Abstract
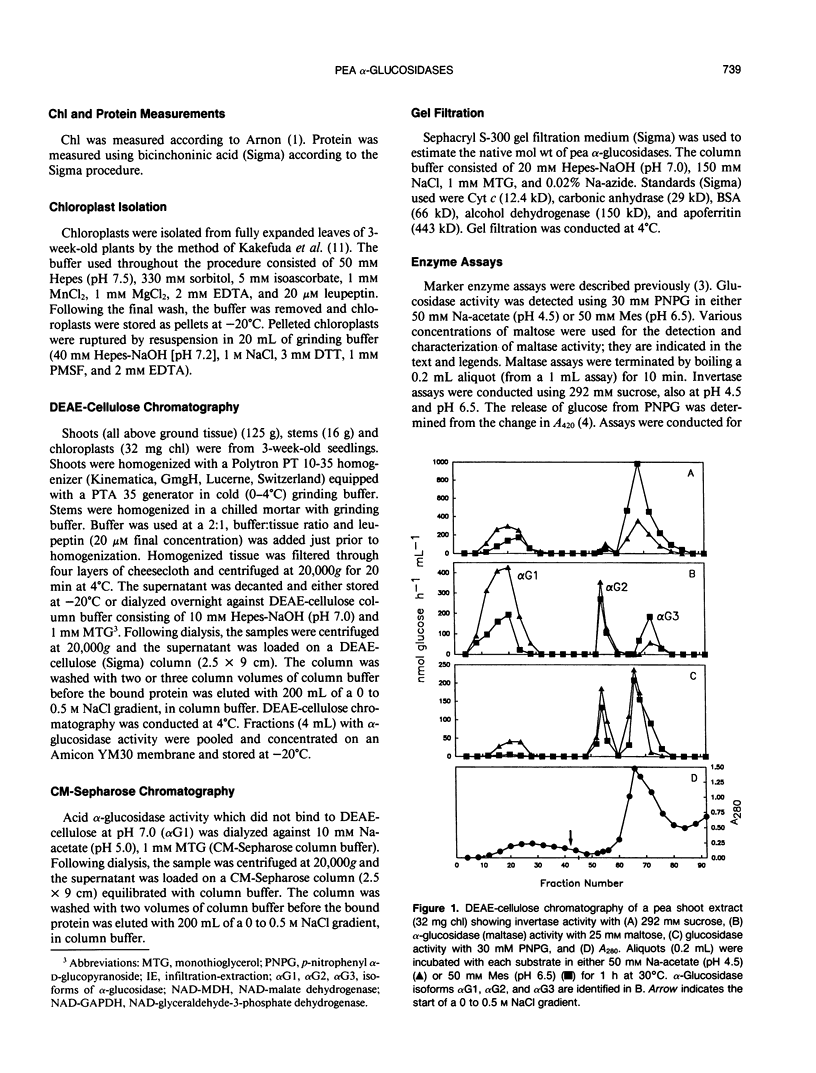
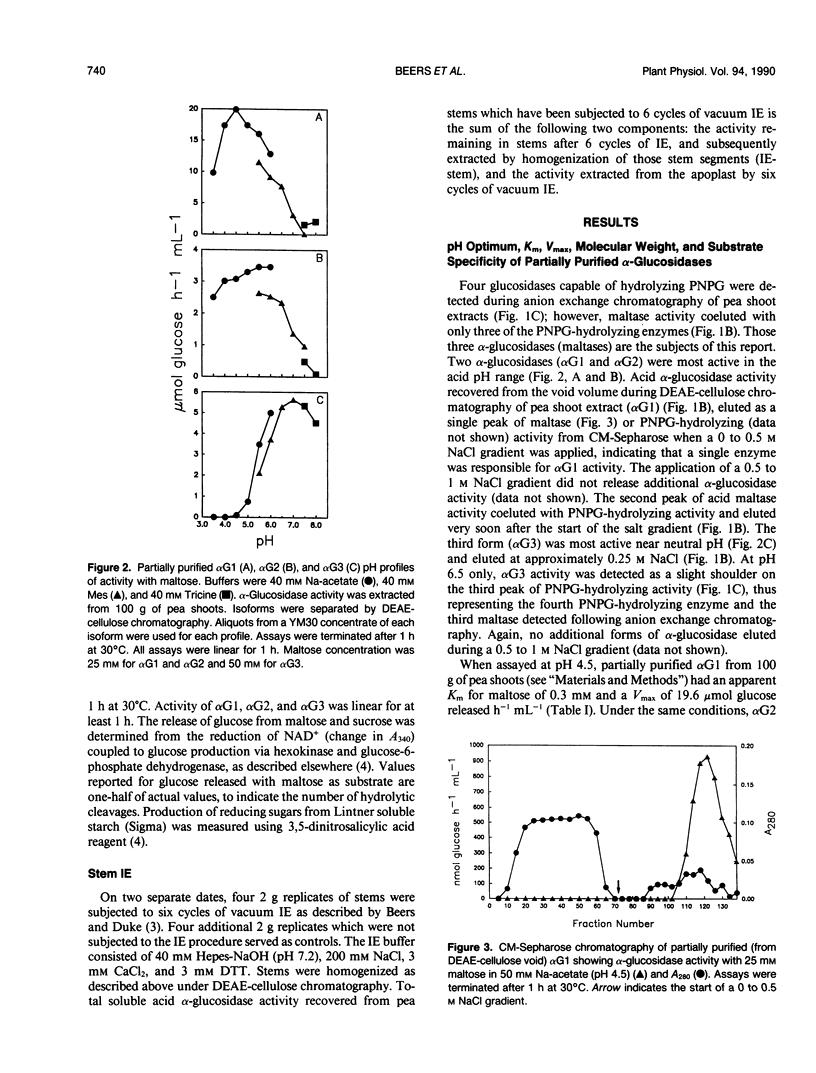
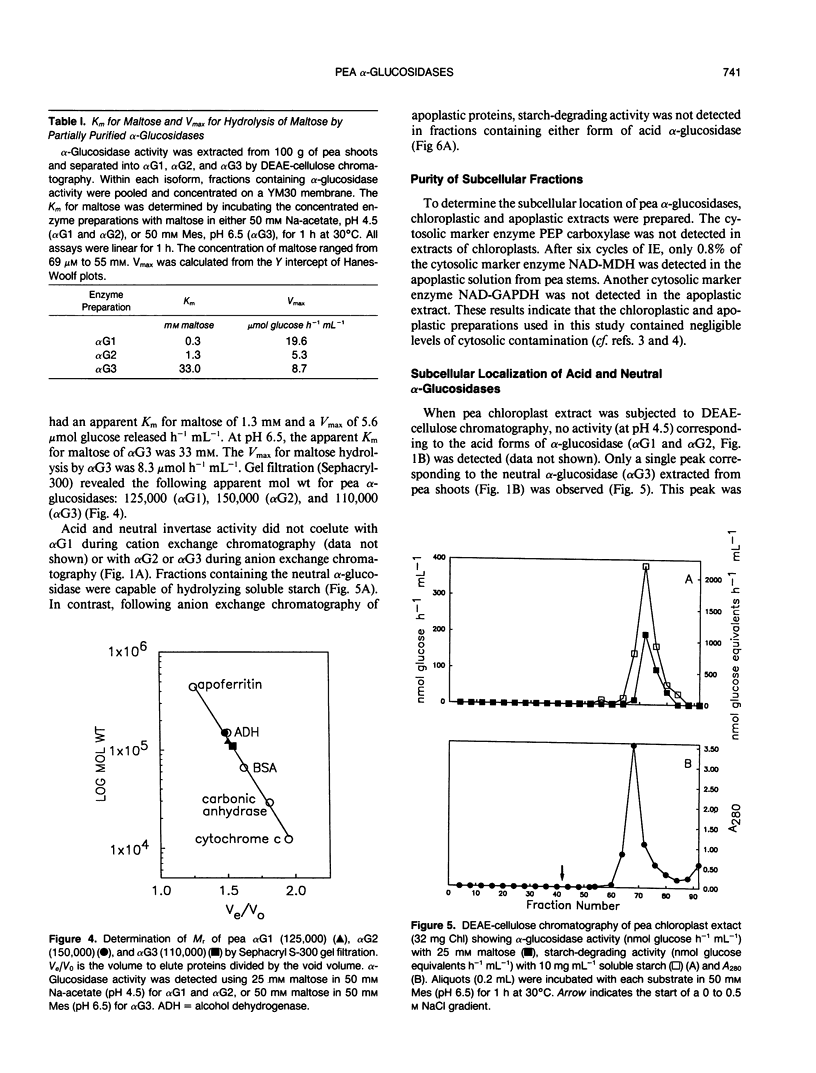
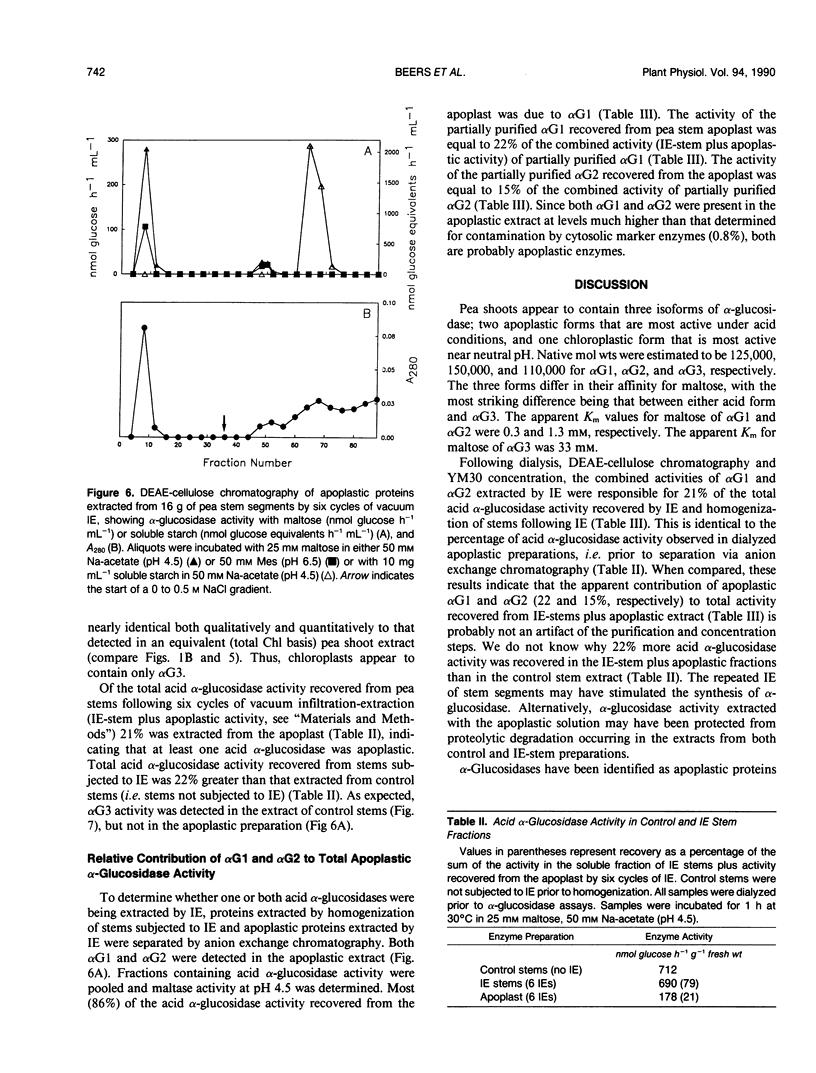
Three isoforms of α-glucosidase (EC 3.2.1.20) have been extracted from pea (Pisum sativum L.) seedlings and separated by DEAE-cellulose and CM-Sepharose chromatography. Two α-glucosidase isoforms (αG1 and αG2) were most active under acid conditions, and appeared to be apoplastic. A neutral form (αG3) was most active near pH 7, and was identified as a chloroplastic enzyme. Together, the activity of αG1 and αG2 in apoplastic preparations accounted for 21% of the total acid α-glucosidase activity recovered from pea stems. The vast majority (86%) of the apoplastic acid α-glucosidase activity was due to αG1. The apparent Km values for maltose of αG1 and αG2 were 0.3 and 1.3 millimolar, respectively. The apparent Km for maltose of αG3 was 33 millimolar. The respective native molecular weights of αG1, αG2, and αG3 were 125,000, 150,000, and 110,000.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beers E. P., Duke S. H. Characterization of alpha-Amylase from Shoots and Cotyledons of Pea (Pisum sativum L.) Seedlings. Plant Physiol. 