Abstract
Screening and testing of potential endocrine-disrupting chemicals for ecological effects is an example of a risk assessment/regulatory activity that can employ adverse outcome pathways (AOPs) to establish linkages between readily measured alterations in endocrine function and whole organism- and population-level responses. Of particular concern are processes controlled by the hypothalamic-pituitary-gonadal/thyroidal axes (HPG/T). However, the availability of AOPs suitable to meet this need is currently limited in terms of species and life-stage representation in the context of the diversity of endpoints influenced by HPG/T function. In this report we describe two novel AOPs that comprise a simple AOP network focused on the effects of chemicals on sex differentiation during early development in fish. The first AOP (346) documents events starting with inhibition of cytochrome P450 aromatase (CYP19), resulting in decreased availability of 17β-estradiol during gonad differentiation, which increases the occurrence of testis formation, resulting in a male-biased sex ratio and consequent population-level declines. The second AOP (376) is initiated by activation of the androgen receptor (AR), also during sexual differentiation, again resulting in a male-biased sex ratio and population-level effects. Both AOPs are strongly supported by existing physiological and toxicological evidence, including numerous fish studies with model CYP19 inhibitors and AR agonists. Accordingly, AOPs 346 and 376 provide a basis for more focused screening and testing of chemicals with the potential to affect HPG function in fish during early development.
Keywords: Adverse Outcome Pathway, Endocrine Effects, Fish, Development, Risk Assessment
Introduction and Background
For almost three decades endocrine-disrupting chemicals (EDCs) have been of concern in both the lay and scientific communities relative to potential effects on human health and the environment (Colborn et al. 1996; Hotchkiss et al. 2008). There have been legislated mandates throughout the world to conduct hazard or risk assessments and institute regulatory controls for chemicals that could adversely affect aspects of development and reproduction by perturbing endocrine function controlled through the hypothalamic-pituitary-gonadal and thyroidal (HPG/T) axes (Hotchkiss et al. 2008). The emphasis on chemicals that cause adverse effects through alterations of specific pathways represents a notable conceptual change for ecological risk assessments, which historically have largely focused on the occurrence of unacceptable apical effects as opposed to their etiology. This emphasis on toxic mechanism(s) has resulted in the development of a relatively extensive suite of assays/tools designed to detect perturbation of HPG/T axes in ecologically relevant species, typically through biochemical, physiological, and histological measurements made using in vitro or in vivo assays (Coady et al. 2017). While many of these types of assays can be remarkably sensitive and efficient in determining whether a chemical is endocrine-active, they typically do not simultaneously collect data that directly pertain to endpoints needed to conduct ecological risk assessments, specifically, changes in development and/or reproduction germane to predicting population-level impacts (Matthiessen et al. 2017).
Development of the adverse outcome pathway (AOP) framework was fueled by the need to establish transparent, causal associations in responses across biological levels of organization, linking defined molecular initiating events (MIEs) to potential apical effects meaningful to risk assessment (Ankley et al. 2010). Consequently, AOPs directly address the challenge faced by scientists and risk assessors seeking to make the connection between chemically induced changes in endocrine function and population-relevant effects. In fact, it was this need that fueled the identification and development of many early AOPs for both human health and ecological effects. For example, of the 350+ author-described AOPs in the AOP-Wiki, approximately 15% focus on some aspect of endocrine function (Organisation for Economic Cooperation and Development [OECD] 2022: Society for the Advancement of Adverse Outcome Pathways [SAAOP] 2022). There has been relatively extensive discussion of the critical role(s) AOPs play in screening and testing of EDCs (Browne et al. 2017; Coady et al. 2017; Noyes et al. 2019; Knapen et al. 2020; McCardle et al. 2020).
The extent of coverage provided by extant AOPs for endocrine pathways of regulatory concern in different life stages of aquatic species is variable. For example, 20 AOPs in the AOP-Wiki capture multiple MIEs and developmental endpoints related to HPT axis function in fish, amphibians, and mammals (Noyes et al. 2019; Knapen et al. 2020). However, in terms of the HPG axis, AOPs for aquatic vertebrates are lacking for critical developmental endpoints such as gonad differentiation and sexual development, which are known to be controlled by the interaction of ligands with estrogen and androgen receptors (ERs, ARs; Norris and Carr 2020; Delbas et al. 2022). In fact, one of the best characterized examples of the effects of EDCs in the environment involves feminization of genetically male fish by exogenous ligands of the ER (Purdom et al. 1994; World Health Organization 2002), an etiology that can arise from organizational events occurring during early developmental (Ankley and Johnson 2004). Although less prominent than estrogen-induced feminization, there also are reports of the masculinization of fish exposed to AR agonists associated, for example, with animal feedlots and pulp and paper mill effluents (Howell et al. 1980; Larsson et al. 2000; Parks et al. 2001; Orlando et al. 2004; Durhan et al. 2006). To address the lack of AOPs relevant to HPG effects in early life-stage fish, the goal of the effort summarized in this paper was to develop two AOPs linking early developmental perturbations to masculinization in later life-stages. Development of the AOPs described herein is complemented by a parallel effort to advance a modeling construct to predict impacts of male-skewed cohorts on fish populations (Miller et al. 2022).
Brief AOP Description
Fish have the largest array of reproductive strategies of any vertebrate class, ranging from gonochorism to synchronous/sequential hermaphrodism to unisex reproduction. Sex determination (i.e., male versus female) and sex differentiation (i.e., development of testes versus ovaries) in gonochoristic species are controlled by a variety of genetic and environmental variables, including chemicals that interact with ERs and ARs (Devlin and Nagahama 2002; Leet et al. 2011). In many species, the default gonadal sex is male, with production of 17β-estradiol (E2) providing the stimulus for differentiation to ovaries (Guigen et al. 2010; Kobayashi et al. 2013). For example, exposure of developing fish to exogenous ER agonists can skew gonad development to ovaries, whereas depression of synthesis of endogenous E2 from testosterone through inhibition of aromatase (cytochrome P450 19 [CYP19]) results in a predominance of animals with testes (Devlin and Nagahama 2002; Guigen et al. 2010; Leet et al. 2011; Kobayashi et al. 2013). Exposure of fish to AR agonists during sexual differentiation also can skew the population toward males, a technique that has been widely employed in the aquaculture industry (Pandian and Sheela 1995; Budd et al. 2015). The exact mechanism(s) through which androgen exposure induces male-biased sex differentiation is not well-defined, although some have hypothesized it may be associated with down-regulation of CYP19 and resultant decreases in estrogen (Kitano et al. 2000).
Given basic knowledge of the endocrinology and physiology of sex differentiation in fish, two novel AOPs are described (Figure 1; Box 1). Both AOPs (AOPs 346 and 376; SAAOP 2022) lead to populations of animals with male-biased sex ratios. There are two different MIEs, one involving inhibition of CYP19 aromatase (Key Event [KE] 36) leading to decreased E2 synthesis (KE 1789), which results in an increased occurrence of animals with testes and a male-biased population (KEs 1790 and 1791, respectively), and culminating in the adverse outcome of decreased population size (KE 360). The MIE for the second AOP is activation of the AR (KE 25) leading directly to an increased occurrence of testes and the other KEs thereafter. Given that the two AOPs share KEs 1790, 1791 and 360, we are describing a simple AOP network (Villeneuve et al. 2014; 2018), which can serve as a basis, for example, for predicting the effects of mixtures of endocrine-active chemicals with differing MIEs (e.g., Ankley et al. 2020).
Figure 1.
