus possesses two NH4+ transport systems, a relatively low-affinity system that appears to be constitutively synthesized and a high-affinity system that appears to be Ntr regulated (20). Both HNL and MNL cultures appeared to be metabolically active, as they showed appreciable rates of NH4+ uptake (23) (Table 1); this, however, does not differentiate between the two NH4+ transport systems. There was a marked difference in the two culture types when the high-affinity system was assayed by using uptake of [14C]methylammonium (20), with HNL cultures showing a much higher rate (Table 1). Since the total NH4+ transport capabilities of the two culture types were nearly the same, NH4+, at the concentrations used here, presumably enters the cell at the same rate in both HNL and MNL cultures. However, the difference in activity for the Ntr-regulated pathway indicates that it is much more active in HNL cultures than in MNL cultures. Treatments which are thought to inhibit this transport system, methionine sulfoximine addition (11) or alkaline pH incubation (12), abolish the switch-off effect (results not shown). The intracellular concentration of free NH4+ in nitrogen-fixing cells of Azotobacter and Rhodobacter spp. is in the range of 0.2 to 2.6 mM (2, 12). However, the minimal concentration of exogenous NH4+ that can induce the switch-off response is about 20 to 60 μM (3, 7, 24, 27), suggesting that only extracellular NH4+ can induce the switch-off response and therefore that the NH4+ uptake system may play an important role in the in vivo response of nitrogenase to added NH4+. Thus, our working hypothesis is that the switch-off process responds to a signal generated by transport of NH4+ by the high-affinity system.
Switch-off and magnitude responses are independent of DRAT and DRAG.
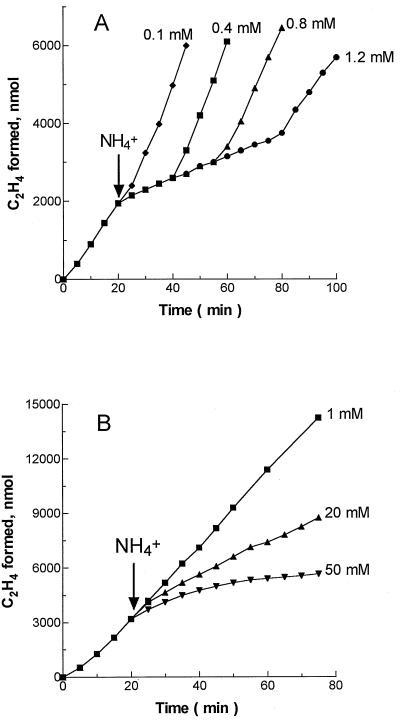
The potential role of the modification system in the magnitude response was examined with mutant strains that are incapable of ADP-ribosylation of Fe protein due to inactivation of the structural genes for DRAT and DRAG (W107I and W107II [16]). We have found that, similarly to the wild-type strain, the addition of NH4+ induced a switch-off response in HNL cultures of these mutants, while the magnitude response to NH4+ addition was observed with MNL cultures (Fig. 3 [data presented only for the W107II mutant]). Western blots confirmed the absence of ADP-ribosylated Fe protein (results not shown). In these mutant strains, both nitrogenase responses were less sensitive to NH4+ since higher NH4+ concentrations were required to induce these responses. Furthermore, incomplete inhibition of in vivo nitrogenase by NH4+ during the switch-off response was observed in HNL cultures (Fig. 3). These differences might be directly due to the absence of in vivo nitrogenase regulation by Fe protein ADP-ribosylation, or they might be an indirect effect.
FIG. 3.
Effects of ammonium on in vivo nitrogenase activity (C2H2 reduction) of HNL and MNL cultures of the R. capsulatus W107II (draT draG) mutant. Cultures were grown to early stationary phase under photoheterotrophic anaerobic conditions on RCV medium without an added nitrogen source (A) or containing 7.6 mM NH4+ as a limiting nitrogen source (B), as described in the legends to Fig. 1 and 2. (A) HNL culture. Optical density at 660 nm (OD660), 1.0; nitrogenase activity, 200 nmol of C2H4 · min−1 · mg of protein−1; extracellular ammonium, ∼10 μM. (B) MNL culture. OD660, 4.4; nitrogenase activity, 75 nmol of C2H4 · min−1 · mg of protein−1; extracellular ammonium, ∼10 μM. Experimental details are as described in the legend to Fig. 1. Ammonium was added at the times indicated by the arrows. Each point represents an average of at least three replicate assays.
