Abstract
The TGF-β regulatory system plays crucial roles in the preservation of organismal integrity. TGF-β signaling controls metazoan embryo development, tissue homeostasis, and injury repair through coordinated effects on cell proliferation, phenotypic plasticity, migration, metabolic adaptation, and immune surveillance of multiple cell types in shared ecosystems. Defects of TGF-β signaling, particularly in epithelial cells, tissue fibroblasts, and immune cells, disrupt immune tolerance, promote inflammation, underlie the pathogenesis of fibrosis and cancer, and contribute to the resistance of these diseases to treatment. Here we review how TGF-β coordinates multicellular response programs in health and disease, and how this knowledge can be leveraged to develop treatments for diseases of the TGF-β system.
Introduction
The development, homeostasis, and repair of metazoan tissues rely on the multipotency and proliferative capacity of rare progenitor cell populations and their progenies, the support of neighboring cells, the surveillance of the immune system, and the input of potent signals. The transforming growth factor β (TGF-β) family of cytokines stands out as the most pleiotropic of these signals and, frequently, also the most dominant. The discovery of TGF-β1 and the elucidation of its signaling pathway from membrane receptors to target genes2 enabled the delineation of the biology of these factors,3-8 the structural basis for TGF-β signaling,9-11 the context-dependent nature of the TGF-β effects,12 and how congenital skeletal, connective and cardiovascular diseases, as well as chronic inflammation, fibrosis, and cancer arise from malfunctions in this pathway.13-17 But as the basis for the different effects of TGF-β on myriad cell types became clear, questions of a higher order emerged: Do the many effects of TGF-β serve a common purpose? How are these effects coordinated? And how can this knowledge be leveraged to treat diseases of the TGF-β system?
Many effects – one overarching role
Among the plethora of TGF-β effects on different cells and tissue environments (Figure 1), some relate to growth control ranging from suppression of proliferation through cell cycle inhibitors in epithelial and hematopoietic cells to stimulation of fibroblast proliferation through the release of mitogens. Other effects of TGF-β relate to the regulation of phenotypic plasticity though genome-wide chromatin changes that modify the developmental state and transcriptional landscape of a cell. Examples include the regulation of pluripotency in stem cells, mesenchymal phenotypic transitions in epithelial and endothelial progenitors, migration and axon formation in neurons, and differentiation in mesenchymal, hematopoietic and epithelial lineages. TGF-β is also a potent fibrogenic signal for fibroblasts, connective tissue, and epithelial cells to produce and remodel the extracellular matrix (ECM). As a key enforcer of immune tolerance and a suppressor of inflammation, TGF-β restricts multiple functions of the adaptive and innate immune systems. The pleiotropic nature of TGF-β distinguishes it from WNT, Hedgehog, Notch, and tyrosine kinase effectors which primarily act to promote organized tissue growth.
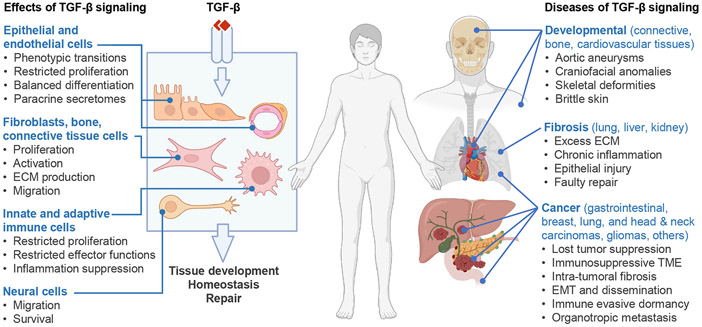
Figure 1. TGF-β in health and disease.
TGF-β guards tissue homeostasis through multiple effects on different cell types. Although TGF-β signals through a common receptor and a set of transcription factors in all cells, it triggers different effects on diverse cellular functions depending on the cell type and tissue environment. Epithelial cells, fibroblasts, immune, vascular, connective, and neural cells are important TGF-β targets, and their coordinated responses determine the overall effect of TGF-β on a tissue. The whole tissue, more than any of the constituent cell types, is the target of TGF-β, and preserving tissue integrity is the ultimate output. TGF-β response programs drive embryo development and promote tissue homeostasis and injury repair in the adult. Congenital defects in TGF-β signaling cause rare yet serious developmental syndromes, and somatic alterations of this pathway underly common forms of fibrosis and cancer.
Notably, TGF-β triggers these diverse effects through a common membrane receptor and a common set of SMAD transcription factors. Although the signaling activity of the TGF-β pathway determines the strength and duration of a response, the nature of this response depends on contextual determinants such as the type and developmental state of the target cell and the presence of response-modifying signals. As a result of these variables, TGF-β can have diverse, sometimes opposite effects. For example, TGF-β can function as an enforcer of homeostasis in a healthy epithelium, as an apoptotic signal in pre-malignant cells arising in this tissue, and as a tumor progression agonist in carcinoma cells that avert this tumor suppressive effect.
The opposite roles of TGF-β as guardian of homeostasis and instigator of pathogenesis have baffled biologists and the pharmaceutical industry, earning TGF-β epithets like “jack of all cytokine trades” and “Jekyll and Hyde growth factor”. However, when taken together, the disparate effects of TGF-β fulfill a common purpose of balancing homeostasis and injury repair. Three cell types – epithelial cells, immune cells, and tissue fibroblasts– are central targets of TGF-β in this overarching function as well as in the most common diseases of TGF-β signaling: chronic inflammation, fibrosis, and cancer (Figure 1). Connective tissue, skeletal, smooth muscle cells and endothelial are also highly responsive to TGF-β, as demonstrated by the consequences of TGF-β malfunctions in these tissues. Yet overall, the whole tissue, more than any of its constituent cell types, is the target of the TGF-β system, and preserving tissue integrity is the ultimate output of multicellular TGF-β responses.
Here, we review the current knowledge on TGF-β signaling, its effects on its principal target cell types, its involvement in common diseases of inflammation, fibrosis and cancer, and efforts to treat TGF-β dysfunctions. The emerging concepts are also relevant to all other members of the TGF-β family, their signaling functions, and their roles in development and homeostasis, as well as other disorders of TGF-β signaling including rare cardiovascular, connective tissue, and skeletal developmental diseases resulting from inherited mutations in TGF-β system components. 4,16,17 Our aim is to distill the basic principles and essential knowledge that inform this vast field.
Active cytokines and latent forms
The TGF-β family of cytokines includes two subfamilies, based on structural and biological criteria (Table 1). In mammals, the TGF-β/Nodal subfamily comprises TGF-β1, TGF-β2 and TGF-β3 (jointly referred to as TGF-β), Nodal, four Activins, and five Growth and Differentiation Factors (GDFs). It also includes the antagonistic ligands Inhibin, which blocks activin receptors, and Lefty1 and Lefty2, which block Nodal co-receptors. The Bone Morphogenetic Protein (BMP) subfamily includes eleven BMPs, four GDFs, and the Anti-Muellerian Hormone (AMH). BMP3 is a BMP receptor antagonist. Members of both subfamilies have pleiotropic effects during development and in adult tissues, although Nodal and AMH play only a few critical roles mostly in development.
Table 1. Mammalian TGF-β family members and receptors.
(*) TGFBR1 is also known as TβRI or ALK5; ACVR1A as ActR1A or ALK2; ACVR1B as ActR1B or ALK4; ACVR1C as ALK7; BMPR1A and BMPR1B as ALK3 and ALK6, respectively; ACVR2 and ACVR2B as ActRII and ActRIIB, respectively; and ACVRL1 as ALK1 or TSR1. (**) Inhibin, lefty, and BMP3 block the receptors for Activins, Nodal, and BMPs, respectively. Not included is GDF15, a distant member of the TGF-β family that binds to GDNF receptor α-like (GFRAL). GFRAL and related receptors for artemin, neurturin, persephin, and glial-derived neurotrophic factor (GDNF) signal through the receptor tyrosine kinase RET257
| Ligand | Type I Receptor | Type II Receptor | Co-receptor | Smad |
|---|---|---|---|---|
| TGFβ-1 | TGFBR1 * | TGFBR2 | Betaglycan | SMAD2/3 |
| TGFβ-2 | TGFBR1 | TGFBR2 | Betaglycan | SMAD2/3 |
| TGFβ-3 | TGFBR1 | TGFBR2 | Betaglycan | SMAD2/3 |
| Activin A | ACVR1B, ACVR1C | ACVR2A, ACVR2B | SMAD2/3 | |
| Activin B | ACVR1B, ACVR1C | ACVR2A, ACVR2B | SMAD2/3 | |
| Activin C | ACVR1B, ACVR1C | ACVR2A, ACVR2B | SMAD2/3 | |
| Activin E | ACVR1B, ACVR1C | ACVR2B | SMAD2/3 | |
| Nodal | ACVR1B, ACVR1C | ACVR2A, ACVR2B | Cripto, Cryptic | SMAD2/3 |
| GDF1 | ACVR1B, ACVR1C | ACVR2A, ACVR2B | Cripto, Cryptic | SMAD2/3 |
| GDF3 | ACVR1B, ACVR1C | ACVR2A, ACVR2B | Cripto, Cryptic | SMAD2/3 |
| GDF8/Myostatin | ACVR1B, ACVR1C | ACVR2A | SMAD2/3 | |
| GDF9 | ACVR1B | BMPR2 | SMAD2/3 | |
| GDF11 | ACVR1B, TGFBR1 | ACVR2A, ACVR2B | SMAD2/3 | |
| Inhibin ** | –– | ACVR2A | Betaglycan | –– |
| Lefty-1 ** | –– | –– | Cripto, Cryptic | –– |
| Lefty-2 ** | –– | –– | Cripto, Cryptic | –– |
| BMP2 | BMPR1A BMPR1B | ACVR2A, ACVR2B, BMPR2 | RGM | SMAD1/5 |
| BMP4 | BMPR1A BMPR1B | ACVR2A, ACVR2B, BMPR2 | SMAD1/5 | |
| BMP5 | ACVR1A, BMPR1A, BMPR1B | ACVR2A, ACVR2B, BMPR2 | SMAD1/5 | |
| BMP6 | ACVR1A, BMPR1A, BMPR1B | ACVR2A, ACVR2B, BMPR2 | RGM | SMAD1/5 |
| BPM7 | ACVR1A, BMPR1A, BMPR1B | ACVR2A, ACVR2B, BMPR2 | SMAD1/5 | |
| BPM8 | ACVR1A, BMPR1A, BMPR1B | ACVR2A, ACVR2B, BMPR2 | SMAD1/5 | |
| BPM8B | BMPR1A, BMPR1B | ACVR2A, BMPR2 | SMAD1/5 | |
| BMP9/GDF2 | ACVRL1 | ACVR2, BMPR2 | Endoglin | SMAD1/5 |
| BMP10 | ACVRL1 | ACVR2, BMPR2 | Endoglin | SMAD1/5 |
| BMP15 | BMPR1B | BMPR2 | SMAD1/5 | |
| GDF5 | BMPR1A, BMPR1B | ACVR2, ACVR2B, BMPR2 | SMAD1/5 | |
| GDF6 | BMPR1A, BMPR1B | ACVR2, ACVR2B, BMPR2 | SMAD1/5 | |
| GDF7 | BMPR1A, BMPR1B | ACVR2, ACVR2B, BMPR2 | SMAD1/5 | |
| GDF10 | BMPR1A, BMPR1B | ACVR2, ACVR2B, BMPR2 | SMAD1/5 | |
| AMH | ACVR1A, BMPR1A, | AMHR2 | SMAD1/5 | |
| BMP3 ** | –– | ACVR2B | –– |
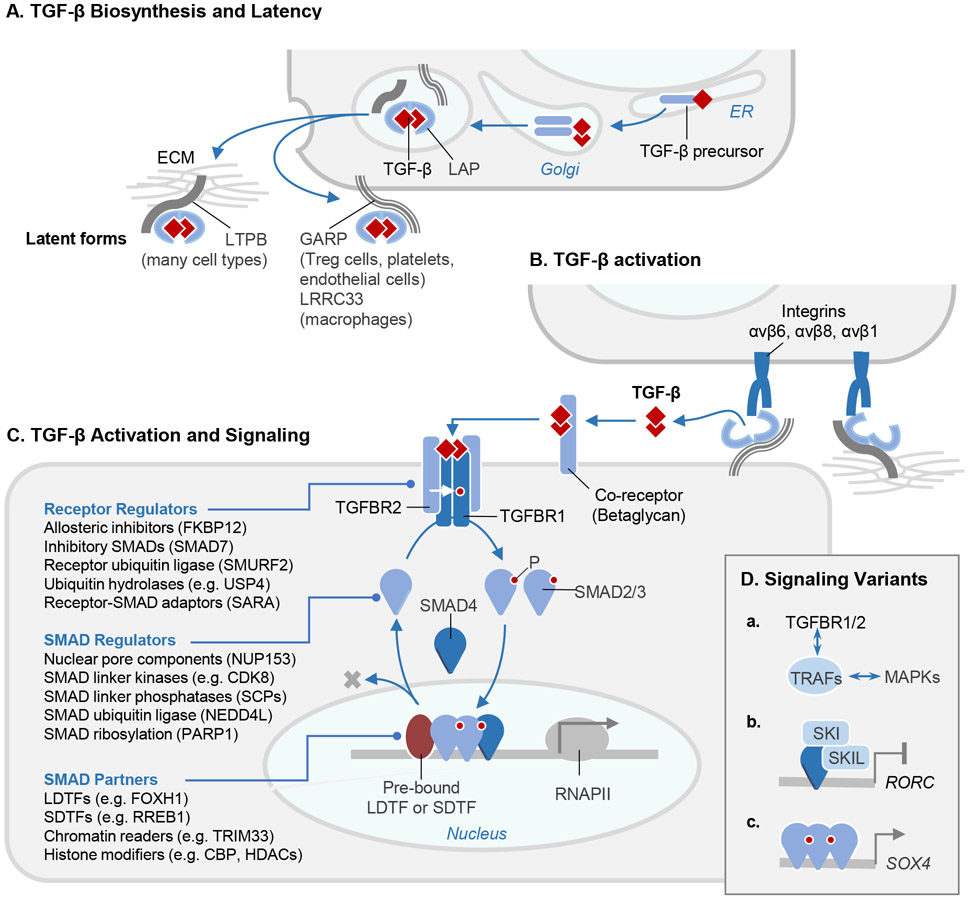
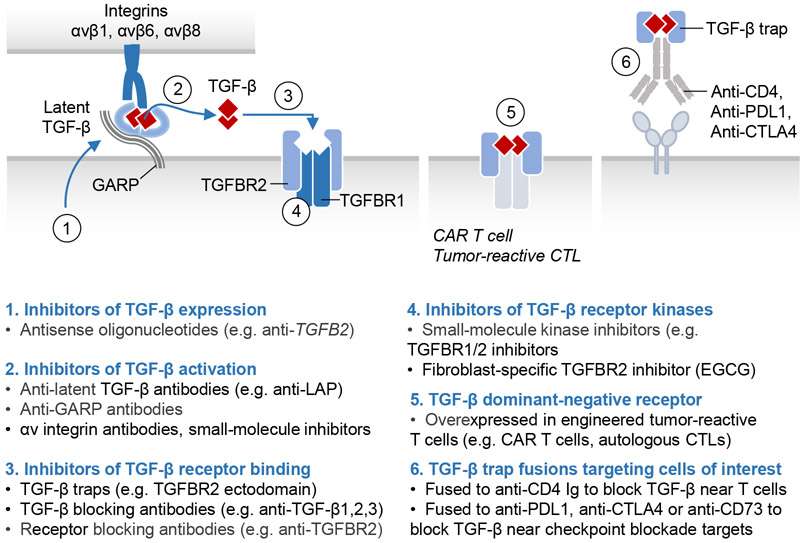
The three TGF-β isoforms are produced by many cell types. Each isoform is synthesized as a disulfide-linked dimeric precursor which is cleaved by the endoprotease furin in the Golgi. The cleaved N-terminal portion of the precursor is called the latency-associated peptide (LAP), and the C-terminal dimeric domain constitutes the mature TGF-β cytokine (Figure 2A). Three intrachain disulfide bonds within each TGF-β monomer form a structurally tight, highly stable “cystine knot” with protruding flexible loops that interact with receptors and ligand regulators. After cleavage, TGF-β remains noncovalently associated with LAP, and multiple contacts with LAP occlude the receptor-binding sites of TGF-β.18 In most cells this complex, called the “small latent TGF-β complex”, is disulfide linked to one of three latent TGF-β binding proteins (LTBPs 1, 3 and 4) or, in certain cells, to transmembrane leucine-rich repeat containing proteins LRRC32, also known as GARP (glycoprotein A repetitions predominant protein)19 or LRRC33.20 After secretion, LTBPs bind to the ECM and can be covalently cross-linked to fibronectin by tissue transglutaminases. GARP/LRRC32 and LRRC33 tether latent TGF-β to the surface of the TGF-β synthesizing cell (Figure 2A).
Figure 2. The TGF-β signaling pathway.
A. TGF-β cytokines are generated by cleavage of the dimeric C-terminal domain of a biosynthetic precursor in the Golgi. The mature cytokine remains sequestered by non-covalent binding to the N-terminal domain of the precursor, or latency-associated peptide (LAP). LAP in this complex becomes disulfide-linked to the latent TGF-β binding protein (LTBP), which is deposited in the extracellular matrix (ECM) after secretion. Alternatively, in the indicated cell types, LAP in the TGF-β complex becomes disulfide-linked to the membrane-anchored proteins GARP or LRRC33 and retained on the cell surface. B. Activation of latent TGF-β involves binding of LAP to αv integrins on adjacent cells, leading to a conformational change that releases the captive TGF-β for binding to receptors. C. The membrane proteoglycan Betaglycan functions as a co-receptor that collects TGF-β for presentation to signaling receptors. TGF-β binds to two pairs of transmembrane serine/threonine protein kinases known as TGFBR1 (type I receptor) and TGFBR2 (type II receptor), to assemble the receptor complex. In this complex, TGFBR2 phosphorylates and activates the TGFBR1 kinase, which binds and phosphorylates (P) the transcription factors SMAD2 and SMAD3. On phosphorylation, these SMADs form trimeric complexes with SMAD4 and accumulate in the nucleus to bind and transcriptionally activate target loci. Recognition of these loci by the SMAD complex frequently requires molecular interaction with lineage-determining transcription factors (LDTF) or signal-driven transcription factors (SDTF). The signaling cycle ends with SMAD dephosphorylation and dissociation from DNA for another round of signaling, or with SMAD polyubiquitination and degradation. Each step in the pathway is controlled by different classes of regulators, the most prominent of which are listed (with examples). D. Variant versions of this pathway include: (a) TGF-β receptors links with MAPKs through TRAF adaptor proteins; (b) SMAD4 recruitment of a SKI-SKIL repressor complex to certain target genes (e.g. RORC in TH17 helper T cells) to prevent leaky transcription in the absence of TGF-β; and (c) SMAD4-independent activation of certain target genes (e.g. SOX4 in pancreatic epithelial progenitors) by SMAD2 and SMAD3.
All other TGF-β family members are also dimers, disulfide-linked in most cases, synthesized as the C-terminal portion of a precursor. Homodimers are the prevalent forms, but natural heterodimers such as TGF-β1.2,21 activin AB22 and BMP2.723,24 further diversify the family. Cells can sense and compute inputs from multiple TGF-β family members and receptors simultaneously.25 Latent forms like those of TGF-β are known for only a few family members. However, several families of secretory molecules bind BMPs and Activins to withhold these ligands from membrane receptors.11
Critical steps in TGF-β activation
Because the association between native TGF-β and LAP is non-covalent, TGF-β can be activated in vitro by heat and extreme pH. However, no convincing data implicate changes in temperature or pH as activators of TGF-β in vivo. LAP presents potential cleavage sites for proteases to release active TGF-β,18 and various serine proteases (e.g. plasmin and cathepsin D) and metalloproteinases (e.g. MMP9 and MMP14) can activate TGF-β in vitro.26,27 However, the phenotypes of mice lacking these proteases do not phenocopy the loss of TGF-β function.28 Thus, the in vivo functional significance of proteolytic activation of TGF-β remains uncertain.
The ECM protein thrombospondin 1 (TSP1) contains an exposed peptide sequence (KRFK) that can bind a conserved sequence (LSKL) in the LAP of all three TGF-β isoforms. This interaction disrupts the association of LAP with the captive TGF-β.29 Mice lacking TSP1 manifest some of the phenotypes of TGF-β1-deficient mice, including inflammation and epithelial hyperplasia in multiple organs.30 TSP1 from infiltrating monocytes is an important mediator of TGF-β activation during vascular remodeling in a model of schistosomiasis-induced pulmonary arterial hypertension.31 However, several allosteric and force-driven processes are now recognized as the main mechanisms for activation of latent TGF-β in vivo. Delineating these mechanisms is a focus of current research and manipulating them is a goal of the pharmaceutical industry and the clinic.
Role of integrins.
The possibility that members of the integrin family of cell adhesion receptors activate latent TGF-β was first suggested by the phenotype of mice lacking the β6 subunit of the integrin αvβ6. αvβ6 is highly induced on epithelial cells in several organs by tissue injury and inflammation.32 Integrin β6 (Itgb6) knockout mice develop exaggerated inflammatory responses in the lungs and skin but are protected from tissue fibrosis in multiple tissues.33-35 Both phenotypic features are consistent with a deficit in TGF-β. Cells expressing αvβ6 can activate TGF-β1 and TGF-β3 by binding the sequence RGD that is present in an exposed loop of their LAP.34,36 Integrin αvβ8, which is expressed in neuroepithelial cells, astrocytes, and in subsets of myeloid cells, T cells, epithelial cells, and fibroblasts, binds to the same RGD site and can also activate TGF-β1.27 Mice lacking Itgb8 die during embryonic development or immediately after birth from intracerebral hemorrhage caused by defective vascular development in the central nervous system.37 Intracerebral hemorrhage in these mice is caused by the absence of αvβ8 on neuroepithelial cells which activates TGF-β for presentation to endothelial cells.38
The integrins αvβ6 and αvβ8 are essential for many of the developmental and homeostatic roles of TGF-β1, as shown by knock-in of a point mutation in TGF-β1 which prevents integrin binding.39 Administration of αvβ6 blocking antibody to Itgb8 knockout mice bred to bypass perinatal mortality, or crossing these mice to mice lacking Itgb6 recapitulates most of the developmental phenotypes of mice lacking TGF-β1 and TGF-β3.40 These phenotypes include severe multiorgan inflammation (a central feature of TGF-β1 knockout mice) and cleft palate (seen in TGF-β3 knockout mice). These observations indicate that integrins αvβ6 and αvβ8 are crucial for TGF-β activity during development and immune homeostasis. Equivalent protection from hepatic and pulmonary fibrosis by deletion of the integrin αv subunit from fibroblasts or treating mice with a small molecule inhibitor to the αvβ1 integrin support the idea that αvβ1 is the main TGF-β activating integrin in fibroblasts.41 In contrast to the TGF-β1 and TGF-β3 LAPs, the TGF-β2 LAP lacks an RGD sequence but contains an alternate sequence in an exposed loop that can bind to αvβ6 for activation.42 TGF-β2 might also be spontaneously active after secretion.43
Mechanisms of activation by integrins.
Integrin αvβ6 activates latent TGF-β1 and TGF-β3 by binding to the RGD sequence in the respective LAPs (Figure 2B). When αvβ6-expressing epithelial cells are induced to contract, physical force deforms the tethered latent complex either releasing free active TGF-β or changing the conformation of the captive cytokine to expose its receptor binding sites. Although expression of this integrin is restricted to epithelial cells, which are not generally considered to be highly contractile, evidence supports an important role for actin-myosin contraction and mechanical deformation of the latent complex in integrin αvβ6–mediated TGF-β activation.34,44
Deletion of LTBP1, required for tethering the latent complex to the extracellular matrix, also inhibits αvβ6-mediated TGF-β activation, and this defect can be rescued by a fusion protein composed of the LAP-tethering and fibronectin-binding domains of LTBP1.45 The crystal structure of the LAP-TGF-β1 complex shows that the cysteine residue in LAP used for tethering to LTBP1, GARP and LRRC33 and the integrin binding loop of LAP are located on opposite poles of the latent complex.18 These findings suggest that force applied across the tethered αvβ6–LAP complex unfolds the latency loop and releases the active cytokine.
Integrin αvβ8 does not seem to activate TGF-β through cell contraction.27 TGF-β activation by integrin αvβ8 is retained even after the entire β8 cytoplasmic domain is deleted. Recent high resolution cryo-EM structural data, together with studies showing that αvβ8 can activate a mutant form of latent TGF-β that cannot release the active cytokine from LAP, suggest that αvβ8 binding to LAP induces a conformational change in the latent complex that allows the captive TGF-β to bind to its receptors without release from LAP.46 The importance of this mechanism for activation by αvβ6 and/or αvβ1 remains to be determined.