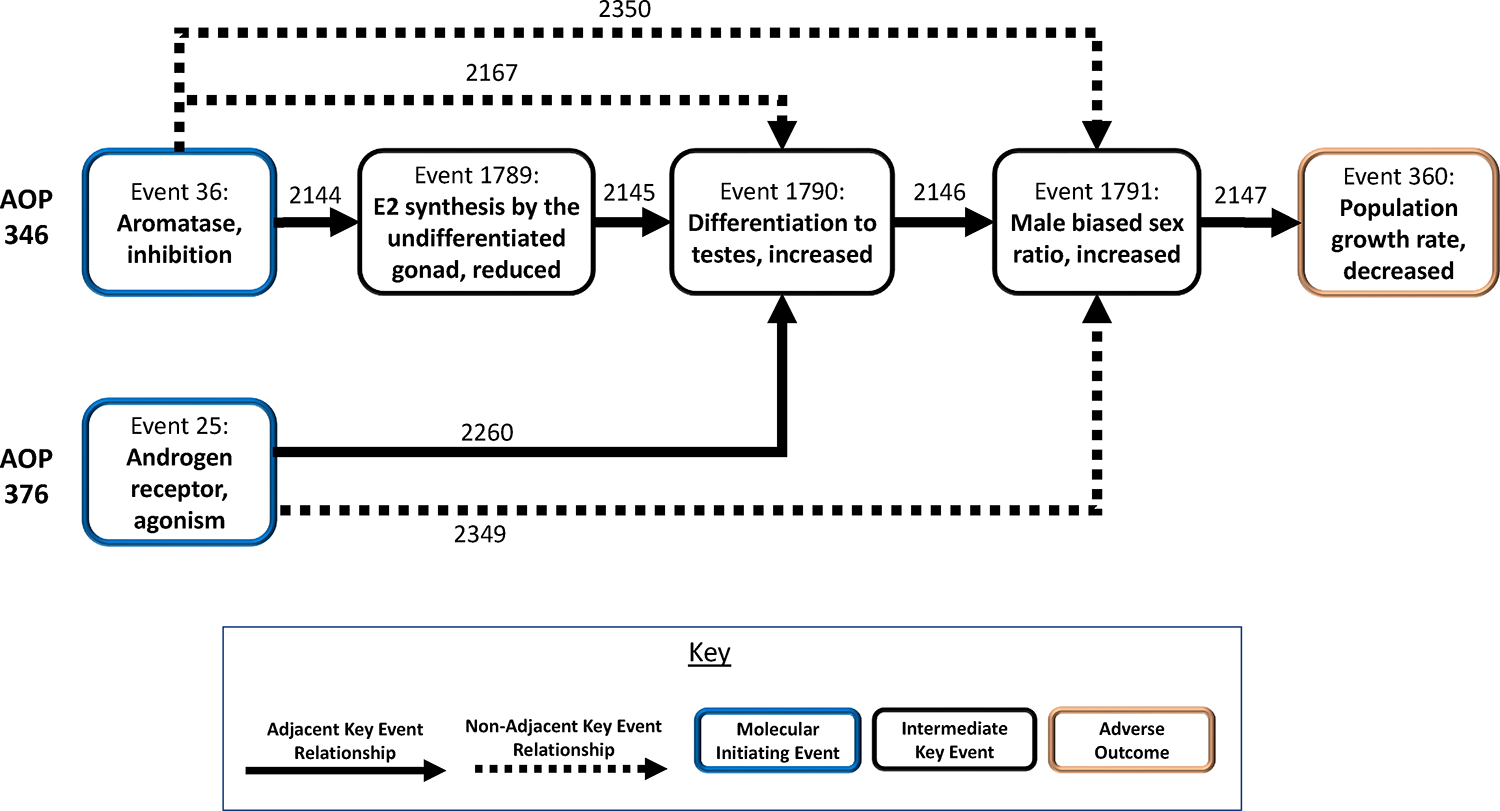
Graphical representation of adverse outcome pathways (AOPs) 346 and 376 (SAAOP 2022).
BOX 1: AOP ID Box.
| Formal AOP Titles | Aromatase inhibition leads to male-biased sex ratio via impacts on gonad differentiation |
| Androgen receptor agonism leading to male-biased sex ratio | |
| AOP Authors | Kelvin J. Santana Rodriguez, Gerald T. Ankley, Kathleen M. Jensen, David H. Miller, Daniel L. Villeneuve |
| AOP Numbers | 346, 376 |
| OECD Work Plan Project | N/A |
List of Key Events (KEs):
| Event ID | KE Title | AOP 346 | AOP 376 |
|---|---|---|---|
|
| |||
| 36 | Inhibition, aromatase1 | MIE | --- |
| 25 | Agonism, androgen receptor1 | --- | MIE |
| 1789 | Reduction, 17β-estradiol synthesis by the undifferentiated gonad2 | KE1 | --- |
| 1790 | Increased, differentiation to testis2 | KE2 | KE1 |
| 1791 | Increased, male biased sex ratio2 | KE3 | KE2 |
| 360 | Decrease, population growth rate3 | KE4 | KE3 |
Previously reviewed and endorsed, minor updates
New KE
Previously reviewed and endorsed, major updates
The various events defined in an AOP need to be directly or indirectly measurable (Villeneuve et al. 2014). It is beyond the scope of this short overview to describe the multitude of approaches that have been/could be used to measure the various KEs in the AOP network (Figure 1). Further detail concerning measurement approaches can be found on the various KE pages associated with AOPs 346 and 376 in the AOP-Wiki (SAAOP 2022). Herein we provide brief illustrative examples specific to the fathead minnow (Pimephales promelas), a small fish species used as a model system for EDC research and testing (Ankley and Johnson 2004). Neither MIE can be directly measured in vivo. However, chemical effects on fathead minnow CYP19 activity and AR binding both can be measured in vitro. For example, Villeneuve et al. (2006) describe the measurement of CYP19 activity in ovarian and brain preparations, as well as the effects of chemical inhibitors on E2 synthesis in ovaries from the fathead minnow (Villeneuve et al. 2007). Wilson et al. (2004) reported the binding of chemicals, including steroidal androgens, to cloned fathead minnow AR. In terms of KE 1789, direct measurements of E2 production by the undifferentiated gonad in fathead minnows have not been conducted, but techniques exist whereby E2 production by tissues could be measured using an ex vivo assay (e.g., Ankley et al. 2007; adapted from McMaster et al. 1995). Alternatively, reductions in E2 synthesis could be indirectly assessed using radioimmunoassay methods optimized to detect E2 concentrations in small volume samples (Jensen et al. 2001). Testes can be easily distinguished from ovaries visually in adult fathead minnows (KE 1790); however, it may be necessary to employ histological and magnification techniques for this assessment in juvenile fish (e.g., Leino et al. 2005). Finally, translation of the consequences of male-skewed populations (KE 1791) on fathead minnow population dynamics (KE 360) can be predicted using the modeling construct described by Miller et al. (2022).
Summary of Scientific Assessment
A weight-of-evidence assessment of robustness of an AOP is based on several considerations, including biological plausibility, demonstration of the essentiality of the KEs, and consistency of AOP-predicted outcomes with extant empirical evidence (Becker et al. 2015). These considerations are each addressed in detail in the AOP 346 and 376 pages in the AOP-Wiki and are briefly summarized below. We also consider the biological domain of applicability of the two AOPs, as well as the environmental occurrence of stressors that can cause effects via the AOPs.
Biological Plausibility
Based on extensive knowledge concerning reproductive physiology and gonad differentiation/development in fish, AOPs 346 and 376 are both considered highly plausible. Aromatase is a rate-limiting enzyme in the conversion of testosterone to E2 (Payne and Hales 2004), so the biological plausibility of aromatase inhibition leading to reductions in available E2 is clear. Since E2 is a major regulator of normal female gonad development (Guiguen et al. 2010), reductions in signaling required for ovarian differentiation is decreased and the bipotential gonad will default to development of testes (Yin et al. 2017; Zhang et al. 2017), thus plausibly resulting in a male-biased sex ratio in a population. A male-biased sex ratio would logically lead to a reduction in the number of breeding females such that, over time, decreases in recruitment result in population decline (Miller et al. 2022). While molecular processes linking AR activation (AOP 376) to increased testis differentiation in fish are less well understood than for CYP19 inhibition (AOP 346), there also is a substantial amount of empirical evidence that show this is a biologically plausible pathway.