These results indicate that in R. capsulatus, ADP-ribosylation of either the Fe protein or other cellular components is not the sole molecular mechanism responsible for in vivo nitrogenase regulation via either the switch-off or the magnitude response. Obviously further investigation is required to determine the molecular mechanisms of the regulation of in vivo nitrogenase activity in R. capsulatus, including determination of the signals for the various regulatory pathways, the switch-off response (ADP-ribosylation dependent and/or ADP-ribosylation independent) and the magnitude response. In this study, we have determined the experimental conditions necessary for the optimal manifestation and differentiation of three possible regulatory processes. This should greatly aid future physiological, biochemical, and genetic dissections of these different responses.
Acknowledgments
This research was supported by grant OGP0036584 from the Natural Sciences and Engineering Research Council of Canada.
W. Klipp is thanked for generously supplying mutants.
REFERENCES
- 1.Alef K, Arp D J, Zumft W G. Nitrogenase switch-off by ammonia in Rhodopseudomonas palustris: loss under nitrogen deficiency and independence from the adenylylation state of glutamine synthetase. Arch Microbiol. 1981;130:138–142. [Google Scholar]
- 2.Cordts M L, Gibson J. Ammonium and methylammonium transport in Rhodobacter sphaeroides. J Bacteriol. 1987;169:1632–1636. doi: 10.1128/jb.169.4.1632-1638.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hallenbeck P C, Meyer C M, Vignais P M. Nitrogenase from the photosynthetic bacterium Rhodopseudomonas capsulata: purification and molecular properties. J Bacteriol. 1982;149:708–717. doi: 10.1128/jb.149.2.708-717.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hallenbeck P C. Mutations affecting nitrogenase switch-off in Rhodobacter capsulatus. Biochim Biophys Acta. 1992;1118:161–168. doi: 10.1016/0167-4838(92)90145-4. [DOI] [PubMed] [Google Scholar]
- 5.Ikeda T P, Schauger A E, Kustu S. Salmonella typhimurium apparently perceives external nitrogen limitation as internal glutamine limitation. J Mol Biol. 1996;259:589–607. doi: 10.1006/jmbi.1996.0342. [DOI] [PubMed] [Google Scholar]
- 6.Johansson B C, Gest H. Adenylylation/deadenylylation control of the glutamine synthetase of Rhodopseudomonas capsulata. Eur J Biochem. 1977;81:365–371. doi: 10.1111/j.1432-1033.1977.tb11960.x. [DOI] [PubMed] [Google Scholar]
- 7.Jones B L, Monty K J. Glutamine as feedback inhibitor of the Rhodopseudomonas sphaeroides nitrogenase system. J Bacteriol. 1979;139:1007–1013. doi: 10.1128/jb.139.3.1007-1013.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jouanneau Y, Meyer C M, Vignais P M. Regulation of nitrogenase activity through iron protein interconversion into an active and inactive form in Rhodopseudomonas capsulata. Biochim Biophys Acta. 1983;749:318–328. [Google Scholar]
- 9.Jouanneau Y, Lebecque S, Vignais P M. Ammonia and light effect on nitrogenase activity in nitrogen-limited continuous culture of Rhodopseudomonas capsulata. Role of glutamine synthetase. Arch Microbiol. 1984;139:326–331. [Google Scholar]
- 10.Jouanneau Y, Roby C, Meyer C M, Vignais P M. ADP-ribosylation of dinitrogenase reductase in Rhodobacter capsulatus. Biochemistry. 1989;28:6524–6530. [Google Scholar]
- 11.Kleiner D, Alef K, Hartman A. Uptake of methionine sulfoximine by some N2 fixing bacteria, and its effect on ammonium transport. FEBS Lett. 1983;164:121–123. doi: 10.1016/0014-5793(83)80032-5. [DOI] [PubMed] [Google Scholar]
