Abstract
Background
Inhaled anticholinergics given in addition to β2‐agonists are effective in reducing hospital admissions in children presenting to the emergency department with a moderate to severe asthma exacerbation. It seems logical to assume a similar beneficial effect in children hospitalised for an acute asthma exacerbation.
Objectives
To assess the efficacy and safety of anticholinergics added to β2‐agonists as inhaled or nebulised therapy in children hospitalised for an acute asthma exacerbation. To investigate the characteristics of patients or therapy, if any, that would influence the magnitude of response attributable to the addition of anticholinergics.
Search methods
We identified trials from the Cochrane Airways Group Specialised Register of trials (CAGR), which is derived through systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED and PsycINFO and through handsearching of respiratory journals and meeting abstracts. The search is current to November 2013.
Selection criteria
Randomised trials comparing the combination of inhaled or nebulised anticholinergics and short‐acting β2‐agonists versus short‐acting β2‐agonists alone in children one to 18 years of age hospitalised for an acute asthma exacerbation were eligible.
Data collection and analysis
Two review authors independently assessed the methodological quality of trials and extracted data; disagreement was resolved by consensus or with the input of a third review author, when needed. Primary outcomes were duration of hospital stay and serious adverse events. Secondary outcomes included admission and duration of stay in the intensive care unit (ICU), ventilation assistance, time to short‐acting β2‐agonists spaced at four hours or longer, supplemental asthma therapy, duration of supplemental oxygen, change from baseline in asthma severity, relapse after discharge, adverse health effects and withdrawals.
Main results
Seven randomised trials were included, four of which reported usable data on 472 children with asthma one to 18 years of age who were admitted to paediatric wards. No trials included patients admitted to the ICU. The anticholinergic used, ipratropium bromide 250 μg, was given every one to eight hours over a period from four hours to the entire length of the hospital stay. Two of four trials (50%) contributing data were deemed of high methodological quality. The addition of anticholinergics to β2‐agonists showed no evidence of effect on the duration of hospital admission (mean difference (MD) ‐0.28 hours, 95% confidence interval (CI) ‐5.07 to 4.52, 3 studies, 327 participants, moderate quality evidence) and no serious or non‐serious adverse events were reported in any included trials. As a result of the similarity of trials, we could not explore the influence of age, admission site, intensity of anticholinergic treatment and co‐interventions on primary outcomes. No statistically significant group difference was noted in other secondary outcomes, including the need for supplemental asthma therapy, time to short‐acting β2‐agonists spaced at four hours or longer, asthma clinical scores, lung function and overall withdrawals for any reason.
Authors' conclusions
In children hospitalised for an acute asthma exacerbation, no evidence of benefit for length of hospital stay and other markers of response to therapy was noted when nebulised anticholinergics were added to short‐acting β2‐agonists. No adverse health effects were reported, yet the small number of trials combined with inadequate reporting prevent firm reassurance regarding the safety of anticholinergics. In the absence of trials conducted in ICUs, no conclusion can be drawn regarding children with impending respiratory failure. These findings support current national and international recommendations indicating that healthcare practitioners should refrain from using anticholinergics in children hospitalised for acute asthma.
Plain language summary
Are inhaled anticholinergics added to β2‐agonists beneficial in children hospitalised with acute asthma?
Background: Anticholinergics (e.g. ipratropium bromide, atropine sulfate) are inhaled drugs. They relax the airway muscles and decrease secretions. Anticholinergics are sometimes used in addition to beta2‐agonists (such as salbutamol and terbutaline), which are potent drugs given to relax smooth muscles in the airways in children with acute asthma. We do not know whether the addition of inhaled anticholinergics to beta2‐agonists is beneficial for children hospitalised with acute asthma.
Review question: We wished to examine the efficacy and safety of inhaled or nebulised (mist inhaled into the lungs) anticholinergics added to beta2‐agonists compared with beta2‐agonists alone in children one to 18 years of age hospitalised for an acute asthma exacerbation.
Study characteristics: In reviewing evidence available until November 2013, we found seven eligible studies of children hospitalised with acute asthma; four of these studies (472 children one to 18 years of age) contributed data to the review. Four studies compared the combination of anticholinergics (ipratropium bromide) and beta2‐agonists versus the same dose of beta2‐agonists alone. Included studies enrolled both girls and boys, with a gender ratio ranging from 59% to 73% males.
Results: No additional benefit was noted by adding anticholinergics to β2‐agonists in terms of duration of hospital stay in patients compared to those who received beta2‐agonists alone. Two of four trials (50%) contributing data were deemed of high methodological quality. No trial reported information on serious adverse events. No statistically significant group difference was noted in other markers of response to therapy, that is, the need for supplemental asthma therapy, time to short‐acting beta2‐agonists spaced at four hours or longer, asthma clinical scores, lung function and overall withdrawals for any reason.
Conclusion: No apparent benefit is derived from adding anticholinergics to beta2‐agonists in children hospitalised for an acute asthma exacerbation, that is, beyond initial treatment in the emergency department. No adverse health effects were reported, yet the small number of trials combined with inadequate reporting prevents firm reassurance regarding the safety of anticholinergics. In the absence of trials conducted in the intensive care unit (ICU), no conclusion can be drawn regarding children with very severe exacerbations who are admitted to the ICU. Our findings support the ongoing recommendations provided by national and international guidelines.
Quality of the results: This review is based on a small number of identified trials conducted in children with acute asthma. All trials contributing to the primary outcome are of high methodological quality, but they are few. As the addition of new trials may change the conclusion, the quality of evidence was downgraded from high to moderate. Additional and larger trials are needed.
Summary of findings
Summary of findings for the main comparison. Combination of anticholinergics + beta2‐agonists versus beta2‐agonists alone.
| Combination of AC + beta2‐agonists versus beta2‐agonists alone | ||||||
| Patient or population: children hospitalised with an acute asthma exacerbation Settings: hospitalised Intervention: combination of anticholinergic + beta2‐agonists Comparison: beta2‐agonists alone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Beta2‐agonistsalone | Combination of AC + beta2‐agonists | |||||
| Duration of the hospital stay (hours) | The mean duration of the hospital stay (hours) in the control groups was 44 hours | The mean reduction in duration of hospital stay (hours) was 0.28 hours in the intervention groups Mean 43.8 (39 to 49) hours |
‐0.28 hours (‐5.07 to 4.52) | 327 (3 studies) | ⊕⊕⊕⊝ moderate1 | |
|
Time to regular dose of short‐acting beta2‐agonists (hours)2 Follow‐up: 4 hours or longer |
The mean time to regular short‐acting beta2‐agonists in the control groups was 33 hours | The mean reduction in time to regular short‐acting beta2‐agonists in the intervention groups was 2.17 hours Mean 31 (26 to 36) hours |
‐2.17 hours (‐7.01 to 2.66) | 269 (2 studies) | ⊕⊕⊕⊝ moderate1 | |
|
Asthma clinical scores Follow‐up: 8 to 36 hours after initial treatment |
See comment | See comment | 0.02 SMD (‐0.34 to 0.38) | 117 (2 studies) | ⊕⊕⊕⊝ moderate1 | Scores were measured on different scales. |
| Admission to the intensive care unit | See comment | See comment | Not estimable | 210 (1 study) | ⊕⊕⊕⊝ moderate3 | Single trial reported admission to the intensive care unit. No events were reported |
| Overall withdrawals | 12 per 100 | 7 per 100 (4 to 15) | OR 0.59 (0.27 to 1.30) | 294 (2 studies) | ⊕⊕⊕⊝ moderate1 | |
| Adverse health events | See comment | See comment | Not estimable | 290 (2 studies) | ⊕⊕⊕⊝ moderate3 | No adverse events were reported in either trial |
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded because of wide confidence intervals.
2This is the time at which emergency dosing with the trial intervention was replaced with regular 4‐hourly treatment with short‐acting beta2‐agonists.
3No events were reported in either arm of the studies.
Background
Description of the condition
Asthma is a chronic inflammatory disorder of the airways that is associated with airway hyperresponsiveness (Ortiz‐Alvarez 2012). Globally the prevalence of severe asthma symptoms, defined as four or more attacks of wheeze or one or more night per week of sleep disturbance from wheeze or wheeze affecting speech in the past 12 months ranged from 0% to 20% in children (Asher 2010; Lai 2009). Asthma exacerbation is defined as an acute or subacute worsening of symptoms and lung function from the individual's usual status or, in some cases, the initial presentation of asthma (GINA 2014). One of the main goals in the management of acute asthma exacerbations in children is to achieve a rapid reversal of airflow obstruction (NAEPP 2011). This is achieved by using inhaled or nebulised short‐acting β2‐agonists (Camargo 2003; Karpel 1997), which are the most effective bronchodilators because of their rapid onset of action and the magnitude of bronchodilation that they achieve (Sears 1992; Svedmyr 1985). Systemic corticosteroids should be added early in the course of treatment for patients who have moderate or severe exacerbations or for children who fail to respond promptly and completely to bronchodilators (Rechelefsky 2003; Rowe 2004).
Description of the intervention
Anticholinergic agents, such as ipratropium bromide and atropine sulfate, have a slower onset of action and a weaker bronchodilating effect but may specifically relieve cholinergic bronchomotor tone and decrease mucosal edema and secretions (Chapman 1996; Gross 1988; Silverman 1990). Thus, with their slower onset but prolonged duration of action, anticholinergics (AC) can work as complementary therapy to β2‐agonists, thereby enhancing bronchodilation.
Several trials have explored the role of ipratropium bromide in the emergency department setting. One systematic review of randomised controlled trials (RCTs) concluded that multiple doses of inhaled anticholinergic agents added to β2‐agonist therapy in the initial treatment of children with acute asthma exacerbations were safe and efficacious, with most of the effect observed in those with severe asthma exacerbations and no apparent benefit noted in children presenting with mild to moderate asthma exacerbations (Plotnick 2000). An updated Cochrane review suggests that the benefit extends to children with moderate and severe exacerbations (Griffiths 2013). Moreover, multiple doses of inhaled anticholinergic agents added to β2‐agonists have been shown to be cost‐effective (Lord 1999).
How the intervention might work
To our knowledge, two trials have assessed the efficacy of ipratropium bromide added to β2‐agonists after the emergency department treatment period, that is, in children hospitalised for an acute asthma exacerbation (Craven 2001; Goggin 2001). Both trials independently demonstrated no benefit conferred by the addition of anticholinergics. Consequently, national and international asthma guidelines currently recommend that inhaled anticholinergics should not be used in hospitalised children with acute asthma (GINA 2014; NAEPP 2011). Yet, in several institutions, anticholinergics are used for a specified period of time after children are admitted, particularly, but not only, those admitted to the intensive care unit (ICU).
Why it is important to do this review
The difference in practice between institutions, termed practice variation, underlines a gap between recommended and observed care, or a care gap. In the absence of an identified systematic review, we believe that a Cochrane review would clarify the evidence accumulated to date regarding the role of anticholinergics in the treatment of children hospitalised for an acute asthma exacerbation.
Objectives
To assess the efficacy and safety of anticholinergics added to β2‐agonists as inhaled or nebulised therapy in children hospitalised for an acute asthma exacerbation. To investigate the characteristics of patients or therapy, if any, that would influence the magnitude of response attributable to the addition of anticholinergics.
Methods
Criteria for considering studies for this review
Types of studies
We included all RCTs.
Types of participants
We included children one to 18 years of age who were hospitalised for an acute asthma exacerbation.
Types of interventions
Intervention: nebulised or inhaled anticholinergics with short‐acting β2‐agonists.
Comparison: nebulised or inhaled short‐acting β2‐agonists alone.
Co‐interventions: Systemic corticosteroids were anticipated and permitted, provided they were similar in the two groups.
Types of outcome measures
Primary outcomes
Duration of hospital stay.
Serious adverse events.
Secondary outcomes
Duration of stay in the ICU (for those admitted to the ICU).
Admission to the ICU (for those admitted on the wards).
Ventilation assistance.
Need for supplemental asthma therapy (e.g. aminophylline).
Duration of need for supplemental oxygen.
Time to short‐acting β2‐agonists spaced at four hours or longer.
Change from baseline in asthma severity measured as lung function, symptoms or clinical scores.
Relapse within 72 hours of discharge from hospital.
Adverse health events.
Withdrawals, namely, overall withdrawals, withdrawals due to poor control of symptoms or deterioration and withdrawals due to adverse effects.
Search methods for identification of studies
Electronic searches
We identified trials from the Cochrane Airways Group Specialised Register of trials (CAGR), which is derived through systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED and PsycINFO, and through handsearching of respiratory journals and meeting abstracts (see Appendix 1 for further details). We searched all records in the CAGR coded as "asthma," using the strategy provided in Appendix 2. The literature search covered all years from inception until November 2013.
No restriction on type or language of publication was applied.
Searching other resources
We checked the reference lists of all identified RCTs to identify potentially relevant citations. We checked the websites of international headquarters of pharmaceutical companies producing anticholinergics for reports of relevant completed trials. We also explored the ClinicalTrials.gov website for relevant clinical trials.
Data collection and analysis
Two review authors (KV and BFC) independently extracted data, and disagreement was resolved by consensus or through the input of a third review author (FMD). When necessary, we contacted authors of included studies to request missing data.
Selection of studies
One review author (KV) independently reviewed each abstract and annotated each as (1) RCT; (2) clearly not an RCT; or (3) unclear. The full‐text publications of citations identified as included RCTs or unclear were reviewed by two review authors independently.
Data extraction and management
Two review authors (KV and BFC) independently extracted data, and disagreement was resolved by consensus or with the input of a third review author (FMD), when needed.
Assessment of risk of bias in included studies
We assessed the methodological quality of included trials by using the 'Risk of bias' tool of The Cochrane Collaboration, which is based on:
random sequence generation;
allocation concealment;
blinding of participants and personnel;
blinding of outcome assessment;
incomplete outcome data;
selective outcome reporting; and
other bias.
Quality assessment was performed independently by two review authors. We resolved disagreement by consensus or with the input of a third review author. The study was deemed to have high methodological quality if reported randomisation procedures and blinding were adequate and there was a low and balanced group attrition, supporting a low risk of bias.
Measures of treatment effect
We calculated treatment effects for dichotomous variables as risk ratio (RR) or risk difference (RD), or both, with 95% confidence intervals (CIs). We assumed equivalence if the RR estimate and its 95% CI fell between 0.9 and 1.1. For continuous outcomes, such as lung function tests, we calculated pooled statistics as mean difference (MD) or standardised mean difference (SMD) with 95% CI.
Unit of analysis issues
The unit of analysis was the participant. If a trial had more than one intervention or control group, we considered additional comparisons, when appropriate.
Dealing with missing data
When possible, we contacted investigators or study sponsors to obtain missing numerical outcome data or data in the required format to allow aggregation in the review. We did not impute missing data.
Assessment of heterogeneity
Homogeneity between included studies for which results were pooled will be tested with the DerSimonian and Laird method, and I2 > 40% was to be used as the cutoff level for significance. In cases of statistically significant heterogeneity, a random‐effects model was applied to the summary estimates. Unless specified otherwise, the fixed‐effect model was used.
