Abstract
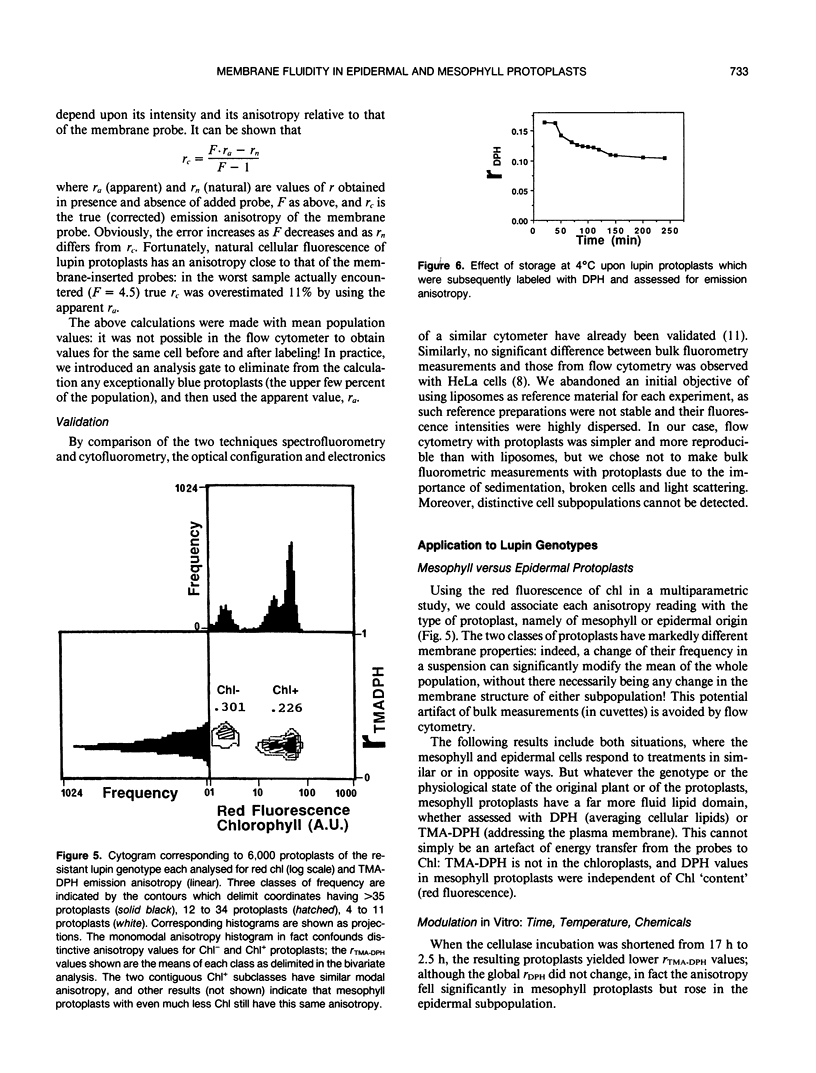
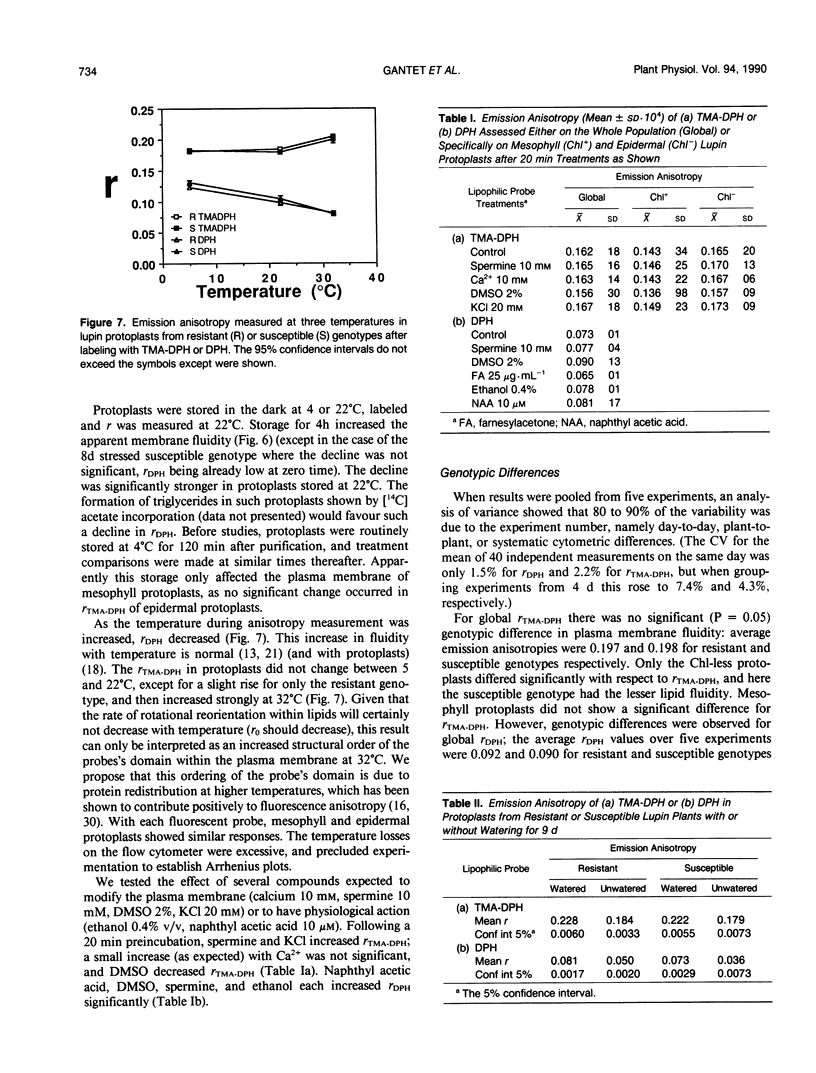
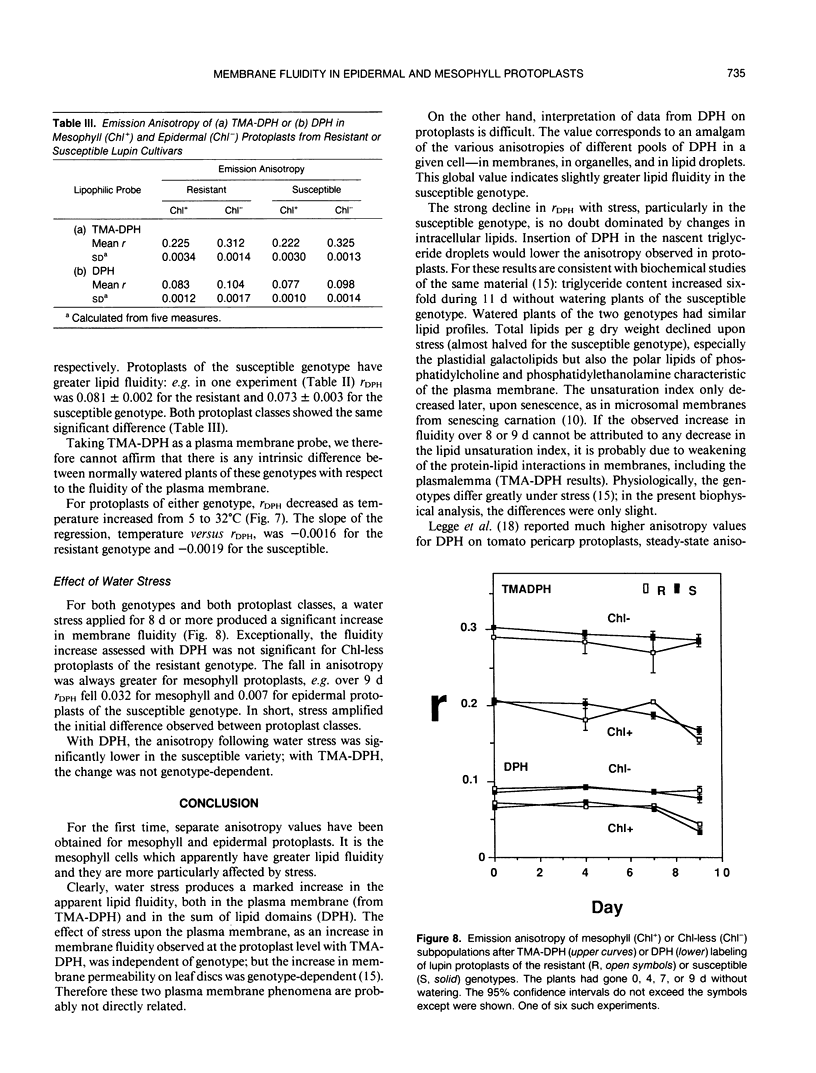
The blue emission anisotropy, r, of two lipophilic probes, diphenylhexatriene (DPH) and its trimethyl-ammonium derivative (TMA-DPH), has been measured in foliar Lupinus albus L. protoplasts for the first time by flow cytometry. Distinctive values have been obtained for protoplasts of epidermal and mesophyll origin, identified by their intensities of chlorophyll fluorescence. Fluorescence microscopy confirmed that TMA-DPH remained in the plasma membrane while DPH penetrated into intracellular lipid domains. Typical emission anisotropy values at 22°C for mesophyll and epidermal protoplasts, respectively, were 0.225 and 0.312 with TMA-DPH, and 0.083 and 0.104 with DPH. This indicates that epidermal cells—and notably their plasma membranes (TMA-DPH)—have higher lipid microviscosity and/or more ordered lipid structure. Two lupin genotypes