Abstract
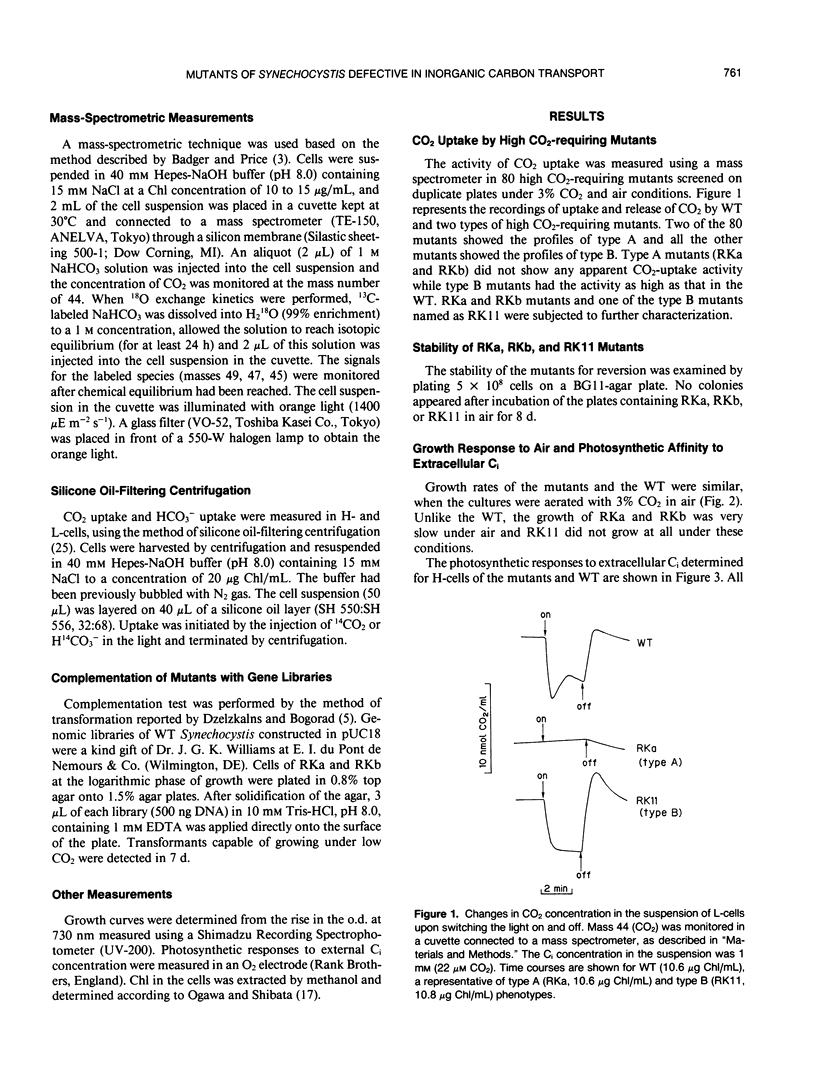
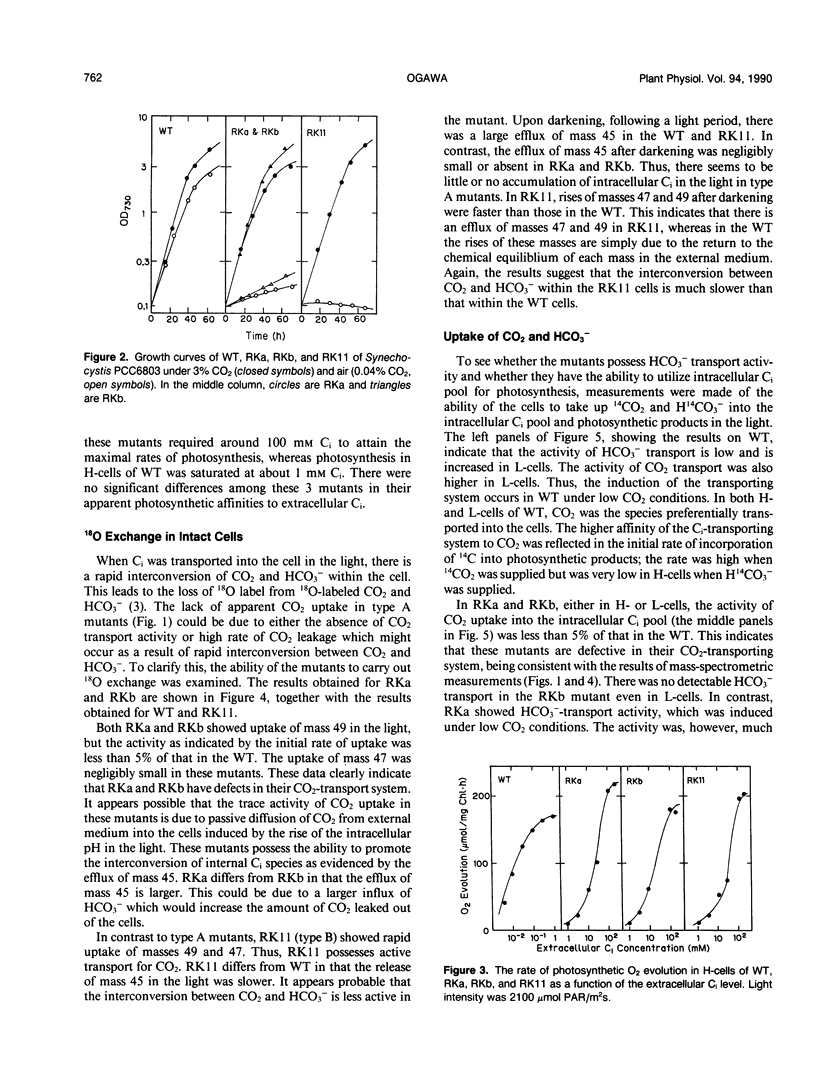
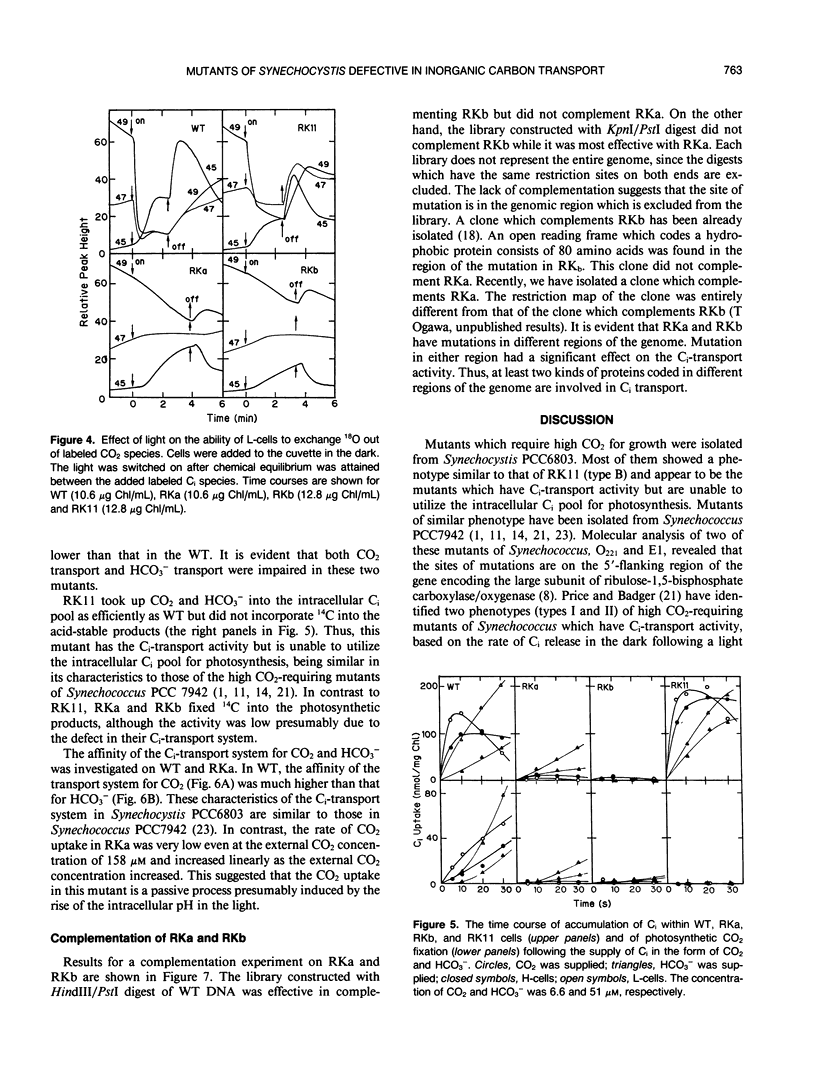
Eighty mutants of Synechocystis PCC6803 that require high CO2 for growth were examined with a mass spectrometer for their ability to take up CO2 in the light. Two of these mutants (type A) did not show any CO2 uptake while the rest of the mutants (type B) took up CO2 actively. Type A mutants (RKa and RKb) and one type B mutant (RK11) were partially characterized. At 3% CO2, growth rates of the mutants and the wild type (WT) were similar. Under air levels of CO2, growth of RKa and RKb was very slow, and RK11 did not grow at all. The photosynthetic affinities for inorganic carbon (Ci) in these three mutants were about 100 times lower than the affinity in WT. The following characteristics of type A mutants indicated that the mutants have a defect in their CO2-transport system: (a) the activity of 13C18O2 uptake in RKa and RKb in the light was less than 5% the activity in WT, and (b) each mutant had only a low level of activity of 14CO2 uptake as measured by the method of silicone oil-filtering centrifugation. The HCO3−-transport system was also impaired in these mutants. The activity of H14CO3− uptake was negligibly low in RKb and was one-third the activity of WT in RKa. On the other hand, the type B mutant, RK11, transported