1990 Apr;92(4):1154–1163. doi: 10.1104/pp.92.4.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beers E. P., Duke S. H. Localization of alpha-Amylase in the Apoplast of Pea (Pisum sativum L.) Stems. Plant Physiol. 1988 Aug;87(4):799–802. doi: 10.1104/pp.87.4.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke S. H., Schrader L. E., Henson C. A., Servaites J. C., Vogelzang R. D., Pendleton J. W. Low root temperature effects on soybean nitrogen metabolism and photosynthesis. Plant Physiol. 1979 May;63(5):956–962. doi: 10.1104/pp.63.5.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frehner M., Conn E. E. The Linamarin beta-Glucosidase in Costa Rican Wild Lima Beans (Phaseolus lunatus L.) Is Apoplastic. Plant Physiol. 1987 Aug;84(4):1296–1300. doi: 10.1104/pp.84.4.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt W. H., Werdan K., Milovancev M., Geller G. Alkalization of the chloroplast stroma caused by light-dependent proton flux into the thylakoid space. Biochim Biophys Acta. 1973 Aug 31;314(2):224–241. doi: 10.1016/0005-2728(73)90137-0. [DOI] [PubMed] [Google Scholar]
- Kakefuda G., Duke S. H. Characterization of Pea Chloroplast D-Enzyme (4-alpha-d-Glucanotransferase). Plant Physiol. 1989 Sep;91(1):136–143. doi: 10.1104/pp.91.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi C., Preiss J. Amylopectin degradation in pea chloroplast extracts. Plant Physiol. 1978 Feb;61(2):218–220. doi: 10.1104/pp.61.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita T. W., Greenberg E., Kuhn D. N., Preiss J. Subcellular localization of the starch degradative and biosynthetic enzymes of spinach leaves. Plant Physiol. 1979 Aug;64(2):187–192. doi: 10.1104/pp.64.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongratz P., Beck E. Diurnal oscillation of amylolytic activity in spinach chloroplasts. Plant Physiol. 1978 Nov;62(5):687–689. doi: 10.1104/pp.62.5.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z., Henson C. A. Degradation of Native Starch Granules by Barley alpha-Glucosidases. Plant Physiol. 1990 Sep;94(1):320–327. doi: 10.1104/pp.94.1.320. [DOI] [PMC free article] [PubMed] [Google Scholar]



