Abstract
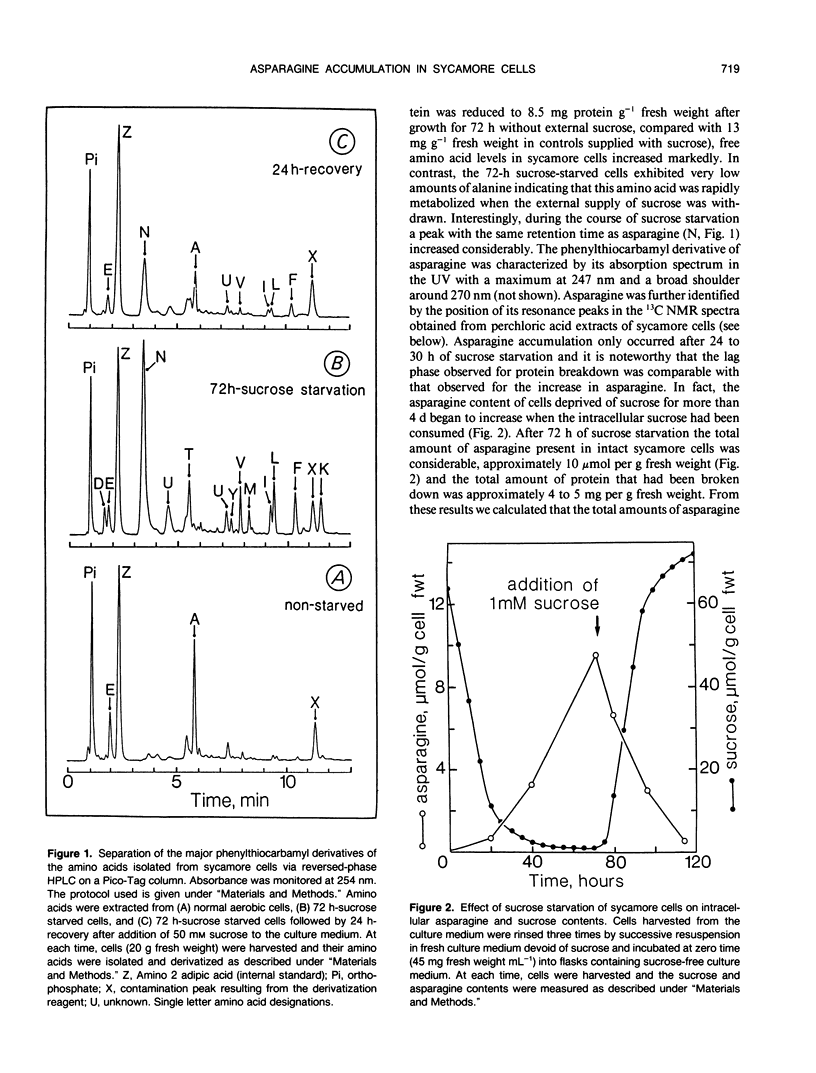
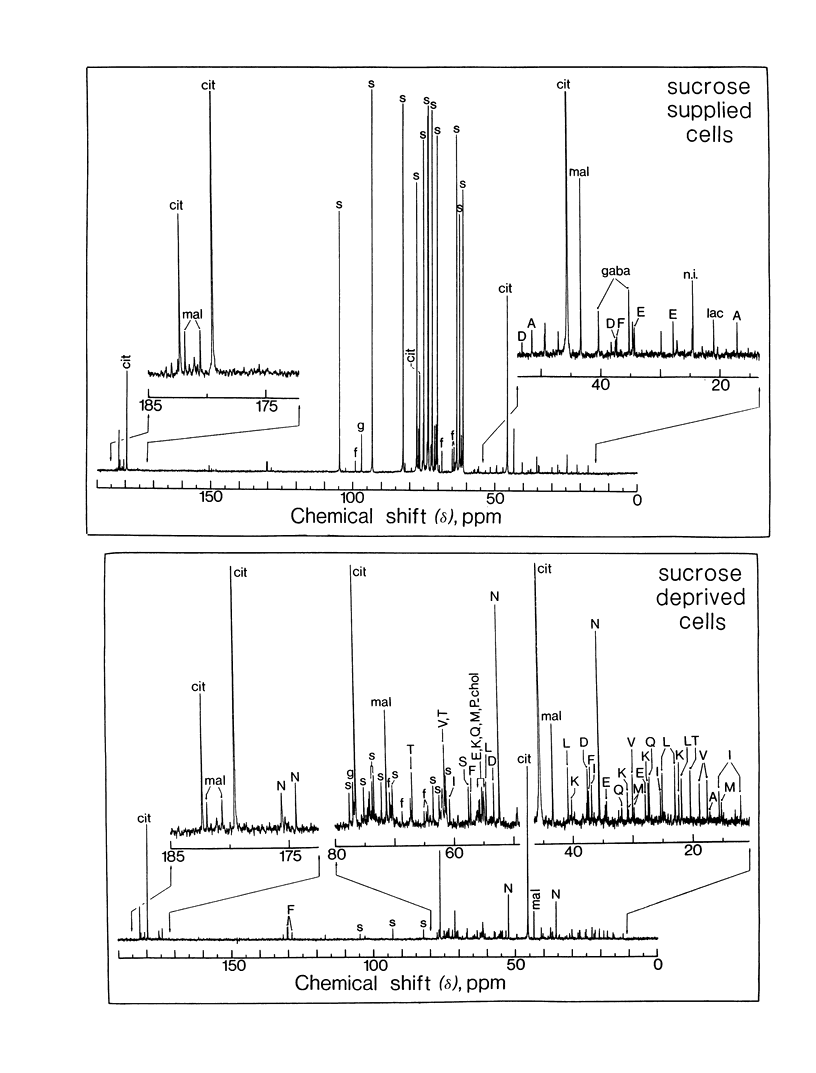
The mobilization of stored carbohydrates (sucrose and starch) and proteins during sucrose starvation was studied with sycamore (Acer pseudoplatanus L.) cells. When almost all the intracellular carbohydrate pools had disappeared, the cell protein content declined progressively whereas asparagine determined by either 13C nuclear magnetic resonance or reversed phase high performance liquid chromatography increased steadily. After a long period of sucrose starvation, the most intense resonances in the 13C nuclear magnetic resonance spectra were from citrate and asparagine. The total amounts of asparagine (expressed as nitrogen) and free amino acids that appeared after a long period of sucrose deprivation corresponded roughly to the total amount of protein (expressed as nitrogen), that disappeared within the same period of time. Addition of sucrose in the culture medium after a long period of sucrose starvation led to a disappearance of asparagine. These results suggest therefore that the presence of asparagine in plant cells in large excess should be considered as a good marker of protein utilization after a long period of sucrose starvation and is very likely related to stress.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bidlingmeyer B. A., Cohen S. A., Tarvin T. L. Rapid analysis of amino acids using pre-column derivatization. J Chromatogr. 1984 Dec 7;336(1):93–104. doi: 10.1016/s0378-4347(00)85133-6. [DOI] [PubMed] [Google Scholar]