Unlike LTBPs, which are widely expressed, GARP and LRRC33 are each expressed on distinct subsets of immune cells and other cell types. GARP is restricted to regulatory T cells, endothelial cells, platelets, and some fibroblasts, whereas LRRC33 is expressed in macrophages and microglia.19,20 GARP and LRRC33 tightly tether latent TGF-β1 to the cell surface, and this tethering plays a critical role in activation of these latent complexes by αvβ6 and αvβ8 integrins (Figure 2B). Integrin-expressing cells can induce TGF-β signaling in adjacent cells. For example, αvβ8 expressed in one cell activates TGF-β signaling in the cell expressing GARP-tethered TGF-β. 46 This pattern fits with the observation that in vivo deletion of TGF-β ligands or TGF-β receptors from the same T cell8 often share many phenotypic features. On the other hand, deletion of Itgb8 from neuroepithelial cells results in a very similar phenotype as deletion of TGF-β receptors in microglia, which do not express Itgb8.47
TGF-β signal transduction
The TGF-β pathway epitomizes membrane-to-nucleus signaling by direct receptor-mediated activation of transcription factors (Figure 2C). The receptor subunit composition, ligand-driven activation mechanism, and signal propagation through SMAD proteins elucidated for TGF-β apply to the rest of the TGF-β family. The composition, function, structural basis, and the many layers of regulators of this pathway have been comprehensively reviewed.9,10,12 Here, we present the key features.
Receptors.
TGF-β ligands bind to pairs of transmembrane serine/threonine protein kinase subunits known as receptors type I and type II. Mammalian genomes include 7 type I receptors and 5 type II receptors9 which are bound in various pairwise combinations by specific ligands (Table 1). In the case of TGF-β1, each monomer contacts one TGF-β type II receptor (TGFBR2) molecule forming a composite ligand-receptor protein surface that is then recognized by one type I receptor molecule (TGFBR1).48 In the case of BMPs and Activins, each monomer contacts independent surfaces of the corresponding type I and type II receptors.49,50 Thus, ligand binding results in the assembly of a hetero-tetrameric receptor complex bound by the dimeric ligand (Figure 2C). The TGFBR2 subunits then phosphorylate a Gly/Ser-rich region (GS region) situated near the kinase domain of the TGFBR1 subunits. Binding of the small protein FKBP12 to the GS region in the unliganded TGFBR1 locks the kinase activity in an inactive state.51 Once phosphorylated by TGFBR2, the GS region is thought to release FKBP12 and serve as a docking site for SMAD proteins as substrates of the TGFBR1 kinase.52 Numerous small-molecule kinase inhibitors have been developed against TGFBR1 and TGFBR2 that block all TGF-β responses.14
Co-receptors.
Co-receptors are crucial for binding of TGF-β and several other family members to the signaling receptors (Table 1). The core protein of the membrane-anchored proteoglycan betaglycan (also known as the type III TGF-β receptor) binds TGF-β for presentation to TGFBR2. 9 This step is particularly important for TGF-β2, which has low intrinsic affinity for the signaling receptors compared to TGF-β1 and TGF-β3.53,54 The transmembrane protein endoglin is an essential co-receptor for BMP9 and BMP10. Other co-receptors are anchored to the cell surface via glycosylphosphatidylinositol tails, including the essential Nodal co-receptors Crypto and Cryptic, and Repulsion Guidance Molecules (RGM) as co-receptors for certain BMPs.
SMAD transcription factors.
SMAD transcription factors are direct substrates of type I receptor kinases (Figure 2C). SMAD proteins consist of N-terminal (or MH1) and C-terminal (or MH2) globular domains connected by a flexible linker region.9,10 The N-terminal domain binds to DNA whereas the C-terminal domain includes sites for SMAD interaction with type I receptors, receptor adaptor proteins, other SMADs, nucleocytoplasmic shuttling factors, DNA binding cofactors, histone acetylases such as p300 and CBP, and chromatin remodeling proteins. The type I receptors for the TGF-β subfamily primarily phosphorylate SMAD2 and SMAD3, whereas those for the BMP subfamily phosphorylate SMADs 1, 5 and 8, with crossover SMAD signaling occurring in certain contexts. These five SMAD proteins are called “receptor-regulated SMADs (R-SMADs).
In the basal state, R-SMAD proteins shuttle between the cytoplasm and the nucleus. Receptor-mediated phosphorylation targets two serine residues at the C-terminus. The resulting pSer-X-pSer-carboxyl group mediates SMAD-SMAD binding for the assembly of trimeric complexes with SMAD4 (R-SMAD–R-SMAD–SMAD4 complexes). SMAD4 is not a receptor substrate nor is it required for R-SMAD nuclear translocation, but it is an essential participant in most SMAD-mediated transcriptional responses. The specific function served by SMAD4 remains unknown. SMAD6 and SMAD7 are inhibitory SMADs which antagonize SMAD4 and the type I receptors, respectively. TGF-β, BMP, interferon-γ, and other signals induce the expression of SMAD7 for negative feedback and antagonistic crosstalk in the pathway.55
Receptor-activated SMAD complexes bind to hundreds of genomic loci in the nucleus, where SMADs undergo phosphorylation at the linker region by the RNA polymerase II (RNAPII) kinases CDK8 and CDK9.56 This stimulates the transcriptional activity of SMAD complexes while leading to further phosphorylation of the linker by glycogen synthase kinase 3β. GSK3β creates binding sites for the HECT domain ubiquitin ligases SMURF1/2 (in SMAD1 and 5) and NEDD4L (in SMAD2 and 3) to mark the activated SMADs for degradation.56-58 Alternatively, SMADs undergo dephosphorylation by SCP1/2 phosphatases59 and dissociation by poly(ADP-ribose) polymerase-1 (PARP-1) mediated ribosylation, to be recycled for new rounds of signaling.60 The regulation of SMADs and RNAPII by related kinases and phosphatases suggests a close coordination in SMAD-dependent activation of RNAPII transcription. The linker region of SMADs is also phosphorylated in the cytoplasm by mitogen-activated protein kinases (MAPKs) and cell cycle CDKs.61,62 Additional regulators of the TGF-β pathway include decoy receptors, mediators of SMAD nucleocytoplasmic shuttling, transcriptional co-activators and co-repressors, non-coding RNAs, and ubiquitination-based receptor and SMAD turnover12 (Figure 2C).
Pathway conservation and mutation.
X-ray crystal structures for ligands, receptors, and SMAD proteins have provided a wealth of insights into the function and specificity of the TGF-β pathway, including the steps of latent TGF-β activation, ligand interactions with traps and receptors, and the interaction of SMAD with receptors, regulators, DNA, and DNA-binding cofactors.9,10,50,63
TGF-β and BMP ligands, receptors, co-receptors, and SMAD proteins, as well as the dichotomy of these two subfamilies are highly conserved across metazoans. The functional complementarity between the two subfamilies is manifest in many contexts, for example, in primordial germ cell development,64 hair follicle progenitor differentiation,65 and epithelial-mesenchymal transitions (EMT).66 Although TGF-β gave name to the entire gene family, TGF-β is restricted to deuterostomes (vertebrates, crinoids, and sea stars) whereas BMPs and Activins are present across all metazoan phyla.63 TGF-β pathway agonists have also emerged by convergent evolution. The rodent intestinal parasitic helminth Heligmosomoides polygyrus encodes a structurally unrelated TGF-β mimic (Hp-TGM) which binds to TGF-β receptors in host T cells to suppress immune attack.67
The central components of the TGF-β system are essential for mammalian development, as shown by gene knockouts in mice. There is no gastrulation without Nodal, and deletion of TGF-β receptors, SMAD2, or SMAD4 is embryonic lethal. As we discuss below, loss-of-function somatic mutations in TGFBR1, TGFBR2, SMAD2, SMAD3 and SMAD4 are frequent in certain types of cancer, and inherited SMAD4 mutations cause a juvenile polyposis and hemorrhagic telangiectasia syndrome with propensity to intestinal cancer. Immune dysregulation, fibrosis, and cancer are the most common diseases involving somatically mutated or otherwise altered TGF-β signaling in the adult, and hence are the focus of this review. Notably, inherited mutations in the TGF-β system in human are the cause of rare if devastating diseases of skeletal, connective, and cardiovascular tissues in human (Table 2). Inherited mutations in TGFB1 encoding a hyperactive TGF-β1 variant cause Camurati-Engelmann disease, a debilitating syndrome characterized by abnormally thick skull and limb bones, joint deformities, and spine curvature.68 Inherited and occasionally spontaneous mutations in TGFB2, TGFB3, TGFBR1, TGFBR2, and SMAD3 cause the five known types of Loeys-Dietz aortic aneurysm syndrome, which is characterized by multiple connective tissue alterations and is highly variable in penetrance, age of manifestation, and severity of the symptoms. These alterations include craniofacial anomalies (e.g. premature skull bone fusion, hypertelorism, bifid uvula), deformities in spine, chest and foot bones, osteoarthritis, a brittle skin prone to bruising, and, most ominously, an enlarged aorta prone to bulging (aneurysm) and rupture.69 Paradoxically, while the mutant alleles causing Loeys-Dietz syndrome encode functionally weakened protein products, the affected tissues show heightened TGF-β signaling activity perhaps resulting from an imbalance in the regulation of other branches of the TGF-β family in these tissues.
Table 2. Congenital conditions associated with TGF-β pathway mutations.
| Mutant gene | Condition | Refs. |
|---|---|---|
| Ligands | ||
| TGFB1 | Camurati-Engelmann disease | 68 |
| TGFB2 | Loeys-Dietz aortic aneurysm syndrome type 4 | 258 |
| TGFB3 | Loeys-Dietz aortic aneurysm syndrome type 5, arrhythmogenic ventricular dysplasia | 259 |
| INHA | Male infertility; Premature ovarian failure | 260,261 |
| NODAL | Heterotaxy | 262 |
| BMP2 | Brachydactyly | 263 |
| BMP6 | Iron overload | 264 |
| BMP10 | Pulmonary arterial hypertension | 265 |
| BMP15 | Ovarian dysgenesis | 266 |
| GDF1 | Congenital cardiovascular malformations | 267 |
| GDF2 (BMP9) | Hereditary hemorrhagic telangiectasia type 5 | 268-270 |
| GDF3 | Microphthalmia, coloboma, skeletal abnormalities | 271 |
| GDF5 | Chondrodysplasia, brachydactyly, symphalangism, acromesomelic dysplasia | 272-274 |
| GDF6 | Klippel-Feil syndrome, microphthalmia, Leber congenital amaurosis | 275,276 |
| MSTN (GDF8) | Increased skeletal muscle mass | 277 |
| GDF9 | Polycystic ovary syndrome | 278 |
| AMH | Persistent Mullerian duct syndrome type 1 | 279 |
| Receptors | ||
| TGFBR1 | Loeys-Dietz aortic aneurysm syndrome type 1 | 280 |
| TGFBR2 | Loeys-Dietz aortic aneurysm syndrome type 2, Marfan syndrome type 2 | 280,281 |
| ACVR1A | Fibrodysplasia ossificans progressiva | 282 |
| ACVR2A | Pre-eclampsia | 283 |
| ACVR2B | Left-right axis malformations | 284 |
| ACVRL1 | Hereditary hemorrhagic telangiectasia type 2, pulmonary arterial hypertension | 285,286 |
| BMPR1B | Pulmonary arterial hypertension, acromesomelic dysplasia, juvenile polyposis | 287-289 |
| BMPR2 | Pulmonary arterial hypertension, pulmonary veno-occlusive disease | 290-292 |
| AMHR2 | Persistent Mullerian duct syndrome type 2 | 293 |
| Co-receptors | ||
| ENG (Endoglin) | Hereditary hemorrhagic telangiectasia type 1, pulmonary arterial hypertension | 286,294 |
| TDGF1 (Cripto) | Forebrain defects | 295 |
| CFC1 (Cryptic) | Autosomal visceral heterotaxy, congenital heart disease | 296,297 |
| SMADs | ||
| SMAD1 | Pulmonary arterial hypertension | 298 |
| SMAD3 | Loeys-Dietz aortic aneurysm syndrome type 3 | 299 |
| SMAD4 | Juvenile polyposis–hereditary hemorrhagic telangiectasia syndrome | 212,298,300 |
| SMAD8 | Pulmonary arterial hypertension | 298 |
Pathway variations.
Although the type I receptors are the main substrates for the type II receptor kinases, TGFBR2 also phosphorylates PAR6 (partition defective 6) to regulate intercellular tight junctions and cell migration in epithelial cells.70,71 A long C-terminal extension in BMPR2 mediates the activation of LIM domain kinase 1 (LIMK1) which phosphorylates cofilin to inhibit actin polymerization. In neurons this phosphorylation regulates neurite outgrowth.72-74
As transducers of TGF-β signals, SMADs primarily mediate transcriptional activation responses with recruited co-activators.12 However, SMADs can also recruit co-repressors. In the absence of TGF-β, SMAD4 forms a complex with SKI (Sloan Kettering Institute proto-oncogene) and the related SKIL (also known as SnoN) which recruit histone deacetylases (HDACs) to prevent the leaky expression of TGF-β target genes under basal conditions (Figure 2D). In CD4+ T cells, the SMAD4-SKI-SKIL complex inhibits expression of RORγt (encoded by RORC) to prevent differentiation into T helper 17 (TH17) cells.75 A failure of SMAD4 with SKI to inhibit intestinal CD8+ T cells triggers chronic inflammatory bowel disease.76 The SMAD4-SKI-SKIL complex is dismantled when TGF-β-activated SMAD2 and SMAD3 join this complex and the E3 ligase Arkadia causes SKI and SKIL polyubiquitination and degradation.77,78 TGF-β activated SMADs increase the expression of SKIL and SMAD7, creating negative feedback loops.79 Other SMAD-binding repressors include TGIF1 and TGIF2, which also interact with retinoid acid receptors and are implicated in the intermodulation of these pathways.80,81
Gene activation by R-SMAD not always requires SMAD4. For example, induction of the transcription factor SOX4 by TGF-β in pancreatic epithelial progenitors requires SMAD2 and SMAD3 but not SMAD4.82 This is in line with the requirement of SMAD2 and SMAD3, but not SMAD4, for pancreas development.83,84 Beyond transcriptional regulation, TGF-β- and BMP-activated SMADs bind pri-miRNA microRNA precursors and the Drosha/DGCR8 microprocessor and enhance pri-miRNA processing into pre-miRNA.85
Early studies on TGF-β and BMP signaling implicated MAPK kinase kinase 7 (MAP3K7), renamed TGF-β activated kinase 1 (TAK1) .86 MAP3K7/TAK1 is a central signal transducer of receptors for major pro-inflammatory signals including interleukin-1β (IL-1β), tumor necrosis factor (TNF), and Toll-like receptors.87 The MAPKs ERK1 and 2, p38MAPK, and JNK, and the phosphatidylinositol 3-kinase (PI3K) can be activated by TGF-β in cell culture.88 The receptor adaptor proteins TRAF4, TRAF6, and SHC have been implicated in TGF-β receptor coupling to MAPK pathways89-91 (Figure 2D). However, genetic evidence and a structural basis for MAPKs and PI3K serving as direct mediators of TGF-β receptor signaling are lacking. TAK1, ERKs, p38, JNK, and PI3K have well-established agonists of their own, including inflammatory signals, receptor tyrosine kinases, cellular stresses, and metabolic sensors. These agonists typically abound in the microenvironment of TGF-β target cells in vivo, raising questions about the significance of TGF-β as an activator of these pathways. In contrast, there is strong genetic and functional evidence that RAS-MAPK activation by canonical agonists or oncogenic RAS mutations is a key collaborator of TGF-β-activated SMADs in the induction of EMTs (see below). The prevailing consensus is that most effects of TGF-β are mediated by the SMAD pathway and influenced by the activity of the MAPK, WNT, and other major pathways.
Basis for contextual responses
The DNA binding activity of SMADs is essential for their role as signal-activated regulators of gene expression. SMAD binding to DNA is mediated by a protruding β-hairpin in the MH1 domain of identical sequence among R-SMADs and SMAD4.92 SMAD2 also binds to this motif but contains a unique flexible loop that occludes the β-hairpin when in the closed conformation.93 Regardless, all R-SMADs and SMAD4 bind with similar affinity to 5-bp GC-rich motif variants including CAGAC, GGCGC, and others.94 Although the DNA binding activity of SMADs is necessary for their function, it is insufficient to dictate pathway-specific and cell-type specific choice of TGF-β target genes.
TGF-β target gene selection depends on the ability of R-SMADs to differentially associate with context-specific transcription factors, forming complexes that combine the DNA binding specificity of the various components. By combining with different partners, TGF-β-activated SMADs and BMP-activated SMADs gain access to different target genes and generate pathway-specific responses. And, by combining with different partners in different cell types, TGF-β-activated SMADs give rise to cell-type specific responses. The paradigm is forkhead box H1 (FOXH1, previously known as Fast1), an essential maternal factor in Nodal-driven mesendoderm differentiation during gastrulation.95 In epiblast cells, which have a relatively nucleosome-dense chromatin, FOXH1 functions as a pioneer transcription factor that binds to cis-regulatory elements in endoderm specification genes (Gsc, Eomes, Mixl, Foxa2) for activation by Nodal-driven SMADs.93,96 FOXH1 selectively binds to SMAD2 and SMAD3, directing these factors to FOXH1-loaded loci. Consistent with a role as a pioneer factor, FOXH1 binds to DNA with extensive interactions over the minor and major grooves and shows higher affinity for its cognate sequence in nucleosomal DNA than in a linear DNA fragment.97
Lineage-determining transcription factors (LDTFs) like FOXH1 act as determinants of cellular responses to TGF-β signaling in many other contexts.12 TGF-β-activated SMADs co-bind the genome with the transcription factor MyoD1 in myoblasts to regulate myogenic differentiation, with PU.1 in pro-B cells to regulate B cell differentiation,98 and with other partners to inhibit cell proliferation and regulate immune cell functions, as mentioned below. BMP-activated SMADs pair with the zinc-finger transcription factor ZFP423 to drive ventral mesoderm specification in Xenopus99 and co-occupy the genome with C/EBPα and GATA1 to drive myeloid and erythroid differentiation in hematopoietic progenitors.100 Genome occupancy by SMADs is also determined by signal-driven transcription factors (SDTFs) as in the case of the RAS-MAPK responsive factor RREB1 discussed below, and by the accessibility of the chromatin at potential SMAD target loci.101 SMADs additionally collaborate with factors that bind poised chromatin marks for gene activation, as is the case of TRIM33 and Nodal-activated SMADs in mesendoderm progenitors.102,103
TGF-β and immune regulation
Fine tuning of adaptive and innate immunity is critical to the maintenance of organismal integrity. Perturbations in this control contribute to disease pathology, and reversing these perturbations is a common therapeutical goal. TGF-β is a key modulator of innate and adaptive immunity, acting as a general enforcer of immune tolerance and a suppressor of inflammation. These functions are fundamental in TGF-β biology. An excess of TGF-β activity causes immunosuppression which supports tumorigenesis, whereas a deficit can result in inflammation leading to fibrosis. These roles were apparent in Tgfb1 knockout mice, which die of multiorgan inflammation early in life.104 This phenotype is substantially rescued by loss of MHC class II,105 suggesting a critical role for TGF-β in constraining adaptive immune responses. T cell–specific deletion of Tgfbr2 early in development causes a similar phenotype,106,107 suggesting that TGF-β acts directly on T cells to suppress excessive adaptive immunity during early post-natal life. TGF-β appears to have a more limited role in the homeostatic regulation of T cells in adult mice. After the immediate perinatal period, TGF-β signaling in T cells is dedicated to dampening responses to pathologic stimuli.108 However, the effects of TGF-β on immune cells are context specific and include cases of enhanced immune cell activity. For example, mice lacking TGFBR2 in T cells have reduced numbers of peripheral naive CD4+ T cells.109 TGF-β suppresses the proliferation and activation of B cells yet it stimulates their IgA class switching function.110 The profound effects of TGF-β signaling on the immune system has been comprehensively reviewed.8,15,111 Here we highlight the most prominent effects of TGF-β on the main components of the immune system (Figure 3).
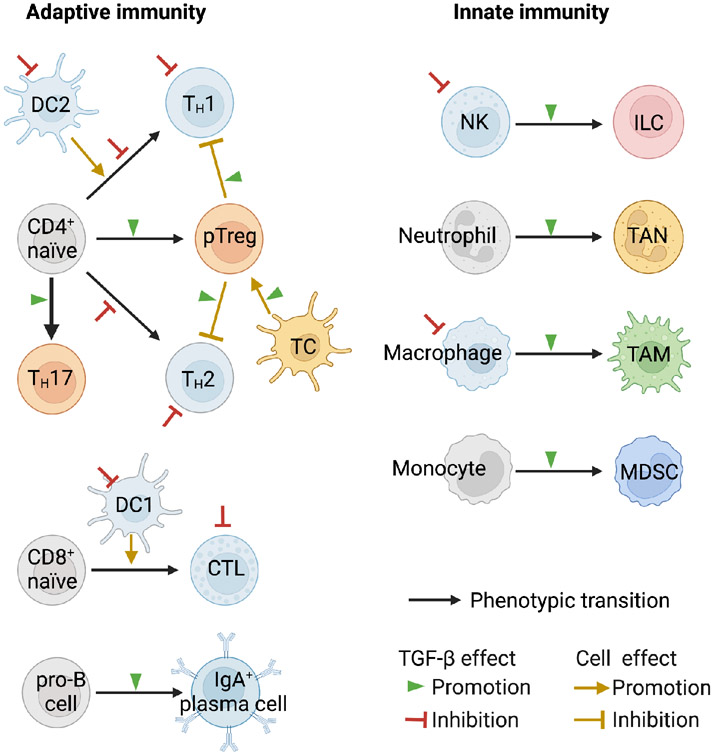
Figure 3. TGF-β and immune regulation.
Scheme of the main classes of immune cells and their regulation by TGF-β in the adult. TGF-β is a critical modulator of both adaptive and innate immunity arms, acting as a general enforcer of immune tolerance and a suppressor of inflammation. In the adaptive arm, TGF-β inhibits the maturation of naïve CD4+ T cells into TH1 and TH2 T helper cells and of naïve CD8+ T cell into cytotoxic T lymphocytes (CTL). TGF-β exerts these effects through direct inhibition of CD4+ and CD8+ maturation and through inhibition of dendritic cell subsets (DC1, DC2) that drive naïve these maturation steps. TGF-β additionally inhibits the helper functions of TH1 and TH2, and the effector functions of CTL cells, and it can do so by acting directly on these cells as well as by promoting the differentiation of CD4+ T cells into peripheral regulatory T cells (pTreg), which inhibit TH1 and TH2 cells partly through TGF-β. A specialized RORγt+ antigen-presenting cell (TC ) activates pTreg cells in the intestinal lymph nodes. TGF-β inhibits B cell proliferation but stimulates IgA class switching in B cells. In the innate immunity arm, TGF-β blunts the effector functions of natural killer (NK) cells, and the inflammatory functions of neutrophils and macrophages while favoring, in the context of tumors, the adoption of tumor-associated neutrophil (TAN) and macrophage (TAM) states which support tumor progression. In chronic infection, inflammation, and cancer, the persistent myelopoiesis includes production of myeloid-derived suppressor cells (MDSC) with TGF-β dependent immunosuppressive functions. These regulatory effects of TGF-β on the immune system occur to different extents in different tissue contexts and depending on whether the circumstance is homeostasis, acute injury or infection, or chronic inflammation, fibrosis, or cancer.
Dendritic cells.
Various subsets of dendritic cells (DCs) play central roles in antigen presentation to CD4+ T cells (by DC2 dendritic cells) and CD8+ T cells (by DC1 dendritic cells) for priming of cytotoxic effector functions as well as regulation of the balance between T helper (TH) and regulatory T cells (Treg)112 (Figure 3). TGF-β regulates the function of these DC subsets. Deletion of Tgfbr2 from DCs in mice leads to multiorgan inflammation and death by 15 weeks of age.113 DCs lacking TGFBR2 express normal levels of MHC class II and costimulatory molecules but produce more interferon γ (IFN-γ), which reduces their ability to induce Treg cells. Adoptive transfer of wild type Treg cells, or inhibition of IFN-γ each only partially rescue this phenotype, suggesting that additional mechanisms are also at play. TGF-β is also important for the development of a subset of skin DCs called Langerhans cells. Targeted deletion of Tgfbr2 of Tgfb1 by langerin-Cre prevents the development of Langerhans cells.114 In contrast to the role of DCs as T cell activators, a subtype of RORγt + antigen-presenting cells, called thetis cells (TC), induce pTreg differentiation in intestinal lymph nodes during early life and require TGF-β-activating integrin αvβ8 for intestinal pTreg differentiation. Loss of Itgb8 in these cells causes colitis, suggesting that this population plays an essential role in tolerogenic antigen presentation.115
T helper cells.