Assessment of reporting biases
If missing or incomplete outcome data were identified, we attempted to contact study authors to obtain the missing data.
Data synthesis
Summary estimates were reported with their 95% CIs. We performed meta‐analysis using RevMan 5.2.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses were planned to explore possible reasons for heterogeneity of study results for primary outcomes. A priori defined subgroups were based on:
age (preschool children vs school‐aged children);
admission site (hospital ward vs intensive care unit);
intensity of anticholinergic treatment; and
co‐intervention with systemic corticosteroids (yes or no).
Sensitivity analysis
For the primary outcomes, we planned to perform sensitivity analyses for publication status by removing the unpublished data, and for methodological quality by removing trials that did not meet the following criteria: random sequence generation, double‐blinding and low and balanced attrition in both groups.
Summary of findings table
We created a 'Summary of findings' table using the primary and secondary outcomes. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of a body of evidence as it relates to the studies contributing data to the meta‐analyses for prespecified outcomes. We used methods and recommendations as described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and GRADE pro software. We justified all decisions to downgrade or upgrade the quality of studies by using footnotes and made comments to aid readers' understanding of the review, when necessary.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies and Characteristics of ongoing studies
Results of the search
The search, conducted until November 2013, yielded a total of 127 citations (Figure 1). Of these, 120 citations were excluded for the following non–mutually exclusive reasons: (1) duplicate references (N = 3), (2) ongoing trials (N = 1), (3) studies not randomised trials (N = 8), (4) participants not asthmatic (N = 7), (5) participants not exclusively children (N = 38), (6) participants not hospitalised for an acute asthma exacerbation or not receiving treatment beyond initial treatment in the emergency department (N = 48), (7) intervention not a combination of anticholinergics and β2‐agonists (N = 11) and (8) control intervention not β2‐agonist alone (N = 4).
1.

Study flow diagram.
Included studies
Seven trials met the inclusion criteria for this review. Of these, three eligible clinical trials did not contribute data to the review because reports were incomplete (Lew 1990; Mirkinson 2000; Ozdemir 2003). The data presented hereafter pertain to only four eligible trials, representing a total of 472 children hospitalised for an acute asthma exacerbation that contributed data to this meta‐analysis (Craven 2001; Goggin 2001; Rayner 1987; Storr 1986). All four trials were published in full text. We describe below the characteristics of trials that contributed data for analysis for this review.
Design
All included trials had a parallel‐group design (Craven 2001; Goggin 2001; Rayner 1987; Storr 1986), and the data on lung function as presented in one trial (Lew 1990) could not be aggregated because of its cross‐over design.
Participants
All four trials involved children one to 18 years of age (Craven 2001; Goggin 2001; Rayner 1987; Storr 1986). The mean (or median) age of children in three studies was five years or younger (Craven 2001; Goggin 2001; Storr 1986) and 6.5 years in Rayner 1987. Most trials described a gender ratio ranging from 59% to 73% males. One study (Goggin 2001) enrolled children with moderate to severe asthma symptoms at baseline, defined as requiring inhaled β2‐agonists at a minimum of every two hours, having forced expiratory volume in one second (FEV1) of 25% to 80% of predicted value or having a clinical asthma score of three to nine on a scale of zero to 10 (higher indicating worse). The other trials did not report asthma severity at baseline (Craven 2001; Rayner 1987; Storr 1986), and no trials provided data that were stratified according to the severity of baseline airway obstruction.
Intervention drugs
Important variability must be noted in the proportion of participants who had received anticholinergics before randomisation. Indeed, in Goggin 2001, children had received intensive ipratropium bromide before randomisation, with a mean of 5.9 doses in the control group and 6.0 doses in the treatment group, but in Craven 2001, only three of 210 participants (all in the treatment group) had received anticholinergics in the emergency department before randomisation. The number of participants who received anticholinergics before enrolment was not reported in the remaining three studies (Lew 1990; Rayner 1987; Storr 1986)
The intervention drug and frequency (Table 2) were as follows: 250 μg ipratropium bromide every four hours, every six hours afterwards and then every eight hours, depending on the asthma care algorithm phase (Craven 2001); 250 μg ipratropium bromide every 30 to 60 minutes initially, progressing to every two hours and then to every four hours as the participant improved clinically (Goggin 2001); 250 μg ipratropium bromide every eight hours (Rayner 1987); or 250 μg ipratropium bromide given within set limits at the discretion of the nursing staff (Storr 1986).
1. Intensity of anticholinergic treatment.
| Studies | Anticholinergic treatment |
| Craven 2001 | 250 μg of ipratropium bromide by jet nebulisation every 4 hours during phase I, every 6 hours during phase II and every 8 hours during phase III of the ACA |
| Goggin 2001 | Nebulised ipratropium bromide inhalation solution 1.0 mL (250 μg) every half an hour to 1 hour at the beginning, progressing to 2 hours and then to 4 hours as the patient improves clinically |
| Lew 1990 | An inhalation of 0.1% atropine sulfate (0.05 mg/kg up to 2 mg in total) at baseline or 4 hours later depending on randomisation |
| Rayner 1987 | Nebulised ipratropium 250 μg 30 minutes after the first dose of salbutamol and every 8 hours afterwards |
| Storr 1986 | Nebuliser with 250 μg ipratropium bromide given within set limits at the discretion of the nursing staff |
Co‐intervention
The use of systemic corticosteroids was variable. All children in two trials received corticosteroids delivered intravenously or orally (Craven 2001; Goggin 2001). However, only 78% of participants in the control group and 74% of those in the treatment group were given oral corticosteroids in one study (Rayner 1987), and fewer participants received systemic steroids (26% and 28% in control and treatment groups, respectively) in the remaining study (Storr 1986). Intravenous aminophylline was used as a rescue therapy in two older studies (Rayner 1987; Storr 1986). If the clinical condition of a child was deteriorating in Craven 2001, subcutaneous epinephrine (0.01 mg/kg, maximum 0.5 mg) was added as part of the rescue therapy.
Outcomes
The primary efficacy outcome, that is, duration of hospital stay, was documented in three trials (Craven 2001; Goggin 2001; Rayner 1987) in a total of 327 children. None of these trials reported the primary safety outcome for our review, that is, serious adverse events. Other reported outcomes included admission to the ICU, need for supplemental asthma therapy, time to short‐acting β2‐agonists spaced at four hours or longer, relapse within 72 hours of discharge from the hospital and change from baseline in asthma severity measured as lung function, symptoms or clinical scores, adverse health effects and withdrawals.
Excluded studies
Risk of bias in included studies
Full details of risk of bias for all seven eligible trials are listed in the Characteristics of included studies table, and a graphical summary is presented in Figure 2. However, the following information pertains only to the four trials contributing data to the review.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
All four trials reported the method of randomisation in sufficient detail to confirm adequacy.
Allocation
Two of four trials contributing data reported details on allocation concealment, and both convincingly reported a valid allocation concealment (Craven 2001; Goggin 2001). Therefore, we judged these two trials to be at low risk of bias and the remaining trials to be at unclear risk of bias (Rayner 1987; Storr 1986).
Blinding
All trials reported double‐blinding, with convincing details indicating low risk of bias.
Incomplete outcome data
All four trials contributing data (Craven 2001; Goggin 2001; Rayner 1987; Storr 1986) reported on missing data; the proportion of withdrawals or missing values was balanced in numbers across intervention groups with similar reasons for missing data across groups, or no missing data were noted at all; thus all four trials were considered at low risk of bias on this criterion.
Selective reporting
Two trials (Craven 2001; Goggin 2001) clearly specified primary and secondary outcomes and reported all outcomes and thus were considered at low risk of bias on this criterion.
Other potential sources of bias
We encountered no other significant sources of bias in the included trials contributing data to the review.
Effects of interventions
See: Table 1
Primary efficacy outcome: duration of hospital stay
Three trials (Craven 2001; Goggin 2001; Rayner 1987) representing 327 children hospitalised with acute asthma contributed to the primary endpoint, that is, duration of hospital stay. The difference in hours of length of hospitalisation between participants treated with the combination of anticholinergics and short‐acting β2‐agonists versus β2‐agonists alone was not statistically significant, with an MD of ‐0.28 hours (95% CI ‐5.07 to 4.52; Analysis 1.1; Figure 3) and no apparent heterogeneity (I2 = 0%). Because of the similarity of trials contributing to the primary outcome with regards to the age of enrolled children, the absence of any trial conducted in the ICU, the use of similar doses of anticholinergics and co‐intervention with systemic corticosteroids in all participants in two trials (Craven 2001; Goggin 2001) and three‐quarters of participants in the remaining trial (Rayner 1987), we could not perform any of the a priori planned subgroup analyses on the primary efficacy outcome.
1.1. Analysis.
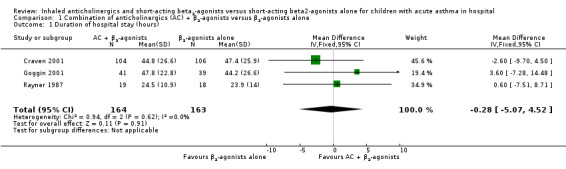
Comparison 1 Combination of anticholinergics (AC) + β2‐agonists versus β2‐agonists alone, Outcome 1 Duration of hospital stay (hours).
3.

Forest plot: Combination of anticholinergics + β2‐agonists versus β2‐agonists alone, outcome: duration of the hospital stay (hours).
As all trials contributing data on the primary outcome were of high methodological quality and were published as full text, we did not perform sensitivity analyses on quality and publication status.
Primary safety outcome: serious adverse events
No trials reported information on serious adverse events.
Secondary outcomes
Duration of stay in ICU
No studies reported on this outcome.
Admission to the ICU
No admission to the ICU was described by the one study reporting on this outcome (Analysis 1.2).
1.2. Analysis.
Comparison 1 Combination of anticholinergics (AC) + β2‐agonists versus β2‐agonists alone, Outcome 2 Admission to the intensive care unit.
Ventilation assistance
No studies reported on this outcome.
Need for supplemental asthma therapy
Four trials (Craven 2001; Goggin 2001; Rayner 1987; Storr 1986) representing 465 children contributed data on this outcome. No statistically significant impact of therapy on the need for supplemental therapy was described (RR 0.77, 95% CI 0.41 to 1.42; Analysis 1.3). The supplemental asthma therapy was intravenous aminophylline in two studies (Rayner 1987; Storr 1986), intravenous aminophylline or oral theophylline in one study (Goggin 2001) and an "intensification regimen," consisting of subcutaneous epinephrine (0.01 mg/kg, maximum 0.5 mg) and a one‐time 500‐μg dose of ipratropium bromide nebulised in combination with higher‐dose albuterol (5.0 mg) in another study (Craven 2001).
1.3. Analysis.

Comparison 1 Combination of anticholinergics (AC) + β2‐agonists versus β2‐agonists alone, Outcome 3 Need for supplemental asthma therapy.
Time to short‐acting β2‐agonists spaced at four hours or longer
Two trials (Craven 2001; Goggin 2001) representing 290 participants contributed to this outcome. No statistically significant group difference (MD ‐2.17, 95% CI ‐7.01 to 2.66; Analysis 1.4) was reported.
1.4. Analysis.

Comparison 1 Combination of anticholinergics (AC) + β2‐agonists versus β2‐agonists alone, Outcome 4 Time to short‐acting β2‐agonists spaced at 4 hours or longer (hours).
Change from baseline in asthma severity measured as lung function, symptoms or clinical scores
Two trials reported on the change from baseline in the asthma clinical score eight to 36 hours after initial treatment (higher is worse). No statistically significant group difference was described (SMD 0.02, 95% CI ‐0.34 to 0.38; Analysis 1.5).
1.5. Analysis.

Comparison 1 Combination of anticholinergics (AC) + β2‐agonists versus β2‐agonists alone, Outcome 5 Asthma clinical scores 8 to 36 hours after initial treatment.
Only a single trial reported on peak expiratory flow rate (PEFR) and described no statistically significant group difference in any of these outcomes (Analysis 1.7). No pooling of data was thus possible.
1.7. Analysis.

Comparison 1 Combination of anticholinergics (AC) + β2‐agonists versus β2‐agonists alone, Outcome 7 Percentages of predicted PEFR at 8 to 36 hours after initial treatment.
Relapse
No relapse was reported in the one study reporting on these outcomes (Analysis 1.6).
1.6. Analysis.
Comparison 1 Combination of anticholinergics (AC) + β2‐agonists versus β2‐agonists alone, Outcome 6 Relapse within 72 hours of discharge from hospital.
Adverse health effects
Two trials (Craven 2001; Goggin 2001) totaling 290 children reported that no adverse health events were observed (Analysis 1.8).
1.8. Analysis.

Comparison 1 Combination of anticholinergics (AC) + β2‐agonists versus β2‐agonists alone, Outcome 8 Adverse health effects.
Withdrawals
Two trials (Craven 2001; Goggin 2001) representing 294 children contributed to this outcome. No statistically significant group difference was noted in overall withdrawals (RR 0.80, 95% CI 0.38 to 1.70; Analysis 1.9) or in the proportion of withdrawals due to deterioration reported by a single trial (Analysis 1.10).
1.9. Analysis.

Comparison 1 Combination of anticholinergics (AC) + β2‐agonists versus β2‐agonists alone, Outcome 9 Overall withdrawals.
1.10. Analysis.

Comparison 1 Combination of anticholinergics (AC) + β2‐agonists versus β2‐agonists alone, Outcome 10 Withdrawals due to deterioration.
Discussion
Summary of main results
In children hospitalised on hospital wards for an acute asthma exacerbation, the combination of nebulised anticholinergics with short‐acting β2‐agonists was associated with no statistically significant reduction in duration of hospital stay. We did not set a priori boundary for equivalence, but after reviewing the literature to determine the cut‐off for a clinically meaningful reduction in length of hospital stay, we proposed that a group difference of 8 hours be considered the minimally clinically important based on various reported length of hospital stay (Cunningham 2008; Smith 2003; Lim 2000). Indeed, the narrow confidence intervals rule out an effect of eight hours or larger of combination therapy over β2‐agonists alone. Because of the similarity of trial designs and participant characteristics (supported by the absence of significant heterogeneity) and incomplete reporting, it was not possible to explore whether characteristics of participants or therapy, such as age, admission site (ward or ICU) or intensity of anticholinergic treatment or co‐interventions, could influence the magnitude of response attributable to the addition of anticholinergics. All trials contributing to the primary outcome were of high methodological quality and were published in full text, thus no bias due to quality or publication status was apparent. Although power was severely limited by the small number of trials, precluding the use of any statistics (Higgins 2011), inspection of the funnel plot did not suggest bias.
This finding was supported by all secondary outcomes, which showed no statistically significant group differences in the need for supplemental asthma therapy, asthma clinical scores, time to short‐acting β2‐agonists spaced at four hours or longer and withdrawals. Although the remaining outcomes could not be pooled because they were reported in only one trial, none showed a statistically significant group difference or effect.
