Abstract
Sterility in male NHP has long been achieved through surgical castration or vasectomy. However, these techniques are irreversible, require a surgical procedure, and have potential consequences such as sperm granulomas and long recovery time. Deslorelin is a gonadotropin-releasing hormone agonist that temporarily and reversibly suppresses sex hormone secretion. Our goal in this study was to investigate the effects of deslorelin on testosterone secretion and testicular volume in male rhesus macaques (Macaca mulatta). Male macaques (n = 4) each received two, 4.7-mg deslorelin implants subcutaneously in the interscapular region. Serum testosterone and testicular volume were then monitored at specific time points until 10 mo after treatment. Testosterone suppression was defined as testosterone levels lower than 0.6 ng/mL for a sustained period of at least 30 d. After implantation, mean testicular volume was significantly reduced by day 121. Testosterone suppression was observed in all subjects. However, the time from implantation to testosterone suppression and duration of suppression varied. Two macaques were hormonally suppressed by day 26 after implantation and remained suppressed for at least 6 mo. The other 2 macaques were hormonally suppressed by 2 mo after implantation; of these two, one remained suppressed for 70 days while the other was suppressed for at least 245 days. We conclude that deslorelin can safely suppress testosterone secretion in male rhesus macaques, but individual variation in onset and duration of action should be considered when establishing reimplantation time points and potential return to reproductive activity.
Abbreviation: GnRH, gonadotropin-releasing hormone
Introduction
Captive animals in research and zoo settings are often reproductively managed to maintain genetic diversity and avoid overpopulation. Historically, contraception in captive settings has been achieved through gonadectomies (i.e., castration or vasectomy in males; ovariohysterectomy or tubal ligation in females) or segregating animals by sex. Gonadectomies are invasive, irreversible, and are associated with perioperative discomfort and stress.12,20 Other methods of population management, such as segregation of animals by sex, may be impractical for some facilities and limit social interactions. These approaches may confound study results by altering neuroendocrine and autonomic responses.32 Oral contraception has been used in female NHP in zoo and research settings with relative success, but adverse effects that include uterine changes and perimenstrual bleeding have been reported.7 Given these concerns, interest in pharmacologic methods of contraception has increased because they may provide useful refinements to the management of reproduction in captive species.
Deslorelin is a gonadotropin-releasing hormone (GnRH) agonist that may provide an affordable, reversible, and noninvasive alternative to surgical methods of contraception. A synthetic nonapeptide of GnRH, deslorelin temporarily suppresses the secretion of sex hormones, including progesterone, estrogen, and testosterone.35 As a reproductive suppressant, deslorelin can be used for temporary sterilization or to medically manage sex hormone-responsive disorders, such as benign prostatic hyperplasia in dogs and adrenocortical disease in ferrets.36,46 Given the well-established link between testosterone and aggression among vertebrate species,3,11 deslorelin may also modulate aggressive behavior among animals in captive populations.17,29,38
Deslorelin is available as a controlled-release, bioresorbable implant (Suprelorin F, Virbac, Fort Worth, TX) that is FDA-approved in the United States to manage adrenocortical gland disease in ferrets. The effects of deslorelin are biphasic. It initially binds to and activates GnRH receptors in the anterior pituitary, inducing a transient stimulatory phase that may last days to weeks.18 During this initial acute phase, there is a surge of gonadotropins (luteinizing hormone and follicle-stimulating hormone) which stimulates sex hormone secretion from the gonads.24 Continuous circulation of gonadotropins leads to the desensitization of pituitary GnRH receptors, resulting in negative feedback on the hypothalamic–pituitary–gonadal axis. This effect leads to a chronic phase that is characterized by the suppression of reproductive hormones and fertility.10,13
Deslorelin has been successful in suppressing reproductive function in male dogs,10,18,37,39,44 cats,15,31 cheetahs,5 coyotes,23 ferrets,41 sea otters,21 turkeys,29 and pigeons.8 Although these studies have shown that deslorelin is easy to use and effective in many species, it has been underutilized in NHP, and previous studies on this topic had varied results. Deslorelin has successfully suppressed testosterone levels and aggression in male lion-tailed macaques (Macaca silenus) receiving 9 to 12 mg of deslorelin33 and olive baboons (Papio anubis) receiving 4.7 mg,26 but did not suppress testosterone concentrations or spermatogenesis in ring-tailed lemurs (Lemur catta) given 4.7 mg.6 No changes in appetite or adverse effects were reported in these 3 studies.6,26,33 A study analyzing the efficacy of deslorelin in captive zoo animals postulated that males primates (including mandrills [Mandrillus sphinx], drills [Mandrillus leucophaeus], baboons [Papio spp.], and lion-tailed macaques [Macaca silensus]) may require higher doses to achieve suppression than do conspecific females.1
Rhesus macaques (Macaca mulatta) are the most commonly used NHP in research.14 Prior studies have confirmed that testosterone suppression occurs in rhesus macaques that are given continuous infusions of GnRH-agonists via osmotic minipumps.2,9,25 In addition, a recent study demonstrated successful hormonal suppression in female rhesus macaques implanted with deslorelin.34 However, at present, no published studies have evaluated the efficacy of deslorelin implants on reproductive function in male rhesus macaques. The goal of this prospective study is to determine the efficacy of deslorelin for reducing reproductive parameters in male rhesus macaques. We hypothesized that two 4.7-mg deslorelin implants would suppress the production of testosterone and decrease testicular volume.