TGF-β plays fundamental roles in regulating and balancing the differentiation of naïve T cells into specific effector subsets (Figure 3). CD4+ T helper (TH) cells support the development and function of CD8+ effector T cells and fall into two subtypes distinguished by their driving transcription factors and secreted cytokines. T-bet and STAT4 drive the differentiation of naïve CD4+ into TH1 cells, which produce IFN-γ and IL-2, and support CD8+ cytotoxic T cells and macrophages. TGF-β-SMAD signaling potently inhibits TH1 differentiation through coordinated effects on at least three levels: inhibition of IL-12 receptors required for TH1 differentiation, inhibition of the expression of T-bet and STAT4; and, inhibition of IFN-γ production by natural killer (NK) cells, thereby interfering with a positive feedback loop through which NK cell-derived IFN-γ amplifies TH1 differentiation.116 TH2 cells are driven by GATA3 and STAT6, and produce IL-4, IL-5 and IL-13 to support B cells and other effector cells. TGF-β inhibits differentiation of TH2 cells by down-regulating GATA3,117 and inhibiting GATA3 function indirectly by inducing the expression of SOX4.118 Balanced inhibition of both TH1 and TH2 cells by TGF-β is important. Although tissue inflammation and damage due to loss of TGF-β signaling in T cells is predominantly mediated by TH1 cells, a loss of the TH1-inducing T-bet led to multiorgan inflammation associated with enhanced TH2 cell differentiation.107 Disabling TGF-β signaling in CD4+ cells in mammary tumor-bearing mice augmented IL-4 production in TH2 cells (but not IFN-γ production in TH1 cells) leading to tumor regression.119
One exception to the general rule of TGF-β as an inhibitor of adaptive immune responses is that TGF-β-activated SMADs cooperate with RORγt to induce the T helper 17 (TH17) phenotype.120 TH17 cells, are important in immune responses to bacteria and fungi and in the development of autoimmunity. Mice lacking TGFBR2 on T cells and mice lacking the TGF-β activating integrin αvβ8 on DCs have reduced numbers of TH17 cells and are protected from developing Experimental Autoimmune Encephalomyelitis (EAE), a disease model that depends on TH17 cells.121 TGF-β-blocking antibody also protects against EAE while overexpression of active TGF-β by T cells exacerbates CNS inflammation and EAE.122
Regulatory T cells.
Treg cells are a subset of T cells that suppress immune responses to enforce tolerance.123 Treg cells perform this role through multiple specialized effects on T helper and effector cells. The transcription factor Foxp3 drives differentiation of naive CD4+ T cells into Treg cells. There are two major subtypes of Treg cells: natural Treg (nTreg), produced in the thymus in early life, and Treg derived from naïve CD4+ T cells in the periphery (pTreg).
TGF-β positively regulates Treg differentiation and activity. TGF-β enhances survival of nTreg cells by suppression of proapoptotic proteins and upregulation of the antiapoptotic protein Bcl2.124 TGF-β-activated SMADs cooperate with STAT5 and nuclear factor of activated T cells (NFAT) to induce expression of FOXP3 in naive CD4+ T cells125 (Figure 3). Mice lacking TGF-β1 or TGFBR2 in T cells display marked reductions in FOXP3+ Treg cell numbers in the periphery, consistent with a role for TGF-β in maintenance of these cells.106,107,126 TGF-β can also promote retention of pTreg cells in specific peripheral tissues such as the large intestine.127 Mice lacking a particular Foxp3 enhancer that is required for TGF-β-mediated pTreg induction do not develop the severe, early-onset multiorgan inflammation seen in mice lacking all Treg cells, suggesting that nTreg cells are sufficient to prevent this phenotype. However, older mice lacking pTreg cells develop TH2-mediated pathology in lung and intestine,128 indicating that pTreg cells do play important roles in controlling immune responses in some peripheral tissues.
Differentiation of naïve CD4+ T cells into pTreg cells or TH17 cells leads to drastically different effects on tissue inflammation. Upon initial sensing of TGF-β, naïve CD4+ T cells upregulate both Foxp3 (critical for Treg differentiation) and RORγt (critical for TH17 cell differentiation). Both the local concentration of active TGF-β and the presence of additional extracellular factors are important determinants of the commitment to either pTreg or TH17 cell fate. Low TGF-β concentrations inhibit the expression of the IL-23 receptor and favor Foxp3 expression, while high concentrations in conjunction with IL-6 upregulate the IL-23 receptor and favor RORγt expression and TH17 cell induction.120 Foxp3 inhibits RORγt function and prevents IL-17 induction, and this is counterbalanced by IL-6, IL-21, and IL-23 to facilitate the formation of TH17 cells. Thus, the Treg, TH17, TH1 and TH2 states are tied to each other through a mutual regulation of their generation and function with TGF-β as a central balancing signal.
Cytotoxic T lymphocytes.
CD8+ T cells mature into effector T cells, also called cytotoxic T lymphocytes (CTLs), which eliminate cancer cells and pathogen-infected cells through the release of cytolytic mediators. TGF-β dampens the proliferation and cytolytic functions of CTLs (Figure 3).8 In vivo, expression of a CD2-driven or CD4-driven dominant-negative TGFBR2 construct, which reduces TGF-β signaling, leads to expansion of CD8+ T cells. However, complete loss of TGF-β signaling in T cells inhibits CD8+ T cell development,107 likely due to the requirement for TGF-β to induce the IL-7 receptor on CD8+ T cells.129 Thus, distinct effects of TGF-β signaling on CD8+ T cell induction, expansion and activation appear to be quantitatively regulated, with low levels of TGF-β signaling acting as an initiator of development in the thymus, but higher levels acting as a brake to inhibit inappropriate expansion and activation in the periphery.
Consistent with a role of TGF-β as a brake on excessive CD8+ T cell function, TGF-β suppresses multiple effector functions of cytotoxic T cells, inhibiting expression of perforin, IFN-γ and granzymes A and B through SMADs in partnership with ATF1.130 Impairment of TGF-β signaling in T cells, or in vivo treatment with inhibitors of TGF-β activation have been consistently shown to enhance CD8+ T cell killing of tumor cells and to reduce tumor growth in multiple in vivo models. TGF-β signaling in CD8+ T cells is important in promoting apoptosis in short-lived effector cells.122 TGF-β also induces a specialized subset of CD8+ T cells residing within the single cell epithelial layer of the intestine that is important for the integrity of mucosal immune responses.131
Natural killer cells.
Natural killer (NK) cells are cytotoxic cells of the innate immune system. NK cells recognize target cells expressing NK cell chemotactic signals and NK receptor ligands upon viral infection or cancer-related genomic alterations. TGF-β blunts innate responses to viral infection and tumors through suppression of NK cell functions.8,111 In addition to inhibiting IFN-γ production by NK cells, TGF-β inhibits the expression of cell-surface receptors NKG2D and NKp30, which NK cells use to recognize and kill stressed and malignant cells.132,133 TGF-β also induces the expression mir-183 in NK cells, which reduces expression of the adaptor protein, DAP12, thus inhibiting responses to cytotoxic NK receptors including NKG2D.134 TGF-β further suppresses NK cell activity by inhibiting activating responses to IL-15.135 Moreover, TGF-β can contribute to immune evasion by inducing trans-differentiation of NK cells into type 1 innate lymphoid cells, which are not cytotoxic.136,137
Neutrophils and macrophages.
Neutrophils (also known as polymorphonuclear leukocytes) are highly prevalent among white blood cells and are responsive to infection and cancer.138 Neutrophils can adopt an anti-tumor phenotype but also a TGF-β-dependent pro-tumorigenic phenotype (tumor associated neutrophils, TAN) that significantly impacts tumor growth and the response to immunotherapy139 (Figure 3).
TGF-β signaling also has dramatic effects on macrophages. In vitro, incubation of tissue macrophages with TGF-β inhibits expression of multiple pro-inflammatory genes, including TNF, IL-12, and inducible nitric oxide synthase which are characteristic of inflammatory macrophages. In parallel, TGF-β induces expression of a suite of genes, including arginase 1 and IL-10 which are characteristic of tumor associated macrophages (TAM). Mice lacking TGFBR2 in myeloid cells demonstrate increased anti-tumor immunity, decreased tumor growth and metastasis and an increased predisposition to stroke.140,141 Although the mechanisms underlying these events differ among models, the decrease in metastases and predisposition to strokes both appear to be explained by increased production of pro-inflammatory cytokines by macrophages that are unable to respond to TGF-β. The persistent stimulation of myelopoiesis that accompanies chronic infection, inflammation, and cancer is associated with the emergence of myeloid-derived suppressor cells (MDSC) displaying TGF-β dependent immunosuppressive ability.142
Microglia are the resident macrophages of the central nervous system. Deletion of αvβ8 from neuroepithelial cells or deletion of Tgfbr2 or Tgfb1 in microglia each lead to the same phenotype of profound and progressive motor defects and persistence of dysmature microglia.47 The motor defects and many of the associated anatomic abnormalities in the brains of these mice can be rescued by post-natal deletion of microglia. This phenotype seems to depend on loss of TGF-β signaling during a limited developmental window, since deletion of Tgfbr2 from macrophages of adult mice results in many of the same changes in macrophage gene expression without dramatic functional impairment.143
TGF-β and fibroblast regulation
TGF-β regulates fibroblast activity in virtually all phases of the early tissue response to injury and the eventual return to normal homeostasis (Figure 4). Fibroblasts are the main producers of connective tissue matrix and play a key role in tissue repair. Fibroblasts are defined by morphological traits combined with a lack of markers for other lineages, and expression of vimentin or platelet-derived growth factor receptor-α.144 Recent data from single cell RNA sequencing show that fibroblasts are markedly heterogeneous with distinct molecular profiles that allow these cells to perform distinct roles in different organs, and different anatomic locations within organs.145,146 Their responses facilitate maintenance of tissue integrity and repair, but when they trigger feed-forward circuits involving TGF-β in response to chronic inflammation, fibroblasts become major contributors to pathologic tissue scarring and organ failure. Here we highlight the shared effects of TGF-β on fibroblasts in these various contexts.
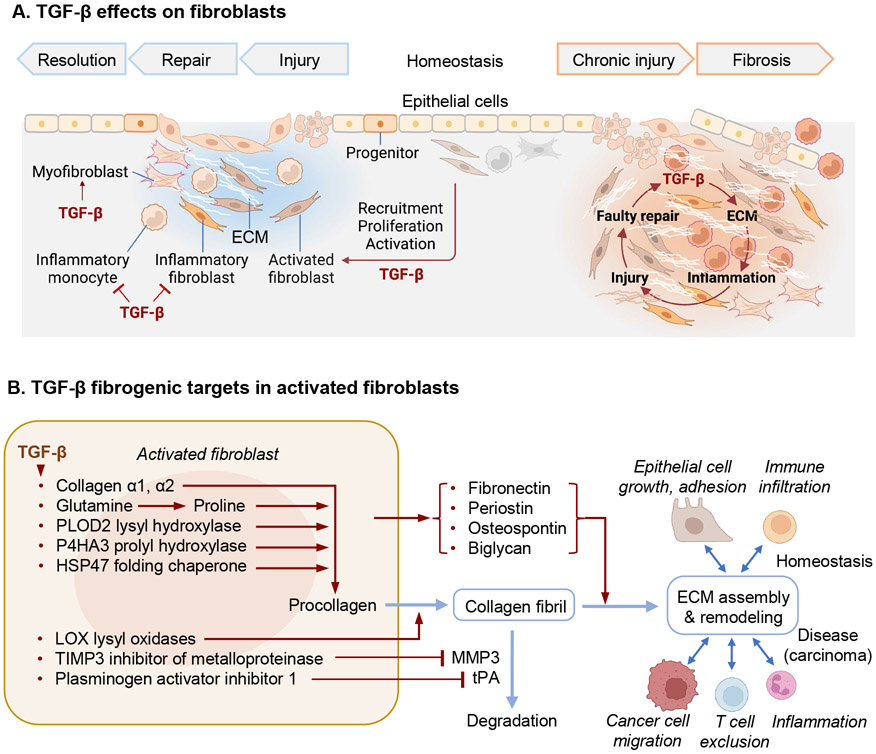
Figure 4. TGF-β regulation of fibroblasts in health and disease.
A. Main effects of TGF-β on fibroblasts during injury repair and chronic fibrosis, and impact on epithelial and immune cells. TGF-β regulates fibroblast activity throughout the tissue response to injury and the return to homeostasis (left side) as well as during chronic fibrosis (right side). TGF-β potently induces the recruitment, proliferation and activation of fibroblast that produce collagens, fibronectin, and other components required for ECM assembly, as well as integrins that mediate cell adhesion to the ECM. Activated fibroblasts additionally establish paracrine communication with epithelial cells, angiogenic progenitors, and local innate and adaptive immune functions. TGF-β also induces a highly contractile myofibroblast phenotype expressing α-smooth muscle actin. These phenotypes appear to emerge at the expense of a pro-inflammatory fibroblast phenotype, while TGF-β additionally restricts inflammatory monocytes. ECM deposition and remodeling is essential for epithelial progenitors to reconstitute the barrier tissue after injury. Tissue fibrosis, characterized by chronic inflammation and accumulation of fibrillar collagens and other ECM components resulting from imbalanced production of ECM by tissue resident fibroblasts. Feed-forward loops involving TGF-β contribute to fibrosis by exaggerating normal physiologic responses and triggering further epithelial injury and inflammation. B. TGF-β potently induces expression of fibrillar collagens as well as the metabolic adaptations, enzymes, and chaperones required for the biosynthesis and ECM deposition of collagen fibrils. TGF-β induces expression of additional ECM components in fibroblasts and epithelial cells. The production and turnover of ECM is a complex process requiring inputs from epithelial cells, innate and adaptive immune cells, and other cell types. Intratumoral fibrosis contributes to the exclusion of T cells from tumors. PLOD2, procollagen-lysine,2-oxoglutarate 5-deoxygenase 2; P4HA3, prolyl-4-hydroxylase 3, catalyzes proline hydroxylation; HSP47, heat-shock protein 47; LOX, lysyl oxidase; TIMP3, tissue inhibitor of metalloproteinase 3.
Fibroblast activation.
In response to tissue injury, fibroblast subsets undergo profound changes in gene expression that either enhance or inhibit tissue inflammation, regeneration, and scarring.145,146 Normally, fibroblast activation results in short term accumulation of fibrillar collagens and other ECM components, together with a coordinated regulation of epithelial, immune, and endothelial cells through immunomodulatory and angiogenic signals.144 This initial response is followed by fibroblast apoptosis and removal of excess collagen to restore normal tissue architecture.147
TGF-β is a potent activator of different fibroblast subsets (Figure 4A). In cell culture, TGF-β induces a highly contractile phenotype associated with the expression of α-smooth muscle actin (αSMA, also known as ACTA2), multiple ECM components, and the enzymes and chaperones required for ECM assembly.13 In this state, fibroblasts are often called “myofibroblasts”, although the expression of ECM proteins and contractile proteins is not highly correlated in vivo. Besides producing and assembling ECM, activated fibroblasts establish paracrine communication with epithelial cells, promote angiogenesis through production of vascular endothelial growth factor A (VEGFA), and mobilize local innate and adaptive immune functions through the secretion of chemokines.144,148 Thus, TGF-β-activated fibroblasts are hubs of ECM production and remodeling and of regulatory signals for epithelial, immune, and endothelial cells.
Coordinated ECM production.
Collagens are comprised of three polypeptide chains organized into a triple helical conformation. Four (of 28) mammalian collagens, (I, II, III and VI) form densely packed fibrils by covalent head-to-tail cross-linking of monomers. After injury, fibroblast-derived collagens I and III are the principal collagens that restore tensile strength and tissue integrity. Fibrillar collagens are produced abundantly in TGF-β activated fibroblasts and have a high content (10%) of proline. TGF-β supports the bioenergetic demands of collagen production by increasing the mitochondrial oxidation of glucose and glutamine. Mitochondrial redox generation promotes proline biosynthesis from glutamine for collagen production while preventing the generation of deleterious reactive oxygen species.149 Collagen monomers undergo extensive lysine and proline hydroxylation for proper folding and assembly. Procollagen-lysine,2-oxoglutarate 5-deoxygenase 2 (PLOD2) catalyzes lysine hydroxylation and prolyl-4-hydroxylase 3 (P4HA3) catalyzes proline hydroxylation. After collagen multimers assemble, the protein folding chaperone HSP47 prevents collagen denaturation or premature fibril formation. TGF-β potently induces expression of each of these fibrillar collagens, enzymes, and chaperones13 (Figure 4B).
After secretion and further proteolytic processing, fibrillar collagens form polymeric fibrils requiring oxidation of lysine residues for fibril cross-linking and stabilization. This step is mediated by a family of five lysyl oxidases, which are all strongly induced by TGF-β.13 TGF-β also induces the expression of plasminogen activator inhibitor 1 (also known as serpin E1) and tissue inhibitor of metalloproteinase 3, which prevent collagen degradation by extracellular proteases. The organization of collagen fibrils is further determined by other TGF-β inducible ECM components including fibronectin, osteopontin, periostin, and biglycan.127 Single cell RNA sequencing data suggest that the genes can be coordinately upregulated in pro-fibrotic fibroblasts in the setting of tissue fibrosis.145,146
Besides modulating ECM production, TGF-β increases expression of integrins on fibroblasts150 and epithelial cells.151 Integrins are the main receptors that cells use to detect and respond to ECM components, providing another example of TGF-β coordinating multi-cellular responses in tissue injury. TGF-β also upregulates expression of the TGF-β activating integrin, αvβ6, a process that may rapidly amplify local TGF-β signaling where needed, but that also contributes to a pathologic feed-forward circuit.
TGF-β and fibrosis.
Tissue fibrosis, characterized by chronic inflammation and accumulation of ECM components impairing organ function in kidney, lungs, liver, colon, and other organs, is a leading cause of morbidity and mortality worldwide.152 The normal production and turnover of ECM is a complex process that requires coordination of inputs from epithelial cells, endothelial cells, innate and adaptive immune cells, and nerves (Figure 4B). Perturbation of inputs from any of these cells can contribute to fibrotic pathology. However, it is primarily through effects on fibroblasts and epithelial cells that TGF-β participates as a prominent player in the initiation, progression, and persistence of fibrosis.
Fibrotic effects through fibroblasts.
Tissue fibrosis results from exaggerated production of collagens and other components of the ECM by tissue resident fibroblasts, often coupled with a reduction in ECM degradation and recycling by these cells. TGF-β promotes fibronectin and collagen production by both mesenchymal and epithelial cells.153 Injection of TGF-β1 into the skin or transgenic or adenovirus-mediated overexpression of TGF-β in the lung cause extensive tissue fibrosis.154,155 TGF-β-blocking antibodies prevent fibrosis in the skin, liver, lung, and kidney.13 TGF-β additionally inhibits multiple secreted proteases that contribute to ECM protein degradation. Recent data from single cell RNA sequencing has identified a subset of fibroblasts that emerge in many tissues in the setting of pathologic fibrosis and are characterized by the highest levels of expression of genes encoding fibrillar collagens and other components of the pathologic ECM.145,146 TGF-β signaling is a major upstream regulator of the gene expression signature that characterizes these cells.
Feed-forward loops involving TGF-β often contribute to fibrosis pathogenesis by exaggerating normal physiologic responses, driving their chronic persistence, and triggering inflammation (Figure 4A). By increasing both ECM production and collagen cross-linking, TGF-β increases tissue stiffness which in turn favors increased collagen production and expression of contractile proteins.156 Activated TGF-β can drive further expression of TGF-β in both autocrine and paracrine fashions. TGF-β is also a potent inducer of the TGF-β activating integrin, αvβ6.151 Furthermore, fibroblasts migrate toward and accumulate at regions of increased stiffness, a process that has been termed durotaxis.157 Increased stiffness facilitates TGF-β activation through αvβ6 on epithelial cells and αvβ1 on fibroblasts, since both activate TGF-β through contraction-dependent effects on the conformation of the latent complex and this process is facilitated when cells are tethered to a stiff substrate.44
Fibrogenic effects through epithelial cells.
Effects of TGF-β on epithelial cells also contribute to fibrosis, as demonstrated by the observation that deletion of TGF-β receptors from epithelial cells inhibits pulmonary fibrosis induced by intratracheal delivery of bleomycin.158 TGF-β stimulates the expression of fibrogenic factors in normal and malignant epithelial cells, which is associated with strong intratumor fibrosis in models of lung metastasis.101 The effects of TGF-β on expression of integrin αvβ6, inhibition of epithelial cell proliferation, and induction of epithelial cell senescence and death may also facilitate fibrosis and perturb normal regeneration in injured epithelial organs.
Considerable attention has been paid to the potential role of epithelial cell senescence as a driver of tissue fibrosis, especially in lung fibrosis. Balanced cell senescence and apoptosis mediate the removal of unwanted cells during homeostasis. However, excessive senescence or apoptosis with a persistent senescence-associated secretory phenotype (SASP) creates an inflammatory microenvironment leading to pathological repair that progresses to fibrosis.159,160
TGF-β in epithelial cell regulation
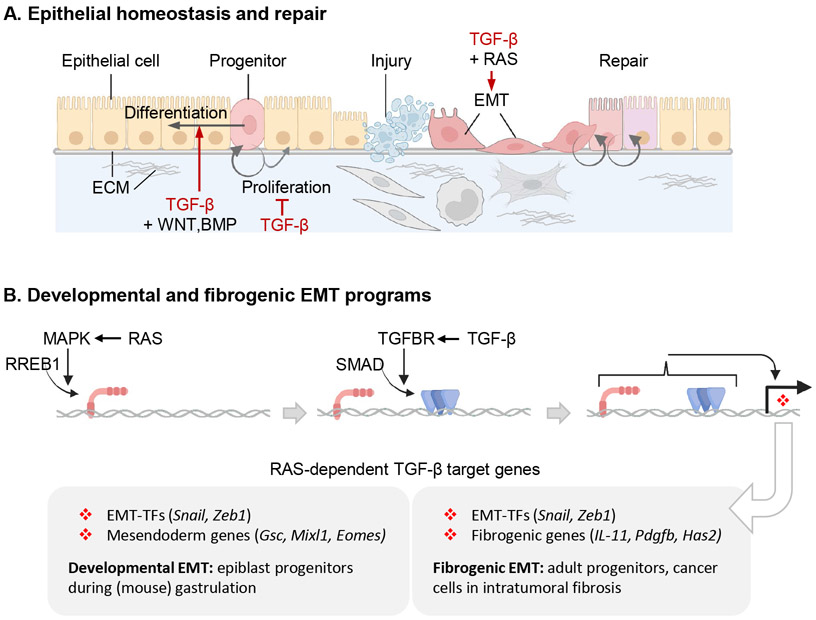
Epithelial barriers protect against noxious agents and fluid loss while supporting respiration, metabolite traffic, secretion, and other specialized functions. Preserving the integrity of epithelial barriers is paramount to metazoan organisms. Epithelial homeostasis and repair involve coordinated interactions between epithelial progenitors and fibroblasts, immune cells, vascular structures, and other stromal components. Adult epithelia and other tissues harbor rare pluripotent progenitors that are poised to proliferate and differentiate to replace the programmed loss of older progeny or accidental losses due to injury. TGF-β regulates the phenotypic plasticity and proliferation of epithelial progenitors and their interactions with other cell types both in health and disease conditions (Figure 5).
Figure 5. TGF-β in epithelial cell regulation.
A. TGF-β regulates the phenotypic plasticity of epithelial progenitors and their interactions with other cell types. TGF-β derived from fibroblasts, immune cells, and from the epithelial cells themselves modulates the proliferation of epithelial progenitors and regulates their differentiation, frequently with countervailing WNT, BMP and other signals. In response to injury, epithelial progenitors undergo EMT for migration to niches that provide appropriate basal lamina ECM support and signals to orchestrate injury repair and eventual resolution. TGF-β is a major inducer of EMTs, which frequently requires the cooperation of RAS-activated MAPK signals. B. RREB1 (RAS-responsive element binding protein 1) links the TGF-β-SMAD and RAS-MARK pathways and coordinates the expression of developmental and fibrogenic EMT programs. MAPK-activated RREB1 binds to target loci including in EMT-TF genes and either mesendoderm specification genes in epiblast cells or fibrogenic genes in adult epithelial progenitors and adenocarcinoma cells. DNA-bound RREB1 then enables TGF-β receptor-activated SMADs to drive expression of these genes.
Regulation of phenotypic plasticity.