Our findings contrast with those of a prior systematic review of RCTs, which concluded on the efficacy of multiple doses of inhaled anticholinergics in combination with β2‐agonist therapy versus β2‐agonist alone in the emergency management of severe asthma exacerbations (Plotnick 2000) and, more recently, on the treatment of children with moderate and severe exacerbations (Griffiths 2013). Recognising that the onset of action of oral corticosteroids is within three to four hours (Rowe 2001), we hypothesise that the beneficial effect of anticholinergics is best observed before the onset of action of systemic corticosteroids, at which point the effect of the latter surpasses that of the former. As hospital admission typically occurs at least three to four hours after oral corticosteroids are administered in the emergency department, perhaps the relative beneficial effect of anticholinergics becomes negligible when compared with systemic corticosteroids.
Only two trials (Craven 2001; Goggin 2001) examined adverse health effects; in both trials, no adverse health effects were observed in either intervention group. In the absence of adverse events in 145 intervention participants in these two trials, the upper limit around this null estimate is 2%. No trial reported any serious adverse health event—our primary safety outcome—possibly because of the absence of events, although we cannot rule out sub‐optimal documentation or reporting. The paucity of data prevents any firm conclusions on the safety of either treatment strategy based on the proportion of adverse health effects or withdrawals, but the proportions would appear to be low. However, use of anticholinergics in the emergency department was not associated with an increase in serious adverse events or adverse events (Griffiths 2013; Plotnick 2000).
Overall completeness and applicability of evidence
The results of this review apply to children one to 18 years old, with a good representation of preschoolers and school‐aged children, as three studies (Craven 2001; Goggin 2001; Storr 1986) included children with a mean or median age younger than five years and one trial (Rayner 1987) included predominantly school‐aged children. Concomitant use of aminophylline and withholding of systemic corticosteroids in participants with acute asthma was noted in two older studies (Rayner 1987; Storr 1986); these approaches contrast to the current standard practice in which systemic corticosteroids was used systematically in all enrolled participants in the two recent studies (Craven 2001; Goggin 2001). Of note, one cannot extrapolate these data to children with impending respiratory failure admitted to the ICU, as the trial was conducted in the ICU.
Quality of the evidence
A very modest number of studies contributed data to the review. Trials contributing most of the data were of high methodological quality. Yet the four studies contributing data totaled only 472 children hospitalised for acute asthma. Clearly the large confidence intervals observed for all outcomes suggest that additional trials could change the conclusion. In addition, the paucity of trials prevented identification of subgroups (e.g. age, intensity of treatment, admission site) for whom the treatment may provide more or less effect. Finally, evidence regarding the safety profile of nebulised anticholinergics is insufficient.
Potential biases in the review process
No evidence of bias due to publication or methodological quality was shown by funnel plot. We recognise that, in view of the small number of studies, our power to identify bias was limited.
Agreements and disagreements with other studies or reviews
On the basis of efficacy, available results support the recommendation of current national and international guidelines (GINA 2014; NAEPP 2011) that anticholinergics should not be used in children hospitalised for an acute asthma exacerbation beyond initial treatment in the emergency department.
Authors' conclusions
Implications for practice.
Among children hospitalised for an acute asthma exacerbation, no evidence suggests that nebulised anticholinergics added to β2‐agonists are effective in reducing length of hospital stay, need for supplemental asthma therapy or time to short‐acting β2‐agonists spaced at four hours or longer, or that this combination improves clinical scores compared with those of β2‐agonists alone. Data are insufficient to reveal whether specific subgroups of patients are more likely to benefit, or if certain characteristics of therapy may influence the magnitude of response attributable to the addition of anticholinergics.
Because of the absence of identified studies conducted in the ICU, no evidence elucidates the possible role of anticholinergics in children with impending respiratory failure.
No adverse health effects were reported; yet the small number of trials combined with inadequate reporting prevented firm reassurance regarding the safety of anticholinergics.
Results support the recommendation of current guidelines that anticholinergics are not indicated in children hospitalised for an acute asthma exacerbation, beyond initial treatment in the emergency department.
Implications for research.
Additional efficacy studies are needed to increase the precision of summary estimates and, urgently, to explore the efficacy of ipratropium bromide in children with impending respiratory failure.
Trials of high methodological quality with adequate documentation of adverse health effects associated with anticholinergics are needed to provide a fair comparison of the safety of these treatment options.
Future trials should aim to incorporate the following design characteristics.
Inclusion of double‐blinding and adequate randomisation with details provided on allocation concealment and complete reporting of withdrawals and dropouts on intention‐to‐treat analysis.
Comparison of different intensities of anticholinergic therapy.
Subgroup data on patients stratified by age group (preschool children vs school‐aged children) and severity of asthma on admission (mild, moderate or severe).
Continuous data provided as means with standard deviations.
Report of changes from baseline (at the time of randomisation) in asthma severity.
Systematic documentation of serious, overall and specific adverse health effects.
Systematic documentation of reasons for withdrawals.
Acknowledgements
We wish to thank the following individuals, who graciously provided additional data, namely, Dr Daniel Craven from Rainbow Babies and Children's Hospital, USA, and Dr Norma Goggin from the Department of Paediatrics, University of Toronto Faculty of Medicine, Canada.
We are indebted to the Cochrane Airways Review Group, namely, Dr Chris Cates, Dr Emma Welsh and Elizabeth Stovold, for performance of the literature search, their ongoing support and their constructive comments.
Chris Cates was the Editor for this review and commented critically on the review.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Group Specialised Register (CAGR)
Electronic searches: core databases
| Database | Frequency of search |
| CENTRAL (The Cochrane Library) | Monthly |
| MEDLINE (Ovid) | Weekly |
| EMBASE (Ovid) | Weekly |
| PsycINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the CAGR
Asthma search
1. exp Asthma/
2. asthma$.mp.
3. (antiasthma$ or anti‐asthma$).mp.
4. Respiratory Sounds/
5. wheez$.mp.
6. Bronchial Spasm/
7. bronchospas$.mp.
8. (bronch$ adj3 spasm$).mp.
9. bronchoconstrict$.mp.
10. exp Bronchoconstriction/
11. (bronch$ adj3 constrict$).mp.
12. Bronchial Hyperreactivity/
13. Respiratory Hypersensitivity/
14. ((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp.
15. ((dust or mite$) adj3 (allerg$ or hypersensitiv$)).mp.
16. or/1‐15
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomised or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases.
Appendix 2. Search strategy for Cochrane Airways Group Register of trials
#1 AST:MISC1
#2 MeSH DESCRIPTOR Asthma Explode All
#3 asthma*:ti,ab
#4 #1 or #2 or #3
#5 ipratropium*
#6 MeSH DESCRIPTOR Ipratropium
#7 MeSH DESCRIPTOR Atropine
#8 atropine*
#9 anticholinergic*
#10 anti‐cholinergic*
#11 anti* NEXT cholinergic*
#12 MeSH DESCRIPTOR Cholinergic Antagonists Explode All
#13 #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12
#14 in‐patient* or inpatient or "in patient"
#15 hospital*
#16 #14 or #15
#17 #4 and #13 and #16
#18 MeSH DESCRIPTOR Child Explode All
#19 MeSH DESCRIPTOR Pediatrics Explode All
#20 MeSH DESCRIPTOR Infant
#21 MeSH DESCRIPTOR Adolescent
#22 paediatric* or paediatric* or child* or adolescen* or infant* or young* or preschool* or pre‐school* or newborn* or new‐born* or neonat* or neo‐nat*
#23 #18 or #19 or #20 or #21 or #22
#24 #17 and #23
[Note: MISC1 denotes the field in the Register where the trial report has been coded for condition, i.e. AST=asthma]
Data and analyses
Comparison 1. Combination of anticholinergics (AC) + β2‐agonists versus β2‐agonists alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of hospital stay (hours) | 3 | 327 | Mean Difference (IV, Fixed, 95% CI) | ‐0.28 [‐5.07, 4.52] |
| 2 Admission to the intensive care unit | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Need for supplemental asthma therapy | 4 | 465 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.41, 1.42] |
| 4 Time to short‐acting β2‐agonists spaced at 4 hours or longer (hours) | 2 | 290 | Mean Difference (IV, Fixed, 95% CI) | ‐2.17 [‐7.01, 2.66] |
| 5 Asthma clinical scores 8 to 36 hours after initial treatment | 2 | 117 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.34, 0.38] |
| 6 Relapse within 72 hours of discharge from hospital | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 Percentages of predicted PEFR at 8 to 36 hours after initial treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Adverse health effects | 2 | 290 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Overall withdrawals | 2 | 294 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.27, 1.30] |
| 10 Withdrawals due to deterioration | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Craven 2001.
| Methods | Design: double‐blind, randomised, placebo‐controlled trial Confirmation of methodology: not obtained |
|
| Participants | Symptomatic participants Randomly assigned: N = 210
Withdrawals: reported Age: median years (interquartile range):
Gender: male (%)
Participants who received systemic corticosteroids before study enrolment (%):
Doses of β2‐agonists before study enrolment: median number (interquartile range)
Participants who received AC before study enrolment: number (total N)
Participants who required supplemental oxygen before study enrolment (%)
Time from first treatment in the emergency department to enrolment in hours (mean ± SD): not reported Eligibility criteria:
Exclusion criteria:
|
|
| Interventions | Protocol Duration
Test group: combination AC + β2‐agonists
Control group: combination placebo + β2‐agonists
Treatment (combination AC + β2‐agonists or combination placebo + β2‐agonists) is given every 4 hours during phase I, every 6 hours during phase II and every 8 hours during phase III of the Asthma Care Algorithm (ACA) Utilisation of the ACA, which consists of 4 stepwise phases of chest assessment performed at prescribed intervals: every 2 hours in phase I, every 3 hours in phase II, every 4 hours in phase III and every 6 hours in phase IV Chest assessment consists of measurement of:
Advancement to the next phase:
Nebulised albuterol (2.5 mg in 2 cc isotonic saline solution, driven by oxygen at 6 L/min) is administered after an assessment only if the child fails to meet advancement criteria If a child's rating deteriorates, then she or he reverts to ACA phase I after receiving the "intensification regimen," which consists of subcutaneous epinephrine (0.01 mg/kg, maximum 0.5 mg) and a 1‐time 500‐μg dose of ipratropium bromide nebulised in combination with higher‐dose albuterol (5.0 mg) Children complete the ACA when they require albuterol no more often than every 6 hours for a minimum of 12 hours All children receive systemic corticosteroids (1‐2 mg/kg/d, maximum dose 60 mg) Crteria for withdrawal from study: reported |
|
| Outcomes | Analysis: ITT Outcomes:
|
|
| Notes | Full paper (2001) Funding information not available “Intensification regimen” consisted of subcutaneous epinephrine (0.01 mg/kg, maximum 0.5 mg) and a 1‐time 500‐μg dose of IB nebulised in combination with higher‐dose albuterol (5.0 mg) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A table of random numbers was used to assign children by simple randomisations in blocks of 10 |
| Allocation concealment (selection bias) | Low risk | Centralised supply of unit dose–coded vials containing study drug or placebo solutions with identical aroma and appearance in both liquid and nebulised states |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind trial |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in quantity across intervention groups with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were specified and all results were presented |
| Other bias | Low risk | No apparent bias was observed |
Goggin 2001.
| Methods | Design: double‐blind, randomised, placebo‐controlled trial Confirmation of methodology: not obtained |
|
| Participants | Symptomatic participants Randomly assigned: N = 84
Withdrawals: reported Age: median years (interquartile range):
Gender: N (male %)
Number of participants who received systemic corticosteroids before study enrolment: not reported Number of doses of β2‐agonists before study enrolment: mean (± SD)
Number of doses of AC before study enrolment: mean (± SD)
Number of participants who required supplemental oxygen before study enrolment: not reported Time from first treatment in the emergency department to enrolment in hours: mean (± SD)
Eligibility criteria:
Exclusion criteria:
|
|
| Interventions | Test group: Combination AC + β2‐agonists
Control group: combination placebo + β2‐agonists
The combination AC + β2‐agonists or the combination placebo + β2‐agonists was given every half an hour to 1 hour at the beginning, progressing to 2 hours and then to 4 hours as the participant improves clinically Device: face mask and nebuliser All children were treated with corticosteroids using intravenous hydrocortisone 4 to 6 mg/kg every 6 hours or oral prednisone 1 mg/kg once daily. The total duration of corticosteroid therapy was a minimum of 5 days Use of supplemental oxygen therapy and other concurrent therapy (e.g. aminophylline) was at the discretion of the attending staff and was recorded Crteria for withdrawal from study: reported |
|
| Outcomes | Analysis: ITT Outcomes:
Each variable is scored 0, 1 or 2 and is summed, with a total possible score of 10
|
|
| Notes | Full paper (2001); contacted the trial author and received additional data Funding information not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was independently performed by a research pharmacist using a table of random numbers. Participants enrolled in the study were stratified at enrolment by 2 criteria (age and number of doses of nebulised ipratropium bromide administered in the emergency department). Children within each stratum were randomly allocated to treatment groups in blocks of 4 |
| Allocation concealment (selection bias) | Low risk | Intervention and placebo solutions were clear, colourless and odourless liquids, and the 2 solutions were indistinguishable from one another in liquid and nebulised states |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in quantity across intervention groups with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | Primary outcome was specified and all outcomes were reported |
| Other bias | Low risk | No apparent bias was noted |
Lew 1990.
| Methods | Design: double‐blind, cross‐over, randomised trial Confirmation of methodology: not obtained |
|
| Participants | Symptomatic participants Randomly assigned: N = 17
Withdrawals: not reported Age: mean years (± SD):
Gender: N (male %)
Number of participants who received systemic corticosteroids before study enrolment: not reported Number of doses of β2‐agonists before study enrolment: not reported Number of doses of AC before study enrolment: not reported Number of participants who required supplemental oxygen before study enrolment: not reported Time from first treatment in the emergency department to enrolment in hours: not reported Eligibility criteria:
Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned in a double‐blind cross‐over fashion to receive as their first therapy either:
Four hours after first inhalation, participants received inhalation of the alternative treatment Device: Nebulised therapy was delivered through a mouthpiece and the nebuliser was attached to tubing with a T‐connection to allow intermittent thumb control during 5‐second maximal inspiration from functional residual capacity to total lung capacity Participants were entered into the study within 72 hours of hospitalisation Inhaled bronchodilator therapy was withheld from all participants for 4 hours before the beginning of the study All participants received maintenance intravenous fluids, continuous infusion of aminophylline at doses sufficient to maintain serum theophylline concentrations between 10 and 20 μg/mL and 1 mg/kg every 6 hours of intravenous methylprednisolone Participants received oxygen supplement via face mask or nasal cannula as needed Criteria for withdrawal from study: not reported |
|
| Outcomes | Analysis: not ITT Outcomes:
FVC FEV1 FEF25‐75%
Signs of atropine side effects at baseline and 30, 60, 120, 180 and 240 minutes after each aerolised treatment
|
|
| Notes | Full paper (1990) Funding information not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on random sequence generation was provided |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment was provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind trial |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Primary outcome was specified and all outcomes were reported |
| Other bias | Low risk | No apparent bias was noted |
Mirkinson 2000.