characterized as resistant or susceptible to drought were analyzed with or without 9 days of water stress shown to increase ion leakage from foliar discs. Water stress greatly increased the apparent fluidity, and more so in the susceptible genotype; the effect was more pronounced in the chlorophyll-containing mesophyll cells than in the epidermal cells.
Full text
PDF
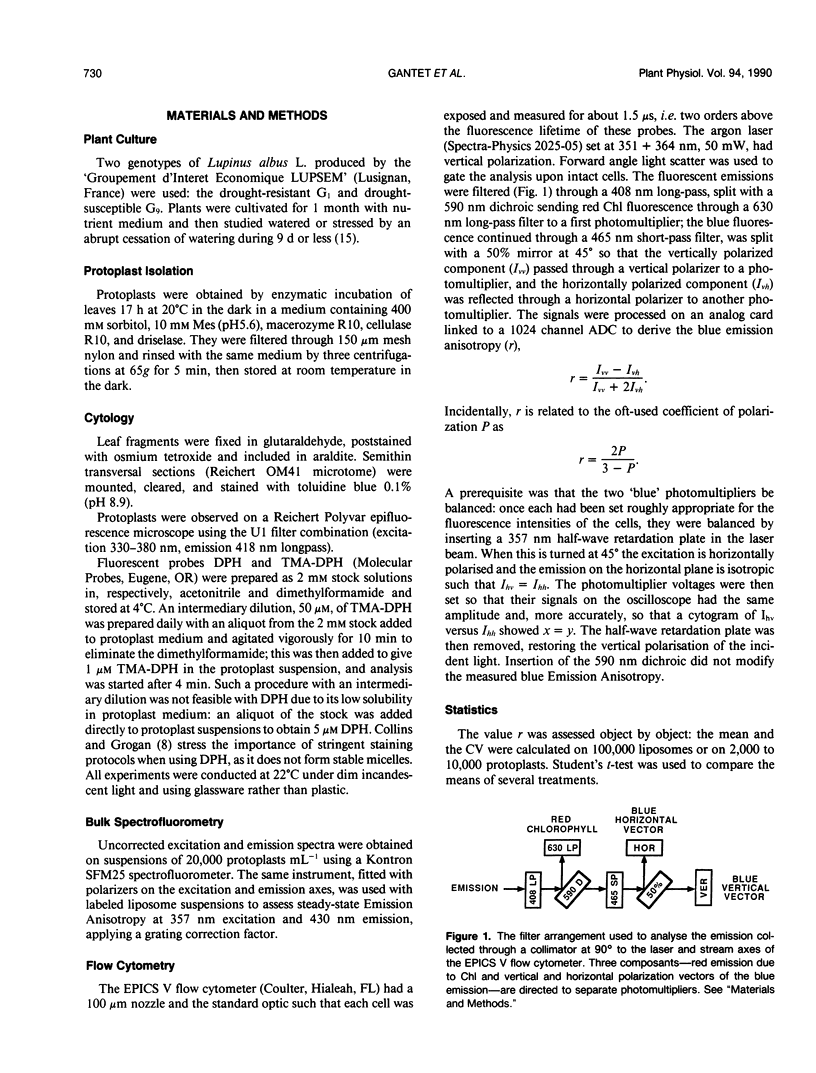
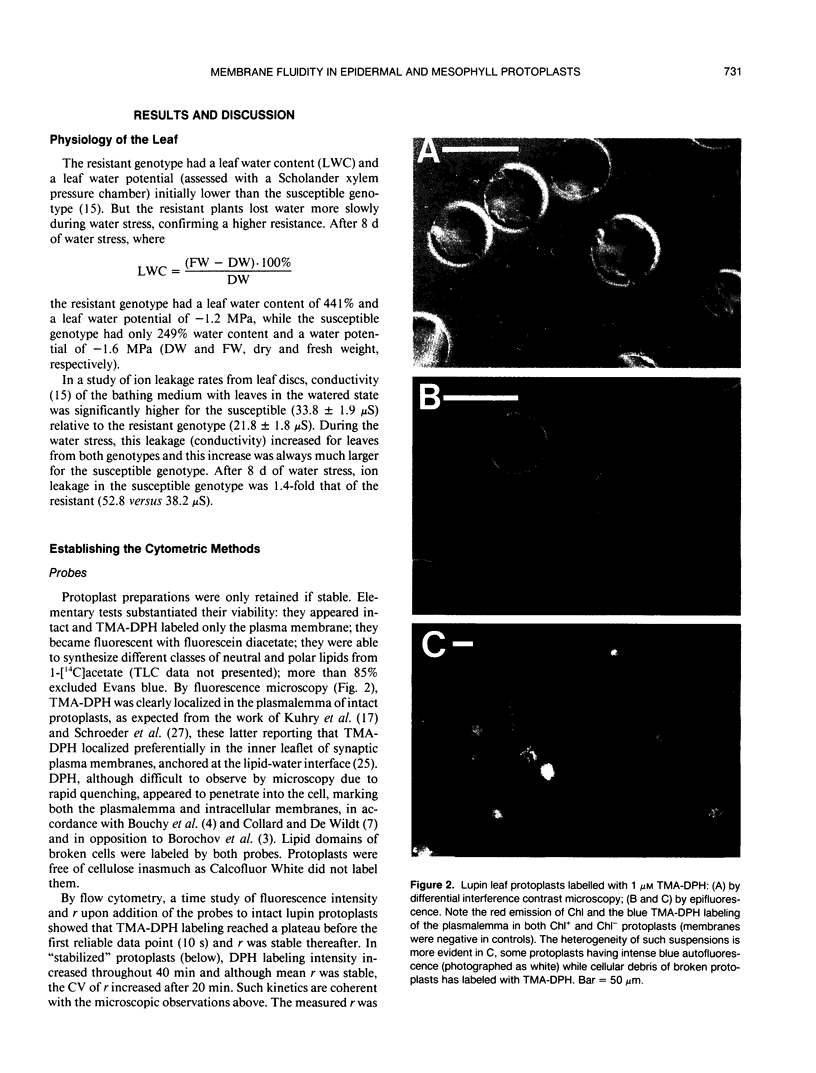
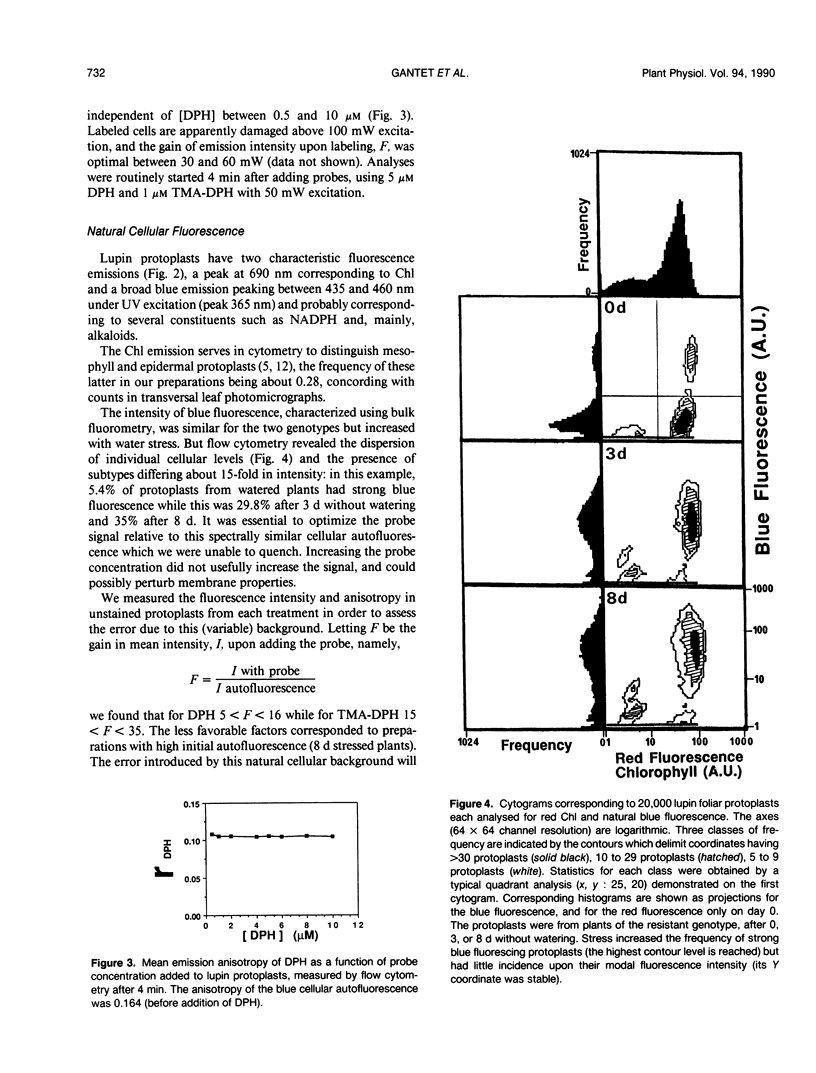


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borochov A., Halevy A. H. Microviscosity of plasmalemmas in rose petals as affected by age and environmental factors. Plant Physiol. 1978 May;61(5):812–815. doi: 10.1104/pp.61.5.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchy M., Donner M., André J. C. Evolution of fluorescence polarization of 1,6-diphenyl-1,3,5-hexatriene (DPH) during the labelling of living cells. Exp Cell Res. 1981 May;133(1):39–46. doi: 10.1016/0014-4827(81)90354-2. [DOI] [PubMed] [Google Scholar]