Phenotypic plasticity refers to the ability of biological systems to change morphology and function in response to environmental and developmental cues. Progenitor cells are adept at responding to such cues during development and injury. TGF-β profoundly influences the phenotypic plasticity of epithelial progenitors, regulating their differentiation and phenotypic transitions during tissue development, morphogenesis, and repair. TGF-β frequently exerts these effects in counterbalance with other inputs, principally from the WNT, BMP, and RAS pathways (Figure 5A). For example, during early development, Nodal-activated SMAD transcriptional complexes and WNT-activated TCF complexes bind to shared target enhancers, activating mesendoderm specification transcription factors.161,162 Postnatal development and adult homeostasis of epithelial tissues provide numerous examples of progenitor differentiation under the control of TGF-β in combination with WNT, BMP, and RAS signaling, such as in mammary ductal differentiation and branching morphogenesis during puberty, pregnancy and lactation; and in lung and kidney morphogenesis, liver regeneration; and intestinal epithelium homeostasis.163-166
Epithelial-mesenchymal transitions.
Another manifestation of phenotypic plasticity is the ability of epithelial progenitors to undergo EMTs. EMTs play critical roles during development, injury repair, and disease.167,168 In an EMT, epithelial cells lose apicobasal polarity and adhesive contacts while gaining actin stress fibers, anteroposterior polarity, motility, and remodeled contacts with neighboring cells and the ECM. EMTs are driven by transcription factors (EMT-TFs) including the zinc-finger proteins Snail (encoded by SNAI1), Slug (SNAI2), ZEB1 and ZEB2, the basic helix-loop-helix proteins Twist1 and Twist2, among others. EMT-TFs cooperatively repress epithelial genes and induce mesenchymal markers. The extent of the mesenchymal traits gained by a cell during an EMT – that is, the “completeness” of an EMT – depends on the range of phenotypic states that a particular epithelial progenitor is programed to access. After undergoing an EMT, cells can revert to an epithelial state through a mesenchymal-to-epithelial transition (MET). However, epithelial progenitors may undergo differentiation during an EMT–MET cycle, emerging from it in a distinct developmental stage.
EMTs are triggered by cell-extrinsic signals, TGF-β being the most widespread and potent of these.167 TGF-β induces EMTs in epithelial cells in mammary, pulmonary, renal, hepatic, and other tissues during development, injury repair, fibrosis, and cancer, whereas Nodal drives EMT in epiblast cells during gastrulation.14 To trigger an EMT, signal-activated SMADs induce the expression of SNAI1/2 and ZEB1/2 to repress epithelial junction proteins such as E-cadherin, occludin and claudin-3, and of epithelial transcription factors such as KLF5.
Developmental and regenerative EMT programs.
TGF-β triggers EMTs as part of broad programs that include coordinated changes in cell proliferation, differentiation, and survival.101,169 In mouse epiblast progenitors, Nodal induces the expression of EMT-TFs and mesendoderm specification transcription factors coordinating EMT and differentiation during gastrulation.170 In adult mammary cells and in lung, breast, and pancreatic carcinoma cells, TGF-β induces the expression of EMT-TFs (e.g. Snail) and fibroblast-activating cytokines (e.g. IL-11, PDGFB), thereby coupling EMT and fibrogenesis.101 Thus, EMTs induced by TGF-β are associated with multiple programs and outcomes in different contexts: mesendodermal differentiation in epiblast progenitors and fibrogenesis in adult epithelial cells and carcinoma cells.
In all these cases, the effects of TGF-β depend on RAS-MAPK activity.82,171-174 The RAS effector RREB1 (RAS-responsive element binding protein 1) plays a central role in this process.101 MAPK-phosphorylates RREB1 in the N-terminal domain to enable its binding to cognate DNA sites in target loci. RREB1 target loci include EMT-TF genes and either mesendoderm specification genes in epiblast cells or fibrogenic genes in adult epithelial and adenocarcinoma cells, depending on cell-specific chromatin accessibility patterns. The MAPK-activated, pre-bound RREB1 then enables TGF-β-activated SMADs to drive expression of these target genes (Figure 5B). RREB1 functions both as a nexus between the TGF-β-SMAD and RAS-MAPK pathways and as a link between EMT and developmental or fibrogenic gene expression programs depending on the cell context. Why these SMAD target genes and not others require RREB1, and what function RREB1 provides to enable transcription of these genes remain open questions.
Growth inhibition and cell senescence.
A strong antiproliferative effect on lung epithelial cell cultures was one of the first identified activities of TGF-β. Dissection of the mechanism led to the identification of the CDK inhibitors p27KIP1,175 p57KIP2,176 and p15INK4B,177 and an interplay between p27KIP1, p15INK4B, and p21CIP1 as TGF-β regulated inhibitors of the cell cycle in lung epithelial cells.178 TGF-β-activated SMADs partner with FOXO transcription factors to activate the expression of p15INK4B and p21CIP1 in keratinocytes and neuroepithelial cells.179,180 Other antiproliferative responses such as the down-regulation of the pleiotropic growth promoting transcription factor MYC frequently accompany the induction of CDK inhibitors by TGF-β.181 The extent of the growth inhibitory effect of TGF-β on epithelial cells depends on the cell type. TGF-β induces a complete arrest of cell cycle in lung epithelial cells in culture, which allowed the isolation of TGF-β receptor mutants to elucidate the mechanism of receptor activation.182-184 However, other epithelial cell types show only mild growth inhibitory responses to TGF-β, and mice with conditional ablation of Tgfbr2 in the skin and gastrointestinal tract do not show extensive hyperplasia except at sites of tissue stress.185
TGF-β induces senescence of epithelial cells in various contexts. Senescence is a process by which cells irreversibly cease to proliferate and typically acquire altered secretory profiles. 186 Senescent cells undergo dramatic transcriptional changes including expression of a suite of secreted proteins, including SASP cytokines, growth factors and proteases. Senescence involves expression of the cell cycle inhibitor p21CIP1, which is induced by TGF-β.187
TGF-β duality in cancer: Tumor suppression
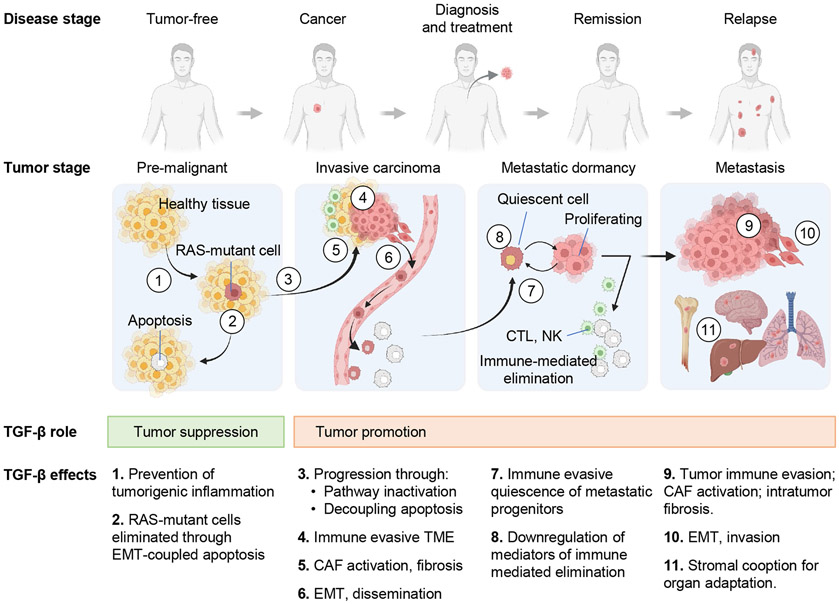
TGF-β has been implicated in the progression of many types of cancer, but the most detailed body of knowledge comes from the analysis of its dual role in breast, colorectal, pancreatic, and lung adenocarcinomas (Figure 6). In these tumors, TGF-β can suppress or promote carcinoma progression depending on the stage of the disease. TGF-β induces apoptosis in early-stage epithelial progenitors harboring oncogenic RAS mutations, but it promotes tumorigenic immunosuppression in cancer cell clones that escape the suppressive effects of TGF-β by inactivating this pathway or decoupling it from apoptosis. Moreover, carcinoma cells that decouple the TGF-β pathway from apoptosis can respond to TGF-β with invasion, dissemination, and metastasis, thus leading to tumor progression instead of tumor suppression.
Figure 6. Roles of TGF-β in cancer.
During the early stages of carcinogenesis, TGF-β exerts tumor suppressive effects by inhibiting tumorigenic inflammation (1 in the graphic) or triggering EMT-coupled apoptosis in pre-malignant progenitors harboring RAS mutations (2). To escape TGF-β dependent apoptosis (3), RAS-mutant cells must acquire TGF-β pathway inactivating mutations or alterations that decouple TGF-β-dependent EMT from apoptosis. This enables carcinoma progression and turns TGF-β into a tumor promoting agonist as the disease progresses. The tumor promoting effects of TGF-β include: (4) generation of an immune evasive TME by excluding or suppressing cytotoxic T cells and NK cells and turning macrophages into TAMs and neutrophils into TANs; (5) activation of CAF fibrogenic and paracrine activities, which favor cancer cell growth, invasion, immune evasion, and angiogenesis; (6) induction of cancer cell EMTs which increase tumor invasion, entry into, and exit from the circulation for tumor dissemination; (7) induction of immune evasive dormancy in disseminated metastatic progenitors; (8) downregulation of mediators of immune clearance in dormant cancer cells; (9, 10) repeated generation of an immune evasive TME, activation of CAFs, and induction of fibrogenic EMT in dormant metastatic progenitors that resume proliferative and survive elimination by the immune system; (11) promotion of metastatic outgrowth by stimulating organ-specific cancer cell-stroma interactions. The cancer cell-intrinsic tumorigenic effects of TGF-β (effects 6, 7, 8, 10 and, partly, 11) are available to carcinoma cells that retain an active TGF-β pathway (though decoupled from apoptosis). The TME effects of TGF-β (effects 4, 5, 9 and, partly, 11) are available to carcinoma cells regardless of how the tumor suppressive effects of TGF-β are cancelled.
Tumor suppressive apoptosis.
Conditional ablation of Tgfbr2 or Smad4 in pancreatic, intestinal, oral mucosa, and skin epithelia in mice does not interfere with the development of these tissues and causes only a mild hyperplasia. However, loss of Tgfbr2 accelerates tumor progression when these tissues harbor KRAS or HRAS oncogenes.83,185,188,189 Genetically engineered mouse models of pancreatic ductal adenocarcinoma (PDAC)83,188 and CRC190-192 showed that the TGF-β pathway interferes with the transition of pre-malignant cells to the carcinoma stage during malignant progression. In line with these observations, pre-malignant pancreatic, intestinal, and skin epithelial progenitors with KRAS or HRAS mutations undergo apoptosis in response to TGF-β, accounting for the tumor suppressive role in these cancer models.82,83,185
TGF-β receptors are not directly coupled to apoptosis effector molecules. How TGF-β becomes a potent inducer of apoptosis was illuminated by studies in normal and malignant pancreatic epithelial progenitors.82,101 In normal progenitors, TGF-β induces expression of SOX4, which pairs with KLF5 as a transcriptional partner to specify a pancreatic epithelial progenitor state. In pre-malignant pancreatic cells with mutant Kras, the dysregulated MAPKs strongly activate RREB1, which pairs with TGF-β-activated SMADs to trigger an intense induction of SNAIL expression and an EMT (Figure 5B). As a transcriptional repressor of epithelial genes, SNAIL inhibits KLF5 expression, driving SOX4 to activate the expression of Bim and other pro-apoptotic genes. Here, an otherwise normal TGF-β/RAS dependent EMT becomes pro-apoptotic owing to a conflict of cell fate signals: a pro-epithelial SOX4 and an overexpressed pro-mesenchymal SNAIL. Of note, RREB1 is frequently downregulated or genetically inactivated in PDAC.193,194
Prevention of tumorigenic inflammation.
In addition to these direct tumor suppressive effects on pre-malignant cells, TGF-β acts as an indirect tumor suppressor by preventing inflammatory responses that cause cancer predisposition. TGF-β is a key suppressor of inflammatory responses through coordinated effects on different immune cell types.15 Disruption of this function can lead to chronic inflammation, which predisposes pre-malignant cells to progress to tumor formation. This is particularly evident in the gastrointestinal tract. TGF-β prevents intestinal inflammatory reactions to the commensal microbiota by exerting tolerogenic effects on immune and epithelial cells.195 Dysregulation of TGF-β signaling is linked to the pathogenesis of ulcerative colitis, a cancer predisposition condition.196 Tgfb1 mutant mice develop a lethal multifocal inflammatory disease as a prominent phenotype and are highly susceptible to developing colitis.104,197 Mice with TGF-β pathway mutations in T cells or dendritic cells also show inflammatory phenotypes8,111 and a higher incidence of gastrointestinal carcinomas.198,199
Is growth inhibition tumor suppressive?
The cytostatic effects of TGF-β through the expression of CDK inhibitors and other factors in epithelial cells have been viewed as tumor suppressive effects. However, this notion is challenged by different lines of evidence. In mouse models of PDAC, the tumor suppressive effect of TGF-β is based on apoptosis, not growth arrest of KRAS-mutant progenitors.83 In mouse models of HER2+ breast cancer, expression of a constitutively active Tgfbr1 transgene in mammary epithelial cells decreased the growth rate of mammary tumors but accelerated lung metastasis from these tumors.200 The growth inhibitory effects of TGF-β on epithelial cells are reversible and unlikely to provide an effective mechanism for the sustained suppression of tumor growth. In fact, disseminated breast cancer cells201 and lung cancer cells202 enter a growth arrested state in response to TGF-β in models of metastatic dormancy. As we discuss below, metastatic dormancy protects stem-like progenitor cells from immune surveillance, preserving these cells for eventual relapse. In effect, TGF-β-mediated immune evasive growth arrest is a strategy for metastatic progression in these models. Although the antiproliferative effects of TGF-β may dampen the growth of some tumors, there is no compelling evidence that these effects suppress tumor development or progression.
Escaping tumor suppression.
Loss-of-function mutations in TGFBR2 were identified in human colon cancer cells with microsatellite instability,203 deletions in SMAD4 in PDAC,204 and missense mutations in SMAD2 in colorectal carcinomas (CRC)205 shortly following the identification of these genes. Subsequent studies identified recurrent genetic alterations in other core components of this pathway, including TGFBR1, SMAD3, and the Activin receptors ACVR1B and ACVR2A which signal through the same SMADs. Analysis of pathway mutations, copy-number changes, and other genetic alterations in 9,125 tumors profiled by The Cancer Genome Atlas in 33 cancer types showed that these alterations are frequent in carcinomas of the pancreas, gastrointestinal tract, lungs, breast, bladder, prostate, endometrium, and head and neck, as well as in low-grade gliomas and diffuse large B-cell lymphomas.206 Most of these alterations are loss-of-function events selected during tumor progression. Gastrointestinal carcinomas and uterine carcinosarcomas with microsatellite instability (MSI) and high mutational loads frequently harbor TGFBR2 and ACVR2A inactivating mutations in prone microsatellite-like sequences.203,206
In the microsatellite-stable common subtype of CRCs, which are initiated by mutations in the WNT pathway and KRAS, alterations in SMAD4 and TGFBR2 occur late during adenoma-carcinoma progression.207 Similarly, SMAD4 inactivating mutations during PDAC progression accumulate after the emergence of early-stage lesions containing KRAS mutations which are indispensable for PDAC initiation.208 This is consistent with the evidence from mouse models of CRC and PDAC mentioned above, showing that TGF-β interferes with the transition to the carcinoma stage during tumorigenesis.
Restoring SMAD4 expression in PDAC tumor cells that developed with Smad4 loss makes these cells undergo massive apoptosis in response to TGF-β.82,209 Although many PDACs elude TGFβ-induced tumor suppression through inactivating mutations in TGF-β receptors and SMADs, nearly half of PDAC tumors and larger proportions of other tumors retain a functional TGF-β pathway. PDACs with intact TGF-β signaling components lack apoptotic responses to TGF-β owing to alterations in the expression of ID1 family members, which are core transcriptional regulators in PDAC progenitors. In these cells, TGF-β-SMAD signaling upregulates ID1 expression, which decouples EMT from apoptosis by neutralizing the effects of SOX4. PI3K/AKT signaling and mechanisms linked to low-frequency genetic events additionally converge with ID1 to prevent TGF-β-dependent apoptosis in PDAC.209
In contrast, human head and neck squamous cell carcinomas (HNSCCs) show loss of SMAD4 at an early-stage, and mice with Smad4 deletion develop spontaneous HNSCCs and genomic instability, suggesting that loss of SMAD4 is a tumor-initiating event in this context.210 In these tumors, SMAD4 loss is associated with decreased expression of BRCA1 and an elevated mutational burden accompanied with sensitivity to PARP inhibitors. These findings, which are also supported by results in a mouse model, suggest that SMAD4-dependent signaling suppresses the emergence of HNSCC tumors by enhancing BRCA1-dependent repair of double-strand DNA breaks.210,211 Of interest, germline SMAD4 mutations give rise to a juvenile polyposis syndrome with inflammatory gastrointestinal polyps that may progress to carcinoma.212,213 SMAD4 haploinsufficiency in this context may predispose to tumorigenic inflammation and/or lead to tumor progression through the eventual loss of the wild type SMAD4 allele.
TGF-β duality in cancer: Tumor progression and metastasis
Cancer cells with the TGF-β pathway decoupled from tumor suppressive effects can respond to TGF-β with effects that promote various phases of the metastatic process, from invasion, dissemination, immune evasive dormancy, and organ-specific colonization (Figure 6). Moreover, independently of the status of this pathway in the cancer cells, TGF-β can additionally promote tumor progression and relapse through effects on the stroma, notably immunosuppressive and desmoplastic effects, which limit the effectiveness of immunotherapy. The effect of TGF-β on these functions varies depending on the tumor type. The extant knowledge comes from mechanistic analysis of mouse models and correlative evidence in human patient samples, showing that TGF-β promotes tumor progression, metastasis, and resistance to therapy.
Immunosuppressive tumor microenvironments.
TGF-β has a multitude of effects on virtually all the adaptive and innate immune cell types and most of these effects enforce tolerance and prevent autoimmune responses. Not surprisingly, one after the other, these effects have been implicated in the generation of an immunosuppressive tumor microenvironment (TME) that favors tumor progression and metastasis and limits the effectiveness of immunotherapy.8,15,111 Immune checkpoint inhibitors, which unleash endogenous anti-tumor immunity by inhibiting pathways that normally restrain CD8+ T cell-mediated tumor cell killing, have revolutionized treatment of a wide variety of cancers, but this approach remains ineffective for most patients. Approximately half of solid tumors can be characterized histologically as “immune excluded”, in which CD8+ T cells accumulate around the periphery of the tumor in a dense collagen-containing band but fail to infiltrate the tumor itself. These tumors are generally resistant to checkpoint inhibitors and exhibit a gene expression pattern suggestive of increased TGF-β signaling. Recent evidence from syngeneic murine models suggests that immune checkpoint sensitivity can be induced in some immune-excluded tumors by treatment with TGF-β inhibitors. This treatment promotes CD8+ T cell infiltration into the core of the tumor, increases granzyme B and interferon production by infiltrating T cells and in many cases leads to induction of long-term anti-tumor immunity.192,214,215 Similar effects can be caused by inhibition of the TGF-β-activating αvβ8 integrin,216-218 which can be expressed on tumor cells themselves or on CD4+ T cells in the tumors.216-218 The mechanisms underlying synergy between checkpoint inhibitors and blockade of TGF-β signaling or activation are the focus of current investigation, and these pre-clinical results have led to active clinical trials evaluating TGF-β or αvβ8 integrin inhibitors in patients with cancer.
Tumorigenic effects through CAFs.
Fibroblasts present in tumors are called cancer-associated fibroblasts (CAFs). CAFs are an important component of the TME and influence tumor progression through ECM remodeling and paracrine signaling which are characteristic of activated fibroblasts. There are no specific markers distinguishing CAFs from the activated fibroblasts participating in wound healing or fibrosis. The consensus is that most CAFs result from the activation, likely dysfunctional, of local fibroblasts.144 Distinct CAF populations distinguished by means of single-cell analysis exhibit either a TGF-β-driven matrix-producing contractile phenotype or an immunomodulating secretome.219-221 Similar to TGF-β activated fibroblasts in wound healing and fibrotic diseases, CAFs are highly effective at producing and remodeling ECM within the TME. This process generates intra-tumoral fibrosis and ECM stiffness, known enhancers of carcinoma cell growth and invasion.222-224 Additionally, CAFs are a source of IL-6 and TGF-β itself, which are immunosuppressive in the TME, and VEGF which drives tumor angiogenesis.144 Beyond these effects on CAFs in carcinomas, TGF-β has been shown to promote growth of other tumor types such as gliomas through the induction of autocrine and paracrine secretomes.225
Metastatic dissemination.
In cancer cells that retain a functional TGF-β pathway, TGF-β can induce stimulate migration into, and out from blood capillaries for metastatic dissemination (Figure 6). Induction of an EMT by TGF-β-SMAD signaling accompanied with increased motility, invasiveness, and metastasis has been noted in HRAS-driven cutaneous squamous cell carcinomas induced by carcinogens226 or by genetic engineering in mice.227 TGF-β signaling in cancer cells in an orthotopic mammary tumor model promoted local invasion and hematogenous dissemination by inducing EMT and a switch from cohesive cell motility to single-cell motility.228 TGF-β can also function as a promoter of extravasation of circulating tumor cells. The activation of TGF-β stored in blood platelets coating colon cancer cells induced EMT and extravasation of cancer cell in the lungs of mice.229 To be determined is Whether TGF-β-induced EMTs promote extravasation by maintaining circulating carcinoma cells in an EMT state or by inducing an EMT in circulating epithelioid carcinoma cell clusters that lodge in capillaries remains to be determined. Additionally, TGF-β-rich breast primary tumors release cancer cells expressing angiopoietin-like 4, a TGF-β-inducible mediator of extravasation and lung metastasis.230,231
Immune evasive dormancy.
Disseminated cancer cells suffer extensive attrition due to immune attack, physical barriers, and metabolic stresses.232 Metastasis typically develops after a dormancy period lasting from months to decades, implying that cancer cells that survive the stress of dissemination enter a period of dormancy.233 In mouse models of metastatic dormancy, disseminated tumor cells localize to perivascular regions where they are able to remain dormant for many months.201,234 Developmentally, these cells correspond to a stem-like early progenitor stage (e.g., SOX2+ stage in the case of LUAD) and are primed to enter quiescence in response to TGF-β and autocrine WNT inhibition.202,234,235 During the dormant phase, these metastasis-initiating progenitors are in equilibrium between an immune-privileged quiescent state and a proliferative state liable to immune-mediated clearance (Figure 6). Quiescent cells downregulate MHC class I molecules,236,237 NK receptor ligands234 and STING (stimulator of interferon genes)202 to evade elimination by the immune system. Dormant cancer cells reentering the cycle re-express these mediators of immune recognition and consequently are cleared by the combined action of T cells and NK cells. Metastatic outbreaks succeed when proliferative clones elude immunity or when immune surveillance subsides.202 Entry into proliferative quiescence in response to TGF-β appears to be a specific property of early-stage malignant progenitors and not of their developmentally more advanced progeny that constitute the bulk of the tumor mass. Thus, TGF-β-induced dormancy may protect disseminated metastatic progenitors from immune surveillance, preserving these cells for long-term relapse. Interestingly, an immune evasive state is also manifest in normal stem cells in hair follicles and muscle, which remain quiescent, but not in intestinal, mammary, or ovarian proliferative stem cells.238 Evolutionarily, immune evasive quiescence of adult stem cells may serve to protect longevity of these cells as they accumulate neoantigens during the organism’s lifespan.
Stromal cooption during metastatic colonization.
After infiltrating distant organs and resisting during dormancy, disseminated cancer cells may initiate metastatic outbreaks. As the metastatic tumors develop, TGF-β in the TME may resume its pro-tumorigenic roles as a mediator of immune suppression, EMT, invasion, and further dissemination.