| Methods | Design: randomised, double‐blind, placebo‐controlled trial Confirmation of methodology: not obtained |
|
| Participants | Symptomatic participants Randomly assigned: N = 42
Withdrawals: not reported Age: mentioned Gender: not reported Number of participants who received systemic corticosteroids before study enrolment: not reported Number of doses of β2‐agonists before study enrolment: not reported Number of doses of AC before study enrolment: not reported Number of participants who required supplemental oxygen before study enrolment: not reported Time from first treatment in the emergency department to enrolment in hours: not reported Eligibility criteria:
Exclusion criteria:
|
|
| Interventions | Participants were treated with an initial standardised protocol consisting of nebulised albuterol 0.15 mg/kg and nebulised ipratropium bromide 250‐500 μg administered together as 3 consecutive doses 20 minutes apart (over 1 hour) All participants continued to receive albuterol at a frequency based on asthma scores and clinical improvement and were then randomly assigned to 2 groups:
All participants additionally received intravenous or oral steroids according to standard practice guidelines and supplemental oxygen and intravenous fluids as needed Clinical status was evaluated at predetermined times, 12 and 24 hours, by assigning asthma scores Criteria for withdrawal from study: not reported in the abstract |
|
| Outcomes | Analysis: ITT not mentioned in the abstract Outcomes:
|
|
| Notes | Abstract only Funding information not mentioned in the abstract |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Ozdemir 2003.
| Methods | Design: randomised, placebo‐controlled trial Confirmation of methodology: not obtained |
|
| Participants | Symptomatic participants Randomly assigned: N = 60
Withdrawals: not reported Age: not reported Gender: N not reported Number of participants who received systemic corticosteroids before study enrolment: not reported Number of doses of β2‐agonists before study enrolment: not reported Number of doses of AC before study enrolment: not reported Number of participants who required supplemental oxygen before study enrolment: not reported Time from first treatment in the emergency department to enrolment in hours: not reported Eligibility criteria:
Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to receive either:
Criteria for withdrawal from study: not reported |
|
| Outcomes | Analysis: ITT not mentioned Outcomes:
PEF rates
|
|
| Notes | Abstract only Funding information not mentioned in the abstract |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
Rayner 1987.
| Methods | Design: randomised clinical study Confirmation of methodology: not obtained |
|
| Participants | Symptomatic patients Randomly assigned: N = 37
Withdrawals: not reported Age: mean 6.5 years
Gender: not reported Number of participants who received systemic corticosteroids before study enrolment: not reported Number of doses of β2‐agonists before study enrolment: not reported Number of doses of AC before study enrolment: not reported Number of participants who required supplemental oxygen before study enrolment: not reported Time from first treatment in the emergency department to enrolment in hours: not reported Eligibility criteria:
Exclusion criteria:
|
|
| Interventions | Test group: nebulised AC
Control group: nebulised placebo
Nebulisers were given 30 minutes after the first dose of salbutamol and every 8 hours afterwards Both groups received nebulised salbutamol (2.5 mg for children ≤ 6 years old and 5 mg for children > 6 years old) on admission and every 4 hours afterwards Steroids were given if good relief was not obtained |
|
| Outcomes | Analysis: not ITT Outcomes:
Peak expiratory flow rate
Based on clinical examination, activity and speech (worst possible score = 25)
Signs of atropine side effects at baseline and 30, 60,120, 180 and 240 minutes after each aerosolised treatment
|
|
| Notes | Full paper (1987) Funding information not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Treatments were randomly allocated, but no information was provided on random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment was provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Doule‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Unclear risk | All specified outcomes were reported, but primary and secondary outcomes were not specified |
| Other bias | Low risk | No apparent bias was observed |
Storr 1986.
| Methods | Design: randomised clinical study Confirmation of methodology: not obtained |
|
| Participants | Symptomatic participants Randomly assigned: N = 138
Withdrawals: not reported Age: mean 5.0 years
Gender: 95 boys (69%)
Number of participants who received systemic corticosteroids before study enrolment: not reported Number of doses of β2‐agonists before study enrolment: not reported Number of doses of AC before study enrolment: not reported Number of participants who required supplemental oxygen before study enrolment: not reported Time from first treatment in the emergency department to enrolment in hours: not reported Eligibility criteria:
Exclusion criteria:
|
|
| Interventions | Test group: combination AC + β2‐agonists
Control group: β2‐agonists alone
Nebulisers were given within set limits at the discretion of the nursing staff Steroids were given to children not responding satisfactorily to nebulised treatment Intravenous aminophylline was given to children in severe respiratory distress Criteria for withdrawal from study: not reported |
|
| Outcomes | Analysis: not ITT Outcomes:
Peak expiratory flow rates immediately before and 20 minutes after treatment (except at night)
|
|
| Notes | Full paper (1986) Funding information not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Treatments were randomly allocated, but no information was provided on random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No adequate information was provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data noted |
| Selective reporting (reporting bias) | Unclear risk | All reported outcomes were presented, but primary and secondary outcomes were not specified |
| Other bias | Low risk | No apparent bias was observed |
AC: anticholinergics; ACA: Asthma Care Algorithm; ACA‐P: Asthma Carepath Progression; FEF25‐75%: forced expiratory flow 25–75%; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; ICU: intensive care unit; ITT: intention‐to‐treat analysis; SD: standard deviation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ahmad 2010 | ONE OF THE GROUPS WAS NOT A COMBINATION OF ANTICHOLINERGICS + β2‐AGONISTS |
| Allen 2005A | PARTICIPANTS WERE NOT HOSPITALISED FOR AN ACUTE ASTHMA EXACERBATION OR DID N OT HAVE TREATMENT BEYOND INITIAL TREATMENT IN THE EMERGENCY DEPARTMENT |
| Andrews 2009 | ONE OF THE GROUPS WAS NOT A COMBINATION OF ANTICHOLINERGICS + β2‐AGONISTS |
| Avital 1992 | PARTICIPANTS WERE NOT HOSPITALISED FOR AN ACUTE ASTHMA EXACERBATION OR DID N OT HAVE TREATMENT BEYOND INITIAL TREATMENT IN THE EMERGENCY DEPARTMENT |
| Azevedo 1990 | PARTICIPANTS WERE NOT EXCLUSIVELY CHILDREN |
| Becker 1999 | ONE OF THE GROUPS WAS NOT A COMBINATION OF ANTICHOLINERGICS + β2‐AGONISTS |
| Benito Fernandez 2000 | PARTICIPANTS WERE NOT HOSPITALISED FOR AN ACUTE ASTHMA EXACERBATION OR DID N OT HAVE TREATMENT BEYOND INITIAL TREATMENT IN THE EMERGENCY DEPARTMENT |
| Berger 2006 | PARTICIPANTS WERE NOT HOSPITALISED FOR AN ACUTE ASTHMA EXACERBATION OR DID NO T HAVE TREATMENT BEYOND INITIAL TREATMENT IN THE EMERGENCY DEPARTMENT |
| Bigham 2010 | ONE OF THE GROUPS WAS NOT A COMBINATION OF ANTICHOLINERGICS + β2‐AGONISTS |
| Boeree 1998 | PARTICIPANTS WERE NOT EXCLUSIVELY CHILDREN |
| Bogie 2007 | ONE OF THE GROUP WAS NOT A COMBINATION OF ANTICHOLINERGICS + β2‐AGONISTS |
| Bradshaw 2008 | PARTICIPANTS WERE NOT EXCLUSIVELY CHILDREN |
| Brenner 1988 | ONE OF THE GROUP WAS NOT A COMBINATION OF ANTICHOLINERGICS + β2‐AGONISTS |
| Browne 2002 | PARTICIPANTS WERE NOT HOSPITALISED FOR AN ACUTE ASTHMA EXACERBATION OR DID NO T HAVE TREATMENT BEYOND INITIAL TREATMENT IN THE EMERGENCY DEPARTMENT |
| Camargo 2010 | PARTICIPANTS WERE NOT EXCLUSIVELY CHILDREN |
| Chen 2008 | ONE OF THE GROUPS WAS NOT A COMBINATION OF ANTICHOLINERGICS + β2‐AGONISTS |
| Chen 2012 | PARTICIPANTS WERE NOT HOSPITALISED FOR AN ACUTE ASTHMA EXACERBATION OR DID NO T HAVE TREATMENT BEYOND INITIAL TREATMENT IN THE EMERGENCY DEPARTMENT |
| Chowdhury 1995 | PARTICIPANTS WERE NOT ASTHMATIC |
| Coulthard 1985 | PARTICIPANTS WERE NOT HOSPITALISED FOR AN ACUTE ASTHMA EXACERBATION OR DID NO T HAVE TREATMENT BEYOND INITIAL TREATMENT IN THE EMERGENCY DEPARTMENT |
| Cydulka 2010 | PARTICIPANTS WERE NOT EXCLUSIVELY CHILDREN |
| Dahlen 2012 | PARTICIPANTS WERE NOT EXCLUSIVELY CHILDREN |
| de Jong 1996 | PARTICIPANTS WERE NOT EXCLUSIVELY CHILDREN |
| Douma 1998 | PARTICIPANTS WERE NOT EXCLUSIVELY CHILDREN |
| Ducharme 1998 | PARTICIPANTS WERE NOT HOSPITALISED FOR AN ACUTE ASTHMA EXACERBATION OR DID NO T HAVE TREATMENT BEYOND INITIAL TREATMENT IN THE EMERGENCY DEPARTMENT |
| Dutt 1990 | PARTICIPANTS WERE NOT HOSPITALISED FOR AN ACUTE ASTHMA EXACERBATION OR DID NO T HAVE TREATMENT BEYOND INITIAL TREATMENT IN THE EMERGENCY DEPARTMENT |
| Freeman 1989A | PARTICIPANTS WERE NOT HOSPITALISED FOR AN ACUTE ASTHMA EXACERBATION OR DID NO T HAVE TREATMENT BEYOND INITIAL TREATMENT IN THE EMERGENCY DEPARTMENT |
| Friberg 1989A | PARTICIPANTS WERE NOT HOSPITALISED FOR AN ACUTE ASTHMA EXACERBATION OR DID NO T HAVE TREATMENT BEYOND INITIAL TREATMENT IN THE EMERGENCY DEPARTMENT |
| Garcia 1998 | PARTICIPANTS WERE NOT ASTHMATIC |
| González 1989 | PARTICIPANTS WERE NOT HOSPITALISED FOR AN ACUTE ASTHMA EXACERBATION OR DID NO T HAVE TREATMENT BEYOND INITIAL TREATMENT IN THE EMERGENCY DEPARTMENT |
| Goodacre 2013 | PARTICIPANTS WERE NOT EXCLUSIVELY CHILDREN |
| Gouin 1999 | STUDY WAS NOT A RANDOMI SED TRIAL |
| Gove 1988 | PARTICIPANTS WERE NOT EXCLUSIVELY CHILDREN |
| Greenough 1986 | PARTICIPANTS WERE NOT HOSPITALISED FOR AN ACUTE ASTHMA EXACERBATION OR DID NO T HAVE TREATMENT BEYOND INITIAL TREATMENT IN THE EMERGENCY DEPARTMENT |
| Groot 1994 | PARTICIPANTS WERE NOT EXCLUSIVELY CHILDREN |
| Haahtela 1991A | PARTICIPANTS WERE NOT EXCLUSIVELY CHILDREN |
| Hardasmalani 2005 | PARTICIPANTS WERE NOT EXCLUSIVELY CHILDREN |
| Hayday 2002E | DUPLICATION OF Goggin 2001 |
| Henry 1989 | PARTICIPANTS WERE NOT HOSPITALISED FOR AN ACUTE ASTHMA EXACERBATION OR DID NO T HAVE TREATMENT BEYOND INITIAL TREATMENT IN THE EMERGENCY DEPARTMENT |
| Iramain 2011 | PARTICIPANTS WERE NOT HOSPITALISED FOR AN ACUTE ASTHMA EXACERBATION OR DID NO T HAVE TREATMENT BEYOND INITIAL TREATMENT IN THE EMERGENCY DEPARTMENT |
| Israel 2004 | PARTICIPANTS WERE NOT EXCLUSIVELY CHILDREN |
| Jiang 2006 | STUDY WAS NOT A RANDOMI SED TRIAL |
| Kaptein 1993 | STUDY WAS NOT A RANDOMI SED TRIAL |
| Kelso 2011 | PARTICIPANTS WERE NOT HOSPITALISED FOR AN ACUTE ASTHMA EXACERBATION OR DID NO T HAVE TREATMENT BEYOND INITIAL TREATMENT IN THE EMERGENCY DEPARTMENT |
| Kerstjens 1992 | PARTICIPANTS WERE NOT EXCLUSIVELY CHILDREN |
| Kerstjens 1993 | PARTICIPANTS WERE NOT EXCLUSIVELY CHILDREN |
| Kerstjens 1994 | PARTICIPANTS WERE NOT EXCLUSIVELY CHILDREN |
| Kerstjens 1995 | PARTICIPANTS WERE NOT EXCLUSIVELY CHILDREN |