- Collard J. G., De Wildt A. Localization of the lipid probe 1,6-diphenyl-1,3,5 hexatriene (DPH) in intact cells by fluorescence microscopy. Exp Cell Res. 1978 Oct 15;116(2):447–450. doi: 10.1016/0014-4827(78)90467-6. [DOI] [PubMed] [Google Scholar]
- Collins J. M., Grogan W. M. Comparison between flow cytometry and fluorometry for the kinetic measurement of membrane fluidity parameters. Cytometry. 1989 Jan;10(1):44–49. doi: 10.1002/cyto.990100108. [DOI] [PubMed] [Google Scholar]
- Fobel M., Lynch D. V., Thompson J. E. Membrane deterioration in senescing carnation flowers : coordinated effects of phospholipid degradation and the action of membranous lipoxygenase. Plant Physiol. 1987 Sep;85(1):204–211. doi: 10.1104/pp.85.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M. H., Delohery T. M. Membrane fluidity measured by fluorescence polarization using an EPICS V cell sorter. Cytometry. 1987 Jan;8(1):20–25. doi: 10.1002/cyto.990080104. [DOI] [PubMed] [Google Scholar]
- Gorvel J. P., Mawas C., Maroux S., Mishal Z. Flow cytometry is a new method for the characterization of intestinal plasma membrane. Biochem J. 1984 Jul 15;221(2):453–457. doi: 10.1042/bj2210453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgerson S. L., Cramer W. A., Morré D. J. Evidence for an increase in microviscosity of plasma membranes from soybean hypocotyls induced by the plant hormone, indole-3-acetic Acid. Plant Physiol. 1976 Oct;58(4):548–551. doi: 10.1104/pp.58.4.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jähnig F. Structural order of lipids and proteins in membranes: evaluation of fluorescence anisotropy data. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6361–6365. doi: 10.1073/pnas.76.12.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhry J. G., Fonteneau P., Duportail G., Maechling C., Laustriat G. TMA-DPH: a suitable fluorescence polarization probe for specific plasma membrane fluidity studies in intact living cells. Cell Biophys. 1983 Jun;5(2):129–140. doi: 10.1007/BF02796139. [DOI] [PubMed] [Google Scholar]