In each tumor type metastasis follows a stereotypic pattern of affected organs and timing.239 The organ distribution of metastasis is a function of many factors including circulation patterns, intrinsic resilience of disseminated cancer cells to diverse tissue microenvironments and their resident immunity, and the ability to express and select for organ-specific colonization traits. Such traits provide cancer cells with the ability to adapt to different metabolic environments, avert hostile surveillance, extract survival signals from the TME, or coopt the host stroma for aggressive outgrowth as a metastatic colony. In the primary tumor and during prolonged dormancy in host organs upon dissemination, the TME selects for organ-specific colonization traits that a cancer cell population may be able to express as a function of its origin. The emergence of organ-specific metastatic traits largely arises from non-genetic changes, although these changes may indirectly result from mutations in epigenetic regulators.240
TGF-β augments the expression of various mediators of organ colonization in cancer cells. This is particularly evident in the case of osteolytic bone metastasis from triple-negative breast cancer, which is driven by TGF-β released during bone matrix resorption.241 TGF-β-SMAD signaling in bone-tropic breast cancer cells stimulates the expression of several mediators of osteolytic bone metastasis242 including parathyroid-related protein,243 IL-11244 and Jagged 1.245 In the lungs, TGF-β- and RAS-dependent activation of a fibrogenic EMT in LUAD cells stimulates lung metastasis,101 whereas TGF-β-dependent expression of ID1 in disseminated breast cancer cells favors the reentry of these cells into the cell cycle.246
Approaches and challenges to therapeutically targeting TGF-β
Pharmaceutical companies have been working for many years to develop inhibitors of TGF-β expression, activation or TGF-β signaling for treatment of a variety of diseases, including cancer, immune dysregulation, fibrosis, and developmental disorders. Numerous TGF-β inhibitors are being tested in ongoing clinical trials and others are in various stages of preclinical development. These inhibitors fall into several classes (Figure 7) including antibodies that prevent the activation of latent TGF-β; antibodies and receptor ectodomain proteins that trap TGF-β or block the TGF-β receptors; small molecule inhibitors of TGF-β receptor kinases. Other approaches seek to mitigate TGF-β interference with immunotherapy by incorporating a dominant-negative TGFBR2 construct or TGF-β antisense oligonucleotides into engineered autologous CTL vaccines or chimeric antigen receptor (CAR) T cells. To specifically target TGF-β near cells of interest, fusion proteins have been created that consist of a TGF-β trapping receptor ectodomains fused to anti-CD4 antibody (to block TGF-β around T cells) or to antibodies against immune checkpoint molecules such as PDL1, CTLA4 or CD37. Detailed accounts on the development and status of these various therapeutic agents are available.8,14,225,247
Figure 7. Approaches to therapeutically targeting TGF-β.
The image summarizes the main points of the TGF-β production, activation, and signaling being targeted by various agents currently under development to treat cancer, fibrosis, and other diseases. TGF-β inhibitory agents include antisense oligonucleotides targeting TGF-β expression, antibodies targeting latent TGF-β, TGF-β-activating integrins, active TGF-β or TGF-β receptors, and small-molecule compounds targeting TGF-β-activating integrins and TGF-β receptors. TGF-β receptor ectodomains fused to immune checkpoint antibodies are engineered to increase the efficacy of immunotherapeutic agents by trapping TGF-β near target cells. For the same purpose, dominant-negative TGF-β receptor constructs are overexpressed in engineered various types of anti-cancer T cells (CAR T cells, autologous CTLs).
Targeting TGF-β ligands and receptors.
Many of the molecules targeting TGF-β or its receptors are highly effective at blocking TGF-β signaling. However, obstacles need to be overcome to realize the therapeutic potential of these strategies. Some obstacles relate to specificity. The small-molecule inhibitors of TGF-β receptors primarily target the ATP-binding pocket of the receptor protein kinase domain. As a result, these inhibitors present the challenge of achieving specificity for TGF-β receptors versus other members of this receptor kinase family. Other obstacles relate to pleiotropy. Because of the many critical roles TGF-β family members play in normal development and tissue homeostasis, potent inhibition of all TGF-β signaling would likely lead to unacceptable toxicity. This possibility is underscored by the embryonic or early post-natal lethal phenotypes of mice with inactivating mutations in each of the three mammalian TGF-β isoforms, and by the severe auto-immune phenotypes of mice with severely impaired TGF-β signaling in T cells or dendritic cells. These dramatic effects might all be consequences of critical developmental roles for TGF-β signaling and do not necessarily preclude treatment of adults with TGF-β inhibitors. Indeed, some of the TGF-β inhibitors currently under development have been given to hundreds of patients without signs of severe toxicity.15,225
Nevertheless, monkeys, rats and mice have developed thickening of cardiac valves and some patients treated with TGF-β inhibitors developed low grade skin cancers, consistent with the known effects of TGF-β in valve development and as a brake on epithelial carcinogenesis, raising concerns about the safety of this approach. Furthermore, because latent forms of each TGF-β isoform are expressed at high concentrations in many tissues of healthy adults, antibodies or other biologics targeting TGF-β isoforms could be limited in effectiveness because of the likelihood they would be sequestered by irrelevant TGF-β tissue stores. This might be one reason for the apparent lack of serious toxicity for several anti-TGF-β biologics that entered clinical trials thus far and for their surprisingly limited efficacy. Cell-permeable small-molecule inhibitors of TGF-β receptor kinases could overcome the challenges of large extracellular stores of latent TGF-β, but those developed so far have generally had quite short in vivo half-lives and would not, in any case, overcome the challenges of the many roles TGF-β signaling plays in maintaining normal tissue homeostasis.
Targeting TGF-β activation.
Renewed confidence on the viability of therapeutically targeting TGF-β comes from the increasing knowledge about the specific mechanisms of TGF-β activation, and the ability to target TGF-β inhibition to a specific cellular context or specific downstream responses. Several strategies to get around these problems are in various stages of development, most aimed at increasing the precision for inhibiting specific pathologic functions of TGF-β without targeting beneficial homeostatic effects. One such strategy has focused on the pathways for integrin-mediated TGF-β activation. As noted above, three integrins, αvβ1, αvβ6 and αvβ8 are involved in the activation of latent TGF-β stored in the ECM or on cell surface.27,34,41 TGF-β can also be activated by integrin-independent pathways, such as binding to thrombospondin, narrowing the scope, and thus the potential toxicity of therapeutic targeting. Importantly, each of these integrins is expressed in a distinct, limited number of cells and at low copy number, further overcoming the challenges of indiscriminately targeting all TGF-β isoforms in every tissue. For example, αvβ6 is restricted in its expression to epithelial cell,32 and is generally expressed at very low levels in healthy epithelia but dramatically upregulated in response to injury and at sites of fibrosis.248 Currently, there are at least three drugs targeting TGF-β activating integrins in active clinical trials: a dual small molecule inhibitor of αvβ1 and αvβ6 in phase 2 clinical trials for treatment of pulmonary fibrosis and primary sclerosing cholangitis, a small molecule inhibitor of avβ1 in a phase 1 trial for liver fibrosis in the setting of non-alcoholic steatohepatitis, and a humanized monoclonal antibody targeting the αvβ8 integrin in a phase 1 study for enhancing responses to immune checkpoint inhibition in cancer. All these studies are in early stages, but recently released data for a 12-week phase 2 study of the αvβ1/αvβ6 inhibitor in 67 patients with idiopathic pulmonary fibrosis showed no significant on-target toxicity and an apparent dose dependent efficacy in slowing the rate of loss of lung function and progression of radiographic evidence of fibrosis. However, other drugs targeting TGF-β activating integrins have been withdrawn because of pre-clinical or clinical adverse events, so conclusions about the safety and efficacy of this approach will need to await the results of longer and larger clinical trials.
Targeting downstream mediators.
Other strategies are based on more precisely targeting inhibition of TGF-β signaling to the cells that drive specific disease pathology. One encouraging example of this approach was the identification of epigallocatechin gallate (EGCG) as a natural product found in several fruits and green teas which protects mice from bleomycin-induced pulmonary fibrosis.249 Efforts to identify its mechanism of action showed that when EGCG covalently inhibits lysyl oxidase homolog 2 (LOXL2), the compound itself is converted into a potent, irreversible inhibitor of TGFBR2. Importantly, LOXL2 is not broadly expressed, with substantial expression in normal tissues restricted to fibroblasts. EGCG is thus effectively a specific inhibitor of TGF-β signaling in fibroblasts. Biopsies from patients who received EGCG showed significant reductions in extractable collagen and other indicators of active fibrosis compared to patients treated with placebo.250
Several efforts are underway to treat TGF-β-driven diseases more precisely by targeting key steps downstream of TGF-β signaling that might contribute more to pathology than to normal homeostatic functions. One example is a monoclonal antibody that blocks connective tissue growth factor, a TGF-β-induced protein that has been proposed to mediate some of the pro-fibrotic effects of TGF-β.251 Another is the development of drugs targeting nicotinamide adenine dinucleotide phosphate oxidase 4 (NOX4), a TGF-β-induced protein that catalyzes the reduction of molecular oxygen to hydrogen peroxide. NOX4 expression is increased in activated fibroblasts and inhibition of NOX4 reduces collagen production from these cells in vitro and inhibits pulmonary fibrosis in vivo.252 Pulmonary fibrosis is a disease associated with aging and in vivo studies in mice suggest that NOX4 upregulation persists longer in fibroblasts from bleomycin-treated aged mice. Aged mice also exhibit more prolonged fibrosis after a single dose of bleomycin than young mice do, and inhibition of NOX4 inhibits fibrosis persistence in aged mice.253 Based on these findings, NOX4 inhibitors are currently under development for treatment of pulmonary fibrosis.
Enhancing cell therapies.
Finally, the advancement of cellular therapeutics opens new avenues to translate what has been learned about TGF-β signaling into novel therapeutic interventions. For example, incorporation of a dominant negative TGFBR2 into CAR-T cells holds promise to overcome the suppressive effects of TGF-β on immune responses within the TME, while minimizing adverse effects of inhibiting the homeostatic roles that TGF-β plays to suppress pathologic immune responses in tissues unaffected by the tumor. Recent early reports suggest that such an approach might be feasible for both hematologic254 and solid tumors.255 Similarly, new advances in synthetic biology, such as the development of synthetic Notch receptors that allow localized and tightly regulated delivery of genetically encoded therapeutics at precise sites of tissue pathology,256 open the possibility for locally presenting TGF-β inhibitors at sites of non-malignant TGF-β-driven diseases such as tissue fibrosis.
Summation and perspectives
We have highlighted how TGF-β functions as a central regulator of tissue homeostasis throughout the lifespan of metazoan organisms. To effectively play this role, the TGF-β pathway engages many other regulatory inputs in distinct cellular contexts, generating an array of molecular and behavioral outputs. Although in most situations these outputs play critical roles in healthy development and maintenance of normal organ function and the effective repair of tissue injury. However, the centrality of TGF-β signaling also sets it up for deviant feed-forward circuits that contribute to progressive pathology and disease. Continued focus on understanding the operating logic of the TGF-β system will identify additional effective strategies to precisely target pathologic roles of TGF-β while preserving its many critical functions.
Recent progress in this field teaches us that the overall response of a tissue to TGF-β is defined by the integrated TGF-β responses of its constituent cell types. The TGF-β response of each cell type in turn consists of multiple coordinated effects on diverse cellular functions –for example, effects on collagen biosynthesis and cytokine production coupled with phenotypic activation and metabolic adaptation in fibroblasts; effects on EMT coupled with fibrogenic signaling, growth inhibition, and differentiation in epithelial progenitors; and, effects on differentiation coupled with immune regulatory functions in T cells. Moreover, variables such as aging, metabolism, endocrine signals, and microbiomes, which impact immunity, inflammation, and stem cell pools, likely influence how tissues read TGF-β signals. How TGF-β response programs are integrated and affected by these variables warrants further investigation.
Out of necessity, this review has focused on fibroblasts, epithelial and immune cells as the most abundant TGF-β target cells, and on fibrosis and cancer as the most common diseases of the TGF-β system. However, in so doing, we have sidestepped other important TGF-β target cells and the diseases that result from defective TGF-β signaling in these cell types (Figure 1). The rare if serious congenital diseases linked to TGF-β and other cytokines in this family (Table 2) substantiate this point. As components of TGF-β target tissues, endothelial, neural, skeletal, connective tissue, and smooth muscle cells also participate in integrated multicellular responses to TGF-β. These cell types and their responses are subject to the same principles discussed above. Advances in understanding the roles of TGF-β in common diseases such as fibrosis and cancer and their potential treatments might be relevant to these other pathologies as well.
The growing understanding of TGF-β signaling in health and disease has created opportunities for intervention in difficult to treat diseases that remain major sources of morbidity and mortality throughout the world. Although systemically administered drugs that broadly inhibit TGF-β signaling are challenging to develop because of the narrow window between efficacy and toxicity, many promising strategies have emerged that use these new biologic insights to more precisely target pathologic TGF-β functions. These strategies nurture considerable optimism about the potential impact of targeted inhibitors of TGF-β activation and signaling for treatment of multiple currently challenging diseases.
Acknowledgemets
The authors thank J.H. Lee for assistance with formatting the manuscript. The primary research work by the authors on the topic of this review is supported by NIH grants R35-CA252978 (JM), P01-CA129243 (JM), R01-HL145037 (DS) and R01-HL142568 (DS), P30-CA008748 (MSKCC), and by the Alan and Sandra Gerry Metastasis and Tumor Ecosystems Center at MSKCC
Footnotes
Declaration of Interests
Joan Massagué holds company stock of Scholar Rock, Inc. Dean Sheppard is a founder of Pliant Therapeutics, a member of the Genentech Scientific Review Board, a member of the Amgen Immunology Scientific Advisory Board, and a member of the Scientific Review Board for Lila Biologics.
References
- 1.Roberts AB, and Sporn MB (1988). Transforming growth factor beta. Adv Cancer Res 51, 107–145. [PubMed] [Google Scholar]
- 2.Massague J. (1998). TGF-beta signal transduction. Annu Rev Biochem 67, 753–791. 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 3.Davis H, Raja E, Miyazono K, Tsubakihara Y, and Moustakas A (2016). Mechanisms of action of bone morphogenetic proteins in cancer. Cytokine Growth Factor Rev 27, 81–92. 10.1016/j.cytogfr.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 4.Goumans MJ, and Ten Dijke P (2018). TGF-β Signaling in Control of Cardiovascular Function. Cold Spring Harb Perspect Biol 10. 10.1101/cshperspect.a022210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katagiri T, and Watabe T (2016). Bone Morphogenetic Proteins. Cold Spring Harb Perspect Biol 8. 10.1101/cshperspect.a021899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyers EA, and Kessler JA (2017). TGF-beta Family Signaling in Neural and Neuronal Differentiation, Development, and Function. Cold Spring Harb Perspect Biol 9. 10.1101/cshperspect.a022244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naka K, and Hirao A (2017). Regulation of Hematopoiesis and Hematological Disease by TGF-β Family Signaling Molecules. Cold Spring Harb Perspect Biol 9. 10.1101/cshperspect.a027987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nixon BG, Gao S, Wang X, and Li MO (2022). TGFβ control of immune responses in cancer: a holistic immuno-oncology perspective. Nat Rev Immunol. 10.1038/s41577-022-00796-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi Y, and Massagué J (2003). Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 113, 685–700. 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 10.Macias MJ, Martin-Malpartida P, and Massagué J (2015). Structural determinants of Smad function in TGF-β signaling. Trends Biochem Sci 40, 296–308. 10.1016/j.tibs.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nolan K, and Thompson TB (2014). The DAN family: modulators of TGF-β signaling and beyond. Protein Sci 23, 999–1012. 10.1002/pro.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.David CJ, and Massagué J (2018). Contextual determinants of TGFβ action in development, immunity and cancer. Nat Rev Mol Cell Biol 19, 419–435. 10.1038/s41580-018-0007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim KK, Sheppard D, and Chapman HA (2018). TGF-beta1 Signaling and Tissue Fibrosis. Cold Spring Harb Perspect Biol 10. 10.1101/cshperspect.a022293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Derynck R, Turley SJ, and Akhurst RJ (2021). TGFβ biology in cancer progression and immunotherapy. Nat Rev Clin Oncol 18, 9–34. 10.1038/s41571-020-0403-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tauriello DVF, Sancho E, and Batlle E (2022). Overcoming TGFβ-mediated immune evasion in cancer. Nat Rev Cancer 22, 25–44. 10.1038/s41568-021-00413-6. [DOI] [PubMed] [Google Scholar]
- 16.MacFarlane EG, Haupt J, Dietz HC, and Shore EM (2017). TGF-β Family Signaling in Connective Tissue and Skeletal Diseases. Cold Spring Harb Perspect Biol 9. 10.1101/cshperspect.a022269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tesseur I, and Wyss-Coray T (2006). A role for TGF-beta signaling in neurodegeneration: evidence from genetically engineered models. Curr Alzheimer Res 3, 505–513. 10.2174/156720506779025297. [DOI] [PubMed] [Google Scholar]
- 18.Shi M, Zhu J, Wang R, Chen X, Mi L, Walz T, and Springer TA (2011). Latent TGF-beta structure and activation. Nature 474, 343–349. 10.1038/nature10152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tran DQ, Andersson J, Wang R, Ramsey H, Unutmaz D, and Shevach EM (2009). GARP (LRRC32) is essential for the surface expression of latent TGF-beta on platelets and activated FOXP3+ regulatory T cells. Proc Natl Acad Sci U S A 106, 13445–13450. 10.1073/pnas.0901944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qin Y, Garrison BS, Ma W, Wang R, Jiang A, Li J, Mistry M, Bronson RT, Santoro D, Franco C, et al. (2018). A Milieu Molecule for TGF-beta Required for Microglia Function in the Nervous System. Cell 174, 156–171 e116. 10.1016/j.cell.2018.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheifetz S, Weatherbee JA, Tsang ML, Anderson JK, Mole JE, Lucas R, and Massagué J (1987). The transforming growth factor-beta system, a complex pattern of cross-reactive ligands and receptors. Cell 48, 409–415. 10.1016/0092-8674(87)90192-9. [DOI] [PubMed] [Google Scholar]
- 22.Ling N, Ying SY, Ueno N, Shimasaki S, Esch F, Hotta M, and Guillemin R (1986). Pituitary FSH is released by a heterodimer of the beta-subunits from the two forms of inhibin. Nature 321, 779–782. 10.1038/321779a0. [DOI] [PubMed] [Google Scholar]
- 23.Little SC, and Mullins MC (2009). Bone morphogenetic protein heterodimers assemble heteromeric type I receptor complexes to pattern the dorsoventral axis. Nat Cell Biol 11, 637–643. 10.1038/ncb1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimmi O, Umulis D, Othmer H, and O'Connor MB (2005). Facilitated transport of a Dpp/Scw heterodimer by Sog/Tsg leads to robust patterning of the Drosophila blastoderm embryo. Cell 120, 873–886. 10.1016/j.cell.2005.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Antebi YE, Linton JM, Klumpe H, Bintu B, Gong M, Su C, McCardell R, and Elowitz MB (2017). Combinatorial Signal Perception in the BMP Pathway. Cell 170, 1184–1196.e1124. 10.1016/j.cell.2017.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Munger JS, Harpel JG, Gleizes PE, Mazzieri R, Nunes I, and Rifkin DB (1997). Latent transforming growth factor-beta: structural features and mechanisms of activation. Kidney Int 51, 1376–1382. 10.1038/ki.1997.188. [DOI] [PubMed] [Google Scholar]
- 27.Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, Kawakatsu H, Sheppard D, Broaddus VC, and Nishimura SL (2002). The integrin alpha(v)beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1. J Cell Biol 157, 493–507. 10.1083/jcb.200109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Worthington JJ, Czajkowska BI, Melton AC, and Travis MA (2011). Intestinal dendritic cells specialize to activate transforming growth factor-beta and induce Foxp3+ regulatory T cells via integrin alphavbeta8. Gastroenterology 141, 1802–1812. 10.1053/j.gastro.2011.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ribeiro SM, Poczatek M, Schultz-Cherry S, Villain M, and Murphy-Ullrich JE (1999). The activation sequence of thrombospondin-1 interacts with the latency-associated peptide to regulate activation of latent transforming growth factor-beta. J Biol Chem 274, 13586–13593. 10.1074/jbc.274.19.13586. [DOI] [PubMed] [Google Scholar]
- 30.Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SM, Lawler J, Hynes RO, Boivin GP, and Bouck N (1998). Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell 93, 1159–1170. 10.1016/s0092-8674(00)81460-9. [DOI] [PubMed] [Google Scholar]
- 31.Kumar R, Mickael C, Kassa B, Gebreab L, Robinson JC, Koyanagi DE, Sanders L, Barthel L, Meadows C, Fox D, et al. (2017). TGF-beta activation by bone marrow-derived thrombospondin-1 causes Schistosoma- and hypoxia-induced pulmonary hypertension. Nat Commun 8, 15494. 10.1038/ncomms15494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Breuss JM, Gillett N, Lu L, Sheppard D, and Pytela R (1993). Restricted distribution of integrin beta 6 mRNA in primate epithelial tissues. J Histochem Cytochem 41, 1521–1527. 10.1177/41.10.8245410. [DOI] [PubMed] [Google Scholar]