| Knöpfli 2005 | STUDY WAS NOT A RANDOMIS ED TRIAL |
| Lanes 1998 | PARTICIPANTS WERE NOT CHILDREN |
| Lowry 1994 | PARTICIPANTS WERE NOT ASTHMATIC |
| Macias 2003A | PARTICIPANTS WERE NOT HOSPITALISED FOR AN ACUTE ASTHMA EXACERBATION OR DID NO T HAVE TREATMENT BEYOND INITIAL TREATMENT IN THE EMERGENCY DEPARTMENT |
| Mallol 1987a | PARTICIPANTS WERE NOT ASTHMATIC |
| Mallol 1987bA | PARTICIPANTS WERE NOT ASTHMATIC |
| Maneechotesuwan 2011 | PARTICIPANTS WERE NOT CHILDREN |
| McDowell 1998 | STUDY WAS NOT A RANDOMI SED TRIAL |
| Meier 1997 | STUDY WAS NOT A RANDOMI SED TRIAL |
| Mitchell 2005A | PARTICIPANTS WERE NOT HOSPITALISED FOR AN ACUTE ASTHMA EXACERBATION OR DID NO T HAVE TREATMENT BEYOND INITIAL TREATMENT IN THE EMERGENCY DEPARTMENT |
| Morris 2010 | PARTICIPANTS WERE NOT HOSPITALISED FOR AN ACUTE ASTHMA EXACERBATION OR DID N OT HAVE TREATMENT BEYOND INITIAL TREATMENT IN THE EMERGENCY DEPARTMENT |
| Morrison 1989 | STUDY WAS NOT A RANDOMI SED TRIAL |
| Nakano 2000 | PARTICIPANTS WERE NOT EXCLUSIVELY CHILDREN |
| Newnham 1995A | PARTICIPANTS WERE NOT EXCLUSIVELY CHILDREN |
| Nibhanipudi 2009 | PARTICIPANTS WERE NOT HOSPITALISED FOR AN ACUTE ASTHMA EXACERBATION OR DID NO T HAVE TREATMENT BEYOND INITIAL TREATMENT IN THE EMERGENCY DEPARTMENT |
| O'Driscoll 1989B | PARTICIPANTS WERE NOT EXCLUSIVELY CHILDREN |
| Overbeek 1996 | PARTICIPANTS WERE NOT EXCLUSIVELY CHILDREN |
| Parkin 1995 | ONE OF THE GROUPS WAS NOT A COMBINATION OF ANTICHOLINERGICS + β2‐AGONISTS |
| Peters 2000 | PARTICIPANTS WERE NOT EXCLUSIVELY CHILDREN |
| Ponce 2009 | PARTICIPANTS WERE NOT HOSPITALISED FOR AN ACUTE ASTHMA EXACERBATION OR DID N OT HAVE TREATMENT BEYOND INITIAL TREATMENT IN THE EMERGENCY DEPARTMENT |
| Powell 2012a | DUPLICATION OF Powell 2013 |
| Powell 2013 | PARTICIPANTS WERE NOT HOSPITALISED FOR AN ACUTE ASTHMA EXACERBATION OR DID N OT HAVE TREATMENT BEYOND INITIAL TREATMENT IN THE EMERGENCY DEPARTMENT |
| Qureshi 1997 | PARTICIPANTS WERE NOT HOSPITALISED FOR AN ACUTE ASTHMA EXACERBATION OR DID N OT HAVE TREATMENT BEYOND INITIAL TREATMENT IN THE EMERGENCY DEPARTMENT |
| Qureshi 1998 | PARTICIPANTS WERE NOT HOSPITALISED FOR AN ACUTE ASTHMA EXACERBATION OR DID N OT HAVE TREATMENT BEYOND INITIAL TREATMENT IN THE EMERGENCY DEPARTMENT |
| Qureshi 2001 | PARTICIPANTS WERE NOT HOSPITALISED FOR AN ACUTE ASTHMA EXACERBATION OR DID N OT HAVE TREATMENT BEYOND INITIAL TREATMENT IN THE EMERGENCY DEPARTMENT |
| Qureshi 2005 | PARTICIPANTS WERE NOT HOSPITALISED FOR AN ACUTE ASTHMA EXACERBATION OR DID N OT HAVE TREATMENT BEYOND INITIAL TREATMENT IN THE EMERGENCY DEPARTMENT |
| Raes 1989 | PARTICIPANTS WERE NOT HOSPITALISED FOR AN ACUTE ASTHMA EXACERBATION OR DID N OT HAVE TREATMENT BEYOND INITIAL TREATMENT IN THE EMERGENCY DEPARTMENT |
| Raissy 2006 | PARTICIPANTS WERE NOT HOSPITALISED FOR AN ACUTE ASTHMA EXACERBATION OR DID N OT HAVE TREATMENT BEYOND INITIAL TREATMENT IN THE EMERGENCY DEPARTMENT |
| Ralston 2005 | PARTICIPANTS WERE NOT HOSPITALISED FOR AN ACUTE ASTHMA EXACERBATION OR DID N OT HAVE TREATMENT BEYOND INITIAL TREATMENT IN THE EMERGENCY DEPARTMENT |
| Ream 2001 | ONE OF THE GROUPS WAS NOT β2‐AGONISTS ALONE |
| Reisman 1988A | PARTICIPANTS WERE NOT HOSPITALISED FOR AN ACUTE ASTHMA EXACERBATION OR DID N OT HAVE TREATMENT BEYOND INITIAL TREATMENT IN THE EMERGENCY DEPARTMENT |
| Richards 1987 | PARTICIPANTS WERE NOT EXCLUSIVELY CHILDREN |
| Roberts 2003 | ONE OF THE GROUPS WAS NOT A COMBINATION OF ANTICHOLINERGICS + β2‐AGONISTS |
| Rodriguez 2008 | PARTICIPANTS WERE NOT HOSPITALISED FOR AN ACUTE ASTHMA EXACERBATION OR DID N OT HAVE TREATMENT BEYOND INITIAL TREATMENT IN THE EMERGENCY DEPARTMENT |
| Rowe 2007 | PARTICIPANTS WERE NOT EXCLUSIVELY CHILDREN |
| Salmun 1999 | PARTICIPANTS WERE NOT EXCLUSIVELY CHILDREN |
| Sano 2000 | PARTICIPANTS WERE NOT ASTHMATIC |
| Schuh 1992 | PARTICIPANTS WERE NOT ASTHMATIC |
| Schuh 1995 | PARTICIPANTS WERE NOT HOSPITALISED FOR AN ACUTE ASTHMA EXACERBATION OR DID N OT HAVE TREATMENT BEYOND INITIAL TREATMENT IN THE EMERGENCY DEPARTMENT |
| Schuh 1997 | PARTICIPANTS WERE NOT HOSPITALISED FOR AN ACUTE ASTHMA EXACERBATION OR DID N OT HAVE TREATMENT BEYOND INITIAL TREATMENT IN THE EMERGENCY DEPARTMENT |
| Self 2002 | ONE OF THE GROUPS WAS NOT A COMBINATION OF ANTICHOLINERGICS + β2‐AGONISTS |
| Sengul 2013 | PARTICIPANTS WERE NOT HOSPITALISED FOR AN ACUTE ASTHMA EXACERBATION OR DID N OT HAVE TREATMENT BEYOND INITIAL TREATMENT IN THE EMERGENCY DEPARTMENT |
| Sienra Monge 2000 | PARTICIPANTS WERE NOT HOSPITALISED FOR AN ACUTE ASTHMA EXACERBATION OR DID N OT HAVE TREATMENT BEYOND INITIAL TREATMENT IN THE EMERGENCY DEPARTMENT |
| Silverman 2012 | PARTICIPANTS WERE NOT EXCLUSIVELY CHILDREN |
| Singh 2008 | PARTICIPANTS WERE NOT EXCLUSIVELY CHILDREN |
| Singhi 2010 | PARTICIPANTS WERE NOT HOSPITALISED FOR AN ACUTE ASTHMA EXACERBATION OR DID N OT HAVE TREATMENT BEYOND INITIAL TREATMENT IN THE EMERGENCY DEPARTMENT |
| Sly 1987 | PARTICIPANTS WERE NOT HOSPITALISED FOR AN ACUTE ASTHMA EXACERBATION OR DID N OT HAVE TREATMENT BEYOND INITIAL TREATMENT IN THE EMERGENCY DEPARTMENT |
| Stewart 2012 | PARTICIPANTS WERE NOT EXCLUSIVELY CHILDREN |
| Stormon 1999 | ONE OF THE GROUPS WAS NOT A COMBINATION OF ANTICHOLINERGICS + β2‐AGONISTS |
| Sur 1990 | PARTICIPANTS WERE NOT EXCLUSIVELY CHILDREN |
| Tasche 1997 | PARTICIPANTS WERE NOT HOSPITALISED FOR AN ACUTE ASTHMA EXACERBATION OR DID N OT HAVE TREATMENT BEYOND INITIAL TREATMENT IN THE EMERGENCY DEPARTMENT |
| Taytard 1987 | PARTICIPANTS WERE NOT EXCLUSIVELY CHILDREN |
| Timsit 2002 | PARTICIPANTS WERE NOT HOSPITALISED FOR AN ACUTE ASTHMA EXACERBATION OR DID N OT HAVE TREATMENT BEYOND INITIAL TREATMENT IN THE EMERGENCY DEPARTMENT |
| Ulrik 1992 | PARTICIPANTS WERE NOT HOSPITALISED FOR AN ACUTE ASTHMA EXACERBATION OR DID N OT HAVE TREATMENT BEYOND INITIAL TREATMENT IN THE EMERGENCY DEPARTMENT |
| UNKNOWN 2011 | PARTICIPANTS WERE NOT HOSPITALISED FOR AN ACUTE ASTHMA EXACERBATION OR DID N OT HAVE TREATMENT BEYOND INITIAL TREATMENT IN THE EMERGENCY DEPARTMENT |
| Upham 2011A | PARTICIPANTS WERE NOT HOSPITALISED FOR AN ACUTE ASTHMA EXACERBATION OR DID N OT HAVE TREATMENT BEYOND INITIAL TREATMENT IN THE EMERGENCY DEPARTMENT |
| Van Asperen 1988 | PARTICIPANTS WERE NOT HOSPITALISED FOR AN ACUTE ASTHMA EXACERBATION OR DID N OT HAVE TREATMENT BEYOND INITIAL TREATMENT IN THE EMERGENCY DEPARTMENT |
| van der Woude 2001A | PARTICIPANTS WERE NOT EXCLUSIVELY CHILDREN |
| Von Berg 2004 | PARTICIPANTS WERE NOT HOSPITALISED FOR AN ACUTE ASTHMA EXACERBATION OR DID N OT HAVE TREATMENT BEYOND INITIAL TREATMENT IN THE EMERGENCY DEPARTMENT |
| Ward 1981 | PARTICIPANTS WERE NOT EXCLUSIVELY CHILDREN |
| Ward 1985 | PARTICIPANTS WERE NOT EXCLUSIVELY CHILDREN |
| Watanasomsiri 2006 | PARTICIPANTS WERE NOT HOSPITALISED FOR AN ACUTE ASTHMA EXACERBATION OR DID N OT HAVE TREATMENT BEYOND INITIAL TREATMENT IN THE EMERGENCY DEPARTMENT |
| Watson 1994 | PARTICIPANTS WERE NOT HOSPITALISED FOR AN ACUTE ASTHMA EXACERBATION OR DID N OT HAVE TREATMENT BEYOND INITIAL TREATMENT IN THE EMERGENCY DEPARTMENT |
| Wilson 1987 | PARTICIPANTS WERE NOT EXCLUSIVELY CHILDREN |
| Wolstenholme 1989 | PARTICIPANTS WERE NOT EXCLUSIVELY CHILDREN |
| Worth 2012 | PARTICIPANTS WERE NOT EXCLUSIVELY CHILDREN |
| Yang 1993 | PARTICIPANTS WERE NOT EXCLUSIVELY CHILDREN |
| Young 1991 | PARTICIPANTS WERE NOT EXCLUSIVELY CHILDREN |
| Youngchaiyud 1989 | PARTICIPANTS WERE NOT EXCLUSIVELY CHILDREN |
| Yung 1998 | ONE OF THE GROUPS WAS NOT β2‐AGONISTS ALONE |
| Zaritsky 1999A | DUPLICATION OF Qureshi 1998 |
| Zorc 1999 | PARTICIPANTS WERE NOT HOSPITALISED FOR AN ACUTE ASTHMA EXACERBATION OR DID N OT HAVE TREATMENT BEYOND INITIAL TREATMENT IN THE EMERGENCY DEPARTMENT |
Characteristics of ongoing studies [ordered by study ID]
Powell 2012.
| Trial name or title | MAGnesium NEbuliser Trial In Children (MAGNETIC) |
| Methods | Randomised, placebo‐controlled, double‐blind multi‐centre trial |
| Participants | Children (aged 2‐16 years) presenting to hospital emergency departments and acute paediatric inpatient units with severe acute asthma Exclusion criteria will be co‐existing respiratory disease such as cystic fibrosis, chronic lung disease of prematurity, severe renal disease, severe liver disease, known to be pregnant |
| Interventions | Nebulised magnesium sulphate compared with placebo along with standard therapy of nebulised salbutamol and ipratropium bromide |
| Outcomes | Two principal outcomes: 1. Yung Asthma Severity Score (ASS) 2. Numbers of participants with 'stepping down' therapy at 1 hour after treatment with the study medication Other outcomes: 3. Number and frequency of additional salbutamol 4. Lung function 5. Length of stay in hospital 6. Requirement for intravenous bronchodilator treatment 7. Admission to a paediatric intensive care unit (PICU) 8. Intubation rate A prospective economic evaluation will be conducted alongside the trial |
| Starting date | December 2007 |
| Contact information | |
| Notes |
Differences between protocol and review
We changed the title of the review.
In the protocol, under Methods, Selection of studies, we had planned the involvement of 2 reviewers in the selection of studies, however only one reviewer performed this task.
In the protocol, we has planned to perform a sensitivity analysis to explore the impact of excluding trials with missing outcomes to evaluate the risk of bias. However due to low number of included trials, we did not perform the sensitivity analysis.
In the protocol, we had mentioned: ''we will create a summary of findings table using the two primary outcomes and the following secondary outcomes: duration of stay in ICU; admission to the ICU; adverse effects; change from baseline in lung function.'' However, as no included trial reported data on the one of two primary outcomes (serious adverse events); no trials included patients admitted to the ICU and consequently no data was available for duration of ICU stay; and only one included trial reported lung function (PEFR), we did not include these outcomes in the Summary of findings table. We thus reported on the primary outcome (duration of hospital stay), and other relevant outcomes namely, time to rescue inhaled short‐acting ß2‐agonists, asthma score and withdrawals.
Contributions of authors
Kevin Vézina wrote the protocol, screened abstracts, selected and ascertained the methodological quality of and extracted data from included studies, conducted the analysis and wrote the first draft of the review.
Bhupendrasinh Chauhan contributed to development of the protocol, abstract selection and study selection, ascertainment of methodological quality and data extraction and reviewed all drafts of the manuscript, responded to reviewers' comments and approved the final manuscript.
Francine M. Ducharme supervised the overall process, approved the protocol, contacted trial authors, interpreted data and approved the final manuscript.
Declarations of interest
Kevin Vézina: none known.
Bhupendrasinh Chuhan received a postdoctoral scholarship through one of Dr Ducharme’s grants from the Canadian Institute of Health Research and reports no conflicts of interest.
Francine M Ducharme has received travel support, research funds and fees for speaking from GlaxoSmithKline, Novartis, Nycomed and/or Merck Frosst Inc.