- Legge R. L., Cheng K. H., Lepock J. R., Thompson J. E. Differential effects of senescence on the molecular organization of membranes in ripening tomato fruit. Plant Physiol. 1986 Aug;81(4):954–959. doi: 10.1104/pp.81.4.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuguchi J., Utsunomiya N., Nakanishi M., Arata Y. Phorbol myristate acetate inhibits increases in membrane fluidity induced by anti-IgM in B cells. J Immunol. 1988 Apr 15;140(8):2495–2499. [PubMed] [Google Scholar]
- Rossignol M., Uso T., Thomas P. Relationship between fluidity and ionic permeability of bilayers from natural mixtures of phospholipids. J Membr Biol. 1985;87(3):269–275. doi: 10.1007/BF01871227. [DOI] [PubMed] [Google Scholar]
- Schaap G. H., de Josselin de Jong J. E., Jongkind J. F. Fluorescence polarization of six membrane probes in embryonal carcinoma cells after differentiation as measured on a FACS II cell sorter. Cytometry. 1984 Mar;5(2):188–193. doi: 10.1002/cyto.990050213. [DOI] [PubMed] [Google Scholar]
- Schroeder F., Morrison W. J., Gorka C., Wood W. G. Transbilayer effects of ethanol on fluidity of brain membrane leaflets. Biochim Biophys Acta. 1988 Dec 8;946(1):85–94. doi: 10.1016/0005-2736(88)90460-9. [DOI] [PubMed] [Google Scholar]
- Van Blitterswijk W. J., Van Hoeven R. P., Van der Meer B. W. Lipid structural order parameters (reciprocal of fluidity) in biomembranes derived from steady-state fluorescence polarization measurements. Biochim Biophys Acta. 1981 Jun 22;644(2):323–332. doi: 10.1016/0005-2736(81)90390-4. [DOI] [PubMed] [Google Scholar]
- Vigh L., Horváth I., Horváth L. I., Dudits D., Farkas T. Protoplast plasmalemma fluidity of hardened wheats correlates with frost resistance. FEBS Lett. 1979 Nov 15;107(2):291–294. doi: 10.1016/0014-5793(79)80393-2. [DOI] [PubMed] [Google Scholar]