CO2 and HCO3− into the intracellular Ci pool as actively as WT but was unable to utilize it for photosynthesis. Complementation analysis of type A mutants indicated that RKa and RKb have mutations in different regions of the genome. These results suggested that at least two kinds of proteins are involved in the Ci-transport system.
Full text
PDF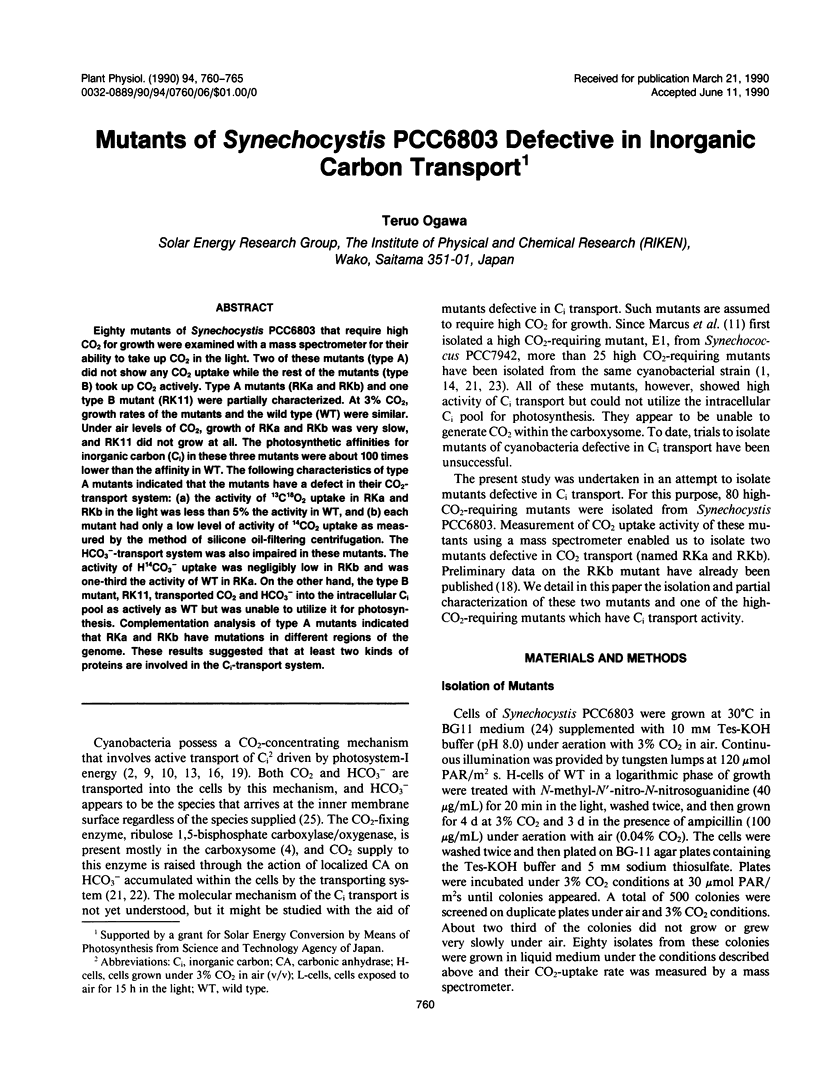

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badger M. R., Price G. D. Carbonic Anhydrase Activity Associated with the Cyanobacterium Synechococcus PCC7942. Plant Physiol. 1989 Jan;89(1):51–60. doi: 10.1104/pp.89.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzelzkalns V. A., Bogorad L. Molecular analysis of a mutant defective in photosynthetic oxygen evolution and isolation of a complementing clone by a novel screening procedure. EMBO J. 1988 Feb;7(2):333–338. doi: 10.1002/j.1460-2075.1988.tb02817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espie G. S., Miller A. G., Birch D. G., Canvin D. T. Simultaneous Transport of CO(2) and HCO(3) by the Cyanobacterium Synechococcus UTEX 625. Plant Physiol. 1988 Jul;87(3):551–554. doi: 10.1104/pp.87.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espie G. S., Miller A. G., Canvin D. T. Characterization of the na-requirement in cyanobacterial photosynthesis. Plant Physiol. 