Materials and Methods
Animals.
The study population comprised 4 sexually mature, adult male rhesus macaques of similar age and weight with no history of reproductive tract abnormalities or trauma (Table 1). These macaques ranged in age from 7 to 9 y (mean ± 1 SD, 8.0 ± 0.7 y), with weights ranging between 11.7 to 19.0 kg (mean, 15.9 ± 2.7 kg; median, 16.5 kg). All animal procedures were approved by the University of California–Davis IACUC. Animals were maintained in full accordance with the standards in the Guide for Care and Use of Laboratory Animals and the Animal Welfare Act and Regulations.45 All animals originated from and were housed at the California National Primate Research Center, an AAALAC-accredited, USDA-registered facility.
Table 1.
Demographics of study animals at the time of enrollment
| Animal | Age (y) | Weight (kg) | Body condition score (1 to 5) | Housing condition |
|---|---|---|---|---|
| M1 | 7 | 19.0 | 4.5 | Single |
| M2 | 8 | 11.7 | 3.0 | Full contact |
| M3 | 9 | 17.0 | 3.5 | Full contact |
| M4 | 8 | 16.0 | 3.5 | Single |
Macaques were fed a commercial monkey chow (Monkey Diet Jumbo 5037, LabDiet, St. Louis, MO), and water was freely available. Two of the study subjects were singly housed, whereas the other 2 were pair-housed with males who were not involved in the current study. Animals were housed indoors in standard caging with controlled temperature and humidity (23 ± 3 °C and 30 to 70%, respectively). Animals were maintained on a 12:12-h light:dark cycle and had visual and auditory contact with conspecifics. The macaques had no outdoor access during the study. Items that could be manipulated were provided for enrichment. Before study initiation, animals were deemed healthy based on physical examination, hematology, and serum biochemistry evaluation.
Study design.
Before treatment, baseline serum testosterone levels and testicular measurements were obtained. Animals were sedated with ketamine (10 mg/kg IM) to facilitate implantation and testicular measurements. Testicular dimensions were measured by using calipers and ultrasonography. Testicular volume was determined by using the formula for an ellipsoid:
Volume (cm3) = 4/3π × length (cm)/2 × width (cm)/2 × thickness (cm)/2
Deslorelin implants are available in 4.7-mg and 9.4-mg formulations and are labeled to provide contraception that lasts for 6 mo and 12 mo, respectively, in dogs.11 The 9.4-mg option was commercially available only in the European Union and Australia at the time of our project initiation.13 A previous study33 that involved deslorelin implantation of male lion-tailed macaques reported a reduction in testosterone with 9 to 12 mg of deslorelin, whereas 6 mg was ineffective.33 Because 9.4-mg implants were not available in the United States at the time of our study, we elected to implant each subject with two 4.7-mg deslorelin implants (Suprelorin F, Virbac) subcutaneously in the interscapular region. The implant matrix of the 4.7-mg formulation that we used is the same as those used previously,33 thereby enabling appropriate matching of doses for comparison.