- Caro L. H., Plomp P. J., Leverve X. M., Meijer A. J. A combination of intracellular leucine with either glutamate or aspartate inhibits autophagic proteolysis in isolated rat hepatocytes. Eur J Biochem. 1989 May 15;181(3):717–720. doi: 10.1111/j.1432-1033.1989.tb14782.x. [DOI] [PubMed] [Google Scholar]
- Gerbling H., Gerhardt B. Peroxisomal degradation of branched-chain 2-oxo acids. Plant Physiol. 1989 Dec;91(4):1387–1392. doi: 10.1104/pp.91.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Journet E. P., Bligny R., Douce R. Biochemical changes during sucrose deprivation in higher plant cells. J Biol Chem. 1986 Mar 5;261(7):3193–3199. [PubMed] [Google Scholar]
- Leverve X. M., Caro L. H., Plomp P. J., Meijer A. J. Control of proteolysis in perifused rat hepatocytes. FEBS Lett. 1987 Jul 27;219(2):455–458. doi: 10.1016/0014-5793(87)80271-5. [DOI] [PubMed] [Google Scholar]
- Roby C., Martin J. B., Bligny R., Douce R. Biochemical changes during sucrose deprivation in higher plant cells. Phosphorus-31 nuclear magnetic resonance studies. J Biol Chem. 1987 Apr 15;262(11):5000–5007. [PubMed] [Google Scholar]
- Urquhart A. A., Joy K. W. Transport, metabolism, and redistribution of xylem-borne amino acids in developing pea shoots. Plant Physiol. 1982 May;69(5):1226–1232. doi: 10.1104/pp.69.5.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]