1988 Nov;88(3):757–763. doi: 10.1104/pp.88.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg D., Kaplan A., Ariel R., Kessel M., Seijffers J. The 5'-flanking region of the gene encoding the large subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase is crucial for growth of the cyanobacterium Synechococcus sp. strain PCC 7942 at the level of CO2 in air. J Bacteriol. 1989 Nov;171(11):6069–6076. doi: 10.1128/jb.171.11.6069-6076.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus Y., Schwarz R., Friedberg D., Kaplan A. High CO(2) Requiring Mutant of Anacystis nidulans R(2). Plant Physiol. 1986 Oct;82(2):610–612. doi: 10.1104/pp.82.2.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. G., Colman B. Active transport and accumulation of bicarbonate by a unicellular cyanobacterium. J Bacteriol. 1980 Sep;143(3):1253–1259. doi: 10.1128/jb.143.3.1253-1259.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T., Kaneda T., Omata T. A Mutant of Synechococcus PCC7942 Incapable of Adapting to Low CO(2) Concentration. Plant Physiol. 1987 Jul;84(3):711–715. doi: 10.1104/pp.84.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T., Kaplan A. The Stoichiometry between CO(2) and H Fluxes Involved in the Transport of Inorganic Carbon in Cyanobacteria. Plant Physiol. 1987 Apr;83(4):888–891. doi: 10.1104/pp.83.4.