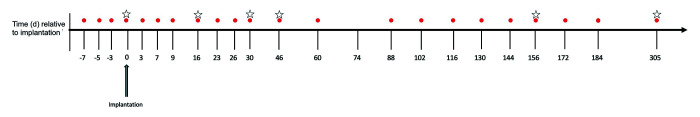
Blood was collected for hormonal analysis at multiple time points before and after implantation and on the day of implantation (Figure 1). All blood collections occurred between 0800 and 1100. Approximately 2 mL of blood was obtained from the cephalic or femoral vein at each time point. Within 15 min of collection, samples were centrifuged (3,000 × g for 10 min) for serum separation. The supernatant was then stored in 1.5-mL microcentrifuge tubes at –80 °C until hormonal assays were performed. Serial testicular measurements were obtained under ketamine sedation on day 0 (implantation day) and on days 16, 30, 63, 93, 121, 156, 184, and 305 after implantation. Macaques were fasted for 8 h prior to sedation, and blood was collected prior to testicular measurements. On dates that did not require testicular measurements, blood collection was performed without sedation via cageside venepuncture. All animals had been acclimated to and trained in this ‘arm-pull’ method prior to the initiation of this study.
Figure 1.
Timeline of blood collection and deslorelin implantation. Numbers correspond to days relative to implantation (day 0). Red circles indicate the days of blood collection. Stars indicate the days of testicular measurements.
Serum testosterone concentrations were measured by the Endocrine Technologies Core at the Oregon National Primate Research Center by using an automated clinical immunoassay platform (Cobas e411, Roche Diagnostics, Indianapolis, IN). The assay range was 0.025 to 15 ng/mL. The intraassay coefficient of variation was 2.3%. Because all samples were measured in a single assay, an interassay coefficient of variation was not determined specifically for this study. However, the interassay coefficient of variation for the testosterone assay in the Endocrine Technologies Core is 6.8%. Hormonal suppression was defined as testosterone levels lower than 0.6 ng/mL for a sustained period of at least 30 d. We chose 0.6 ng/mL as the threshold because this value is comparable to that of castrated rhesus macaques and is consistent with previously reported basal testosterone levels of rhesus macaques receiving GnRH agonists.9,34
Statistics and data visualization.
We used a Kruskal–Wallis equality-of-populations rank test (kwallis function; Stata/MP 14.0, Stata Corporation, College Station, TX) to investigate the difference in means across different points in time. Data visualizations were performed in JMP 16 (SAS Institute, Cary, NC). Descriptive statistics are expressed as mean with SEM.
Results
Effects of deslorelin on testosterone secretion within 3 d of implantation.
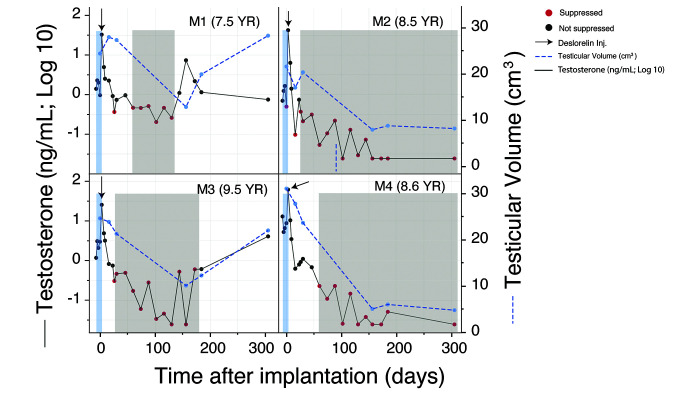
In the week prior to implantation, testosterone levels varied widely among subjects (Figure 2) and subject M2’s testosterone concentration was below the threshold of suppression on the day of implantation. On day 3 after implantation, all subjects showed at least a 7-fold rise in testosterone from their individual averaged preimplantation concentrations. One animal (M2) had a 42-fold increase from baseline, whereas M1, M3, and M4 had 20-, 11- and 7.5-fold increases, respectively. At 1 wk after implantation, all subjects showed a decline in testosterone as compared with day 3, but levels remained at least twice their average preimplantation levels in all macaques but one (M4).
Figure 2.
Testicular volume over time before and after implantation (log10 transformation) and serum testosterone concentrations before and after implantation in macaques M1, M2, M3, and M4. The arrow indicates the day of deslorelin implantation. Red dots indicate testosterone concentrations lower than 0.6 ng/mL. The shaded grey regions indicate suppression of testosterone to lower than 0.6 ng/mL for at least 30 d. The shaded blue regions indicate baseline testosterone values obtained prior to implantation.
Duration of deslorelin-induced suppression of testosterone levels.