New
References
References to studies included in this review
Craven 2001 {published data only}
- Craven D, Kercsmar CM, Myers TR, O'Riordan MA, Golonka G, Moore S, et al. Ipratropium bromide plus nebulized albuterol for the treatment of hospitalized children with acute asthma. Journal of Pediatrics 2001;138(1):51‐8. [] [DOI] [PubMed] [Google Scholar]
Goggin 2001 {published data only}
- Goggin N, Macarthur C, Parkin PC. Randomized trial of the addition of ipratropium bromide to albuterol and corticosteroid therapy in children hospitalized because of an acute asthma exacerbation. Archives of Pediatrics and Adolescent Medicine 2001;155(12):1329‐34. [DOI] [PubMed] [Google Scholar]
Lew 1990 {published data only}
- Lew DB, Herrod HG, Crawford LV. Combination of atropine and isoetharine aerosol therapy in pediatric acute asthma. Annals of Allergy 1990;64(2 Pt 2):195‐200. [] [PubMed] [Google Scholar]
Mirkinson 2000 {published data only}
- Mirkinson LJ, Ottolini MM. The evaluation of the use of ipratropium bromide in children hospitalized with acute asthma. Pediatric Research 2000;47(4):479A. [; CN‐00401958] [Google Scholar]
Ozdemir 2003 {published data only}
- Ozdemir M, Akcakaya N, Camioglu Y, Cokugras H. Ipratropium bromide plus nebulized ß‐2 agonist for the treatment of hospitalized children with acute asthma attack [Abstract]. Allergy and Clinical Immunology International: Journal of the World Allergy Organization 2003;1(Suppl):Abstract No: P‐2‐7. [] [Google Scholar]
Rayner 1987 {published data only}
- Rayner RJ, Cartlidge PH, Upton CJ. Salbutamol and ipratropium in acute asthma. Archives of Disease in Childhood 1987;62(8):840‐1. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Storr 1986 {published data only}
- Storr J, Lenney W. Nebulised ipratropium and salbutamol in asthma. Archives of Disease in Childhood 1986;61(6):602‐3. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Ahmad 2010 {published data only}
- Ahmad J, Ali Khan R, Malik MA. A study of Nigella sativa oil in the management of wheeze associated lower respiratory tract illness in children. African Journal of Pharmacy and Pharmacology 2010;4(7):436‐9. [] [Google Scholar]
Allen 2005A {published data only}
- Allen JY, MacIas CG, Allen Joseph Y, Macias Charles G. The efficacy of ketamine in pediatric emergency department patients who present with acute severe asthma. Annals of Emergency Medicine 2005;46(1):43‐50. [] [DOI] [PubMed] [Google Scholar]
Andrews 2009 {published data only}
- Andrews T, McGintee E, Mittal MK, Tyler L, Chew A, Zhang X, et al. High‐dose continuous nebulized levalbuterol for pediatric status asthmaticus: a randomized trial. Journal of Pediatrics 2009;155(2):205‐10.e1. [; CN‐00699100] [DOI] [PubMed] [Google Scholar]
Avital 1992 {published data only}
- Avital A, Sanchez I, Chernick V. Efficacy of salbutamol and ipratropium bromide in decreasing bronchial hyperreactivity in children with cystic fibrosis. Pediatric Pulmonology 1992;13(1):34‐7. [] [DOI] [PubMed] [Google Scholar]
Azevedo 1990 {published data only}
- Azevedo M, Costa JT, Fontes P, Silva JP, Araujo O. Effect of terfenadine and ipratropium bromide on ultrasonically nebulized distilled water‐induced asthma. Journal of International Medical Research 1990;18(1):37‐49. [] [DOI] [PubMed] [Google Scholar]
Becker 1999 {published data only}
- Becker JM, Arora A, Scarfone RJ, Spector ND, Fontana‐Penn ME, Gracely E, et al. Oral versus intravenous corticosteroids in children hospitalized with asthma. Journal of Allergy and Clinical Immunology 1999;103(4):586‐90. [] [DOI] [PubMed] [Google Scholar]
Benito Fernandez 2000 {published data only}
- Benito Fernández J, Mintegui Raso S, Sánchez Echaniz J, Vázquez Ronco MA, Pijoan Zubizarreta JI, Benito Fernandez J, et al. [Efficacy of early administration of nebulized ipratropium bromide in children with asthmatic crisis]. [Spanish] [Eficacia de la administracion precoz de bromuro de ipratropio nebulizado en ninos con crisis asmatica]. Anales Espanoles de Pediatria 2000;53(3):217‐22. [; CN‐00372775] [PubMed] [Google Scholar]
Berger 2006 {published data only}
- Berger WE, Milgrom H, Skoner DP, Tripp K, Parsey MV, Baumgartner RA, et al. Evaluation of levalbuterol metered dose inhaler in pediatric patients with asthma: a double‐blind, randomized, placebo‐ and active‐controlled trial. Current Medical Research and Opinion 2006;22(6):1217‐26. [] [DOI] [PubMed] [Google Scholar]
Bigham 2010 {published data only}
- Bigham MT, Jacobs BR, Monaco MA, Brilli RJ, Wells D, Conway EM, et al. Helium/oxygen‐driven albuterol nebulization in the management of children with status asthmaticus: a randomized, placebo‐controlled trial. Pediatric Critical Care Medicine 2010;11(3):356‐61. [] [PubMed] [Google Scholar]
Boeree 1998 {published data only}
- Boeree MJ, Peters FTM, Postma DS, Kleibeuker JH. No effects of high‐dose omeprazole in patients with severe airway hyperresponsiveness and (a)symptomatic gastro‐oesophageal reflux. European Respiratory Journal 1998;11(5):1070‐4. [] [DOI] [PubMed] [Google Scholar]
Bogie 2007 {published data only}
- Bogie AL, Towne D, Luckett PM, Abramo TJ, Wiebe RA. Comparison of intravenous terbutaline versus normal saline in pediatric patients on continuous high‐dose nebulized albuterol for status asthmaticus. Pediatric Emergency Care 2007;23(6):355‐61. [] [DOI] [PubMed] [Google Scholar]
Bradshaw 2008 {published data only}
- Bradshaw TA, Matusiewicz SP, Crompton GK, Alastair Innes J, Greening AP. Intravenous magnesium sulphate provides no additive benefit to standard management in acute asthma. Respiratory Medicine 2008;102(1):143‐9. [] [DOI] [PubMed] [Google Scholar]
Brenner 1988 {published data only}
- Brenner M, Berkowitz R, Marshall N, Strunk RC. Need for theophylline in severe steroid‐requiring asthmatics. Clinical Allergy 1988;18(2):143‐50. [] [DOI] [PubMed] [Google Scholar]
Browne 2002 {published data only}
- Browne GJ, Trieu L, Asperen P. Randomized, double‐blind, placebo‐controlled trial of intravenous salbutamol and nebulized ipratropium bromide in early management of severe acute asthma in children presenting to an emergency department. Critical Care Medicine 2002;30(2):448‐53. [] [DOI] [PubMed] [Google Scholar]
Camargo 2010 {published data only}
- Camargo CA, Gurner DM, Smithline HA, Chapela R, Fabbri LM, Green SA, et al. A randomized placebo‐controlled study of intravenous montelukast for the treatment of acute asthma. Journal of Allergy and Clinical Immunology 2010;125(2):374‐80. [] [DOI] [PubMed] [Google Scholar]
Chen 2008 {published data only}
- Chen ZG, Li M, Chen H, Chen YF, Chen FH, Ji JZ. [Efficacy of pulmicort suspension plus salbutamol and ipratropium bromide for management of acute asthma exacerbation in children: a comparative study]. [Chinese]. Nan Fang Yi Ke Da Xue Xue Bao [Journal of Southern Medical University] 2008;28(3):470‐2. [] [PubMed] [Google Scholar]
Chen 2012 {published data only}
- Chen AH, Chen RC, Zhan JY, Huang S, Lin YN, Chen DH, et al. [The efficacy of nebulized budesonide in acute moderate to severe exacerbations of asthma in children]. [Chinese]. Tuberculosis and Respiratory Diseases 2012;35(4):269‐74. [] [PubMed] [Google Scholar]
Chowdhury 1995 {published data only}
- Chowdhury D, al Howasi M, Khalil M, al‐Frayh AS, Chowdhury S, Ramia S. The role of bronchodilators in the management of bronchiolitis: a clinical trial. Annals of Tropical Paediatrics 1995;15(1):77‐84. [] [DOI] [PubMed] [Google Scholar]
Coulthard 1985 {published data only}
- Coulthard K, Lines D, Rossi S, Cressi N. Inhaled ipratropium bromide (atrovent) in asthmatic children [abstract]. Australian Journal of Hospital Pharmacy 1985, (Suppl):52. [; CN‐00279610] [Google Scholar]
Cydulka 2010 {published data only}
- Cydulka RK, Emerman CL, Muni A. Levalbuterol versus levalbuterol plus ipratropium in the treatment of severe acute asthma. Journal of Asthma 2010;47(10):1094‐100. [] [DOI] [PubMed] [Google Scholar]
Dahlen 2012 {published data only}
- Dahlen B, Gomez FP, Casas A, Howarth PH, Dahlen SE, Rodriguez‐Roisin R. Salbutamol but not ipratropium abolishes leukotriene D4‐induced gas exchange abnormalities in asthma. European Journal of Clinical Pharmacology 2012;68(10):1375‐83. [] [DOI] [PubMed] [Google Scholar]
de Jong 1996 {published data only}
- Jong JW, Mark TW, Koëter GH, Postma DS. Rebound airway obstruction and responsiveness after cessation of terbutaline: effects of budesonide. American Journal of Respiratory and Critical Care Medicine 1996;153(1):70‐5. [] [DOI] [PubMed] [Google Scholar]
Douma 1998 {published data only}
- Douma WR, Kerstjens HA, Rooyackers JM, Koëter GH, Postma DS. Risk of over treatment with current peak flow criteria in self‐management plans. Dutch CNSLD Study Group. Chronic Non‐Specific Lung Disease. European Respiratory Journal 1998;12(4):848‐52 [CRS‐ID: 4900100000006167]. [DOI] [PubMed] [Google Scholar]
Ducharme 1998 {published data only}
- Ducharme FM, Davis GM. Randomized controlled trial of ipratropium bromide and frequent low doses of salbutamol in the management of mild and moderate acute pediatric asthma. Journal of Pediatrics 1998;133(4):479‐85. [] [DOI] [PubMed] [Google Scholar]
Dutt 1990 {published data only}
- Dutt NS, Reddy TC, Raghuveer TS, Yeshwanth M, Sunil Dutt N, Chinnapa Reddy T, et al. A comparative study of atropine sulphate, terbutaline and their combination in asthmatic children. Lung India 1990;8(2):73‐5. [; INDMED: TB687] [Google Scholar]
Freeman 1989A {published data only}
- Freeman J, Landau LI. The effects of ipratropium bromide and fenoterol nebulizer solutions in children with asthma. Clinical Pediatrics 1989;28(12):556‐60. [] [DOI] [PubMed] [Google Scholar]
Friberg 1989A {published data only}
- Friberg S, Graff‐Lonnevig V. Ipratropium bromide (Atrovent) in childhood asthma: a cumulative dose‐response study. Annals of Allergy 1989;62(2):131‐4. [] [PubMed] [Google Scholar]
Garcia 1998 {published data only}
- Garcia J, Roure P, Hayem C, Dupont D. [Bronchial endoscopy under local anesthesia and pain in children. The value of a nitrous oxide‐oxygen combination] [Endoscopie bronchique sous anesthésie locale et douleur chez l'enfant. Intérêt du mélange protoxyde d'azote‐oxygène]. Revue Des Maladies Respiratoires 1998;15(2):179‐83. [] [PubMed] [Google Scholar]
González 1989 {published data only}
- González R, Girardi G. [Therapy with ketotifen in breast‐fed infants with asthma] [Terapia con ketotifeno en el asma del lactante]. Boletin Medico Del Hospital Infantil de Mexico 1989;46(6):395‐8. [; CN‐00061291] [PubMed] [Google Scholar]
Goodacre 2013 {published data only}
- Goodacre S, Cohen J, Bradburn M, Gray A, Benger J, Coats T. Intravenous or nebulised magnesium sulphate versus standard therapy for severe acute asthma (3Mg trial): a double‐blind, randomised controlled trial. Lancet Respiratory Medicine 2013;1(4):293‐300. [] [DOI] [PubMed] [Google Scholar]
Gouin 1999 {published data only}
- Gouin S, Patel H. Utilization analysis of an observation unit for children with asthma [English]. Pediatric Emergency Care 1999;15(2):79‐83. [] [DOI] [PubMed] [Google Scholar]
Gove 1988 {published data only}
- Gove RI, Burge PS, Stableforth DE, Skinner C. The effects of ketotifen on beta‐adrenergic activity in asthmatics. European Journal of Clinical Pharmacology 1988;34(6):585‐9. [] [DOI] [PubMed] [Google Scholar]
Greenough 1986 {published data only}
- Greenough A, Yuksel B, Everett L, Price JF. Inhaled ipratropium bromide and terbutaline in asthmatic children. Respiratory Medicine 1993;87(2):111‐4. [] [DOI] [PubMed] [Google Scholar]
Groot 1994 {published data only}
- Groot CA, Lammers JW, Festen J, Herwaarden CL. The protective effects of ipratropium bromide and terbutaline on distilled water‐induced bronchoconstriction. Pulmonary Pharmacology 1994;7(1):59‐63. [] [DOI] [PubMed] [Google Scholar]
Haahtela 1991A {published data only}
- Haahtela T, Ahokas I, Ahonen A, Assendelft A, Havu M, Havu K, et al. Inhaled bronchodilators in asthma: low or high dose?. Annals of Allergy 1991;66(2):175‐80. [] [PubMed] [Google Scholar]
Hardasmalani 2005 {published data only}
- Hardasmalani MD, DeBari V, Bithoney WG, Gold N. Levalbuterol versus racemic albuterol in the treatment of acute exacerbation of asthma in children. Pediatric Emergency Care 2005;21(7):415‐9. [] [DOI] [PubMed] [Google Scholar]
Hayday 2002E {published data only}
- Hayday K, Stevermer JJ. In children hospitalized for asthma exacerbations, does adding ipratropium bromide to albuterol and corticosteroids improve outcome?. Journal of Family Pracitice 2002;51(3):280. [] [PubMed] [Google Scholar]
Henry 1989 {published data only}
- Henry RL, Hankin RG, Abramson R. Comparison of preservative‐free and preservative‐containing ipratropium bromide. Australian Paediatric Journal 1989;25(2):86‐8. [] [DOI] [PubMed] [Google Scholar]
Iramain 2011 {published data only}
- Iramain R, López‐Herce J, Coronel J, Spitters C, Guggiari J, Bogado N. Inhaled salbutamol plus ipratropium in moderate and severe asthma crises in children. Journal of Asthma 2011;48(3):298‐303. [] [DOI] [PubMed] [Google Scholar]
Israel 2004 {published data only}
- Israel E, Chinchilli VM, Ford JG, Boushey HA, Cherniack R, Craig TJ, et al. Use of regularly scheduled albuterol treatment in asthma: genotype‐stratified, randomised, placebo‐controlled cross‐over trial.[see comment]. Lancet 2004;364(9444):1505‐12. [] [DOI] [PubMed] [Google Scholar]
Jiang 2006 {published data only}
- Jiang W‐H, Deng L, Wen H‐H, Yu J‐L, Zeng Q. [Effects of salbutamol and ipratropium bromide inhalation on pulmonary function in young children with asthmatoid bronchitis]. [Chinese]. Zhongguo Dang Dai Er Ke Za Zhi [Chinese Journal of Contemporary Pediatrics] 2006;8(4):295‐7. [] [PubMed] [Google Scholar]
Kaptein 1993 {published data only}