Essentiality
Essentiality provides a powerful demonstration of the veracity of a proposed AOP, in that the concept demonstrates that if an early KE (such as the MIE) is blocked, subsequent KEs (including adverse outcomes) do not occur. There is good evidence of essentiality for AOP 346 based on gene modification studies in fish. Lau et al. (2016) generated insertion/deletion mutations in the zebrafish (Danio rerio) gonadal CYP19 gene (cyp19a1a) using TALEN (transcription activator-like effector nuclease) and CRISPR (clustered regularly interspaced short palindromic repeats)/Cas9 approaches. All mutant cyp19a1a−/− fish developed as males. Histological examination (at 120 days post-fertilization [dpf]) of the cyp1a1a−/− mutants showed that they exhibited normal spermatogenesis in the testis with no observable difference between the wild type (+/+) and heterozygous (+/−) males. Further, to confirm the necessity of E2 synthesis for ovarian differentiation, they performed an experiment to “rescue” the phenotype of cyp19a1a mutants by exposure to E2 during the period of gonadal differentiation (15–30 dpf). This intervention resulted in normal functioning ovaries, even in some individuals at the lowest E2 concentration tested. In a similar study also with zebrafish, Yin et al. (2017) generated cyp19a1a and cyp19a1b (brain form of aromatase) mutant lines and a cyp19a1a;cyp19a1b double-knockout line using TALENs. All cyp19a1a mutants and cyp19a1a;cyp19a1b double mutants developed as males, whereas the cyp19a1b mutants (−/−) had a 1:1 sex ratio similar to the wild type controls. This again supports the essentiality of gonadal aromatase inhibition for testis differentiation that would lead to a male biased sex ratio. Additionally, a small rescue experiment performed with E2 on all male mutant cyp1a1a−/− fish indicated that exposure to the estrogen led to transformation of some males to females. Finally, similar experiments in Nile tilapia (Oreochromis niloticus) were described by Zhang et al. (2017), who worked with female mutants for cypa19a1a. Results showed that all cyp19a1a+/− XX and cyp19a1a+/+ XX fish developed as females, whereas all cyp19a1a−/− XX and cyp19a1a−/− XY fish developed as males, based on gonad differentiation. The cyp19a1a−/− XX tilapia shifted to the male pathway as early as 5 days post hatch (dph) and ultimately were fertile.
There is also strong evidence demonstrating essentiality for AOP 376 based on gene manipulation and chemical inhibition studies. Golan and Levavi-Sivian (2014) exposed genetic Nile tilapia females to 17α-methyltestosterone (a synthetic steroid) and showed that the AR agonist produced a 100% male-biased sex ratio. However, in combined exposures with the model AR antagonist flutamide (a pharmaceutical), the sex inversion potency was reduced, highlighting the critical nature of the AR as the MIE. In a gene manipulation study, Crowder et al. (2018) generated zebrafish with a mutation in the AR gene, most of which developed ovaries and displayed female secondary sexual characteristics. The small percentage of mutants that developed as males displayed female secondary sexual characteristics with structurally disorganized testes and were unable to produce normal levels of sperm. Finally, in a similar study also with zebrafish, Yu et al. (2018) generated an AR gene mutant line using CRISPR/Cas9. The number of female offspring was increased, and the resulting AR-null males had female secondary sex characteristics and were infertile due to defective spermatogenesis, again directly demonstrating importance of the AR in testis differentiation and development.
Empirical Evidence
Data from a variety of studies with fish provide critical insights as to the concordance and consistency of the proposed AOPs. Much of this work has been done with known chemical inhibitors of CYP19 (AOP 346, KE 36) or AR agonists (AOP 376, KE 25) in the context of EDC testing or aquaculture research. Studies that employ multiple doses of the test chemical(s) and evaluate more than one KE are most useful in terms of determining concordance of a given AOP, but more limited studies also can be very important in demonstrating consistency of AOP predicted outcomes. This type of information is captured in concordance tables associated with the AOP 346 and 376 pages in the AOP-Wiki (SAAOP 2022). Following we present a subset of illustrative information from the tables.
AOP 346 Empirical Evidence Summary
Chemical inhibition of aromatase is most easily measured in vitro. Doering et al. (2019) determined the effects of different concentrations of several established CYP19 inhibitors (e.g., the pharmaceutical fadrozole, the fungicide prochloraz) on brain aromatase activity in a taxonomically diverse set of fish species. Activity was inhibited in a dose-dependent manner and while absolute potency of the chemicals varied across species, rank order potency of the test chemicals was generally similar. The same aromatase inhibitors assessed by Doering et al. (2019) in vitro were also used in many the in vivo studies presented below.
Several in vivo studies with fish have evaluated the effects of varying degrees of aromatase inhibition on different KEs in AOP 346. However, there are limitations to these studies in the context of determining dose-dependency across all KEs in the AOP. Most studies measure dose-dependent effects of a model chemical on only one KE, however, there are a couple that have considered multiple KEs (albeit at a limited number of time points). For example, Ruksana et al. (2010) exposed Nile tilapia from 9–35 days dph to the aromatase inhibitor exemestane at 100, 500, 1000 and 2000 μg/g feed, and used an immunohistochemical analysis to show there was no cross-reactivity with CYP19 at the three highest doses. However, gonad tissue samples exhibited a strong immuno-positive response at the lowest dose of exemestane (100 μg/g feed) at a level similar to control fish. No ovarian development was noted in fish in the 1000 and 2000 μg/g treatments, with the animals exhibiting complete testicular differentiation. Uchida et al. (2004) evaluated two KEs in AOP 346 in an experiment with zebrafish genetic females produced by mating of wild-type females with gynogenetic sex-reversed males. In fish exposed to fadrozole from 15–40 dph via the diet ovarian transition to testis was observed, culminating in 62.5, 100 and 100% males in 10, 100 and 1000 μg/g treatments, respectively.
The most frequently reported dose-response relationship for AOP 346 is for non-adjacent relationships between aromatase inhibition (the MIE) and one of two different downstream KEs, increased differentiation to testes or an increased male-biased sex ratio. For example, Muth-Köhne et al. (2016) showed a dose-dependent rate of male gonad maturity index in zebrafish exposed from 0–63 dph to different concentrations of fadrozole via the water. Studies with Nile tilapia, zebrafish, fathead minnows, bluegill (Lepomis macrochirus), yellow catfish (Pelteobagrus fulvidraco) and Japanese flounder (Paralichthys olivaceus) exposed during development to different concentrations/doses of known aromatase inhibitors (fadrozole, letrozole, prochloraz) via the diet or water reported dose-dependent increases in the relative number of males (Kwon et al. 2000; Kitano et al. 2000; Thorpe et al. 2011; Holbech et al. 2012; Gao et al. 2010; Shen et al. 2013). All these studies demonstrate consistency of the AOP relative to expected outcomes in the context of different intensity of the perturbation of the MIE.
AOP 376 Empirical Evidence Summary
There is a significant amount of empirical evidence from studies with fish supporting AOP 376. The dose-dependence of the responses relative to the concentration of exogenous AR agonists has been established in vivo for some key events in the AOP. As for CYP19 inhibition, activation of the AR (the MIE) is difficult to directly measure in vivo. However, relative AR binding affinity of several of the agonists tested in vivo has been documented using in vitro studies with fish ARs (e.g., Wilson et al. 2004). This in vitro evidence can reliably be used to identify specific chemicals as AR agonists (i.e., able to activate the MIE). That is, dependence of the downstream in vivo responses on concentration and potency of chemicals activating the AR in vitro can be used as indirect evidence of dose-response concordance between the MIE and later KEs. Wilson et al. (2004) used an assay with stably transfected fathead minnow AR in whole cells for competitive binding experiments with several natural and synthetic steroids. The model compounds bound to the AR in a concentration-dependent manner, with rank affinity of synthetic steroids R1881›17α-methytestosterone›17α- and 17β-trenbolone. Of the natural steroids evaluated, dihydrotestosterone was the most potent followed by the fish-specific androgen, 11- ketotestosterone, then testosterone and androstenedione. The different androgenic steroids tested by Wilson et al. (2004) in vitro have been used in various in vivo experiments selected to support our weight-of-evidence assessment of AOP 376.