- 12.Kleiner D. Bacterial ammonium transport. FEMS Microbiol Rev. 1985;32:87–100. [Google Scholar]
- 13.Liang J, Nielsen G M, Lies D P, Burris R H, Roberts G P, Ludden P W. Mutations in the draT and draG genes of Rhodospirillum rubrum result in loss of regulation of nitrogenase by reversible ADP-ribosylation. J Bacteriol. 1991;173:6903–6909. doi: 10.1128/jb.173.21.6903-6909.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lowery R G, Saari L L, Ludden P W. Reversible regulation of the nitrogenase iron protein from Rhodospirillum rubrum by ADP-ribosylation in vitro. J Bacteriol. 1986;166:513–518. doi: 10.1128/jb.166.2.513-518.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ludden P W, Roberts G P. The biochemistry and genetics of nitrogen fixation by photosynthetic bacteria. In: Blankenship R E, Madigan M T, Bauer C E, editors. Anoxygenic photosynthetic bacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 929–947. [Google Scholar]
- 16.Masepohl B, Krey R, Klipp W. The draTG gene region of Rhodobacter capsulatus is required for post-translational regulation of both the molybdenum and the alternative nitrogenase. J Gen Microbiol. 1993;139:2667–2675. doi: 10.1099/00221287-139-11-2667. [DOI] [PubMed] [Google Scholar]
- 17.Michalski W P, Nicholas D J D, Vignais P M. 14C-labelling of glutamine synthetase and Fe-protein of nitrogenase in toluene-treated cells of Rhodopseudomonas capsulata. Biochim Biophys Acta. 1983;743:136–148. [Google Scholar]
- 18.Murrell J C. The rapid switch-off of nitrogenase activity in obligate methane-oxidizing bacteria. Arch Microbiol. 1988;150:489–495. [Google Scholar]
- 19.Pierrard J, Ludden P W, Roberts G P. Posttranslational regulation of nitrogenase in Rhodobacter capsulatus: existence of two independent regulatory effects of ammonium. J Bacteriol. 1993;175:1358–1366. doi: 10.1128/jb.175.5.1358-1366.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rapp B J, Landrum D C, Wall J D. Methylammonium uptake by Rhodobacter capsulatus. Arch Microbiol. 1986;146:134–141. [Google Scholar]
- 21.Reich S, Almon H, Böger P. Short-term effect of ammonia on nitrogenase activity of Anabaena variabilis (ATCC 29413) FEMS Microbiol Lett. 1986;34:53–62. [Google Scholar]
- 22.Shapiro B M, Stadtman E R. Glutamine synthetase (Escherichia coli) Methods Enzymol. 1970;17A:910–922. doi: 10.1016/s0076-6879(85)13032-6. [DOI] [PubMed] [Google Scholar]
- 23.Solorzano L. Determination of ammonia in natural sea water by the phenolhypochlorite method. Limnol Oceanogr. 1969;14:799–801. [Google Scholar]
- 24.Sweet W J, Burris R H. Inhibition of nitrogenase activity by NH4+ in Rhodospirillum rubrum. J Bacteriol. 1981;145:824–831. doi: 10.1128/jb.145.2.824-831.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yakunin A F, Troshina O Y, Jha M, Gogotov I N. Effect of ammonium on nitrogenase activity in the heterocystous cyanobacterium Anabaena variabilis. Mikrobiologiya. 1992;61:256–260. [Google Scholar]
- 26.Yakunin A F, Hallenbeck P C. A luminol/iodophenol chemiluminescent detection system for Western immunoblots. Anal Biochem. 1998;258:146–149. doi: 10.1006/abio.1998.2571. [DOI] [PubMed] [Google Scholar]
- 27.Zumft W G, Castillo F. Regulatory properties of the nitrogenase from Rhodopseudomonas palustris. Arch Microbiol. 1978;117:53–60. doi: 10.1007/BF00689351. [DOI] [PubMed] [Google Scholar]