Two macaques (M2 and M3) showed testosterone suppression by day 26 after implantation, whereas the other 2 macaques (M1 and M4) were not suppressed until 2 mo after implantation. M2 and M1 showed suppression at as early as days 16 and 26, respectively. However, both demonstrated a transitory escape from suppression before maintaining basal levels for a sustained time period (at least 279 d for M2 and 70 d for M1). Similarly, the testosterone level of M4 was just over the threshold of suppression at day 16, after which testosterone concentrations increased for about a month before reaching borderline suppression at day 46 and complete suppression between days 60 and 305.
The duration of suppression varied substantially between subjects. Testosterone levels in 2 animals (M2 and M4) were suppressed for at least 8 mo (Table 2); those in M3 and M1 were suppressed for at least 5 mo and 70 d, respectively.
Table 2.
Duration of testosterone suppression
| Animal | Duration of testosterone suppression (d) |
|---|---|
| M1 | 70 |
| M2 | ≥ 280 |
| M3 | 158 |
| M4 | ≥ 250 |
Duration of testosterone suppression was defined as the number of days after implantation during which testosterone was less 0.6 ng/mL for at least 30 d.
Deslorelin significantly reduces mean testicular size.
At 5% significance, we rejected the null hypothesis of equality of population means at t = 0 and t = 121 (P = 0.0209). To test for differences in means between t = 0 and t = 305, we used a Kruskal–Wallis equality-of-populations rank test and failed to reject the null hypothesis of equality of population means at t = 0 and t = 305 at 5% significance (P = 0.248). Thus, overall testicular volume was significantly lower at 121 d but not 305 d after implantation.
Discussion
Reproductive control is an important component in managing captive species because it ensures that population numbers remain appropriate for the allotted space and budget of a given facility. Deslorelin is a GnRH agonist that has been shown to reliably suppress hormonal activity in several species.7,22 In our current investigation, we measured testicular volume and testosterone levels in male macaques that each received two 4.7-mg subcutaneous deslorelin implants.
Preimplantation testosterone concentrations were above basal levels in all subjects but M2 on implantation day. On days 7, 5, and 3 before implantation, testosterone in M2 exceeded basal levels but was below the threshold of suppression on day 0. A possible explanation for this pattern is that testosterone secretion in adult male rhesus macaques follows a diurnal pattern, with the nadir of secretion in the morning and elevated levels in the evening.27 In addition, testosterone secretion occurs in a pulsatile fashion, and adult male macaques have been shown to have 7 to 12 peaks in testosterone over a 24-h period (each preceded by peaks of serum luteinizing hormone), with the lowest pulse frequency in morning hours.40 In an effort to reduce the effect of circadian variation in testosterone secretion, we performed all blood collections between 0800 and 1100. Monitoring serial testosterone concentrations over a 24-h period, along with measurement of luteinizing hormone concentrations, would enable better characterization of the circadian rhythm of hormonal secretion and its potential effects as a confounding variable. Although ketamine sedation was utilized to obtain samples for hormonal analysis, ketamine is not thought to alter testosterone levels since a previous study found no significant difference in serum testosterone concentrations in conscious and ketamine-sedated rhesus macaques.47
In this study, the hormonal suppression threshold was defined as 0.6 ng/mL, which is consistent with basal levels of rhesus macaques receiving GnRH agonists9 and comparable to that of castrated rhesus macaques.34 After implantation, all macaques showed a transient androgen surge at day 3, consistent with the initial stimulatory (‘flare up’) phase of the GnRH agonist.18 Deslorelin successfully suppressed testosterone in all subjects, although the time to and duration of suppression varied. Latency to effectiveness ranged between 26 to 60 d, with M2 and M3 achieving consistent suppression by day 26 and M1 and M4 by day 60. Both M1 and M2 had basal testosterone levels on days 26 and 16, respectively. However, testosterone levels escaped suppression for a period of 34 d for M1 and 10 d for M2 before returning to basal levels for more than 30 d. The observed differences in onset of action suggests that the stimulatory phase may be subject to individual biologic variation. This factor is an important consideration, given that animals in this phase may exhibit normal or even enhanced fertility. As such, alternate population control methods such as sex segregation should be enacted during the first 1 to 2 mo after implantation to prevent unintended pregnancy.