Studies with zebrafish and Japanese medaka (Oryzias latipes) exposed to 17β-trenbolone or methyltestosterone during development resulted in gonadal masculinization in a concentration-dependent manner. This was evidenced by increased maturation of testes, including (in some of the studies) increased spermatozoa abundance or amount of testicular tissue (Seki et al. 2004; Örn et al. 2003; 2016; Morthorst et al. 2010; Baumann et al. 2014). Dietary or water-borne exposures during early development to known steroidal androgens (17β-trenbolone, methyltestosterone, methyldihydrotestosterone or 11-ketotestosterone) have been shown to produce male-skewed sex ratios in a concentration-dependent manner in different fish species, including channel catfish (Ictalurus punctatus), Chinook salmon (Oncorhynchus tshawytscha), fathead minnow, and zebrafish (Piferrer et al. 1993; Galvez et al. 1995; Morthorst et al. 2010; Holbech et al. 2012; Baumann et al. 2014).
Evaluation of empirical data can also identify where there may be inconsistencies relative to a proposed AOP. In the case of AOP 376, the preponderance of evidence indicates that exposure of a wide variety of teleost fishes to known AR agonists during sexual differentiation will produce male-skewed fish cohorts. For example, Pandian and Sheela (1995) provided a comprehensive overview of effects of steroidal hormones on sex inversion in the context of aquacultural practices and reported that methyltestosterone had been used to successfully produce male-biased sex ratios in 25 different teleost species. There are, however, reports in which exposure to aromatizable androgens such as methyltestosterone led both to masculinization and feminization of fish (e.g., Piferrer et al. 1993; Örn et al 2003). This most likely is due to conversion of the androgen to its corresponding estrogen analogue (i.e., methylestradiol; Hornung et al. 2004). In other instances, non-aromatizable androgens (e.g., dihydrotestosterone) have been reported to feminize fish exposed during early development (e.g., Davis et al. 1992; Bogers et al. 2006). The mechanism underlying this is uncertain, but plausibly could involve binding to the estrogen receptor which is known to interact with a variety of steroids, including androgens at comparatively high exposure concentrations.
Applicability Domain
A critical component of the development and documentation of an AOP involves identification of its biological domain of applicability in terms of life-stage, sex, and species. An important application of AOPs involves prediction of potential effects of a given chemical or non-chemical stressor in an organism in scenarios where empirical data are lacking. As such, definition of applicability domain dictates the degree to which effects extrapolation is supportable. In terms of AOPs 346 and 376, the applicable life-stage is developing embryos and juveniles prior to- or during gonadal development. These AOPs are not applicable to sexually differentiated adults. The MIEs for the two AOPs, inhibition of CYP19 and AR activation, occur prior to gonad differentiation. Therefore, the AOPs are only applicable to sexually undifferentiated individuals.
Definition of the taxonomic domain applicability of AOPs 346 and 376 is more challenging. Phylogenetic analysis and evaluation of protein sequence data using the publicly available software package SeqAPASS (sequence alignment for across species susceptibility) has shown that the structure of the MIE proteins, CYP19 and the AR, are well conserved among most jawed vertebrates (LaLone et al. 2018). However, the two AOPs are not expected to apply to mammals, birds, or other jawed vertebrates with genetic sex determination. They may, however, be broadly applicable to fishes, amphibians, and reptiles with environmental sex determination, although outcomes may differ across physiologically different taxa. From a pragmatic perspective, most physiological and toxicological evidence supporting the two AOPs is derived from fish in class Osteichthyes (bony fishes). AOP 376 is not considered relevant to Agnathans since the AR appears not to be present in jawless fishes (Thornton 2001). However, given the substantial diversity of sex determination and differentiation strategies even within Osteichthyes (Angelopoulou et al. 2012), quantitative sensitivity and taxonomic domain of applicability of the two AOPs are hard to generalize, although it does seem reasonable to suppose they would have broad applicability in bony fishes. Pragmatic support for this hypothesis comes from analyses such as conducted by Pandian and Sheela (1995) who found that developmental exposure to different steroidal AR agonists does indeed produce male-biased sex ratios in a taxonomically diverse group of fish species important to aquaculture.
Known Chemical and Nonchemical Stressors
There are many environmentally relevant chemicals capable of triggering the MIEs for AOPs 346 or 376. Human pharmaceuticals (e.g., fadrozole, letrozole) that specifically inhibit CYP19 (KE 36) theoretically could occur in aquatic environments, although it is unlikely that contamination by these types of compounds would be widespread. Also relevant to the AOP are various conazole (triazole, imidazole) fungicides used as veterinary and human drugs and, more substantively, for a variety of agricultural applications worldwide (see Bhagat et al. 2021 and references therein). Conazole fungicides act through the inhibition of CYP51, which is involved in the synthesis of ergosterol used in formation of fungal cell walls; however, most are not specific to CYP51 and can bind to and inhibit different CYP isozymes, including those involved in steroidogenesis in vertebrates (Kjaerstad et al. 2010; Bhagat et al. 2021). In fact, some conazoles (e.g., prochloraz, propiconazole) are used as model EDCs based on their ability to inhibit CYP19 in both mammalian and fish species (Kjaerstad et al. 2010; Holbech et al. 2012; Dang and Kienzler 2019). Matthiessen and Weltje (2015) conducted an eco-epidemiological analysis comparing effects from lab studies with fish and conazoles to occurrence of the same fungicides from field monitoring studies and concluded that rarely would environmental concentrations of the conazoles be sufficient to cause masculinization. However, monitoring data used for that analysis were somewhat limited.
While vertebrate ARs are notably less “promiscuous” than various ERs in terms of the structural diversity of chemicals that can act as agonists, there are known environmental contaminants that activate fish ARs (KE 25). Specifically, both natural and synthetic steroidal androgens enter aquatic environments in conjunction with human and animal wastes. For example, the synthetic growth promotor 17β-trenbolone has been hypothesized as contributing to masculinization of fish at sites in the vicinity of livestock feeding operations (Orlando et al. 2004; Durhan et al. 2006; Ankley et al. 2018). Masculinized fish from the field also have been observed at sites receiving pulp/paper mill effluent (e.g., Howell et al. 1980; Larsson et al. 2000; Parks et al. 2001), which typically contain a complex mixture of plant-derived sterols. Although known steroidal AR agonists have been detected in pulp mill effluent (e.g., androstenedione), specific identities of chemicals causing androgenic activity remains uncertain (e.g., Durhan et al. 2002). Also, there are a wide variety of steroidal androgens used to purposely masculinize fish that could enter aquatic environments associated with aquacultural operations (Pandian and Sheela 1995). Finally, there is the potential for environmental occurrence of steroidal progestins used as pharmaceuticals in humans, some of which can act as potent AR agonists in fish (e.g., levonorgestrel), producing masculinization in developing animals (Dang and Kienzler 2019).
There is at least one nonchemical stressor that can influence AOP 346, namely temperature, which could be very consequential in the context of climate change. Temperature-dependent sex determination has been clearly demonstrated in various fish, as well as reptile species (Ramsey et al. 2007; Yamaguchi et al. 2007 Kobayashi et al. 2013; Norris and Carr 2020). In at least some species the effects of temperature on sex ratio appear to be controlled via differential expression of aromatase. For example, D’Cotta et al. (2001) exposed Nile tilapia to temperatures of 27.8 versus 35.8 C and found that the higher temperature decreased aromatase expression while increasing the male sex ratio. A similar observation was made by Uchida et al. (2004) in zebrafish.