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price G. D., Badger M. R. Ethoxyzolamide Inhibition of CO(2) Uptake in the Cyanobacterium Synechococcus PCC7942 without Apparent Inhibition of Internal Carbonic Anhydrase Activity. Plant Physiol. 1989 Jan;89(1):37–43. doi: 10.1104/pp.89.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price G. D., Badger M. R. Isolation and Characterization of High CO(2)-Requiring-Mutants of the Cyanobacterium Synechococcus PCC7942 : Two Phenotypes that Accumulate Inorganic Carbon but Are Apparently Unable to Generate CO(2) within the Carboxysome. Plant Physiol. 1989 Oct;91(2):514–525. doi: 10.1104/pp.91.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz R., Friedberg D., Kaplan A. Is there a role for the 42 kilodalton polypeptide in inorganic carbon uptake by cyanobacteria? Plant Physiol. 1988 Oct;88(2):284–288. doi: 10.1104/pp.88.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Kunisawa R., Mandel M., Cohen-Bazire G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol Rev. 1971 Jun;35(2):171–205. doi: 10.1128/br.35.2.171-205.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volokita M., Zenvirth D., Kaplan A., Reinhold L. Nature of the Inorganic Carbon Species Actively Taken Up by the Cyanobacterium Anabaena variabilis. Plant Physiol. 1984 Nov;76(3):599–602. doi: 10.1104/pp.76.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]