- Kaptein AA, Brand PLP, Dekker FW, Kerstjens HAM, Postma DS, Sluiter HJ. Quality‐of‐life in a long‐term multicentre trial in chronic nonspecific lung disease: assessment at baseline. European Respiratory Journal 1993;6(10):1479‐84. [PubMed] [Google Scholar]
Kelso 2011 {published data only}
- Kelso JM. Effect of addition of single dose of oral montelukast to standard treatment in acute moderate to severe asthma in children between 5 and 15 years of age: a randomised, double‐blind, placebo controlled trial. Pediatrics 2011;128(Suppl 3):S128‐9. [] [DOI] [PubMed] [Google Scholar]
Kerstjens 1992 {published data only}
- Kerstjens HA, Brand PL, Hughes MD, Robinson NJ, Postma DS, Sluiter HJ, et al. A comparison of bronchodilator therapy with or without inhaled corticosteroid therapy for obstructive airways disease. Dutch Chronic Non‐Specific Lung Disease Study Group [see comments]. New England Journal of Medicine 1992;327(20):1413‐9. [] [DOI] [PubMed] [Google Scholar]
Kerstjens 1993 {published data only}
- Kerstjens HA, Brand PL, Quanjer PH, Bruggen‐Bogaarts BA, Koëter GH, Postma DS. Variability of bronchodilator response and effects of inhaled corticosteroid treatment in obstructive airways disease. Dutch CNSLD Study Group. Thorax 1993;48(7):722‐9. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kerstjens 1994 {published data only}
- Kerstjens HA, Brand PL, Jong PM, Koëter GH, Postma DS. Influence of treatment on peak expiratory flow and its relation to airway hyperresponsiveness and symptoms. The Dutch CNSLD Study Group. Thorax 1994;49(11):1109‐15. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kerstjens 1995 {published data only}
- Kerstjens HAM, Schouten JP, Brand PLP, Schoonbrood DFME, Sterk PJ, Postma DS, et al. Importance of total serum IgE for improvement in airways hyperresponsiveness with inhaled corticosteroids in asthma and chronic obstructive pulmonary disease. The Dutch CNSLD Study Group. American Journal of Respiratory and Critical Care Medicine 1995;151(2 Pt 1):360‐8. [; CN‐00365546] [DOI] [PubMed] [Google Scholar]
Knöpfli 2005 {published data only}
- Knöpfli BH, Bar‐Or O, Araújo CG. Effect of ipratropium bromide on EIB in children depends on vagal activity. Medicine and Science in Sports and Exercise 2005;37(3):354‐9. [] [DOI] [PubMed] [Google Scholar]
Lanes 1998 {published data only}
- Lanes SF, Garrett JE, Wentworth CE3, Fitzgerald JM, Karpel JP. The effect of adding ipratropium bromide to salbutamol in the treatment of acute asthma: a pooled analysis of three trials [English]. Chest 1998;114(2):365‐72. [] [DOI] [PubMed] [Google Scholar]
Lowry 1994 {published data only}
- Lowry R, Wood A, Higenbottam T. The effect of anticholinergic bronchodilator therapy on cough during upper respiratory tract infections. British Journal of Clinical Pharmacology 1994;37(2):187‐91. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Macias 2003A {published data only}
- Macias CG, Felner EI, Gan V, Macias CG, Felner EI, Gan V. Inhaled corticosteroids may be superior to systemic corticosteroids in children with moderate‐to‐severe acute asthma . Pediatric Asthma Allergy and Immunology 2003;16(3):121‐8. [] [Google Scholar]
Mallol 1987a {published data only}
- Mallol J, Barrueto L, Girardi G, Toro O. Bronchodilator effect of fenoterol and ipratropium bromide in infants with acute wheezing: use of MDI with a spacer device. Pediatric Pulmonology 1987;3(5):352‐6. [] [DOI] [PubMed] [Google Scholar]
Mallol 1987bA {published data only}
- Mallol J, Barrueto L, Girardi G, Muñoz R, Puppo H, Ulloa V, et al. Use of nebulized bronchodilators in infants under 1 year of age: analysis of four forms of therapy. Pediatric Pulmonology 1987;3(5):298‐303. [] [DOI] [PubMed] [Google Scholar]
Maneechotesuwan 2011 {published data only}
- Maneechotesuwan K, Suthamsmai T, Ratanasaenglert K, Pipopsuthipaiboon S. Bronchodilator effect of Ipraterol on methacholine‐induced bronchoconstriction in asthmatic patients. Journal of the Medical Association of Thailand 2011;94(Suppl 1):S66‐71. [] [PubMed] [Google Scholar]
McDowell 1998 {published data only}
- McDowell KM, Chatburn RL, Myers TR, O'Riordan MA, Kercsmar CM. A cost‐saving algorithm for children hospitalized for status asthmaticus. Archives of Pediatrics and Adolescent Medicine 1998;152(10):977‐84. [; CN‐00156088] [DOI] [PubMed] [Google Scholar]
Meier 1997 {published data only}
- Meier CR, Jick H. Drug use and pulmonary death rates in increasingly symptomatic asthma patients in the UK. Thorax 1997;52(7):612‐7. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mitchell 2005A {published data only}
- Mitchell EA, Didsbury PB, Kruithof N, Robinson E, Milmine M, Barry M, et al. A randomized controlled trial of an asthma clinical pathway for children in general practice. Acta Paediatrica 2005;94(2):226‐33. [] [DOI] [PubMed] [Google Scholar]
Morris 2010 {published data only}
- Morris CR, Becker AB, Piñieiro A, Massaad R, Green SA, Smugar SS, et al. A randomized, placebo‐controlled study of intravenous montelukast in children with acute asthma. Annals of Allergy, Asthma and Immunology 2010;104(2):161‐71. [] [DOI] [PubMed] [Google Scholar]
Morrison 1989 {published data only}
- Morrison JF, Pearson SB. The effect of the circadian rhythm of vagal activity on bronchomotor tone in asthma. British Journal of Clinical Pharmacology 1989;28(5):545‐9. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nakano 2000 {published data only}
- Nakano Y, Enomoto N, Kawamoto A, Hirai R, Chida K. Efficacy of adding multiple doses of oxitropium bromide to salbutamol delivered by means of a metered‐dose inhaler with a spacer device in adults with acute severe asthma. Journal of Allergy and Clinical Immunology 2000;106(3):472‐8. [] [DOI] [PubMed] [Google Scholar]
Newnham 1995A {published data only}
- Newnham DM, Grove A, McDevitt DG, Lipworth BJ. Subsensitivity of bronchodilator and systemic beta 2 adrenoceptor responses after regular twice daily treatment with eformoterol dry powder in asthmatic patients. Thorax 1995;50(5):497‐504. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nibhanipudi 2009 {published data only}
- Nibhanipudi K, Hassen GW, Smith A. Beneficial effects of warmed humidified oxygen combined with nebulized albuterol and ipratropium in pediatric patients with acute exacerbation of asthma in winter months. Journal of Emergency Medicine 2009;37(4):446‐50. [] [DOI] [PubMed] [Google Scholar]
O'Driscoll 1989B {published data only}
- O'Driscoll BR, Taylor RJ, Horsley MG, Chambers DK, Bernstein A. Nebulised salbutamol with and without ipratropium bromide in acute airflow obstruction. Lancet 1989;1(8652):1418‐20. [] [DOI] [PubMed] [Google Scholar]
Overbeek 1996 {published data only}
- Overbeek SE, Kerstjens HA, Bogaard JM, Mulder PG, Postma DS. Is delayed introduction of inhaled corticosteroids harmful in patients with obstructive airways disease (asthma and COPD)? The Dutch CNSLD Study Group. The Dutch Chronic Nonspecific Lung Disease Study Groups. Chest 1996;110(1):35‐41 [CRS‐ID: 4900100000005270]. [DOI] [PubMed] [Google Scholar]
Parkin 1995 {published data only}
- Parkin PC, Saunders NR, Diamond SA, Winders PM, Macarthur C. Randomised trial spacer v nebuliser for acute asthma. Archives of Disease in Childhood 1995;72(3):239‐40. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peters 2000 {published data only}
- Peters JI, Shelledy DC, Jones APJ, Lawson RW, Davis CP, LeGrand TS. A randomized, placebo‐controlled study to evaluate the role of salmeterol in the in‐hospital management of asthma. Chest 2000;118(2):313‐20. [] [DOI] [PubMed] [Google Scholar]
Ponce 2009 {published data only}
- Ponce Castro H, Rodríguez Gaytán R, Rodríguez Orozco AR. Efficacy of two methods of administration of salbutamol‐ipratropium bromide in asthma crises [Eficacia de dos métodos de administración de salbutamol‐bromuro de ipratropio en crisis asmática]. Revista Alergia Mexico (Tecamachalco, Puebla, Mexico : 1993) 2009;56(5):149‐53. [] [PubMed] [Google Scholar]
Powell 2012a {published data only}
- Powell CVE. A randomised, double blind, placebo controlled study of nebulised magnesium sulphate in acute asthma in children—the magnetic study [Abstract]. Archives of Disease in Childhood 2012;97(Suppl 1):A2‐3. [] [Google Scholar]
Powell 2013 {published data only}
- Powell C, Kolamunnage‐Dona R, Lowe J, Boland A, Petrou S, Doull I, et al. Magnesium sulphate in acute severe asthma in children (MAGNETIC): a randomised, placebo‐controlled trial. Lancet Respiratory Medicine 2013;1(4):301‐8. [] [DOI] [PubMed] [Google Scholar]
Qureshi 1997 {published data only}
- Qureshi FA, Zaritsky A, Lakkis H. Efficacy of nebulized ipratropium in severely asthmatic children. Annals of Emergency Medicine 1997;29(2):205‐11. [; CN‐00136296] [DOI] [PubMed] [Google Scholar]
Qureshi 1998 {published data only}
- Qureshi F, Pestian J, Davis P, Zaritsky A. Effect of nebulized ipratropium on the hospitalization rates of children with asthma. New England Journal of Medicine 1998;339(15):1030‐5. [; CN‐00155153] [DOI] [PubMed] [Google Scholar]
Qureshi 2001 {published data only}
- Qureshi F, Zaritsky A, Poirier MP, Saritsky A, Poirier M. Comparative efficacy of oral dexamethasone versus oral prednisone in acute pediatric asthma. Journal of Pediatrics 2001;139(1):20‐6. [] [DOI] [PubMed] [Google Scholar]
Qureshi 2005 {published data only}
- Qureshi F, Zaritsky A, Welch C, Meadows T, Burke BL. Clinical efficacy of racemic albuterol versus levalbuterol for the treatment of acute pediatric asthma. Annals of Emergency Medicine 2005;46(1):29‐36. [] [DOI] [PubMed] [Google Scholar]
Raes 1989 {published data only}
- Raes M, Mulder P, Kerrebijn KF. Long‐term effect of ipratropium bromide and fenoterol on the bronchial hyperresponsiveness to histamine in children with asthma. Journal of Allergy and Clinical Immunology 1989;84(6 Pt 1):874‐9. [] [DOI] [PubMed] [Google Scholar]
Raissy 2006 {published data only}
- Raissy HH, Davies L, Marshik P, Kelly HW. Inspiratory flow through dry‐powder inhalers (DPIs) in asthmatic children 2 to 12 years old. Pediatric Asthma, Allergy and Immunology 2006;19(4):223‐30. [] [Google Scholar]
Ralston 2005 {published data only}
- Ralston ME, Euwema MS, Knecht KR, Ziolkowski TJ, Coakley TA, Cline SM. Comparison of levalbuterol and racemic albuterol combined with ipratropium bromide in acute pediatric asthma: a randomized controlled trial. Journal of Emergency Medicine 2005;29(1):29‐35. [] [DOI] [PubMed] [Google Scholar]
Ream 2001 {published data only}
- Ream RS, Loftis LL, Albers GM, Becker BA, Lynch RE, Mink RB. Efficacy of IV theophylline in children with severe status asthmaticus. Chest 2001;119(5):1480‐8. [] [DOI] [PubMed] [Google Scholar]
Reisman 1988A {published data only}
- Reisman J, Galdes‐Sebaldt M, Kazim F, Canny G, Levison H. Frequent administration by inhalation of salbutamol and ipratropium bromide in the initial management of severe acute asthma in children. Journal of Allergy and Clinical Immunology 1988;81(1):16‐20. [] [DOI] [PubMed] [Google Scholar]
Richards 1987 {published data only}
- Richards R, Tattersfield AE. Comparison of the airway response to eye drops of timolol and its isomer L‐714,465 in asthmatic subjects. British Journal of Clinical Pharmacology 1987;24(4):485‐91. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Roberts 2003 {published data only}
- Roberts G, Newsom D, Gomez K, Raffles A, Saglani S, Begent J, et al. Intravenous salbutamol bolus compared with an aminophylline infusion in children with severe asthma: a randomised controlled trial. Thorax 2003;58(4):306‐10. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rodriguez 2008 {published data only}
- Rodriguez E, Vera V, Perez‐Puigbo A, Capriles‐Hulett A, Ferro S, Manrique J, et al. Equivalence of a single saline nebulised dose of formoterol powder vs three doses of nebulised albuterol every twenty minutes in acute asthma in children: a suitable cost effective approach for developing nations. Allergologia Et Immunopathologia 2008;36(4):196‐200. [] [DOI] [PubMed] [Google Scholar]
Rowe 2007 {published data only}
- Rowe BH, Wong E, Blitz S, Diner B, Mackey D, Ross S, et al. Adding long‐acting beta‐agonists to inhaled corticosteroids after discharge from the emergency department for acute asthma: a randomized controlled trial. Academic Emergency Medicine 2007;14(10):833‐40. [] [DOI] [PubMed] [Google Scholar]
Salmun 1999 {published data only}
- Salmun LM, Barlan I, Wolf HM, Eibl M, Twarog FJ, Geha RS, et al. Effect of intravenous immunoglobulin on steroid consumption in patients with severe asthma: a double‐blind, placebo‐controlled, randomized trial. Journal of Allergy and Clinical Immunology 1999;103(5 Pt 1):810‐5. [] [DOI] [PubMed] [Google Scholar]
Sano 2000 {published data only}
- Sano F, Cortez GK, Solé D, Naspitz CK. Inhaled budesonide for the treatment of acute wheezing and dyspnea in children up to 24 months old receiving intravenous hydrocortisone. Journal of Allergy and Clinical Immunology 2000;105(4):699‐703. [; CN‐00277091] [DOI] [PubMed] [Google Scholar]
Schuh 1992 {published data only}
- Schuh S, Johnson D, Canny G, Reisman J, Shields M, Kovesi T, et al. Efficacy of adding nebulized ipratropium bromide to nebulized albuterol therapy in acute bronchiolitis. Pediatrics 1992;90(6):920‐3. [] [PubMed] [Google Scholar]
Schuh 1995 {published data only}
- Schuh S, Johnson DW, Callahan S, Canny G, Levison H. Efficacy of frequent nebulized ipratropium bromide added to frequent high‐dose albuterol therapy in severe childhood asthma. Journal of Pediatrics 1995;126(4):639‐45. [] [DOI] [PubMed] [Google Scholar]
Schuh 1997 {published data only}
- Schuh S, Johnson D, Stephens D, Callahan S, Canny G. Hospitalization patterns in severe acute asthma in children. Pediatric Pulmonology 1997;23(3):184‐92. [; CN‐00138364] [DOI] [PubMed] [Google Scholar]
Self 2002 {published data only}