Potential Applications
The AOP framework directly supports hazard and risk assessments for EDCs. While specific strategies employed by different authorities tasked with assessing risks of EDCs vary somewhat regionally, virtually all use some variation of a tiered screening and testing approach, as exemplified by a framework developed by the OECD (OECD 2012). Chemical hazard in prospective assessments (i.e., new, or untested chemicals) initially is evaluated using existing knowledge, computational predictions, and/or measurements from in vitro assays to determine potential for interactions with HPG/T axes. The tiered assessment then proceeds to short-term in vivo tests and, when deemed necessary, long-term (including full lifecycle) assays in taxa of concern. Critical to this process is identifying in vivo assays and endpoints suitable for assessing risk, a need that AOPs address.
By way of specifically illustrating the role of AOPs it is useful to consider the EDSP (Endocrine Disruptor Screening Program) in the US (USEPA 2019). The USEPA EDSP aims to assess up to 10,000 chemicals in terms of potential effects on HPG/T function in humans and nonmammalian species. This clearly is not a task achievable solely through in vivo testing, so an initial proposed step involves the use of models based on data from in vitro high throughput (HTP) assays to identify chemicals likely to interact with endocrine pathways of regulatory concern. For example, systems have been developed to identify chemicals with the potential to affect ERs (Judson et al. 2015), ARs (Kleinstreuer et al. 2017) and enzymes involved in steroid synthesis, including CYP19 (Haggard et al. 2018). Results of these assays essentially capture AOP MIEs, which allows for the identification of assays/endpoints suitable for higher tier testing and risk assessment (Browne et al. 2017; McCardle et al. 2020). These assays may need to be developed or may already be available. For example, if HTP-derived data/models suggest that a chemical is an AR agonist or a CYP19 inhibitor, the AOPs described herein would indicate the desirability for higher tier testing employing an early-life stage assay with fish that could capture the potential for male-biased populations. In fact, such an assay is available; OECD (2018) describes a 42-d fish sexual development test focused on gonad differentiation in multiple small fish species that would be suitable for testing chemicals flagged as AR agonists or CYP19 inhibitors.
There also are applications for the two AOPs in diagnostic (sometimes termed retrospective) assessments of potential effects of complex mixtures of chemicals in the environment. For example, there are in vitro assay systems (some in an HTP format) that measure endocrine-associated bioactivities in environmental samples such as surface water (e.g., Escher et al. 2013; Blackwell et al. 2019). Alternatively, it is possible to crosswalk analytical data from complex environmental samples with in vitro (e.g., HTP) results from single chemical studies to determine the presence of compounds in complex mixtures at concentrations possibly sufficient to affect biological pathways of concern, including those related to HPG/T function (e.g., Blackwell et al. 2017; Corsi et al. 2019). These direct or indirect determinations of bioactivity in many instances can be used as proxies for MIEs, such as activation of ERs or ARs, or inhibition of CYPs which, when interpreted in the context of relevant AOPs, enables insights into potential effects at higher biological levels of organization in exposed organisms (e.g., Blackwell et al. 2019; Corsi et al. 2019). This, in turn, provides the basis for species and endpoint selection for additional testing and monitoring. In the context of the AOPs described herein, if elevated AR activation or CYP19 inhibition is measured or inferred at a given field site(s), subsequent monitoring could be focused on the occurrence of male-biased fish populations.
In conclusion, assessment of potential ecological hazards of EDCs represents a deviation from approaches typically used in the past for environmental contaminants in that often it is necessary not only to establish the potential for adverse effects on survival, growth, or reproduction, but demonstrate that effects occur via perturbation of the HPG/T axes (Matthiessen et al. 2017). The AOP framework is ideal for meeting this challenge because causal linkages can be established between the types of molecular, biochemical, and histological endpoints associated with disruption of specific endocrine pathways and apical responses in individuals that can be translated into population-level impacts (Coady et al. 2017; Knapen et al. 2020; McCardle et al. 2020). However, for the framework to play a prominent role in assessing risks and regulating EDCs there is a pressing need for a “library” of AOPs that capture relevant MIEs and adverse outcomes. The two AOPs described herein contribute to populating this type of knowledgebase.
Supplementary Material
Acknowledgement
The authors declare no conflicts of interest. Support for the work was provided entirely by the US Environmental Protection Agency (USEPA). We thank Prarthana Shankar and Katie O’Shaughnessy for helpful comments on an earlier version of the manuscript. We also thank Dries Knapen, the Associate Editor coordinating the review for Environmental Toxicology and Chemistry and the review team who provided important feedback and advice on the report and associated AOP-Wiki content: Reginald (Rex) Fitzgerald, Henrik Holbech and Ioanna Katsiadaki.
Footnotes
Disclaimer
The viewpoints expressed are those of the authors and do not necessarily reflect opinions/policies of the USEPA. Mention of trade names and commercial products does not constitute endorsement or recommendation for use. This paper has been reviewed and approved for publication in accordance with USEPA guidelines.
Data Availability
No new data were generated for this review manuscript.
References
- Angelopoulou R, Lavranos G, Manolakou P. 2012. Sex determination strategies in 2012: Towards a common regulatory model? Repro. Biol. Endocrinol. 10, 13. 10.1186/1477-7827-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankley GT, Johnson RD. 2004. Small fish models for identifying and assessing the effects of endocrine-disrupting chemicals. Inst. Lab. Anim. Res. J. 45:469–483. [DOI] [PubMed] [Google Scholar]
- Ankley GT, Jensen KM, Kahl MD, Makynen EA, Blake LS, Greene KJ, Johnson RD, Villeneuve DL. 2007. Ketoconazole in the fathead minnow (Pimephales promelas): Reproductive toxicity and biological compensation. Environ. Toxicol. Chem. 26:1214–1223. [DOI] [PubMed] [Google Scholar]
- Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD, Mount DR, Nichols JW, Russom CL, Schmieder PK, Serrano JA, Tietge JE, Villeneuve DL. 2010. Adverse outcome pathways: A conceptual framework to support ecotoxicology research and risk assessment. Environ. Toxicol. Chem. 29:730–741. [DOI] [PubMed] [Google Scholar]