Outdoor-housed male rhesus macaques are seasonal breeders whose testosterone levels typically fluctuate widely across a 12-mo period, with highest levels observed during the early and middle parts of the mating season (October and November).16,30 The reproduction of indoor-housed rhesus macaques is less influenced by season, and macaques can breed all year in captive settings.4,19 In our current study, subjects were implanted in early November, which corresponds to the middle of the breeding season.16,30 Hormonal suppression was observed roughly 1 to 2 mo after implantation, during December to January, corresponding with the end of the breeding season, when testosterone levels naturally decline in outdoor-housed macaques.16,30 Although these observations may be interpreted as confounding, the decrease in testosterone we observed was of greater magnitude than the androgen decline that occurs naturally at the end of the breeding season. In our study, testosterone was lower than 0.6 ng/mL, whereas reported plasma testosterone concentrations in outdoor-housed macaques in the nonmating season are 3.9 to 4.0 ng/mL.16,28 Given this difference, we speculate that the androgen suppression we saw occurred secondary to the effect of the GnRH agonist rather than seasonal hormonal variation. Future studies may investigate the effects of deslorelin implanted in different seasons to confirm that hormonal suppression occurs independent of seasonal hormonal fluctuations.
Testicular volume was of interest because it directly correlates with tubular size and gonadal function.42,43 Mean testicular volume was significantly reduced in all subjects at 3 mo after treatment. Conversely, mean testicular volume at days 0 and 305 were not significantly different, suggesting that reduction in testicular volume was transient and likely correlated to hormonal suppression. M1 had a higher testicular volume at day 16 after implantation, perhaps reflecting the stimulatory phase with upregulation of the hypothalamic–pituitary–gonadal axis. The testicular size of 2 subjects, M1 and M3, began to increase gradually at around 6 mo after implantation, and by month 10, both subjects had testicular size that was comparable to the preimplantation volume. M2 and M4 also maintained basal testosterone levels at 10 mo after implantation whereas M1 and M3 did not, suggesting that hormonal suppression and testicular size are correlated.
Because deslorelin alters sex hormone concentrations, changes in behavior and social dynamics may occur. Although a formal behavioral analysis was not included in this study, we observed a change in the social dynamics between M2 and his pair mate. M2 was singly housed at the start of the study and was paired with a male macaque (unrelated to this study) on day 3 after implantation. At their social introduction, M2 asserted dominance over his partner, but the pair was otherwise affiliative and compatible. At 3 mo after implantation, M2 sustained minor injuries inflicted by his partner, and a behavior analysis reported a clear change in the dominance status between the pair. This reversion from dominance to submission has previously been reported in an olive baboon (Papio anubis) treated with deslorelin.26 For this reason, caution should be exercised when cohousing desloresin-treated and untreated male NHP.
Despite interindividual variation in the onset and duration of action of deslorelin, our results suggest that deslorelin suppresses testosterone to basal levels in male rhesus macaques and may offer a safe and noninvasive method of contraception. As such, deslorelin may enable the cohousing of animals of opposite sexes or those whose aggressive behaviors previously precluded them from group housing. Due to the drug’s reversibility, animals receiving deslorelin can be bred successfully once the hormonal component of the implant is depleted.15 This feature maximizes the animal’s potential reproductive capabilities, which is particularly important when genetically valuable animals are managed for conservation or research. Future studies may include longer post-implantation hormonal monitoring, measurement of luteinizing hormone levels, and behavioral analyses.
Acknowledgments
The Endocrine Technologies Core (ETC) at Oregon National Primate Research Center (ONPRC) is supported (in part) by NIH grant P51 OD011092 for operation of the Oregon National Primate Research Center.