- 33.Hahm K, Lukashev ME, Luo Y, Yang WJ, Dolinski BM, Weinreb PH, Simon KJ, Chun Wang L, Leone DR, Lobb RR, et al. (2007). Alphav beta6 integrin regulates renal fibrosis and inflammation in Alport mouse. Am J Pathol 170, 110–125. 10.2353/ajpath.2007.060158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, et al. (1999). The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 96, 319–328. 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 35.Huang XZ, Wu JF, Cass D, Erle DJ, Corry D, Young SG, Farese RV Jr., and Sheppard D (1996). Inactivation of the integrin beta 6 subunit gene reveals a role of epithelial integrins in regulating inflammation in the lung and skin. J Cell Biol 133, 921–928. 10.1083/jcb.133.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Annes JP, Rifkin DB, and Munger JS (2002). The integrin alphaVbeta6 binds and activates latent TGFbeta3. FEBS Lett 511, 65–68. S001457930103280X [pii]. [DOI] [PubMed] [Google Scholar]
- 37.Proctor JM, Zang K, Wang D, Wang R, and Reichardt LF (2005). Vascular development of the brain requires beta8 integrin expression in the neuroepithelium. J Neurosci 25, 9940–9948. 10.1523/JNEUROSCI.3467-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arnold TD, Niaudet C, Pang MF, Siegenthaler J, Gaengel K, Jung B, Ferrero GM, Mukouyama YS, Fuxe J, Akhurst R, et al. (2014). Excessive vascular sprouting underlies cerebral hemorrhage in mice lacking alphaVbeta8-TGFbeta signaling in the brain. Development 141, 4489–4499. 10.1242/dev.107193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang Z, Mu Z, Dabovic B, Jurukovski V, Yu D, Sung J, Xiong X, and Munger JS (2007). Absence of integrin-mediated TGFbeta1 activation in vivo recapitulates the phenotype of TGFbeta1-null mice. J Cell Biol 176, 787–793. 10.1083/jcb.200611044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aluwihare P, Mu Z, Zhao Z, Yu D, Weinreb PH, Horan GS, Violette SM, and Munger JS (2009). Mice that lack activity of alphavbeta6- and alphavbeta8-integrins reproduce the abnormalities of Tgfb1- and Tgfb3-null mice. J Cell Sci 122, 227–232. 10.1242/jcs.035246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reed NI, Jo H, Chen C, Tsujino K, Arnold TD, DeGrado WF, and Sheppard D (2015). The alphavbeta1 integrin plays a critical in vivo role in tissue fibrosis. Sci Transl Med 7, 288ra279. 10.1126/scitranslmed.aaa5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Le VQ, Zhao B, Ramesh S, Toohey C, DeCosta A, Mintseris J, Liu X, Gygi S, and Springer TA (2023). A specialized integrin-binding motif enables proTGF-β2 activation by integrin αVβ6 but not αVβ8. Proc Natl Acad Sci U S A 120, e2304874120. 10.1073/pnas.2304874120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun T, Huang Z, Liang WC, Yin J, Lin WY, Wu J, Vernes JM, Lutman J, Caplazi P, Jeet S, et al. (2021). TGFbeta2 and TGFbeta3 isoforms drive fibrotic disease pathogenesis. Sci Transl Med 13. 10.1126/scitranslmed.abe0407. [DOI] [PubMed] [Google Scholar]
- 44.Giacomini MM, Travis MA, Kudo M, and Sheppard D (2012). Epithelial cells utilize cortical actin/myosin to activate latent TGF-beta through integrin alpha(v)beta(6)-dependent physical force. Exp Cell Res 318, 716–722. 10.1016/j.yexcr.2012.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Annes JP, Chen Y, Munger JS, and Rifkin DB (2004). Integrin alphaVbeta6-mediated activation of latent TGF-beta requires the latent TGF-beta binding protein-1. J Cell Biol 165, 723–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Campbell MG, Cormier A, Ito S, Seed RI, Bondesson AJ, Lou J, Marks JD, Baron JL, Cheng Y, and Nishimura SL (2020). Cryo-EM Reveals Integrin-Mediated TGF-beta Activation without Release from Latent TGF-beta. Cell 180, 490–501 e416. 10.1016/j.cell.2019.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arnold TD, Lizama CO, Cautivo KM, Santander N, Lin L, Qiu H, Huang EJ, Liu C, Mukouyama YS, Reichardt LF, et al. (2019). Impaired alphaVbeta8 and TGFbeta signaling lead to microglial dysmaturation and neuromotor dysfunction. J Exp Med 216, 900–915. 10.1084/jem.20181290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Groppe J, Hinck CS, Samavarchi-Tehrani P, Zubieta C, Schuermann JP, Taylor AB, Schwarz PM, Wrana JL, and Hinck AP (2008). Cooperative assembly of TGF-beta superfamily signaling complexes is mediated by two disparate mechanisms and distinct modes of receptor binding. Mol Cell 29, 157–168. 10.1016/j.molcel.2007.11.039. [DOI] [PubMed] [Google Scholar]
- 49.Gipson GR, Goebel EJ, Hart KN, Kappes EC, Kattamuri C, McCoy JC, and Thompson TB (2020). Structural perspective of BMP ligands and signaling. Bone 140, 115549. 10.1016/j.bone.2020.115549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goebel EJ, Hart KN, McCoy JC, and Thompson TB (2019). Structural biology of the TGFβ family. Exp Biol Med (Maywood) 244, 1530–1546. 10.1177/1535370219880894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huse M, Chen YG, Massagué J, and Kuriyan J (1999). Crystal structure of the cytoplasmic domain of the type I TGF beta receptor in complex with FKBP12. Cell 96, 425–436. 10.1016/s0092-8674(00)80555-3. [DOI] [PubMed] [Google Scholar]
- 52.Huse M, Muir TW, Xu L, Chen YG, Kuriyan J, and Massagué J (2001). The TGF beta receptor activation process: an inhibitor- to substrate-binding switch. Mol Cell 8, 671–682. 10.1016/s1097-2765(01)00332-x. [DOI] [PubMed] [Google Scholar]
- 53.Henen MA, Mahlawat P, Zwieb C, Kodali RB, Hinck CS, Hanna RD, Krzysiak TC, Ilangovan U, Cano KE, Hinck G, et al. (2019). TGF-beta2 uses the concave surface of its extended finger region to bind betaglycan's ZP domain via three residues specific to TGF-beta and inhibin-alpha. J Biol Chem 294, 3065–3080. 10.1074/jbc.RA118.005210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lopez-Casillas F, Wrana JL, and Massague J (1993). Betaglycan presents ligand to the TGF beta signaling receptor. Cell 73, 1435–1444. 10.1016/0092-8674(93)90368-z. [DOI] [PubMed] [Google Scholar]
- 55.Miyazawa K, and Miyazono K (2017). Regulation of TGF-β Family Signaling by Inhibitory Smads. Cold Spring Harb Perspect Biol 9. 10.1101/cshperspect.a022095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alarcón C, Zaromytidou AI, Xi Q, Gao S, Yu J, Fujisawa S, Barlas A, Miller AN, Manova-Todorova K, Macias MJ, et al. (2009). Nuclear CDKs drive Smad transcriptional activation and turnover in BMP and TGF-beta pathways. Cell 139, 757–769. 10.1016/j.cell.2009.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aragón E, Goerner N, Zaromytidou AI, Xi Q, Escobedo A, Massagué J, and Macias MJ (2011). A Smad action turnover switch operated by WW domain readers of a phosphoserine code. Genes Dev 25, 1275–1288. 10.1101/gad.2060811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gao S, Alarcón C, Sapkota G, Rahman S, Chen PY, Goerner N, Macias MJ, Erdjument-Bromage H, Tempst P, and Massagué J (2009). Ubiquitin ligase Nedd4L targets activated Smad2/3 to limit TGF-beta signaling. Mol Cell 36, 457–468. 10.1016/j.molcel.2009.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sapkota G, Knockaert M, Alarcón C, Montalvo E, Brivanlou AH, and Massagué J (2006). Dephosphorylation of the linker regions of Smad1 and Smad2/3 by small C-terminal domain phosphatases has distinct outcomes for bone morphogenetic protein and transforming growth factor-beta pathways. J Biol Chem 281, 40412–40419. 10.1074/jbc.M610172200. [DOI] [PubMed] [Google Scholar]
- 60.Lönn P, van der Heide LP, Dahl M, Hellman U, Heldin CH, and Moustakas A (2010). PARP-1 attenuates Smad-mediated transcription. Mol Cell 40, 521–532. 10.1016/j.molcel.2010.10.029. [DOI] [PubMed] [Google Scholar]
- 61.Kretzschmar M, Doody J, and Massagué J (1997). Opposing BMP and EGF signalling pathways converge on the TGF-beta family mediator Smad1. Nature 389, 618–622. 10.1038/39348. [DOI] [PubMed] [Google Scholar]
- 62.Matsuura I, Denissova NG, Wang G, He D, Long J, and Liu F (2004). Cyclin-dependent kinases regulate the antiproliferative function of Smads. Nature 430, 226–231. 10.1038/nature02650. [DOI] [PubMed] [Google Scholar]
- 63.Hinck AP, Mueller TD, and Springer TA (2016). Structural Biology and Evolution of the TGF-β Family. Cold Spring Harb Perspect Biol 8. 10.1101/cshperspect.a022103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Senft AD, Bikoff EK, Robertson EJ, and Costello I (2019). Genetic dissection of Nodal and Bmp signalling requirements during primordial germ cell development in mouse. Nat Commun 10, 1089. 10.1038/s41467-019-09052-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oshimori N, and Fuchs E (2012). Paracrine TGF-β signaling counterbalances BMP-mediated repression in hair follicle stem cell activation. Cell Stem Cell 10, 63–75. 10.1016/j.stem.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zeisberg M, Hanai J, Sugimoto H, Mammoto T, Charytan D, Strutz F, and Kalluri R (2003). BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med 9, 964–968. 10.1038/nm888. [DOI] [PubMed] [Google Scholar]
- 67.Johnston CJC, Smyth DJ, Kodali RB, White MPJ, Harcus Y, Filbey KJ, Hewitson JP, Hinck CS, Ivens A, Kemter AM, et al. (2017). A structurally distinct TGF-β mimic from an intestinal helminth parasite potently induces regulatory T cells. Nat Commun 8, 1741. 10.1038/s41467-017-01886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kinoshita A, Saito T, Tomita H, Makita Y, Yoshida K, Ghadami M, Yamada K, Kondo S, Ikegawa S, Nishimura G, et al. (2000). Domain-specific mutations in TGFB1 result in Camurati-Engelmann disease. Nat Genet 26, 19–20. 10.1038/79128. [DOI] [PubMed] [Google Scholar]
- 69.Loeys BL, and Dietz HC (1993). Loeys-Dietz Syndrome. In GeneReviews((R)), Adam MP, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, Gripp KW, and Amemiya A, eds. [Google Scholar]
- 70.Ozdamar B, Bose R, Barrios-Rodiles M, Wang HR, Zhang Y, and Wrana JL (2005). Regulation of the polarity protein Par6 by TGFbeta receptors controls epithelial cell plasticity. Science 307, 1603–1609. 10.1126/science.1105718. [DOI] [PubMed] [Google Scholar]
- 71.Yi JJ, Barnes AP, Hand R, Polleux F, and Ehlers MD (2010). TGF-beta signaling specifies axons during brain development. Cell 142, 144–157. 10.1016/j.cell.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Foletta VC, Lim MA, Soosairajah J, Kelly AP, Stanley EG, Shannon M, He W, Das S, Massague J, and Bernard O (2003). Direct signaling by the BMP type II receptor via the cytoskeletal regulator LIMK1. J Cell Biol 162, 1089–1098. 10.1083/jcb.200212060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee-Hoeflich ST, Causing CG, Podkowa M, Zhao X, Wrana JL, and Attisano L (2004). Activation of LIMK1 by binding to the BMP receptor, BMPRII, regulates BMP-dependent dendritogenesis. Embo j 23, 4792–4801. 10.1038/sj.emboj.7600418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Podkowa M, Christova T, Zhao X, Jian Y, and Attisano L (2013). p21-Activated kinase (PAK) is required for Bone Morphogenetic Protein (BMP)-induced dendritogenesis in cortical neurons. Mol Cell Neurosci 57, 83–92. 10.1016/j.mcn.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 75.Zhang S, Takaku M, Zou L, Gu AD, Chou WC, Zhang G, Wu B, Kong Q, Thomas SY, Serody JS, et al. (2017). Reversing SKI-SMAD4-mediated suppression is essential for T(H)17 cell differentiation. Nature 551, 105–109. 10.1038/nature24283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Igalouzene R, Hernandez-Vargas H, Benech N, Guyennon A, Bauché D, Barrachina C, Dubois E, Marie JC, and Soudja SM (2022). SMAD4 TGF-β-independent function preconditions naive CD8+ T cells to prevent severe chronic intestinal inflammation. J Clin Invest 132. 10.1172/jci151020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Levy L, Howell M, Das D, Harkin S, Episkopou V, and Hill CS (2007). Arkadia activates Smad3/Smad4-dependent transcription by triggering signal-induced SnoN degradation. Mol Cell Biol 27, 6068–6083. 10.1128/mcb.00664-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nagano Y, Mavrakis KJ, Lee KL, Fujii T, Koinuma D, Sase H, Yuki K, Isogaya K, Saitoh M, Imamura T, et al. (2007). Arkadia induces degradation of SnoN and c-Ski to enhance transforming growth factor-beta signaling. J Biol Chem 282, 20492–20501. 10.1074/jbc.M701294200. [DOI] [PubMed] [Google Scholar]
- 79.Tecalco-Cruz AC, Ríos-López DG, Vázquez-Victorio G, Rosales-Alvarez RE, and Macías-Silva M (2018). Transcriptional cofactors Ski and SnoN are major regulators of the TGF-β/Smad signaling pathway in health and disease. Signal Transduct Target Ther 3, 15. 10.1038/s41392-018-0015-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shah A, Melhuish TA, Fox TE, Frierson HF Jr., and Wotton D (2019). TGIF transcription factors repress acetyl CoA metabolic gene expression and promote intestinal tumor growth. Genes Dev 33, 388–402. 10.1101/gad.320127.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wotton D, Lo RS, Lee S, and Massagué J (1999). A Smad transcriptional corepressor. Cell 97, 29–39. 10.1016/s0092-8674(00)80712-6. [DOI] [PubMed] [Google Scholar]
- 82.David CJ, Huang YH, Chen M, Su J, Zou Y, Bardeesy N, Iacobuzio-Donahue CA, and Massagué J (2016). TGF-β Tumor Suppression through a Lethal EMT. Cell 164, 1015–1030. 10.1016/j.cell.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bardeesy N, Cheng KH, Berger JH, Chu GC, Pahler J, Olson P, Hezel AF, Horner J, Lauwers GY, Hanahan D, and DePinho RA (2006). Smad4 is dispensable for normal pancreas development yet critical in progression and tumor biology of pancreas cancer. Genes Dev 20, 3130–3146. 10.1101/gad.1478706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wiater E, and Vale W (2012). Roles of activin family in pancreatic development and homeostasis. Mol Cell Endocrinol 359, 23–29. 10.1016/j.mce.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 85.Davis BN, Hilyard AC, Lagna G, and Hata A (2008). SMAD proteins control DROSHA-mediated microRNA maturation. Nature 454, 56–61. 10.1038/nature07086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yamaguchi K, Shirakabe K, Shibuya H, Irie K, Oishi I, Ueno N, Taniguchi T, Nishida E, and Matsumoto K (1995). Identification of a member of the MAPKKK family as a potential mediator of TGF-beta signal transduction. Science 270, 2008–2011. 10.1126/science.270.5244.2008. [DOI] [PubMed] [Google Scholar]
- 87.Mihaly SR, Ninomiya-Tsuji J, and Morioka S (2014). TAK1 control of cell death. Cell Death Differ 21, 1667–1676. 10.1038/cdd.2014.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Heldin CH, and Moustakas A (2016). Signaling Receptors for TGF-β Family Members. Cold Spring Harb Perspect Biol 8. 10.1101/cshperspect.a022053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sorrentino A, Thakur N, Grimsby S, Marcusson A, von Bulow V, Schuster N, Zhang S, Heldin CH, and Landström M (2008). The type I TGF-beta receptor engages TRAF6 to activate TAK1 in a receptor kinase-independent manner. Nat Cell Biol 10, 1199–1207. 10.1038/ncb1780. [DOI] [PubMed] [Google Scholar]
- 90.Yamashita M, Fatyol K, Jin C, Wang X, Liu Z, and Zhang YE (2008). TRAF6 mediates Smad-independent activation of JNK and p38 by TGF-beta. Mol Cell 31, 918–924. 10.1016/j.molcel.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee MK, Pardoux C, Hall MC, Lee PS, Warburton D, Qing J, Smith SM, and Derynck R (2007). TGF-beta activates Erk MAP kinase signalling through direct phosphorylation of ShcA. Embo j 26, 3957–3967. 10.1038/sj.emboj.7601818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shi Y, Wang YF, Jayaraman L, Yang H, Massagué J, and Pavletich NP (1998). Crystal structure of a Smad MH1 domain bound to DNA: insights on DNA binding in TGF-beta signaling. Cell 94, 585–594. 10.1016/s0092-8674(00)81600-1. [DOI] [PubMed] [Google Scholar]
- 93.Aragón E, Wang Q, Zou Y, Morgani SM, Ruiz L, Kaczmarska Z, Su J, Torner C, Tian L, Hu J, et al. (2019). Structural basis for distinct roles of SMAD2 and SMAD3 in FOXH1 pioneer-directed TGF-β signaling. Genes Dev 33, 1506–1524. 10.1101/gad.330837.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Martin-Malpartida P, Batet M, Kaczmarska Z, Freier R, Gomes T, Aragón E, Zou Y, Wang Q, Xi Q, Ruiz L, et al. (2017). Structural basis for genome wide recognition of 5-bp GC motifs by SMAD transcription factors. Nat Commun 8, 2070. 10.1038/s41467-017-02054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen X, Weisberg E, Fridmacher V, Watanabe M, Naco G, and Whitman M (1997). Smad4 and FAST-1 in the assembly of activin-responsive factor. Nature 389, 85–89. 10.1038/38008. [DOI] [PubMed] [Google Scholar]
- 96.Charney RM, Forouzmand E, Cho JS, Cheung J, Paraiso KD, Yasuoka Y, Takahashi S, Taira M, Blitz IL, Xie X, and Cho KW (2017). Foxh1 Occupies cis-Regulatory Modules Prior to Dynamic Transcription Factor Interactions Controlling the Mesendoderm Gene Program. Dev Cell 40, 595–607.e594. 10.1016/j.devcel.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pluta R, Aragón E, Prescott NA, Ruiz L, Mees RA, Baginski B, Flood JR, Martin-Malpartida P, Massagué J, David Y, and Macias MJ (2022). Molecular basis for DNA recognition by the maternal pioneer transcription factor FoxH1. Nat Commun 13, 7279. 10.1038/s41467-022-34925-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mullen AC, Orlando DA, Newman JJ, Lovén J, Kumar RM, Bilodeau S, Reddy J, Guenther MG, DeKoter RP, and Young RA (2011). Master transcription factors determine cell-type-specific responses to TGF-β signaling. Cell 147, 565–576. 10.1016/j.cell.2011.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hata A, Seoane J, Lagna G, Montalvo E, Hemmati-Brivanlou A, and Massagué J (2000). OAZ uses distinct DNA- and protein-binding zinc fingers in separate BMP-Smad and Olf signaling pathways. Cell 100, 229–240. 10.1016/s0092-8674(00)81561-5. [DOI] [PubMed] [Google Scholar]
- 100.Trompouki E, Bowman TV, Lawton LN, Fan ZP, Wu DC, DiBiase A, Martin CS, Cech JN, Sessa AK, Leblanc JL, et al. (2011). Lineage regulators direct BMP and Wnt pathways to cell-specific programs during differentiation and regeneration. Cell 147, 577–589. 10.1016/j.cell.2011.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Su J, Morgani SM, David CJ, Wang Q, Er EE, Huang YH, Basnet H, Zou Y, Shu W, Soni RK, et al. (2020). TGF-β orchestrates fibrogenic and developmental EMTs via the RAS effector RREB1. Nature 577, 566–571. 10.1038/s41586-019-1897-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Luo M, Bai J, Liu B, Yan P, Zuo F, Sun H, Sun Y, Xu X, Song Z, Yang Y, et al. (2019). H3K18ac Primes Mesendodermal Differentiation upon Nodal Signaling. Stem Cell Reports 13, 642–656. 10.1016/j.stemcr.2019.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xi Q, Wang Z, Zaromytidou AI, Zhang XH, Chow-Tsang LF, Liu JX, Kim H, Barlas A, Manova-Todorova K, Kaartinen V, et al. (2011). A poised chromatin platform for TGF-β access to master regulators. Cell 147, 1511–1524. 10.1016/j.cell.2011.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, and et al. (1992). Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature 359, 693–699. 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Letterio JJ, Geiser AG, Kulkarni AB, Dang H, Kong L, Nakabayashi T, Mackall CL, Gress RE, and Roberts AB (1996). Autoimmunity associated with TGF-beta1-deficiency in mice is dependent on MHC class II antigen expression. J Clin Invest 98, 2109–2119. 10.1172/JCI119017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Marie JC, Liggitt D, and Rudensky AY (2006). Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-beta receptor. Immunity 25, 441–454. 10.1016/j.immuni.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 107.Li MO, Sanjabi S, and Flavell RA (2006). Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity 25, 455–471. 10.1016/j.immuni.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 108.Zhang N, and Bevan MJ (2012). TGF-beta signaling to T cells inhibits autoimmunity during lymphopenia-driven proliferation. Nat Immunol 13, 667–673. 10.1038/ni.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li MO, Wan YY, Sanjabi S, Robertson AK, and Flavell RA (2006). Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol 24, 99–146. 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 110.Park SR, Seo GY, Choi AJ, Stavnezer J, and Kim PH (2005). Analysis of transforming growth factor-beta1-induced Ig germ-line gamma2b transcription and its implication for IgA isotype switching. Eur J Immunol 35, 946–956. 10.1002/eji.200425848. [DOI] [PubMed] [Google Scholar]
- 111.Batlle E, and Massagué J (2019). Transforming Growth Factor-β Signaling in Immunity and Cancer. Immunity 50, 924–940. 10.1016/j.immuni.2019.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brown CC, and Rudensky AY (2023). Spatiotemporal regulation of peripheral T cell tolerance. Science 380, 472–478. 10.1126/science.adg6425. [DOI] [PubMed] [Google Scholar]
- 113.Ramalingam R, Larmonier CB, Thurston RD, Midura-Kiela MT, Zheng SG, Ghishan FK, and Kiela PR (2012). Dendritic cell-specific disruption of TGF-beta receptor II leads to altered regulatory T cell phenotype and spontaneous multiorgan autoimmunity. J Immunol 189, 3878–3893. 10.4049/jimmunol.1201029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kaplan DH, Li MO, Jenison MC, Shlomchik WD, Flavell RA, and Shlomchik MJ (2007). Autocrine/paracrine TGFbeta1 is required for the development of epidermal Langerhans cells. J Exp Med 204, 2545–2552. 10.1084/jem.20071401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Brown CC, Gudjonson H, Pritykin Y, Deep D, Lavallée VP, Mendoza A, Fromme R, Mazutis L, Ariyan C, Leslie C, et al. (2019). Transcriptional Basis of Mouse and Human Dendritic Cell Heterogeneity. Cell 179, 846–863.e824. 10.1016/j.cell.2019.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.O'Garra A, Gabrysova L, and Spits H (2011). Quantitative events determine the differentiation and function of helper T cells. Nat Immunol 12, 288–294. 10.1038/ni.2003. [DOI] [PubMed] [Google Scholar]
- 117.Gorelik L, Fields PE, and Flavell RA (2000). Cutting edge: TGF-beta inhibits Th type 2 development through inhibition of GATA-3 expression. J Immunol 165, 4773–4777. 10.4049/jimmunol.165.9.4773. [DOI] [PubMed] [Google Scholar]
- 118.Kuwahara M, Yamashita M, Shinoda K, Tofukuji S, Onodera A, Shinnakasu R, Motohashi S, Hosokawa H, Tumes D, Iwamura C, et al. (2012). The transcription factor Sox4 is a downstream target of signaling by the cytokine TGF-β and suppresses T(H)2 differentiation. Nat Immunol 13, 778–786. 10.1038/ni.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu M, Kuo F, Capistrano KJ, Kang D, Nixon BG, Shi W, Chou C, Do MH, Stamatiades EG, Gao S, et al. (2020). TGF-β suppresses type 2 immunity to cancer. Nature 587, 115–120. 10.1038/s41586-020-2836-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, et al. (2008). TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature 453, 236–240. 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Melton AC, Bailey-Bucktrout SL, Travis MA, Fife BT, Bluestone JA, and Sheppard D (2010). Expression of alphavbeta8 integrin on dendritic cells regulates Th17 cell development and experimental autoimmune encephalomyelitis in mice. J Clin Invest 120, 4436–4444. 10.1172/JCI43786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Travis MA, and Sheppard D (2014). TGF-beta activation and function in immunity. Annu Rev Immunol 32, 51–82. 10.1146/annurev-immunol-032713-120257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Akagbosu B, Tayyebi Z, Shibu G, Paucar Iza YA, Deep D, Parisotto YF, Fisher L, Pasolli HA, Thevin V, Elmentaite R, et al. (2022). Novel antigen-presenting cell imparts T(reg)-dependent tolerance to gut microbiota. Nature 610, 752–760. 10.1038/s41586-022-05309-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ouyang W, Beckett O, Ma Q, and Li MO (2010). Transforming growth factor-beta signaling curbs thymic negative selection promoting regulatory T cell development. Immunity 32, 642–653. 10.1016/j.immuni.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tone Y, Furuuchi K, Kojima Y, Tykocinski ML, Greene MI, and Tone M (2008). Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat Immunol 9, 194–202. 10.1038/ni1549. [DOI] [PubMed] [Google Scholar]