- Ankley GT, Coady KK, Gross M, Holbech H, Levine SL, Maack G, Williams M. 2018. A critical review of the environmental occurrence and potential effects in aquatic vertebrates of the potent androgen receptor agonist 17β-trenbolone. Environ. Toxicol. Chem. 37: 2064–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankley GT, Blackwell BR, Cavallin JE, Doering JA, Feifarek DJ, Jensen KM, Kahl MD, LaLone CA, Poole ST, Randolph EC, Saari TW, Villeneuve DL. 2020. Adverse outcome pathway network-based assessment of the interactive effects of an androgen receptor agonist and an aromatase inhibitor on fish endocrine function. Environ. Toxicol. Chem. 39: 913–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann L, Knörr S, Keiter S, Nagel T, Rehberger K, Volz S, Oberrauch S, Schiller V, Fenske M, Holbech H, Segner H, Braunbeck T. 2014. Persistence of endocrine disruption in zebrafish (Danio rerio) after discontinued exposure to the androgen 17β-trenbolone. Environ. Toxicol. Chem. 33:2488–2496. [DOI] [PubMed] [Google Scholar]
- Becker RA, Ankley GT, Edwards SW, Kennedy SW, Linkov I, Meek B, Sachana M, Segner H, Van Der Burg B, Villeneuve DL, Watanabe H, Barton-Maclaren TS. 2015. Increasing scientific confidence in adverse outcome pathways: Application of tailored Bradford-Hill considerations for evaluating weight-of-evidence. Regul. Toxicol. Pharmacol. 72:514–537. [DOI] [PubMed] [Google Scholar]
- Bhagat J, Singh N, Nishimura N, Shimada Y. 2021. A comprehensive review on environmental toxicity of azole compounds in fish. Chemosphere, 10.1016/j.chemosphere.2020.128335. [DOI] [PubMed] [Google Scholar]
- Blackwell BR, Ankley GT, Corsi SR, DeCicco LA, Houck KA, Judson RS, Li S, Martin MT, Murphy E, Schroeder AL, Smith ER, Swintek J, Villeneuve DL. 2017. An “EAR” on environmental surveillance and monitoring: A case study on the use of exposure-activity ratios (EARs) to prioritize sites, chemicals and bioactivities of concern in the Great Lakes. Environ. Sci. Technol. 51:8713–8724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell BR, Ankley GT, Bradley PM, Houck KA, Schroeder AL, Swintek J, Villeneuve DL. 2019. Potential toxicity of complex mixtures in surface waters from a nationwide survey of United States streams: Identifying in vitro bioactivities and causative chemicals. Environ. Sci. Technol. 53:973–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogers R, De Vries-Buitenweg S, Van Gils M, Baltussen E, Hargreaves A, van de Waart B, De Roode D, Legler J, Murk A. 2006. Development of chronic tests for endocrine active chemicals. Part 2: An extended fish early-life stage test with an androgenic chemical in the fathead minnow (Pimephales promelas). Aquat. Toxicol. 80:119–130. [DOI] [PubMed] [Google Scholar]
- Browne P, Noyes PD, Casey W, Dix DJ. 2017. Application of adverse outcome pathways to US EPA’s endocrine disruptor screening program. Environ. Health Perspect. 125 (9).096001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budd AM, Banh QQ, Domingos JA, Jerry DR. 2015. Sex control in fish: Challenges and opportunities for aquaculture. J. Mar. Sci. Engineer. 3:329–355. [Google Scholar]
- Coady KK, Biever RC, Denslow ND, Gross M, Guiney PD, Holbech H, Karouna-Renier NK, Katsiadaki I, Kruger H, Levine SL, Maack G, Williams M, Wolf JC, Ankley GT. 2017. Current limitations and recommendations to improve testing for environmental assessment of endocrine active chemicals. Integ. Environ. Assess. Manag. 13:302–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colborn T, Dumanoski D, Myers JP. 1996. Our Stolen Future: Are We Threatening Our Fertility, Intelligence, and Survival? A Scientific Detective Story. Dutton, New York. 306 pp. [Google Scholar]
- Corsi SR, De Cicco LA, Villeneuve DL, Blackwell BR, Fay KA, Ankley GT, Baldwin AK. 2019. Prioritizing chemicals of ecological concern in Great Lakes tributaries using high-throughput screening data and adverse outcome pathways. Sci. Total Environ. 686:995–1009. [DOI] [PubMed] [Google Scholar]
- Crowder CM, Lassiter CS, Gorelick DA. 2018. Nuclear androgen receptor regulates testes organization and oocyte maturation in zebrafish. Endocrinology 159:980–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Cotta H, Fostier A, Guiguen Y, Govoroun M, Baroiller JF. 2001. Aromatase plays a key role during normal and temperature-induced sex differentiation of tilapia Oreochromis niloticus. Mol. Repro. Dev 59:265–276. [DOI] [PubMed] [Google Scholar]
- Dang Z, Kienzler A. 2019. Changes in fish sex ratio as a basis for regulating endocrine disruptors. Environ. International 130:1–22. [DOI] [PubMed] [Google Scholar]
- Davis KB, Goudie CA, Simco BA, Tiersch TR, Carmichael GJ. 1992. Influence of dihydrotestosterone on sex determination in channel catfish and blue catfish: Period of developmental sensitivity. Gen. Comp. Endocrinol. 86:147–151. [DOI] [PubMed] [Google Scholar]
- Delbas G, Blazquez M, Fernandino JI, Grigorova P, Hales BF, Metcalfe C, Navarro-Martin L, Parent L, Robaire B, Rwigemera A, Van Der Kraak G, Wade M, Marlatt V. 2022. Effects of endocrine disrupting chemicals on gonad development: Mechanistic insights from fish and mammals. Environ. Res. 204:112040, 10.1016/j.envres.2021.112040 [DOI] [PubMed] [Google Scholar]
- Devlin RH, Nagahama Y. 2002. Sex determination and sex differentiation in fish: An overview of genetic, physiological and environmental influences. Aquaculture 208:191–364. [Google Scholar]
- Doering JA, Villeneuve DL, Fay KA, Randolph EC, Jensen KM, Kahl MD, LaLone CL, Ankley GT. 2019. Differential sensitivity to in vitro inhibition of cytochrome P450 aromatase (CYP19) activity among 18 freshwater fishes. Toxicol. Sci. 170:394–403. [DOI] [PubMed] [Google Scholar]
- Durhan EJ, Lambright C, Wilson V, Butterworth BC, Kuehl DW, Orlando EF, Guillette LJ, Gray LE, Ankley GT. 2002. Evaluation of androstenedione as an androgenic component of river water downstream of a pulp and paper mill effluent. Environ. Toxicol. Chem. 21:1973–1976. [PubMed] [Google Scholar]
- Durhan EJ, Lambright CS, Makynen EA, Lazorchak J, Hartig PC, Wilson VS, Gray LE, Ankley GT. 2006. Identification of metabolites of trenbolone acetate in androgenic runoff from a beef feedlot. Environ. Health Perspect. 114 (Suppl. 1):65–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escher BI, Allinson M, Altenburger R, Bain PA, Balaguer P, Busch W, Crago J, Denslow ND, Dopp E, Hilscherova K. 2013. Benchmarking organic micropollutants in wastewater, recycled water and drinking water with in vitro bioassays. Environ. Sci. Technol. 48:1940–1956. [DOI] [PubMed] [Google Scholar]
- Galvez J, Mazik P, Phelps R, Mulvaney D. 1995. Masculinization of Channel catfish Ictalurus punctatus by oral administration of trenbolone acetate. World Aquacult. Soc. 26:378–383. [Google Scholar]
- Gao ZX, Wang HP, Wallat G, Yao H, Rapp D, O’Bryant P, MacDonald R, Wang W. 2010. Effects of a non-steroidal aromatase inhibitor on gonadal differentiation of bluegill sunfish Lepomis macrochirus. Aquacult. Res. 41:1282–1289. [Google Scholar]
- Golan M, Levavi-Sivan B. 2014. Artificial masculinization in tilapia involves androgen receptor activation. Gen. Comp. Endocrinol. 207:50–55. [DOI] [PubMed] [Google Scholar]
- Guiguen Y, Fostier A, Piferrer F, Chang CF. 2010. Ovarian aromatase and estrogens: A pivotal role for gonadal sex differentiation and sex change in fish. Gen. Comp. Endocrinol. 165:352–366. [DOI] [PubMed] [Google Scholar]
- Haggard DE, Karmaus AL, Martin MT, Judson RS, Setzer RW, Paul-Friedman K. 2018. High-throughput H295R steroidogenesis assay: Utility of an alternative and statistical approach to characterize effects on steroidogenesis. Tox. Sci. 162:509–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbech H, Kinnberg KL, Brande-Lavridsen N, Bjerregaard P, Petersen GI, Norrgren L, Orn S, Braunbeck T, Baumann L, Bomke C, Dorgerloh M, Bruns E, Ruehl-Fehlert C, Green JW, Springer TA, Gourmelon A. 2012. Comparison of zebrafish (Danio rerio) and fathead minnow (Pimephales promelas) as test species in the Fish Sexual Development Test (FSDT). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 155:407–415. [DOI] [PubMed] [Google Scholar]