References
- 1.Agnew MK, Asa CS, Franklin AD, McDonald MM, Cowl VB. 2021. Deslorelin (Suprelorin) use in North American and European zoos and aquariums: Taxonomic scope, dosing, and efficacy. J Zoo Wildl Med 52:427–436. 10.1638/2020-0217. [DOI] [PubMed] [Google Scholar]
- 2.Akhtar FB, Marshall GR, Wickings EJ, Nieschlag E. 1983. Reversible induction of azoospermia in rhesus monkeys by constant infusion of a gonadotropin-releasing hormone agonist using osmotic minipumps. J Clin Endocrinol Metab 56:534–540. 10.1210/jcem-56-3-534. [DOI] [PubMed] [Google Scholar]
- 3.Archer J. 1988. The Behavioural Biology of Aggression, vol 1. Cambridge (UK): Cambridge University Press. [Google Scholar]
- 4.Beck RT, Lubach GR, Coe CL. 2020. Feasibility of successfully breeding rhesus macaques (Macaca mulatta) to obtain healthy infants yearround. Am J Primatol 82:e23085. 10.1002/ajp.23085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertschinger HJ, Jago M, Nöthling J, Human A. 2006. Repeated use of the GnRH analogue deslorelin to down-regulate reproduction in male cheetahs (Acinonyx jubatus). Theriogenology 66:1762–1767. 10.1016/j.theriogenology.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 6.Carbajal A, Tallo-Parra O, Sabes-Alsina M, et al. 2018. Effect of deslorelin implants on the testicular function in male ring-tailed lemurs (Lemur catta): Deslorelin effect in Lemur catta. J Zoo Aquar Res 6:37–40. [Google Scholar]
- 7.Carroll KE, Mackiewicz AL, Ardeshir A, Alber SA, Christe KL. 2022. Hormonal suppression in female rhesus macaques (Macaca mulatta) implanted subcutaneously with deslorelin. J Am Assoc Lab Anim Sci 61:226–233. 10.30802/AALAS-JAALAS-21-000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cowan ML, Martin GB, Monks DJ, Johnston SD, Doneley RJT, Blackberry MA. 2014. Inhibition of the reproductive system by deslorelin in male and female pigeons (Columba livia). J Avian Med Surg 28:102–108. 10.1647/2013-027. [DOI] [PubMed] [Google Scholar]
- 9.Davis-daSilva M, Wallen K. 1989. Suppression of male rhesus testicular function and sexual behavior by a gonadotropin-releasing hormone agonist. Physiol Behav 45:963–968. 10.1016/0031-9384(89)90222-9. [DOI] [PubMed] [Google Scholar]
- 10.Driancourt MA, Briggs JR. 2020. Gonadotropin-releasing hormone (GnRH) agonist implants for male dog fertility suppression: A review of mode of action, efficacy, safety, and uses. Front Vet Sci 7:7. 10.3389/fvets.2020.00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ehrenkranz J, Bliss E, Sheard MH. 1974. Plasma testosterone: correlation with aggressive behavior and social dominance in man. Psychosom Med 36:469–475. 10.1097/00006842-197411000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Firth AM, Haldane SL. 1999. Development of a scale to evaluate postoperative pain in dogs. J Am Vet Med Assoc 214:651–659. [PubMed] [Google Scholar]
- 13.Fontaine C. 2015. Long-term contraception in a small implant: A review of Suprelorin (deslorelin) studies in cats. J Feline Med Surg 17:766–771. 10.1177/1098612X15594990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibbs RA, Rogers J, Katze MG, Bumgarner R, Weinstock GM, Mardis ER, Remington KA, Strausberg RL, Venter JC, Wilson RK, Batzer MA, Bustamante CD, Eichler EE, Hahn MW, Hardison RC, Makova KD, Miller W, Milosavljevic A, Palermo RE, Siepel A, Sikela JM, Attaway T, Bell S, Bernard KE, Buhay C, Chandrabose MN, Dao M, Davis C, Delehaunty KD, Ding Y, Dinh HH, Dugan-Rocha S, Fulton LA, Gabisi RA. 2007. Evolutionary and biomedical insights from the rhesus macaque genome. Science 316:222–234. [DOI] [PubMed] [Google Scholar]
- 15.Goericke-Pesch S, Georgiev P, Antonov A, Albouy M, Wehrend A. 2011. Clinical efficacy of a GnRH-agonist implant containing 4.7-mg deslorelin, Suprelorin, regarding suppression of reproductive function in tomcats. Theriogenology 75:803–810. 10.1016/j.theriogenology.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 16.Gordon TP, Rose RM, Bernstein IS. 1976. Seasonal rhythm in plasma testosterone levels in the rhesus monkey (Macaca mulatta): A 3-year study. Horm Behav 7:229–243. 10.1016/0018-506X(76)90050-7. [DOI] [PubMed] [Google Scholar]