- 126.Marie JC, Letterio JJ, Gavin M, and Rudensky AY (2005). TGF-beta1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med 201, 1061–1067. 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kim SV, Xiang WV, Kwak C, Yang Y, Lin XW, Ota M, Sarpel U, Rifkin DB, Xu R, and Littman DR (2013). GPR15-mediated homing controls immune homeostasis in the large intestine mucosa. Science 340, 1456–1459. 10.1126/science.1237013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Josefowicz SZ, Niec RE, Kim HY, Treuting P, Chinen T, Zheng Y, Umetsu DT, and Rudensky AY (2012). Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature 482, 395–399. 10.1038/nature10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ouyang W, Oh SA, Ma Q, Bivona MR, Zhu J, and Li MO (2013). TGF-beta cytokine signaling promotes CD8+ T cell development and low-affinity CD4+ T cell homeostasis by regulation of interleukin-7 receptor alpha expression. Immunity 39, 335–346. 10.1016/j.immuni.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Thomas DA, and Massague J (2005). TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell 8, 369–380. 10.1016/j.ccr.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 131.Konkel JE, Maruyama T, Carpenter AC, Xiong Y, Zamarron BF, Hall BE, Kulkarni AB, Zhang P, Bosselut R, and Chen W (2011). Control of the development of CD8αα+ intestinal intraepithelial lymphocytes by TGF-β. Nat Immunol 12, 312–319. 10.1038/ni.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Castriconi R, Cantoni C, Della Chiesa M, Vitale M, Marcenaro E, Conte R, Biassoni R, Bottino C, Moretta L, and Moretta A (2003). Transforming growth factor beta 1 inhibits expression of NKp30 and NKG2D receptors: consequences for the NK-mediated killing of dendritic cells. Proc Natl Acad Sci U S A 100, 4120–4125. 10.1073/pnas.0730640100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lazarova M, and Steinle A (2019). Impairment of NKG2D-Mediated Tumor Immunity by TGF-β. Front Immunol 10, 2689. 10.3389/fimmu.2019.02689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Donatelli SS, Zhou JM, Gilvary DL, Eksioglu EA, Chen X, Cress WD, Haura EB, Schabath MB, Coppola D, Wei S, and Djeu JY (2014). TGF-β-inducible microRNA-183 silences tumor-associated natural killer cells. Proc Natl Acad Sci U S A 111, 4203–4208. 10.1073/pnas.1319269111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Viel S, Marçais A, Guimaraes FS, Loftus R, Rabilloud J, Grau M, Degouve S, Djebali S, Sanlaville A, Charrier E, et al. (2016). TGF-β inhibits the activation and functions of NK cells by repressing the mTOR pathway. Sci Signal 9, ra19. 10.1126/scisignal.aad1884. [DOI] [PubMed] [Google Scholar]
- 136.Gao Y, Souza-Fonseca-Guimaraes F, Bald T, Ng SS, Young A, Ngiow SF, Rautela J, Straube J, Waddell N, Blake SJ, et al. (2017). Tumor immunoevasion by the conversion of effector NK cells into type 1 innate lymphoid cells. Nat Immunol 18, 1004–1015. 10.1038/ni.3800. [DOI] [PubMed] [Google Scholar]
- 137.Cortez VS, Ulland TK, Cervantes-Barragan L, Bando JK, Robinette ML, Wang Q, White AJ, Gilfillan S, Cella M, and Colonna M (2017). SMAD4 impedes the conversion of NK cells into ILC1-like cells by curtailing non-canonical TGF-β signaling. Nat Immunol 18, 995–1003. 10.1038/ni.3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.McFarlane AJ, Fercoq F, Coffelt SB, and Carlin LM (2021). Neutrophil dynamics in the tumor microenvironment. J Clin Invest 131. 10.1172/jci143759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, and Albelda SM (2009). Polarization of tumor-associated neutrophil phenotype by TGF-beta: "N1" versus "N2" TAN . Cancer Cell 16, 183–194. 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Novitskiy SV, Pickup MW, Chytil A, Polosukhina D, Owens P, and Moses HL (2012). Deletion of TGF-beta signaling in myeloid cells enhances their anti-tumorigenic properties. J Leukoc Biol 92, 641–651. 10.1189/jlb.1211639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hollander MC, Latour LL, Yang D, Ishii H, Xiao Z, Min Y, Ray-Choudhury A, Munasinghe J, Merchant AS, Lin PC, et al. (2017). Attenuation of Myeloid-Specific TGFbeta Signaling Induces Inflammatory Cerebrovascular Disease and Stroke. Circ Res 121, 1360–1369. 10.1161/CIRCRESAHA.116.310349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gabrilovich DI (2017). Myeloid-Derived Suppressor Cells. Cancer Immunol Res 5, 3–8. 10.1158/2326-6066.Cir-16-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zoller T, Schneider A, Kleimeyer C, Masuda T, Potru PS, Pfeifer D, Blank T, Prinz M, and Spittau B (2018). Silencing of TGFbeta signalling in microglia results in impaired homeostasis. Nat Commun 9, 4011. 10.1038/s41467-018-06224-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sahai E, Astsaturov I, Cukierman E, DeNardo DG, Egeblad M, Evans RM, Fearon D, Greten FR, Hingorani SR, Hunter T, et al. (2020). A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer 20, 174–186. 10.1038/s41568-019-0238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Buechler MB, Pradhan RN, Krishnamurty AT, Cox C, Calviello AK, Wang AW, Yang YA, Tam L, Caothien R, Roose-Girma M, et al. (2021). Cross-tissue organization of the fibroblast lineage. Nature 593, 575–579. 10.1038/s41586-021-03549-5. [DOI] [PubMed] [Google Scholar]
- 146.Tsukui T, Sun KH, Wetter JB, Wilson-Kanamori JR, Hazelwood LA, Henderson NC, Adams TS, Schupp JC, Poli SD, Rosas IO, et al. (2020). Collagen-producing lung cell atlas identifies multiple subsets with distinct localization and relevance to fibrosis. Nat Commun 11, 1920. 10.1038/s41467-020-15647-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Beers MF, and Morrisey EE (2011). The three R's of lung health and disease: repair, remodeling, and regeneration. J Clin Invest 121, 2065–2073. 10.1172/JCI45961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Buechler MB, Fu W, and Turley SJ (2021). Fibroblast-macrophage reciprocal interactions in health, fibrosis, and cancer. Immunity 54, 903–915. 10.1016/j.immuni.2021.04.021. [DOI] [PubMed] [Google Scholar]
- 149.Schwörer S, Berisa M, Violante S, Qin W, Zhu J, Hendrickson RC, Cross JR, and Thompson CB (2020). Proline biosynthesis is a vent for TGFβ-induced mitochondrial redox stress. Embo j 39, e103334. 10.15252/embj.2019103334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Heino J, Ignotz RA, Hemler ME, Crouse C, and Massagué J (1989). Regulation of cell adhesion receptors by transforming growth factor-beta. Concomitant regulation of integrins that share a common beta 1 subunit. J Biol Chem 264, 380–388. [PubMed] [Google Scholar]
- 151.Sheppard D, Cohen DS, Wang A, and Busk M (1992). Transforming growth factor beta differentially regulates expression of integrin subunits in guinea pig airway epithelial cells. J Biol Chem 267, 17409–17414. [PubMed] [Google Scholar]
- 152.Friedman SL, Sheppard D, Duffield JS, and Violette S (2013). Therapy for fibrotic diseases: nearing the starting line. Sci Transl Med 5, 167sr161. 10.1126/scitranslmed.3004700. [DOI] [PubMed] [Google Scholar]
- 153.Ignotz RA, and Massague J (1986). Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem 261, 4337–4345. [PubMed] [Google Scholar]
- 154.Roberts AB, Sporn MB, Assoian RK, Smith JM, Roche NS, Wakefield LM, Heine UI, Liotta LA, Falanga V, Kehrl JH, and et al. (1986). Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A 83, 4167–4171. 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sime PJ, Xing Z, Graham FL, Csaky KG, and Gauldie J (1997). Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J Clin Invest 100, 768–776. 10.1172/JCI119590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Liu F, Mih JD, Shea BS, Kho AT, Sharif AS, Tager AM, and Tschumperlin DJ (2010). Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J Cell Biol 190, 693–706. 10.1083/jcb.201004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lo CM, Wang HB, Dembo M, and Wang YL (2000). Cell movement is guided by the rigidity of the substrate. Biophys J 79, 144–152. 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Li M, Krishnaveni MS, Li C, Zhou B, Xing Y, Banfalvi A, Li A, Lombardi V, Akbari O, Borok Z, and Minoo P (2011). Epithelium-specific deletion of TGF-beta receptor type II protects mice from bleomycin-induced pulmonary fibrosis. J Clin Invest 121, 277–287. 10.1172/JCI42090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lehmann M, Korfei M, Mutze K, Klee S, Skronska-Wasek W, Alsafadi HN, Ota C, Costa R, Schiller HB, Lindner M, et al. (2017). Senolytic drugs target alveolar epithelial cell function and attenuate experimental lung fibrosis ex vivo. Eur Respir J 50. 10.1183/13993003.02367-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yao C, Guan X, Carraro G, Parimon T, Liu X, Huang G, Mulay A, Soukiasian HJ, David G, Weigt SS, et al. (2021). Senescence of Alveolar Type 2 Cells Drives Progressive Pulmonary Fibrosis. Am J Respir Crit Care Med 203, 707–717. 10.1164/rccm.202004-1274OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang Q, Zou Y, Nowotschin S, Kim SY, Li QV, Soh CL, Su J, Zhang C, Shu W, Xi Q, et al. (2017). The p53 Family Coordinates Wnt and Nodal Inputs in Mesendodermal Differentiation of Embryonic Stem Cells. Cell Stem Cell 20, 70–86. 10.1016/j.stem.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Singh AM, Reynolds D, Cliff T, Ohtsuka S, Mattheyses AL, Sun Y, Menendez L, Kulik M, and Dalton S (2012). Signaling network crosstalk in human pluripotent cells: a Smad2/3-regulated switch that controls the balance between self-renewal and differentiation. Cell Stem Cell 10, 312–326. 10.1016/j.stem.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Oshimori N, and Fuchs E (2012). The harmonies played by TGF-β in stem cell biology. Cell Stem Cell 11, 751–764. 10.1016/j.stem.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kahata K, Maturi V, and Moustakas A (2018). TGF-β Family Signaling in Ductal Differentiation and Branching Morphogenesis. Cold Spring Harb Perspect Biol 10. 10.1101/cshperspect.a031997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sancho E, Batlle E, and Clevers H (2004). Signaling pathways in intestinal development and cancer. Annu Rev Cell Dev Biol 20, 695–723. 10.1146/annurev.cellbio.20.010403.092805. [DOI] [PubMed] [Google Scholar]
- 166.Saito A, Horie M, and Nagase T (2018). TGF-β Signaling in Lung Health and Disease. Int J Mol Sci 19. 10.3390/ijms19082460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Nieto MA, Huang RY, Jackson RA, and Thiery JP (2016). EMT: 2016. Cell 166, 21–45. 10.1016/j.cell.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 168.Yang J, Antin P, Berx G, Blanpain C, Brabletz T, Bronner M, Campbell K, Cano A, Casanova J, Christofori G, et al. (2020). Guidelines and definitions for research on epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 21, 341–352. 10.1038/s41580-020-0237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lee JH, and Massagué J (2022). TGF-β in developmental and fibrogenic EMTs. Semin Cancer Biol 86, 136–145. 10.1016/j.semcancer.2022.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bardot ES, and Hadjantonakis AK (2020). Mouse gastrulation: Coordination of tissue patterning, specification and diversification of cell fate. Mech Dev 163, 103617. 10.1016/j.mod.2020.103617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Horiguchi K, Shirakihara T, Nakano A, Imamura T, Miyazono K, and Saitoh M (2009). Role of Ras signaling in the induction of snail by transforming growth factor-beta. J Biol Chem 284, 245–253. 10.1074/jbc.M804777200. [DOI] [PubMed] [Google Scholar]
- 172.Janda E, Lehmann K, Killisch I, Jechlinger M, Herzig M, Downward J, Beug H, and Grünert S (2002). Ras and TGF[beta] cooperatively regulate epithelial cell plasticity and metastasis: dissection of Ras signaling pathways. J Cell Biol 156, 299–313. 10.1083/jcb.200109037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sun X, Meyers EN, Lewandoski M, and Martin GR (1999). Targeted disruption of Fgf8 causes failure of cell migration in the gastrulating mouse embryo. Genes Dev 13, 1834–1846. 10.1101/gad.13.14.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yamaguchi TP, Harpal K, Henkemeyer M, and Rossant J (1994). fgfr-1 is required for embryonic growth and mesodermal patterning during mouse gastrulation. Genes Dev 8, 3032–3044. 10.1101/gad.8.24.3032. [DOI] [PubMed] [Google Scholar]
- 175.Polyak K, Lee MH, Erdjument-Bromage H, Koff A, Roberts JM, Tempst P, and Massagué J (1994). Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell 78, 59–66. 10.1016/0092-8674(94)90572-x. [DOI] [PubMed] [Google Scholar]
- 176.Lee MH, Reynisdóttir I, and Massagué J (1995). Cloning of p57KIP2, a cyclin-dependent kinase inhibitor with unique domain structure and tissue distribution. Genes Dev 9, 639–649. 10.1101/gad.9.6.639. [DOI] [PubMed] [Google Scholar]
- 177.Hannon GJ, and Beach D (1994). p15INK4B is a potential effector of TGF-beta-induced cell cycle arrest. Nature 371, 257–261. 10.1038/371257a0. [DOI] [PubMed] [Google Scholar]
- 178.Reynisdóttir I, Polyak K, Iavarone A, and Massagué J (1995). Kip/Cip and Ink4 Cdk inhibitors cooperate to induce cell cycle arrest in response to TGF-beta. Genes Dev 9, 1831–1845. 10.1101/gad.9.15.1831. [DOI] [PubMed] [Google Scholar]
- 179.Seoane J, Le HV, Shen L, Anderson SA, and Massagué J (2004). Integration of Smad and forkhead pathways in the control of neuroepithelial and glioblastoma cell proliferation. Cell 117, 211–223. 10.1016/s0092-8674(04)00298-3. [DOI] [PubMed] [Google Scholar]
- 180.Gomis RR, Alarcón C, He W, Wang Q, Seoane J, Lash A, and Massagué J (2006). A FoxO-Smad synexpression group in human keratinocytes. Proc Natl Acad Sci U S A 103, 12747–12752. 10.1073/pnas.0605333103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Seoane J, Pouponnot C, Staller P, Schader M, Eilers M, and Massagué J (2001). TGFbeta influences Myc, Miz-1 and Smad to control the CDK inhibitor p15INK4b. Nat Cell Biol 3, 400–408. 10.1038/35070086. [DOI] [PubMed] [Google Scholar]
- 182.Laiho M, Weis MB, and Massagué J (1990). Concomitant loss of transforming growth factor (TGF)-beta receptor types I and II in TGF-beta-resistant cell mutants implicates both receptor types in signal transduction. J Biol Chem 265, 18518–18524. [PubMed] [Google Scholar]
- 183.Wrana JL, Attisano L, Cárcamo J, Zentella A, Doody J, Laiho M, Wang XF, and Massagué J (1992). TGF beta signals through a heteromeric protein kinase receptor complex. Cell 71, 1003–1014. 10.1016/0092-8674(92)90395-s. [DOI] [PubMed] [Google Scholar]
- 184.Wrana JL, Attisano L, Wieser R, Ventura F, and Massagué J (1994). Mechanism of activation of the TGF-beta receptor. Nature 370, 341–347. 10.1038/370341a0. [DOI] [PubMed] [Google Scholar]
- 185.Guasch G, Schober M, Pasolli HA, Conn EB, Polak L, and Fuchs E (2007). Loss of TGFbeta signaling destabilizes homeostasis and promotes squamous cell carcinomas in stratified epithelia. Cancer Cell 12, 313–327. 10.1016/j.ccr.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Campisi J. (2013). Aging, cellular senescence, and cancer. Annu Rev Physiol 75, 685–705. 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Munoz-Espin D, Canamero M, Maraver A, Gomez-Lopez G, Contreras J, Murillo-Cuesta S, Rodriguez-Baeza A, Varela-Nieto I, Ruberte J, Collado M, and Serrano M (2013). Programmed cell senescence during mammalian embryonic development. Cell 155, 1104–1118. 10.1016/j.cell.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 188.Ijichi H, Chytil A, Gorska AE, Aakre ME, Fujitani Y, Fujitani S, Wright CV, and Moses HL (2006). Aggressive pancreatic ductal adenocarcinoma in mice caused by pancreas-specific blockade of transforming growth factor-beta signaling in cooperation with active Kras expression. Genes Dev 20, 3147–3160. 10.1101/gad.1475506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lu SL, Herrington H, Reh D, Weber S, Bornstein S, Wang D, Li AG, Tang CF, Siddiqui Y, Nord J, et al. (2006). Loss of transforming growth factor-beta type II receptor promotes metastatic head-and-neck squamous cell carcinoma. Genes Dev 20, 1331–1342. 10.1101/gad.1413306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Takaku K, Oshima M, Miyoshi H, Matsui M, Seldin MF, and Taketo MM (1998). Intestinal tumorigenesis in compound mutant mice of both Dpc4 (Smad4) and Apc genes. Cell 92, 645–656. 10.1016/s0092-8674(00)81132-0. [DOI] [PubMed] [Google Scholar]
- 191.Muñoz NM, Upton M, Rojas A, Washington MK, Lin L, Chytil A, Sozmen EG, Madison BB, Pozzi A, Moon RT, et al. (2006). Transforming growth factor beta receptor type II inactivation induces the malignant transformation of intestinal neoplasms initiated by Apc mutation. Cancer Res 66, 9837–9844. 10.1158/0008-5472.Can-06-0890. [DOI] [PubMed] [Google Scholar]
- 192.Tauriello DVF, Palomo-Ponce S, Stork D, Berenguer-Llergo A, Badia-Ramentol J, Iglesias M, Sevillano M, Ibiza S, Cañellas A, Hernando-Momblona X, et al. (2018). TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 554, 538–543. 10.1038/nature25492. [DOI] [PubMed] [Google Scholar]
- 193.Costello LC, Zou J, Desouki MM, and Franklin RB (2012). Evidence for changes in RREB-1, ZIP3, and Zinc in the early development of pancreatic adenocarcinoma. J Gastrointest Cancer 43, 570–578. 10.1007/s12029-012-9378-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.CancerGenomeAtlasResearchNetwork. (2017). Integrated Genomic Characterization of Pancreatic Ductal Adenocarcinoma. Cancer Cell 32, 185–203 e113. 10.1016/j.ccell.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Schmitt M, and Greten FR (2021). The inflammatory pathogenesis of colorectal cancer. Nat Rev Immunol 21, 653–667. 10.1038/s41577-021-00534-x. [DOI] [PubMed] [Google Scholar]
- 196.Ihara S, Hirata Y, and Koike K (2017). TGF-β in inflammatory bowel disease: a key regulator of immune cells, epithelium, and the intestinal microbiota. J Gastroenterol 52, 777–787. 10.1007/s00535-017-1350-1. [DOI] [PubMed] [Google Scholar]
- 197.Kulkarni AB, Huh CG, Becker D, Geiser A, Lyght M, Flanders KC, Roberts AB, Sporn MB, Ward JM, and Karlsson S (1993). Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci U S A 90, 770–774. 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hahn JN, Falck VG, and Jirik FR (2011). Smad4 deficiency in T cells leads to the Th17-associated development of premalignant gastroduodenal lesions in mice. J Clin Invest 121, 4030–4042. 10.1172/jci45114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kim BG, Li C, Qiao W, Mamura M, Kasprzak B, Anver M, Wolfraim L, Hong S, Mushinski E, Potter M, et al. (2006). Smad4 signalling in T cells is required for suppression of gastrointestinal cancer. Nature 441, 1015–1019. 10.1038/nature04846. [DOI] [PubMed] [Google Scholar]
- 200.Siegel PM, Shu W, Cardiff RD, Muller WJ, and Massagué J (2003). Transforming growth factor beta signaling impairs Neu-induced mammary tumorigenesis while promoting pulmonary metastasis. Proc Natl Acad Sci U S A 100, 8430–8435. 10.1073/pnas.0932636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ghajar CM, Peinado H, Mori H, Matei IR, Evason KJ, Brazier H, Almeida D, Koller A, Hajjar KA, Stainier DY, et al. (2013). The perivascular niche regulates breast tumour dormancy. Nat Cell Biol 15, 807–817. 10.1038/ncb2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Hu J, Sánchez-Rivera FJ, Wang Z, Johnson GN, Ho YJ, Ganesh K, Umeda S, Gan S, Mujal AM, Delconte RB, et al. (2023). STING inhibits the reactivation of dormant metastasis in lung adenocarcinoma. Nature 616, 806–813. 10.1038/s41586-023-05880-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Markowitz S, Wang J, Myeroff L, Parsons R, Sun L, Lutterbaugh J, Fan RS, Zborowska E, Kinzler KW, Vogelstein B, and et al. (1995). Inactivation of the type II TGF-beta receptor in colon cancer cells with microsatellite instability. Science 268, 1336–1338. 10.1126/science.7761852. [DOI] [PubMed] [Google Scholar]
- 204.Hahn SA, Schutte M, Hoque AT, Moskaluk CA, da Costa LT, Rozenblum E, Weinstein CL, Fischer A, Yeo CJ, Hruban RH, and Kern SE (1996). DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science 271, 350–353. 10.1126/science.271.5247.350. [DOI] [PubMed] [Google Scholar]
- 205.Eppert K, Scherer SW, Ozcelik H, Pirone R, Hoodless P, Kim H, Tsui LC, Bapat B, Gallinger S, Andrulis IL, et al. (1996). MADR2 maps to 18q21 and encodes a TGFbeta-regulated MAD-related protein that is functionally mutated in colorectal carcinoma. Cell 86, 543–552. 10.1016/s0092-8674(00)80128-2. [DOI] [PubMed] [Google Scholar]
- 206.Sanchez-Vega F, Mina M, Armenia J, Chatila WK, Luna A, La KC, Dimitriadoy S, Liu DL, Kantheti HS, Saghafinia S, et al. (2018). Oncogenic Signaling Pathways in The Cancer Genome Atlas. Cell 173, 321–337.e310. 10.1016/j.cell.2018.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Fearon ER (2011). Molecular genetics of colorectal cancer. Annu Rev Pathol 6, 479–507. 10.1146/annurev-pathol-011110-130235. [DOI] [PubMed] [Google Scholar]
- 208.Maitra A, and Hruban RH (2008). Pancreatic cancer. Annu Rev Pathol 3, 157–188. 10.1146/annurev.pathmechdis.3.121806.154305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Huang YH, Hu J, Chen F, Lecomte N, Basnet H, David CJ, Witkin MD, Allen PJ, Leach SD, Hollmann TJ, et al. (2020). ID1 Mediates Escape from TGFβ Tumor Suppression in Pancreatic Cancer. Cancer Discov 10, 142–157. 10.1158/2159-8290.Cd-19-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Bornstein S, White R, Malkoski S, Oka M, Han G, Cleaver T, Reh D, Andersen P, Gross N, Olson S, et al. (2009). Smad4 loss in mice causes spontaneous head and neck cancer with increased genomic instability and inflammation. J Clin Invest 119, 3408–3419. 10.1172/jci38854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hernandez AL, Young CD, Bian L, Weigel K, Nolan K, Frederick B, Han G, He G, Devon Trahan G, Rudolph MC, et al. (2020). PARP Inhibition Enhances Radiotherapy of SMAD4-Deficient Human Head and Neck Squamous Cell Carcinomas in Experimental Models. Clin Cancer Res 26, 3058–3070. 10.1158/1078-0432.Ccr-19-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Howe JR, Roth S, Ringold JC, Summers RW, Järvinen HJ, Sistonen P, Tomlinson IP, Houlston RS, Bevan S, Mitros FA, et al. (1998). Mutations in the SMAD4/DPC4 gene in juvenile polyposis. Science 280, 1086–1088. 10.1126/science.280.5366.1086. [DOI] [PubMed] [Google Scholar]
- 213.Houlston R, Bevan S, Williams A, Young J, Dunlop M, Rozen P, Eng C, Markie D, Woodford-Richens K, Rodriguez-Bigas MA, et al. (1998). Mutations in DPC4 (SMAD4) cause juvenile polyposis syndrome, but only account for a minority of cases. Hum Mol Genet 7, 1907–1912. 10.1093/hmg/7.12.1907. [DOI] [PubMed] [Google Scholar]