- Hornung MW, Jensen KM, Korte JJ, Kahl MD, Durhan EJ, Denny JS, Henry TR, Ankley GT. 2004. Mechanistic basis for estrogenic effects in fathead minnow (Pimephales promelas) following exposure to the androgen 17alpha-methyltestosterone: Conversion of 17α-methyltestosterone to 17α-methylestradiol. Aquat. Toxicol. 66:15–23. [DOI] [PubMed] [Google Scholar]
- Hotchkiss AK, Rider CV, Blystone CR, Wilson VS, Hartig PC, Ankley GT, Foster PM, Gray CL, Gray LE. 2008. Fifteen years after “Wingspread”-environmental endocrine disrupters and human and wildlife health: Where we are today and where we need to go. Toxicol. Sci. 105:235–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell WM, Black DA, Bortone SA. 1980. Abnormal expression of secondary sex characters in a population of mosquitofish Gambusia affinis holbrooki: evidence for environmentally-induced masculinization. Copeia 1980, 676–81. [Google Scholar]
- Jensen KM, Korte JJ, Kahl MD, Ankley GT. 2001. Aspects of basic reproductive biology and endocrinology in the fathead minnow (Pimephales promelas). Comp. Biochem. Physiol. 128C:127–141. [DOI] [PubMed] [Google Scholar]
- Judson RS, Magpantay FM, Chickarmane V, Haskell C, Tania N, Taylor J, Xia M, Huang R, Rotroff DM, Filer DL, Houck KA, Martin MT, Sipes N, Richard AM, Mansouri K, Setzer RW, Knudsen TB, Crofton KM, Thomas RS. 2015. Integrated model of chemical perturbations of a biological pathway using 18 in vitro high-throughput screening assays for the estrogen receptor. Toxicol. Sci. 148:137–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitano T, Takamune K, Nagahama Y, Abe S-I. 2000. Aromatase inhibition and 17α- methyltestosterone cause sex reversal from genetical females to phenotypic males and suppression of P450 aromatase gene expression in Japanese flounder (Paralichthys olivaceus). Mol. Repro. Develop. 56:1–5. [DOI] [PubMed] [Google Scholar]
- Kjaerstad MB, Taxvig C, Nellemann C, Vinggaard AM, Anderson HR. 2010. Endocrine disrupting effects in vitro of conazole antifungals used as pesticides and pharmaceuticals. Repro. Toxicol. 30:573–582. [DOI] [PubMed] [Google Scholar]
- Kleinstreuer NC, Ceger P, Watt ED, Martin M, Houck K, Browne P, Thomas RS, Casey WM, Dix DJ, Allen D, Sakamuru S, Xia M, Huang R, Judson R. 2017. Development and validation of a computational model for androgen receptor activity. Chem. Res. Toxicol. 30:946–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapen D, Stinckens E, Cavallin JE, Ankley GT, Holbech H, Villeneuve DL, Vergauwen L. 2020. Toward an AOP network-based tiered testing strategy for the assessment of thyroid hormone disruption. Environ. Sci. Technol. 54: 8491–8499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Nagahama Y, Nakamura M. 2013. Diversity and plasticity of sex determination and differentiation in fishes. Sex. Develop. 7:115–125. [DOI] [PubMed] [Google Scholar]
- Kwon JY, Haghpanah V, Kogson-Hurtado LM, McAndrew BJ, Penman DJ. 2000. Masculinization of genetic female Nile tilapia (Oreochromis niloticus) by dietary administration of an aromatase inhibitor during sexual differentiation. J. Experimental Zool 287:46–53. [PubMed] [Google Scholar]
- LaLone CA, Villeneuve DL, Doering JA, Blackwell BR, Transue TR, Simmons CW, Swintek J, Degitz SJ, Williams AJ, Ankley GT. 2018. Evidence for cross-species extrapolation of mammalian-based high-throughput screening assay results. Environ. Sci. Technol. 52:13960–13971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson DGJ, Hällman H, Förlin L. 2000. More male fish embryos near a pulp mill. Environ. Toxicol. Chem. 19:2911–2917. [Google Scholar]
- Lau ES, Zhang Z, Qin M, Ge W. 2016. Knockout of zebrafish ovarian aromatase gene (cyp19a1a) by TALEN and CRISPR/Cas9 leads to all-male offspring due to failed ovarian differentiation. Scientific Rep., 6, 37357. 10.1038/srep37357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leet JK, Gall HE, Sepulveda MS. 2011. A review of studies on androgen and estrogen exposure in fish early life stages: Effects on gene and hormone control of sex differentiation. J Appl Toxicol 31:379–398. [DOI] [PubMed] [Google Scholar]
- Leino RL, Jensen KM, Ankley GT. 2005. Gonadal histology and characteristic histopathology associated with endocrine disruption in the fathead minnow (Pimephales promelas). Environ. Toxicol. Pharmacol. 19:85–98. [DOI] [PubMed] [Google Scholar]
- Matthiessen P, Weltje L. 2015. A review of the effects of azole compounds in fish and their possible involvement in masculinization of wild fish populations. Crit. Rev. Toxicol. 45: 453–467. [DOI] [PubMed] [Google Scholar]
- Matthiessen P, Ankley GT, Biever R, Bjerregaard P, Borgert C, Brugger K, Blankenship A, Chambers J, Coady K, Constantine L, Dang Z, Denslow N, Dreier DA, Dungey S, Gray LE, Gross M, Guiney P, Hecker M, Holbech H, Iguchi T, Kadlec S, Karouna-Renier N, Katsiadaki I, Kawashima Y, Kloas W, Krueger H, Lagadic L, Leopold A, Levine S, Maack G, Marty S, Meador J, Mihaich E, Odum J, Ortego L, Parrott J, Pickford D, Roberts M, Schaefers C, Swartz T, Solomon K, Verslycke T, Weltje L, Wheeler J, Williams M, Wolf JC, Yamazaki K. 2017. Recommended approaches to the scientific evaluation of ecotoxicological hazards and risks of endocrine-active substances. Integ. Environ. Assess. Manag. 13:267–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCardle M, Freeman EL, Stavely J, Ortega L, Coady K, Weltjie L, Weyers A, Wheeler JR, Bone AJ. 2020. Critical review of read across potential in testing for endocrine related effects in vertebrate ecological receptors. Environ. Toxicol. Chem. 39:739–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster ME, Munkittrick KR, Jardine JJ, Robinson RD, Van Der Kraak GJ. 1995. Protocol for measuring in vitro steroid production by fish gonadal tissue. Canadian Technical Report of Fisheries and Aquatic Sciences 1961:1–78. [Google Scholar]
- Miller DH, Villeneuve DL, Santana-Rodriguez KJ, Ankley GT. 2022. A multidimensional matrix model for predicting the effects of male biased sex ratios on fish populations. Environ. Toxicol. Chem. 41:1066–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morthorst JE, Holbech H, Bjerregaard P. 2010. Trenbolone causes irreversible masculinization of zebrafish at environmentally relevant concentrations. Aquat. Toxicol. 98:336–343. [DOI] [PubMed] [Google Scholar]
- Muth-Köhne E, Westphal-Settele K, Brückner J, Konradi S, Schiller V, Schäfers C, Teigeler M, Fenske M. 2016. Linking the response of endocrine regulated genes to adverse effects on sex differentiation improves comprehension of aromatase inhibition in a Fish Sexual Development Test. Aquat. Toxicol. 176:116–127. [DOI] [PubMed] [Google Scholar]
- Norris D, Carr J. 2020. Vertebrate Endocrinology, 6th Edition. Academic Press, 656 pp. [Google Scholar]
- Noyes PD, Paul-Friedman K, Browne P, Haselman JT, Gilbert ME, Hornung MW, Barone S, Crofton KM, Laws SC, Stoker TE, Simmons SO, Tietge JE, Degitz SJ. 2019. Evaluating chemicals for thyroid disruption: Opportunities and challenges with in vitro testing and adverse outcome pathway approaches. Environ. Health Perspect. 127, 10.1289/EHP5297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organisation for Economic Cooperation and Development (OECD). 2012. OECD Conceptual Framework for Testing and Assessment of Endocrine Disruptors. OECD, Paris, France. [Google Scholar]