- 17.Harley JJ, Power A, Stack JD. 2019. Investigation of the efficacy of the GnRH agonist deslorelin in mitigating intraspecific aggression in captive male Amur leopards (Panthera pardus orientalis). Zoo Biol 38:214–219. 10.1002/zoo.21475. [DOI] [PubMed] [Google Scholar]
- 18.Junaidi A, Williamson P, Martin G, et al. 2007. Pituitary and testicular endocrine responses to exogenous gonadotrophin-releasing hormone (GnRH) and luteinising hormone in male dogs treated with GnRH agonist implants. Reprod Fertil Dev 19:891–898. 10.1071/RD07088. [DOI] [PubMed] [Google Scholar]
- 19.Kaumanns W, Singh M, Schwibbe M. 2013. Environmental change and housing conditions result in disappearance and return of reproductive seasonality in rhesus macaques (Macaca mulatta). Curr Sci 105:517–521. [Google Scholar]
- 20.Kraicer J, Beraud G, Lywood D. 1977. Pars intermedia ACTH and MSH content: effect of adrenalectomy, gonadectomy, and a neurotropic (noise) stress. Neuroendocrinology 23:352–367. 10.1159/000122684. [DOI] [PubMed] [Google Scholar]
- 21.Larson S, Belting T, Rifenbury K, Fisher G, Boutelle S. 2013. Preliminary findings of fecal gonadal hormone concentrations in 6 captive sea otters (Enhydra lutris) after deslorelin implantation. Zoo Biol 32:307–315. 10.1002/zoo.21032. [DOI] [PubMed] [Google Scholar]
- 22.Lucas X. 2014. Clinical use of deslorelin (GnRH agonist) in companion animals: A review. Reprod Domest Anim 49:64–71. 10.1111/rda.12388. [DOI] [PubMed] [Google Scholar]
- 23.MacGregor MJ, Asa CS, Skinner DC. 2017. Variable duration of reproductive suppression in male coyotes (Canis latrans) treated with a high dose of the gonadotrophin-releasing hormone agonist deslorelin. Reprod Fertil Dev 29:1271–1279. 10.1071/RD15253. [DOI] [PubMed] [Google Scholar]
- 24.Maddison JE, Page SW, Church DB. 2008. Small Animal Clinical Pharmacology, vol. 5. Hertfordshire (UK): Elsevier Health Sciences. [Google Scholar]
- 25.Mann DR, Gould KG, Collins DC. 1984. Influence of continuous gonadotropin-releasing hormone (GnRH) agonist treatment on luteinizing hormone and testosterone secretion, the response to GnRH, and the testicular response to human chorionic gonadotropin in male rhesus monkeys. J Clin Endocrinol Metab 58:262–267. 10.1210/jcem-58-2-262. [DOI] [PubMed] [Google Scholar]
- 26.Martinez G, Lacoste R, Dumasy M, Garbit S, Brouillet S, Coutton C, Arnoult C, Druelle F, Molina-Vila P. 2020. Deslorelin acetate implant induces transient sterility and behavior changes in male olive baboon (Papio anubis): A case study. J Med Primatol 49:344–348. 10.1111/jmp.12479. [DOI] [PubMed] [Google Scholar]
- 27.Mattern LG, Helmreich DL, Cameron JL. 1993. Diurnal pattern of pulsatile luteinizing hormone and testosterone secretion in adult male rhesus monkeys (Macaca mulatta): Influence of the timing of daily meal intake. Endocrinology 132:1044–1054. 10.1210/endo.132.3.8440171. [DOI] [PubMed] [Google Scholar]
- 28.Mehlman PT, Higley JD, Fernald BJ, Sallee FR, Suomi SJ, Linnoila M. 1997. CSF 5-HIAA, testosterone, and sociosexual behaviors in free-ranging male rhesus macaques in the mating season. Psychiatr Res 72:89–102. 10.1016/S0165-1781(97)00084-X. [DOI] [PubMed] [Google Scholar]
- 29.Molter CM, Fontenot DK, Terrell SP. 2015. Use of deslorelin acetate implants to mitigate aggression in two adult male domestic turkeys (Meleagris gallopavo) and correlating plasma testosterone concentrations. J Avian Med Surg 29:224–230. 10.1647/2014-041. [DOI] [PubMed] [Google Scholar]
- 30.Muroyama Y, Shimizu K, Sugiura H. 2007. Seasonal variation in fecal testosterone levels in free-ranging male Japanese macaques. Am J Primatol 69:603–610. 10.1002/ajp.20366. [DOI] [PubMed] [Google Scholar]
- 31.Novotny R, Cizek P, Vitasek R, Bartoskova A, Prinosilova P, Janosovska M. 2012. Reversible suppression of sexual activity in tomcats with deslorelin implant. Theriogenology 78:848–857. 10.1016/j.theriogenology.2012.03.035. [DOI] [PubMed] [Google Scholar]