- 214.Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, Kadel EE III, Koeppen H, Astarita JL, Cubas R, et al. (2018). TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 554, 544–548. 10.1038/nature25501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Dodagatta-Marri E, Meyer DS, Reeves MQ, Paniagua R, To MD, Binnewies M, Broz ML, Mori H, Wu D, Adoumie M, et al. (2019). α-PD-1 therapy elevates Treg/Th balance and increases tumor cell pSmad3 that are both targeted by α-TGFβ antibody to promote durable rejection and immunity in squamous cell carcinomas. J Immunother Cancer 7, 62. 10.1186/s40425-018-0493-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Takasaka N, Seed RI, Cormier A, Bondesson AJ, Lou J, Elattma A, Ito S, Yanagisawa H, Hashimoto M, Ma R, et al. (2018). Integrin αvβ8-expressing tumor cells evade host immunity by regulating TGF-β activation in immune cells. JCI Insight 3. 10.1172/jci.insight.122591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Dodagatta-Marri E, Ma HY, Liang B, Li J, Meyer DS, Chen SY, Sun KH, Ren X, Zivak B, Rosenblum MD, et al. (2021). Integrin αvβ8 on T cells suppresses anti-tumor immunity in multiple models and is a promising target for tumor immunotherapy. Cell Rep 36, 109309. 10.1016/j.celrep.2021.109309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Lainé A, Labiad O, Hernandez-Vargas H, This S, Sanlaville A, Léon S, Dalle S, Sheppard D, Travis MA, Paidassi H, and Marie JC (2021). Regulatory T cells promote cancer immune-escape through integrin αvβ8-mediated TGF-β activation. Nat Commun 12, 6228. 10.1038/s41467-021-26352-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Philippeos C, Telerman SB, Oulès B, Pisco AO, Shaw TJ, Elgueta R, Lombardi G, Driskell RR, Soldin M, Lynch MD, and Watt FM (2018). Spatial and Single-Cell Transcriptional Profiling Identifies Functionally Distinct Human Dermal Fibroblast Subpopulations. J Invest Dermatol 138, 811–825. 10.1016/j.jid.2018.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Croft AP, Campos J, Jansen K, Turner JD, Marshall J, Attar M, Savary L, Wehmeyer C, Naylor AJ, Kemble S, et al. (2019). Distinct fibroblast subsets drive inflammation and damage in arthritis. Nature 570, 246–251. 10.1038/s41586-019-1263-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Öhlund D, Handly-Santana A, Biffi G, Elyada E, Almeida AS, Ponz-Sarvise M, Corbo V, Oni TE, Hearn SA, Lee EJ, et al. (2017). Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med 214, 579–596. 10.1084/jem.20162024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Winkler J, Abisoye-Ogunniyan A, Metcalf KJ, and Werb Z (2020). Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat Commun 11, 5120. 10.1038/s41467-020-18794-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Hayward MK, Muncie JM, and Weaver VM (2021). Tissue mechanics in stem cell fate, development, and cancer. Dev Cell 56, 1833–1847. 10.1016/j.devcel.2021.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Takai K, Le A, Weaver VM, and Werb Z (2016). Targeting the cancer-associated fibroblasts as a treatment in triple-negative breast cancer. Oncotarget 7, 82889–82901. 10.18632/oncotarget.12658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Ciardiello D, Elez E, Tabernero J, and Seoane J (2020). Clinical development of therapies targeting TGFbeta: current knowledge and future perspectives. Ann Oncol 31, 1336–1349. 10.1016/j.annonc.2020.07.009. [DOI] [PubMed] [Google Scholar]
- 226.Oft M, Akhurst RJ, and Balmain A (2002). Metastasis is driven by sequential elevation of H-ras and Smad2 levels. Nat Cell Biol 4, 487–494. 10.1038/ncb807. [DOI] [PubMed] [Google Scholar]
- 227.Oshimori N, Oristian D, and Fuchs E (2015). TGF-β promotes heterogeneity and drug resistance in squamous cell carcinoma. Cell 160, 963–976. 10.1016/j.cell.2015.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Giampieri S, Manning C, Hooper S, Jones L, Hill CS, and Sahai E (2009). Localized and reversible TGFbeta signalling switches breast cancer cells from cohesive to single cell motility. Nat Cell Biol 11, 1287–1296. 10.1038/ncb1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Labelle M, Begum S, and Hynes RO (2011). Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell 20, 576–590. 10.1016/j.ccr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, Viale A, Olshen AB, Gerald WL, and Massagué J (2005). Genes that mediate breast cancer metastasis to lung. Nature 436, 518–524. 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Padua D, Zhang XH, Wang Q, Nadal C, Gerald WL, Gomis RR, and Massagué J (2008). TGFbeta primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell 133, 66–77. 10.1016/j.cell.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Massagué J, and Ganesh K (2021). Metastasis-Initiating Cells and Ecosystems. Cancer Discov 11, 971–994. 10.1158/2159-8290.Cd-21-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Risson E, Nobre AR, Maguer-Satta V, and Aguirre-Ghiso JA (2020). The current paradigm and challenges ahead for the dormancy of disseminated tumor cells. Nat Cancer 1, 672–680. 10.1038/s43018-020-0088-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Malladi S, Macalinao DG, Jin X, He L, Basnet H, Zou Y, de Stanchina E, and Massagué J (2016). Metastatic Latency and Immune Evasion through Autocrine Inhibition of WNT. Cell 165, 45–60. 10.1016/j.cell.2016.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Laughney AM, Hu J, Campbell NR, Bakhoum SF, Setty M, Lavallée VP, Xie Y, Masilionis I, Carr AJ, Kottapalli S, et al. (2020). Regenerative lineages and immune-mediated pruning in lung cancer metastasis. Nat Med 26, 259–269. 10.1038/s41591-019-0750-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Pommier A, Anaparthy N, Memos N, Kelley ZL, Gouronnec A, Yan R, Auffray C, Albrengues J, Egeblad M, Iacobuzio-Donahue CA, et al. (2018). Unresolved endoplasmic reticulum stress engenders immune-resistant, latent pancreatic cancer metastases. Science 360. 10.1126/science.aao4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Pantel K, Schlimok G, Kutter D, Schaller G, Genz T, Wiebecke B, Backmann R, Funke I, and Riethmüller G (1991). Frequent down-regulation of major histocompatibility class I antigen expression on individual micrometastatic carcinoma cells. Cancer Res 51, 4712–4715. [PubMed] [Google Scholar]
- 238.Agudo J, Park ES, Rose SA, Alibo E, Sweeney R, Dhainaut M, Kobayashi KS, Sachidanandam R, Baccarini A, Merad M, and Brown BD (2018). Quiescent Tissue Stem Cells Evade Immune Surveillance. Immunity 48, 271–285.e275. 10.1016/j.immuni.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Massagué J, and Obenauf AC (2016). Metastatic colonization by circulating tumour cells. Nature 529, 298–306. 10.1038/nature17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Nguyen B, Fong C, Luthra A, Smith SA, DiNatale RG, Nandakumar S, Walch H, Chatila WK, Madupuri R, Kundra R, et al. (2022). Genomic characterization of metastatic patterns from prospective clinical sequencing of 25,000 patients. Cell 185, 563–575.e511. 10.1016/j.cell.2022.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Yin JJ, Selander K, Chirgwin JM, Dallas M, Grubbs BG, Wieser R, Massagué J, Mundy GR, and Guise TA (1999). TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J Clin Invest 103, 197–206. 10.1172/jci3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordón-Cardo C, Guise TA, and Massagué J (2003). A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 3, 537–549. 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 243.Kakonen SM, Selander KS, Chirgwin JM, Yin JJ, Burns S, Rankin WA, Grubbs BG, Dallas M, Cui Y, and Guise TA (2002). Transforming growth factor-beta stimulates parathyroid hormone-related protein and osteolytic metastases via Smad and mitogen-activated protein kinase signaling pathways. J Biol Chem 277, 24571–24578. 10.1074/jbc.M202561200. [DOI] [PubMed] [Google Scholar]
- 244.Kang Y, He W, Tulley S, Gupta GP, Serganova I, Chen CR, Manova-Todorova K, Blasberg R, Gerald WL, and Massagué J (2005). Breast cancer bone metastasis mediated by the Smad tumor suppressor pathway. Proc Natl Acad Sci U S A 102, 13909–13914. 10.1073/pnas.0506517102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Sethi N, Dai X, Winter CG, and Kang Y (2011). Tumor-derived JAGGED1 promotes osteolytic bone metastasis of breast cancer by engaging notch signaling in bone cells. Cancer Cell 19, 192–205. 10.1016/j.ccr.2010.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Stankic M, Pavlovic S, Chin Y, Brogi E, Padua D, Norton L, Massagué J, and Benezra R (2013). TGF-β-Id1 signaling opposes Twist1 and promotes metastatic colonization via a mesenchymal-to-epithelial transition. Cell Rep 5, 1228–1242. 10.1016/j.celrep.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Tauriello DV, Calon A, Lonardo E, and Batlle E (2017). Determinants of metastatic competency in colorectal cancer. Mol Oncol 11, 97–119. 10.1002/1878-0261.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Horan GS, Wood S, Ona V, Li DJ, Lukashev ME, Weinreb PH, Simon KJ, Hahm K, Allaire NE, Rinaldi NJ, et al. (2008). Partial inhibition of integrin alpha(v)beta6 prevents pulmonary fibrosis without exacerbating inflammation. Am J Respir Crit Care Med 177, 56–65. 10.1164/rccm.200706-805OC. [DOI] [PubMed] [Google Scholar]
- 249.Wei Y, Kim TJ, Peng DH, Duan D, Gibbons DL, Yamauchi M, Jackson JR, Le Saux CJ, Calhoun C, Peters J, et al. (2017). Fibroblast-specific inhibition of TGF-beta1 signaling attenuates lung and tumor fibrosis. J Clin Invest 127, 3675–3688. 10.1172/JCI94624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Chapman HA, Wei Y, Montas G, Leong D, Golden JA, Trinh BN, Wolters PJ, Le Saux CJ, Jones KD, Hills NK, et al. (2020). Reversal of TGFbeta1-Driven Profibrotic State in Patients with Pulmonary Fibrosis. N Engl J Med 382, 1068–1070. 10.1056/NEJMc1915189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Lasky JA, Ortiz LA, Tonthat B, Hoyle GW, Corti M, Athas G, Lungarella G, Brody A, and Friedman M (1998). Connective tissue growth factor mRNA expression is upregulated in bleomycin-induced lung fibrosis. Am J Physiol 275, L365–371. 10.1152/ajplung.1998.275.2.L365. [DOI] [PubMed] [Google Scholar]
- 252.Hecker L, Vittal R, Jones T, Jagirdar R, Luckhardt TR, Horowitz JC, Pennathur S, Martinez FJ, and Thannickal VJ (2009). NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med 15, 1077–1081. 10.1038/nm.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Bernard K, and Thannickal VJ (2019). NADPH Oxidases and Aging Models of Lung Fibrosis. Methods Mol Biol 1982, 487–496. 10.1007/978-1-4939-9424-3_29. [DOI] [PubMed] [Google Scholar]
- 254.Bollard CM, Tripic T, Cruz CR, Dotti G, Gottschalk S, Torrano V, Dakhova O, Carrum G, Ramos CA, Liu H, et al. (2018). Tumor-Specific T-Cells Engineered to Overcome Tumor Immune Evasion Induce Clinical Responses in Patients With Relapsed Hodgkin Lymphoma. J Clin Oncol 36, 1128–1139. 10.1200/JCO.2017.74.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Narayan V, Barber-Rotenberg JS, Jung IY, Lacey SF, Rech AJ, Davis MM, Hwang WT, Lal P, Carpenter EL, Maude SL, et al. (2022). PSMA-targeting TGFbeta-insensitive armored CAR T cells in metastatic castration-resistant prostate cancer: a phase 1 trial. Nat Med 28, 724–734. 10.1038/s41591-022-01726-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Roybal KT, Williams JZ, Morsut L, Rupp LJ, Kolinko I, Choe JH, Walker WJ, McNally KA, and Lim WA (2016). Engineering T Cells with Customized Therapeutic Response Programs Using Synthetic Notch Receptors. Cell 167, 419–432 e416. 10.1016/j.cell.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Rochette L, Zeller M, Cottin Y, and Vergely C (2020). Insights Into Mechanisms of GDF15 and Receptor GFRAL: Therapeutic Targets. Trends Endocrinol Metab 31, 939–951. 10.1016/j.tem.2020.10.004. [DOI] [PubMed] [Google Scholar]
- 258.Lindsay ME, Schepers D, Bolar NA, Doyle JJ, Gallo E, Fert-Bober J, Kempers MJ, Fishman EK, Chen Y, Myers L, et al. (2012). Loss-of-function mutations in TGFB2 cause a syndromic presentation of thoracic aortic aneurysm. Nat Genet 44, 922–927. 10.1038/ng.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Rienhoff HY Jr., Yeo CY, Morissette R, Khrebtukova I, Melnick J, Luo S, Leng N, Kim YJ, Schroth G, Westwick J, et al. (2013). A mutation in TGFB3 associated with a syndrome of low muscle mass, growth retardation, distal arthrogryposis and clinical features overlapping with Marfan and Loeys-Dietz syndrome. Am J Med Genet A 161a, 2040–2046. 10.1002/ajmg.a.36056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Arslan Ates E, Eltan M, Sahin B, Gurpinar Tosun B, Seven Menevse T, Geckinli BB, Greenfield A, Turan S, Bereket A, and Guran T (2022). Homozygosity for a novel INHA mutation in two male siblings with hypospadias, primary hypogonadism, and high-normal testicular volume. Eur J Endocrinol 186, K25–k31. 10.1530/eje-21-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Dixit H, Deendayal M, and Singh L (2004). Mutational analysis of the mature peptide region of inhibin genes in Indian women with ovarian failure. Hum Reprod 19, 1760–1764. 10.1093/humrep/deh342. [DOI] [PubMed] [Google Scholar]
- 262.Mohapatra B, Casey B, Li H, Ho-Dawson T, Smith L, Fernbach SD, Molinari L, Niesh SR, Jefferies JL, Craigen WJ, et al. (2009). Identification and functional characterization of NODAL rare variants in heterotaxy and isolated cardiovascular malformations. Hum Mol Genet 18, 861–871. 10.1093/hmg/ddn411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Dathe K, Kjaer KW, Brehm A, Meinecke P, Nürnberg P, Neto JC, Brunoni D, Tommerup N, Ott CE, Klopocki E, et al. (2009). Duplications involving a conserved regulatory element downstream of BMP2 are associated with brachydactyly type A2. Am J Hum Genet 84, 483–492. 10.1016/j.ajhg.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Daher R, Kannengiesser C, Houamel D, Lefebvre T, Bardou-Jacquet E, Ducrot N, de Kerguenec C, Jouanolle AM, Robreau AM, Oudin C, et al. (2016). Heterozygous Mutations in BMP6 Pro-peptide Lead to Inappropriate Hepcidin Synthesis and Moderate Iron Overload in Humans. Gastroenterology 150, 672–683.e674. 10.1053/j.gastro.2015.10.049. [DOI] [PubMed] [Google Scholar]
- 265.Eyries M, Montani D, Nadaud S, Girerd B, Levy M, Bourdin A, Tresorier R, Chaouat A, Cottin V, Sanfiorenzo C, et al. (2019). Widening the landscape of heritable pulmonary hypertension mutations in paediatric and adult cases. Eur Respir J 53. 10.1183/13993003.01371-2018. [DOI] [PubMed] [Google Scholar]
- 266.Galloway SM, McNatty KP, Cambridge LM, Laitinen MP, Juengel JL, Jokiranta TS, McLaren RJ, Luiro K, Dodds KG, Montgomery GW, et al. (2000). Mutations in an oocyte-derived growth factor gene (BMP15) cause increased ovulation rate and infertility in a dosage-sensitive manner. Nat Genet 25, 279–283. 10.1038/77033. [DOI] [PubMed] [Google Scholar]
- 267.Karkera JD, Lee JS, Roessler E, Banerjee-Basu S, Ouspenskaia MV, Mez J, Goldmuntz E, Bowers P, Towbin J, Belmont JW, et al. (2007). Loss-of-function mutations in growth differentiation factor-1 (GDF1) are associated with congenital heart defects in humans. Am J Hum Genet 81, 987–994. 10.1086/522890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Balachandar S, Graves TJ, Shimonty A, Kerr K, Kilner J, Xiao S, Slade R, Sroya M, Alikian M, Curetean E, et al. (2022). Identification and validation of a novel pathogenic variant in GDF2 (BMP9) responsible for hereditary hemorrhagic telangiectasia and pulmonary arteriovenous malformations. Am J Med Genet A 188, 959–964. 10.1002/ajmg.a.62584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Graf S, Haimel M, Bleda M, Hadinnapola C, Southgate L, Li W, Hodgson J, Liu B, Salmon RM, Southwood M, et al. (2018). Identification of rare sequence variation underlying heritable pulmonary arterial hypertension. Nat Commun 9, 1416. 10.1038/s41467-018-03672-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Wooderchak-Donahue WL, McDonald J, O'Fallon B, Upton PD, Li W, Roman BL, Young S, Plant P, Fülöp GT, Langa C, et al. (2013). BMP9 mutations cause a vascular-anomaly syndrome with phenotypic overlap with hereditary hemorrhagic telangiectasia. Am J Hum Genet 93, 530–537. 10.1016/j.ajhg.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Ye M, Berry-Wynne KM, Asai-Coakwell M, Sundaresan P, Footz T, French CR, Abitbol M, Fleisch VC, Corbett N, Allison WT, et al. (2010). Mutation of the bone morphogenetic protein GDF3 causes ocular and skeletal anomalies. Hum Mol Genet 19, 287–298. 10.1093/hmg/ddp496. [DOI] [PubMed] [Google Scholar]
- 272.Thomas JT, Lin K, Nandedkar M, Camargo M, Cervenka J, and Luyten FP (1996). A human chondrodysplasia due to a mutation in a TGF-beta superfamily member. Nat Genet 12, 315–317. 10.1038/ng0396-315. [DOI] [PubMed] [Google Scholar]
- 273.Kjaer KW, Eiberg H, Hansen L, van der Hagen CB, Rosendahl K, Tommerup N, and Mundlos S (2006). A mutation in the receptor binding site of GDF5 causes Mohr-Wriedt brachydactyly type A2. J Med Genet 43, 225–231. 10.1136/jmg.2005.034058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Martinez-Garcia M, Garcia-Canto E, Fenollar-Cortes M, Aytes AP, and Trujillo-Tiebas MJ (2016). Characterization of an acromesomelic dysplasia, Grebe type case: novel mutation affecting the recognition motif at the processing site of GDF5. J Bone Miner Metab 34, 599–603. 10.1007/s00774-015-0693-z. [DOI] [PubMed] [Google Scholar]
- 275.Tassabehji M, Fang ZM, Hilton EN, McGaughran J, Zhao Z, de Bock CE, Howard E, Malass M, Donnai D, Diwan A, et al. (2008). Mutations in GDF6 are associated with vertebral segmentation defects in Klippel-Feil syndrome. Hum Mutat 29, 1017–1027. 10.1002/humu.20741. [DOI] [PubMed] [Google Scholar]
- 276.Asai-Coakwell M, March L, Dai XH, Duval M, Lopez I, French CR, Famulski J, De Baere E, Francis PJ, Sundaresan P, et al. (2013). Contribution of growth differentiation factor 6-dependent cell survival to early-onset retinal dystrophies. Hum Mol Genet 22, 1432–1442. 10.1093/hmg/dds560. [DOI] [PubMed] [Google Scholar]
- 277.Schuelke M, Wagner KR, Stolz LE, Hübner C, Riebel T, Kömen W, Braun T, Tobin JF, and Lee SJ (2004). Myostatin mutation associated with gross muscle hypertrophy in a child. N Engl J Med 350, 2682–2688. 10.1056/NEJMoa040933. [DOI] [PubMed] [Google Scholar]
- 278.Takebayashi K, Takakura K, Wang H, Kimura F, Kasahara K, and Noda Y (2000). Mutation analysis of the growth differentiation factor-9 and −9B genes in patients with premature ovarian failure and polycystic ovary syndrome. Fertil Steril 74, 976–979. 10.1016/s0015-0282(00)01539-9. [DOI] [PubMed] [Google Scholar]
- 279.Knebelmann B, Boussin L, Guerrier D, Legeai L, Kahn A, Josso N, and Picard JY (1991). Anti-Müllerian hormone Bruxelles: a nonsense mutation associated with the persistent Müllerian duct syndrome. Proc Natl Acad Sci U S A 88, 3767–3771. 10.1073/pnas.88.9.3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Loeys BL, Chen J, Neptune ER, Judge DP, Podowski M, Holm T, Meyers J, Leitch CC, Katsanis N, Sharifi N, et al. (2005). A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat Genet 37, 275–281. 10.1038/ng1511. [DOI] [PubMed] [Google Scholar]
- 281.Mizuguchi T, Collod-Beroud G, Akiyama T, Abifadel M, Harada N, Morisaki T, Allard D, Varret M, Claustres M, Morisaki H, et al. (2004). Heterozygous TGFBR2 mutations in Marfan syndrome. Nat Genet 36, 855–860. 10.1038/ng1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Shore EM, Xu M, Feldman GJ, Fenstermacher DA, Cho TJ, Choi IH, Connor JM, Delai P, Glaser DL, LeMerrer M, et al. (2006). A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet 38, 525–527. 10.1038/ng1783. [DOI] [PubMed] [Google Scholar]
- 283.Fitzpatrick E, Johnson MP, Dyer TD, Forrest S, Elliott K, Blangero J, Brennecke SP, and Moses EK (2009). Genetic association of the activin A receptor gene (ACVR2A) and pre-eclampsia. Mol Hum Reprod 15, 195–204. 10.1093/molehr/gap001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Kosaki R, Gebbia M, Kosaki K, Lewin M, Bowers P, Towbin JA, and Casey B (1999). Left-right axis malformations associated with mutations in ACVR2B, the gene for human activin receptor type IIB. Am J Med Genet 82, 70–76. . [DOI] [PubMed] [Google Scholar]
- 285.Johnson DW, Berg JN, Baldwin MA, Gallione CJ, Marondel I, Yoon SJ, Stenzel TT, Speer M, Pericak-Vance MA, Diamond A, et al. (1996). Mutations in the activin receptor-like kinase 1 gene in hereditary haemorrhagic telangiectasia type 2. Nat Genet 13, 189–195. 10.1038/ng0696-189. [DOI] [PubMed] [Google Scholar]
- 286.Peacock AJ, Murphy NF, McMurray JJ, Caballero L, and Stewart S (2007). An epidemiological study of pulmonary arterial hypertension. Eur Respir J 30, 104–109. 10.1183/09031936.00092306. [DOI] [PubMed] [Google Scholar]
- 287.Chida A, Shintani M, Nakayama T, Furutani Y, Hayama E, Inai K, Saji T, Nonoyama S, and Nakanishi T (2012). Missense mutations of the BMPR1B (ALK6) gene in childhood idiopathic pulmonary arterial hypertension. Circ J 76, 1501–1508. 10.1253/circj.cj-11-1281. [DOI] [PubMed] [Google Scholar]
- 288.Ullah A, Umair M, Muhammad D, Bilal M, Lee K, Leal SM, and Ahmad W (2018). A novel homozygous variant in BMPR1B underlies acromesomelic dysplasia Hunter-Thompson type. Ann Hum Genet 82, 129–134. 10.1111/ahg.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Howe JR, Bair JL, Sayed MG, Anderson ME, Mitros FA, Petersen GM, Velculescu VE, Traverso G, and Vogelstein B (2001). Germline mutations of the gene encoding bone morphogenetic protein receptor 1A in juvenile polyposis. Nat Genet 28, 184–187. 10.1038/88919. [DOI] [PubMed] [Google Scholar]
- 290.Deng Z, Morse JH, Slager SL, Cuervo N, Moore KJ, Venetos G, Kalachikov S, Cayanis E, Fischer SG, Barst RJ, et al. (2000). Familial primary pulmonary hypertension (gene PPH1) is caused by mutations in the bone morphogenetic protein receptor-II gene. Am J Hum Genet 67, 737–744. 10.1086/303059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Lane KB, Machado RD, Pauciulo MW, Thomson JR, Phillips JA 3rd, Loyd JE, Nichols WC, and Trembath RC (2000). Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. Nat Genet 26, 81–84. 10.1038/79226. [DOI] [PubMed] [Google Scholar]
- 292.Runo JR, Vnencak-Jones CL, Prince M, Loyd JE, Wheeler L, Robbins IM, Lane KB, Newman JH, Johnson J, Nichols WC, and Phillips JA 3rd (2003). Pulmonary veno-occlusive disease caused by an inherited mutation in bone morphogenetic protein receptor II. Am J Respir Crit Care Med 167, 889–894. 10.1164/rccm.200208-861OC. [DOI] [PubMed] [Google Scholar]
- 293.Imbeaud S, Faure E, Lamarre I, Mattéi MG, di Clemente N, Tizard R, Carré-Eusèbe D, Belville C, Tragethon L, Tonkin C, et al. (1995). Insensitivity to anti-müllerian hormone due to a mutation in the human anti-müllerian hormone receptor. Nat Genet 11, 382–388. 10.1038/ng1295-382. [DOI] [PubMed] [Google Scholar]
- 294.McAllister KA, Grogg KM, Johnson DW, Gallione CJ, Baldwin MA, Jackson CE, Helmbold EA, Markel DS, McKinnon WC, Murrell J, and et al. (1994). Endoglin, a TGF-beta binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat Genet 8, 345–351. 10.1038/ng1294-345. [DOI] [PubMed] [Google Scholar]
- 295.de la Cruz JM, Bamford RN, Burdine RD, Roessler E, Barkovich AJ, Donnai D, Schier AF, and Muenke M (2002). A loss-of-function mutation in the CFC domain of TDGF1 is associated with human forebrain defects. Hum Genet 110, 422–428. 10.1007/s00439-002-0709-3. [DOI] [PubMed] [Google Scholar]
- 296.Bamford RN, Roessler E, Burdine RD, Saplakoğlu U, dela Cruz J, Splitt M, Goodship JA, Towbin J, Bowers P, Ferrero GB, et al. (2000). Loss-of-function mutations in the EGF-CFC gene CFC1 are associated with human left-right laterality defects. Nat Genet 26, 365–369. 10.1038/81695. [DOI] [PubMed] [Google Scholar]
- 297.Goldmuntz E, Bamford R, Karkera JD, dela Cruz J, Roessler E, and Muenke M (2002). CFC1 mutations in patients with transposition of the great arteries and double-outlet right ventricle. Am J Hum Genet 70, 776–780. 10.1086/339079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Nasim MT, Ogo T, Ahmed M, Randall R, Chowdhury HM, Snape KM, Bradshaw TY, Southgate L, Lee GJ, Jackson I, et al. (2011). Molecular genetic characterization of SMAD signaling molecules in pulmonary arterial hypertension. Hum Mutat 32, 1385–1389. 10.1002/humu.21605. [DOI] [PubMed] [Google Scholar]
- 299.van de Laar IM, Oldenburg RA, Pals G, Roos-Hesselink JW, de Graaf BM, Verhagen JM, Hoedemaekers YM, Willemsen R, Severijnen LA, Venselaar H, et al. (2011). Mutations in SMAD3 cause a syndromic form of aortic aneurysms and dissections with early-onset osteoarthritis. Nat Genet 43, 121–126. 10.1038/ng.744. [DOI] [PubMed] [Google Scholar]
- 300.Gallione CJ, Repetto GM, Legius E, Rustgi AK, Schelley SL, Tejpar S, Mitchell G, Drouin E, Westermann CJ, and Marchuk DA (2004). A combined syndrome of juvenile polyposis and hereditary haemorrhagic telangiectasia associated with mutations in MADH4 (SMAD4). Lancet 363, 852–859. 10.1016/s0140-6736(04)15732-2. [DOI] [PubMed] [Google Scholar]