- Organisation for Economic Cooperation and Development (OECD). 2018. Revised Guidance Document 150 on Standardized Test Guidelines for Evaluation of Chemicals for Endocrine Disruption. C.2.9. Fish Sexual Development Test (FDST), OECD TG 234. OECD; Paris, France. [Google Scholar]
- Organisation for Economic Cooperation and Development (OECD). 2022. OECD AOP Knowledgebase. OECD, Paris, France. https://aopkb.oecd.org/background.html. Accessed 16 December 2022. [Google Scholar]
- Orlando EF, Kolok AS, Binzcik GA, Gates JL, Horton MK, Lambright CS, Gray LE, Soto AM, Guillette LJ. 2004. Endocrine-disrupting effects of cattle feedlot effluent on an aquatic sentinel species, the fathead minnow. Environ. Health Perspect.112:353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Örn S, Holbech H, Madsen TH, Norrgren L, Petersen GI. 2003. Gonad development and vitellogenin production in zebrafish (Danio rerio) exposed to ethinylestradiol and methyltestosterone. Aquat. Toxicol. 65:397–411. [DOI] [PubMed] [Google Scholar]
- Örn S, Holbech H, Norrgren L. 2016. Sexual disruption in zebrafish (Danio rerio) exposed to mixtures of 17α-ethinylestradiol and 17β-trenbolone. Environ. Toxicol. Chem. 41:225–231. [DOI] [PubMed] [Google Scholar]
- Pandian TJ, Sheela SG. 1995. Hormonal induction of sex reversal in fish. Aquaculture 138:1–22. [Google Scholar]
- Parks LG, Lambright CS, Orlando EF, Guillette LJ, Ankley GT, Gray LE. 2001. Masculinization of female mosquitofish in kraft mill effluent contaminated Fenholloway River water is associated with androgen receptor agonist activity. Toxicol. Sci. 62:257–267. [DOI] [PubMed] [Google Scholar]
- Payne AH, Hales DB. 2004. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocrine Rev 25: 947–970. [DOI] [PubMed] [Google Scholar]
- Piferrer F, Baker IJ, Donaldson EM. 1993. Effects of natural, synthetic, aromatizable, and nonaromatizable androgens in inducing male sex differentiation in genotypic female chinook salmon (Oncorhynchus tshawytscha). Gen. Comp. Endocrinol. 91:59–65 [DOI] [PubMed] [Google Scholar]
- Purdom CE, Harriman PA, Bye VVJ, Eno NC, Tyler CR, Sumpter JP. 1994. Estrogenic effects of effluent from sewage treatment works. Chem. Ecol. 8:275–285. [Google Scholar]
- Ramsey M, Shoemaker C, Crews D. 2007. Gonadal expression of Sf1 and aromatase during sex determination in the red-eared slider turtle (Trachemys scripta), a reptile with temperature-dependent sex determination. Differentiation 75:978–991. [DOI] [PubMed] [Google Scholar]
- Ruksana S, Pandit NP, Nakamura M. 2010. Efficacy of exemestane, a new generation of aromatase inhibitor, on sex differentiation in a gonochoristic fish. Comp. Biochem. Physiol.- Toxicol. Pharmacol. 152D:69–74. [DOI] [PubMed] [Google Scholar]
- Seki M, Yokota H, Matsubara H, Maeda M, Tadokoro H, Kobayashi K. 2004. Fish full life-cycle testing for androgen methyltestosterone on medaka (Oryzias latipes). Environ. Toxicol. Chem. 23:774–781. [DOI] [PubMed] [Google Scholar]
- Shen ZG, Fan QX, Yang W, Zhang YL, Hu PP, Xie CX. 2013. Effects of non-steroidal aromatase inhibitor letrozole on sex inversion and spermatogenesis in yellow catfish Pelteobagrus fulvidraco. Biol. Bull. 225:18–23. [DOI] [PubMed] [Google Scholar]
- Society for the Advancement of Adverse Outcome Pathways (SAAOP). 2022. Welcome to the Collaborative Adverse Outcome Pathway Wiki (AOP-Wiki). Available from: https://aopwiki.org/ and http://www.saaop.org/. Accessed 2 August 2022.
- Thornton JW. 2001. Evolution of vertebrate steroid receptors from an ancestral estrogen receptor by ligand exploitation and serial genome expansions. PNAS 98:5671–5676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe KL, Marca Pereira ML, Schiffer H, Burkhardt-Holm P, Weber K, Wheeler JR. 2011. Mode of sexual differentiation and its influence on the relative sensitivity of the fathead minnow and zebrafish in the fish sexual development test. Aquat. Toxicol. 105:412–420. [DOI] [PubMed] [Google Scholar]
- Uchida D, Yamashita M, Kitano K, Iguchi T. 2004. An aromatase inhibitor or high water temperature induce oocyte apoptosis and depletion of P450 aromatase activity in the gonads of genetic female zebrafish during sex-reversal. Comp. Biochem. Physiol.-Mol. Integrat. Physiol. 137A:11–20. [DOI] [PubMed] [Google Scholar]
- US Environmental Protection Agency (USEPA). 2019. Endocrine disruptor screening program (EDSP) overview. Washington, DC. https://www.epa.gov/endocrine-disruption/endocrine-disruptor-screening-program-edsp-overview. Accessed 2 August 2022.
- Villeneuve DL, Knoebl I, Kahl MD, Jensen KM, Hammermeister DE, Greene KJ, Blake LS, Ankley GT. 2006. Relationship between brain and ovary aromatase activity and isoform-specific aromatase mRNA expression in the fathead minnow (Pimephales promelas). Aquat. Toxicol. 76:353–368. [DOI] [PubMed] [Google Scholar]
- Villeneuve DL, Ankley GT, Makynen EA, Blake LS, Greene KJ, Higley EB, Newsted JL, Giesy JP, Hecker M. 2007. Comparison of fathead minnow ovary explant and H295R cell-based steroidogenesis assays for identifying endocrine active chemicals. Ecotoxicol. Environ. Safety 68:20–32. [DOI] [PubMed] [Google Scholar]
- Villeneuve DL, Crump D, Garcia-Reyero N, Hecker M, Hutchinson TH, LaLone CA, Landesmann B, Lettieri T, Munn S, Nepelska M, Ottinger MA, Vergauwen L, Whelan M. 2014. Adverse outcome pathway (AOP) development I: Strategies and principles. Toxicol. Sci. 142:312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve DL, Angrish MM, Fortin MC, Katsiadaki I, Leonard M, Margiotta-Casaluci L, Munn S, O’Brien JM, Pollesch N, Smith LC, Zhang X, Knapen D. 2018. Adverse outcome pathways II: Network analytics. Environ. Toxicol. Chem. 37:1734–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson VS, Cardon MC, Thornton J, Korte JJ, Ankley GT, Welch J, Gray LE, Hartig PC. 2004. Cloning, expression and characterization of the androgen receptor and isolation of the estrogen receptor alpha from the fathead minnow (Pimephales promelas). Environ. Sci. Technol. 38:6314–6321. [DOI] [PubMed] [Google Scholar]
- World Health Organization. 2002. ICPS Global Assessment of the State-of-the-Science of Endocrine Disruptors. International Programme on Chemical Safety. https://www.who.int/publications/i/item/WHO-PSC-EDC-02.2. Accessed 14 November 2022. [Google Scholar]
- Yamaguchi T, Yamaguchi S, Hirai T, Kitano T. 2007. Follicle-stimulating hormone signaling and Fox12 are involved in transcriptional regulation of aromatase gene during gonadal sex differentiation in Japanese flounder, Paralichthys olivaceus. Biochem. Biophys. Res. Comm. 359:935–940. [DOI] [PubMed] [Google Scholar]
- Yin Y, Tang H, Liu Y, Chen Y, Li G, Liu X, H. Lin H 2017. Targeted disruption of aromatase reveals dual functions of cyp19a1a during sex differentiation in zebrafish. Endocrinology 158:3030–3041. [DOI] [PubMed] [Google Scholar]
- Yu G, Zhang D, Liu W, Wang J, Liu X, Zhou C, Gui J, Xiao W. 2018. Zebrafish androgen receptor is required for spermatogenesis and maintenance of ovarian function. Oncotarget 9: 24320–24334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Li M, Ma H, Liu X, Shi H, Li M, Wang D. 2017. Mutation of foxl2 or cyp19a1a results in female to male sex reversal in XX Nile tilapia. Endocrinology 158:2634–2647. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No new data were generated for this review manuscript.