- Self TH, Redmond AM, Nguyen WT. Reassessment of theophylline use for severe asthma exacerbation: is it justified in critically ill hospitalized patients?. Journal of Asthma 2002;39(8):677‐86. [] [DOI] [PubMed] [Google Scholar]
Sengul 2013 {published data only}
- Sengul Gokalp A, Bicer S, Siraneci R. Efficacy of nebulized ipratropium bromide in the treatment of acute asthma in children: randomised, double blind, placebo‐controlled trial [Cocukluk cagi akut astim atagi tedavisinde nebulize ipratropium bromurun etkinlgi: cift kor randomize kontrollu calisma]. Nobel Medicus 2013;9(1):67‐75. [] [Google Scholar]
Sienra Monge 2000 {published data only}
- Sienra Monge JJ, Bermejo Guevara MA, Rio Navarro BE, Rosas Vargas MA, Reyes Ruiz NI, Rio Navarro BE. [Degree and duration of bronchodilatation with an agonist beta 2 administered alone versus an agonist beta 2 administered with ipratropium bromide in children with acute asthma] [Grado y duracion de la bronchodilatatacion mediante la administracion de un agonista beta 2 solo vs un agonista beta 2 mas bromuro de ipratropio en ninos con asma aguda]. Revista Alergia Mexico (Tecamachalco, Puebla, Mexico : 1993) 2000;47(1):26‐9. [; CN‐00423875] [PubMed] [Google Scholar]
Silverman 2012 {published data only}
- Silverman RA, Foley F, Dalipi R, Kline M, Lesser M. The use of rhDNAse in severely ill, non‐intubated adult. Respiratory Medicine 2012;106(8):1096‐102. [] [DOI] [PubMed] [Google Scholar]
Singh 2008 {published data only}
- Singh AK, Gaur S, Kumar R. A randomized controlled trial of intravenous magnesium sulphate as an adjunct to standard therapy in acute severe asthma. Iranian Journal of Allergy, Asthma, and Immunology 2008;7(4):221‐9. [] [PubMed] [Google Scholar]
Singhi 2010 {published data only}
- Singhi S, Bansal A, Chopra K, Grover S. Randomized comparison of magnesium sulfate, terbutaline and aminophylline infusion in acute severe asthma in children [Abstract]. Critical Care Medicine 2010;38(12 Suppl):Poster 371. [] [Google Scholar]
Sly 1987 {published data only}
- Sly PD, Landau LI, Olinsky A. Failure of ipratropium bromide to modify the diurnal variation of asthma in asthmatic children. Thorax 1987;42(5):357‐60. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stewart 2012 {published data only}
- Stewart SL, Martin AL, Davis BE, Cockcroft DW. Salbutamol tolerance to bronchoprotection: course of onset. Annals of Allergy, Asthma and Immunology 2012;109(6):454‐7. [] [DOI] [PubMed] [Google Scholar]
Stormon 1999 {published data only}
- Stormon MO, Mellis CM, Asperen PP, Kilham HA. Outcome evaluation of early discharge of asthmatic children from hospital: a randomized control trial. Journal of Quality in Clinical Practice 1999;19(3):149‐54. [] [DOI] [PubMed] [Google Scholar]
Sur 1990 {published data only}
- Sur S, Mohiuddin AA, Vichyanond P, Nelson HS. A random double‐blind trial of the combination of nebulized atropine methylnitrate and albuterol in nocturnal asthma. Annals of Allergy 1990;65(5):384‐8. [] [PubMed] [Google Scholar]
Tasche 1997 {published data only}
- Tasche MJ, Wouden JC, Uijen JH, Ponsioen BP, Bernsen RM, Suijlekom‐Smit LW, et al. Randomised placebo‐controlled trial of inhaled sodium cromoglycate in 1‐4‐year‐old children with moderate asthma. Lancet 1997;350(9084):1060‐4. [] [DOI] [PubMed] [Google Scholar]
Taytard 1987 {published data only}
- Taytard A, Vergeret J, Guenard H, Vaida P, Bellvert P, Freour P. Prevention of exercise‐induced asthma by oxitropium bromide. European Journal of Clinical Pharmacology 1987;33(5):455‐8. [] [DOI] [PubMed] [Google Scholar]
Timsit 2002 {published data only}
- Timsit S, Sannier N, Bocquet N, Cojocaru B, Wille C, Boursiquot C, et al. Benefit of ipratropium bromide for the treatment of childhood asthma in the emergency department [Apport du bromure d'ipratropium dans la prise en charge des crises d'asthme aux urgences]. Archives de Pediatrie 2002;9(2):117‐24. [; CN‐00442961] [DOI] [PubMed] [Google Scholar]
Ulrik 1992 {published data only}
- Ulrik CS, Backer V, Bach Mortensen N. Bronchodilating effect of ipratropium bromide inhalation powder and aerosol in children and adolescents with stable bronchial asthma. Allergy 1992;47(2 Pt 2):133‐7. [] [DOI] [PubMed] [Google Scholar]
UNKNOWN 2011 {published data only}
- Unknnown. Nebulized budesonide added to standard therapy does not improve asthma outcomes. Journal of the National Medical Association 2011;103(9‐10):1005. [] [Google Scholar]
Upham 2011A {published data only}
- Upham BD, Mollen CJ, Scarfone RJ, Seiden J, Chew A, Zorc JJ. Nebulized budesonide added to standard pediatric emergency department treatment of acute asthma: a randomized, double‐blind trial. Academic Emergency Medicine 2011;18(7):665‐73. [] [DOI] [PubMed] [Google Scholar]
Van Asperen 1988 {published data only}
- Asperen PP, Manglick P, Allen H. Mechanisms of bronchial hyperreactivity in cystic fibrosis. Pediatric Pulmonology 1988;5(3):139‐44. [] [DOI] [PubMed] [Google Scholar]
van der Woude 2001A {published data only}
- Woude HJ, Winter TH, Aalbers R. Decreased bronchodilating effect of salbutamol in relieving methacholine induced moderate to severe bronchoconstriction during high dose treatment with long acting beta2 agonists. Thorax 2001;56(7):529‐35. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Von Berg 2004 {published data only}
- Berg A, Jeena PM, Soemantri PA, Vertruyen A, Schmidt P, Gerken F, et al. Efficacy and safety of ipratropium bromide plus fenoterol inhaled via Respimat Soft Mist Inhaler vs. a conventional metered dose inhaler plus spacer in children with asthma. Pediatric Pulmonology 2004;37(3):264‐72. [] [DOI] [PubMed] [Google Scholar]
Ward 1981 {published data only}
- Ward MJ, Fentem PH, Smith WH, Davies D. Ipratropium bromide in acute asthma. British Medical Journal (Clinical Research Ed.) 1981;282(6264):598‐600. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ward 1985 {published data only}
- Ward MJ, Macfarlane JT, Davies D. A place for ipratropium bromide in the treatment of severe acute asthma. British Journal of Diseases of the Chest 1985;79(4):374‐8. [] [DOI] [PubMed] [Google Scholar]
Watanasomsiri 2006 {published data only}
- Watanasomsiri A, Phipatanakul W. Comparison of nebulized ipratropium bromide with salbutamol vs salbutamol alone in acute asthma exacerbation in children. Annals of Allergy, Asthma and Immunology 2006;96(5):701‐6. [] [DOI] [PubMed] [Google Scholar]
Watson 1994 {published data only}
- Watson WT, Shuckett EP, Becker AB, Simons FE. Effect of nebulized ipratropium bromide on intraocular pressures in children. Chest 1994;105(5):1439‐41. [] [DOI] [PubMed] [Google Scholar]
Wilson 1987 {published data only}
- Wilson NM, Green S, Coe C, Barnes PJ. Duration of protection by oxitropium bromide against cholinergic challenge. European Journal of Respiratory Diseases 1987;71(5):455‐8. [] [PubMed] [Google Scholar]
Wolstenholme 1989 {published data only}
- Wolstenholme RJ, Shettar SP. Comparison of a combination of fenoterol with ipratropium bromide (Duovent) and salbutamol in young adults with nocturnal asthma. Respiration; International Review of Thoracic Diseases 1989;55(3):152‐7. [] [DOI] [PubMed] [Google Scholar]
Worth 2012 {published data only}
- Worth H, Dethlefsen U. Patients with asthma benefit from concomitant therapy with cineole: a placebo‐controlled, double‐blind trial. Journal of Asthma 2012;49(8):849‐53. [] [DOI] [PubMed] [Google Scholar]
Yang 1993 {published data only}
- Yang SC, Lee TS, Chen GC. [Effects of ipratropium bromide as a nebulized solution on respiratory function in mechanically ventilated patients]. Journal of the Formosan Medical Association 1993;92 Suppl 1:S19‐24. [] [PubMed] [Google Scholar]
Young 1991 {published data only}
- Young GP, Freitas P. A randomized comparison of atropine and metaproterenol inhalational therapies for refractory status asthmaticus.[erratum appears in Ann Emerg Med 1991 Sep;20(9):1031]. Annals of Emergency Medicine 1991;20(5):513‐9. [; CN‐00075095] [DOI] [PubMed] [Google Scholar]
Youngchaiyud 1989 {published data only}
- Youngchaiyud P, Charoenratanakul S, Suthamsmai T, Sriwatanakul K. Comparison of fenoterol, ipratropium bromide and their combination in asthma. Siriraj Hospital Gazette 1989;41(4):197‐201. [] [Google Scholar]
Yung 1998 {published data only}
- Yung M, South M. Randomised controlled trial of aminophylline for severe acute asthma. Archives of Disease in Childhood 1998;79(5):405‐10. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zaritsky 1999A {published data only}
- Zaritsky A, Qureshi F, Zaritsky A, Qureshi F. Ipratropium does indeed reduce admissions to hospital with severe asthma. BMJ (Clinical Research Ed.) 1999;318(7185):738. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zorc 1999 {published data only}
- Zorc JJ, Pusic MV, Ogborn CJ, Lebet R, Duggan AK. Ipratropium bromide added to asthma treatment in the pediatric emergency department. Pediatrics 1999;103(4 Pt 1):748‐52. [] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Powell 2012 {published data only}
- Powell C. MAGnesium NEbuliser Trial In Children (MAGNETIC). NIHR Health Technology Assessment Programme 2012. []
Additional references
Asher 2010
- Asher MI. Recent perspectives on global epidemiology of asthma in childhood. Allergology and Immunolopathology 2010;38(2):83‐7. [DOI] [PubMed] [Google Scholar]
Camargo 2003
- Camargo CA Jr, Emond SD, Boulet L, Gibson PG, Kolbe J, Wagner CW, et al. Emergency department‐asthma plan. Developed at: Asthma Education in the Adult Emergency Department: A Multidisciplinary Consensus Conference. New York, 2001.
Chapman 1996
- Chapman K. An international perspective on anticholinergic therapy. American Journal of Medicine 1996;100:2‐4S. [DOI] [PubMed] [Google Scholar]
Cunningham 2008
- Cunningham S, Logan C, Lockerbie L, Dunn MJG, McMurry A, Prescott RJ. Effect of an Integrated Care Pathway on Acute Asthma/Wheezein Children Attending Hospital: Cluster Randomized Trial. J Pediatr 2008;152:315‐20. [DOI] [PubMed] [Google Scholar]
GINA 2014
- Global Initiative for Asthma. From the Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma (GINA) 2014. http://www.ginasthma.org/local/uploads/files/GINA_Report_2014.pdf (accessed 8 January 2014).
Griffiths 2013
- Griffiths B, Ducharme FM. Combined inhaled anticholinergics and short‐acting beta2‐agonists for initial treatment of acute asthma in children. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD000060.pub2] [DOI] [PubMed] [Google Scholar]
Gross 1988
- Gross NJ. Ipratropium bromide. New England Journal of Medicine 1988;8:486–94. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated September 2011]. The Cochrane Collaboration. www.cochrane‐handbook.org, 2011. [Google Scholar]
Karpel 1997
- Karpel JP, Aldrich TK, Prezant DJ, Guguchev K, Gaitan‐Salas A, Pathiparti R. Emergency treatment of acute asthma with albuterol metered‐dose inhaler plus holding chamber: how often should treatments be administered?. Chest 1997;112(2):348‐56. [DOI] [PubMed] [Google Scholar]
Lai 2009
- Lai CKW, Beasley R, Crane J, Foliaki S, Shah J, Weiland S, the ISAAC Phase Three Study Group. Global variation in the prevalence and severity of asthma symptoms: Phase Three of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax 2009;64:476‐83. [DOI] [PubMed] [Google Scholar]
Lim 2000
- Lim TK, Chin NK, Lee KH, Stebbings AM. Early discharge of patients hospitalizedwith acute asthma: a controlled study. RESPIRATORY MEDICINE 2000;94:1234‐1240. [DOI] [PubMed] [Google Scholar]
Lord 1999
- Lord J, Ducharme FM, Stamp RL, Littlejohns P, Churchill R. Cost effectiveness analysis of inhaled anticholinergics for acute childhood and adolescent asthma. BMJ 1999;319:1470‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
NAEPP 2011
- National Asthma education and Prevention Program. NAEPP Expert Panel Report Guidelines for the Diagnosis and Management of Asthma. http://www.nhlbi.nih.gov/guidelines/asthma/ (accessed 8 January 2014).
Ortiz‐Alvarez 2012
- Ortiz‐Alvarez O, Mikrogianakis A, Canadian Paediatric Society, Acute Care Committee. Managing the paediatric patient with an acute asthma exacerbation. Paediatr Child Health 2012;17(5):251‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Plotnick 2000
- Plotnick L, Ducharme F. Combined inhaled anticholinergics and beta2‐agonists for initial treatment of acute asthma in children. Cochrane Database of Systematic Reviews 2000, Issue 3. [DOI: 10.1002/14651858.CD000060] [DOI] [PubMed] [Google Scholar]
Rechelefsky 2003
- Rachelefsky G. Treating exacerbations of asthma in children: the role of systemic corticosteroids. Pediatrics 2003;112(2):382‐97. [DOI] [PubMed] [Google Scholar]
RevMan 5.2 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Rowe 2001
- Rowe BH, Spooner C, Ducharme F, Bretzlaff J, Bota G. Early emergency department treatment of acute asthma with systemic corticosteroids. Cochrane Database of Systematic Reviews 2001, Issue 1. [DOI: 10.1002/14651858.CD002178] [DOI] [PubMed] [Google Scholar]
Rowe 2004
- Rowe BH, Edmonds ML, Spooner CH, Diner B, Camargo CA Jr. Corticosteroid therapy for acute asthma. Respiratory Medicine 2004;98(4):275‐84. [DOI] [PubMed] [Google Scholar]
Sears 1992
- Sears MR. Clinical application of beta‐agonists. Practical Allergy and Immunology 1992;7:98‐100. [Google Scholar]
Silverman 1990
- Silverman M. The role of anticholinergic antimuscarinic bronchodilator therapy in children. Lung 1990;168:304‐9. [DOI] [PubMed] [Google Scholar]
Smith 2003
- SmithM, Iqbal SMSI, Rowe BH, N’Diaye T. Corticosteroids for hospitalised children with acute asthma. Cochrane Database of Systematic Reviews 2003, Issue 110.1002/14651858.CD002886.. [DOI: 10.1002/14651858.CD002886] [DOI] [PMC free article] [PubMed] [Google Scholar]
Svedmyr 1985
- Svedmyr N. A beta2‐adrenergic agonist for use in asthma pharmacology, pharmacokinetics, clinical efficacy and adverse effects. Pharmacotherapy 1985;5:109‐26. [DOI] [PubMed] [Google Scholar]