- 32.Pekow C. 2005. Defining, measuring, and interpreting stress in laboratory animals. Contemp Top Lab Anim Sci 44:41–45. [PubMed] [Google Scholar]
- 33.Penfold LM, Norton T, Asa CS. 2021. Effects of GnRH agonists on testosterone and testosterone-stimulated parameters for contraception and aggression reduction in male lion-tailed macaques (Macaca silenus). Zoo Biol 40:541–550. 10.1002/zoo.21635. [DOI] [PubMed] [Google Scholar]
- 34.Perachio AA, Alexander M, Marr L, Collins D. 1977. Diurnal variations of serum testosterone levels in intact and gonadectomized male and female rhesus monkeys. Steroids 29:21–33. 10.1016/0039-128X(77)90106-4. [DOI] [PubMed] [Google Scholar]
- 35.Pinto CR, Meyers PJ. 2007. Control and Synchronization of the Estrous Cycle and Ovulation, p 91–98. In: Current Therapy in Large Animal Theriogenology. Philadelphia (PA): Elsevier. [Google Scholar]
- 36.Polisca A, Orlandi R, Troisi A, Brecchia G, Zerani M, Boiti C, Zelli R. 2013. Clinical efficacy of the GnRH agonist (deslorelin) in dogs affected by benign prostatic hyperplasia and evaluation of prostatic blood flow by Doppler ultrasound. Reprod Domest Anim 48:673–680. 10.1111/rda.12143. [DOI] [PubMed] [Google Scholar]
- 37.Ponglowhapan S. 2011. Clinical applications of GnRH agonist deslorelin in dogs and cats. Wetchasan Sattawaphaet 41:59. [Google Scholar]
- 38.Raines JA, Fried JJ. 2016. Use of deslorelin acetate implants to control aggression in a multi-male group of rock hyrax (Procavia capensis). Zoo Biol 35:201–204. [DOI] [PubMed] [Google Scholar]
- 39.Romagnoli S, Siminica A, Sontas B, Milani C, Mollo A, Stelletta C. 2012. Semen quality and onset of sterility following administration of a 4.7-mg deslorelin implant in adult male dogs. Reprod Domest Anim 47:389–392. 10.1111/rda.12058. [DOI] [PubMed] [Google Scholar]
- 40.Schlatt S, Pohl CR, Ehmcke J, Ramaswamy S. 2008. Age-related changes in diurnal rhythms and levels of gonadotropins, testosterone, and inhibin B in male rhesus monkeys (Macaca mulatta). Biol Reprod 79:93–99. 10.1095/biolreprod.107.066126. [DOI] [PubMed] [Google Scholar]
- 41.Schoemaker NJ, Van Deijk R, Muijlaert B, Kik MJ, Kuijten AM, de Jong FH, Trigg TE, Kruitwagen CL, Mol JA. 2008. Use of a gonadotropin-releasing hormone agonist implant as an alternative for surgical castration in male ferrets (Mustela putorius furo). Theriogenology 70:161–167. 10.1016/j.theriogenology.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 42.Sotos JF, Tokar NJ. 2012. Testicular volumes revisited: A proposal for a simple clinical method that can closely match the volumes obtained by ultrasound and its clinical application. Int J Pediatr Endocrinol 2012:17. 10.1186/1687-9856-2012-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takihara H, Cosentino MJ, Sakatoku J, Cockett AT. 1987. Significance of testicular size measurement in andrology. II. Correlation of testicular size with testicular function. J Urol 137:416–419. 10.1016/S0022-5347(17)44053-5. [DOI] [PubMed] [Google Scholar]
- 44.Trigg TE, Wright P, Armour A, et al. 2001. Use of a GnRH analogue implant to produce reversible long-term suppression of reproductive function in male and female domestic dogs. J Reprod Fertil Suppl 57:255–261. [PubMed] [Google Scholar]
- 45.US Department of Agriculture. Animal Welfare Act as amended. 7 USC §2131–21592013.
- 46.Wagner RA, Piché CA, Jöchle W, Oliver JW. 2005. Clinical and endocrine responses to treatment with deslorelin acetate implants in ferrets with adrenocortical disease. Am J Vet Res 66:910–914. 10.2460/ajvr.2005.66.910. [DOI] [PubMed] [Google Scholar]
- 47.Zaidi P, Wickings EJ, Nieschlag E. 1982. The effects of ketamine HC1 and barbiturate anaesthesia on the metabolic clearance and production rates of testosterone in the male rhesus monkey, Macaca mulatta. J Steroid Biochem 16:463–466. 10.1016/0022-4731(82)90061-9. [DOI] [PubMed] [Google Scholar]