Abstract
A 3′ -phosphoadenosine 5′ -phosphosulfate (PAPS):desulfoglucosinolate sulfotransferase (EC 2.8.2-) was extensively purified from light-grown cress (Lepidium sativum L.) seedlings by gel filtration and concanavalin A-Sepharose 4B, Matrex Gel Green A, and Mono Q fast protein liquid chromatography. The purified enzyme, which required bovine serum albumin for stabilization, had a native molecular weight of 31,000 ± 5,000 and an apparent isoelectric point of 5.2. Using PAPS (Km 60 micromolar) as sulfur donor, it catalyzed the sulfation of desulfobenzylglucosinolate (Km 82 micromolar), desulfo-p-hydroxybenzylglucosinolate (Km 670 micromolar), and desulfoallylglucosinolate (Km 6.5 millimolar) at an optimal pH of 9.0. All other potential substrates tested, including flavonoids, flavonoid glycosides, cinnamic acids, and phenylacetaldoxime, were not sulfated. Sulfotransferase activity was stimulated by MgCl2, MnCl2 and reducing agents and inhibited by ZnCl2, PbNO3 NiCl2 and the reaction product PAP. The thiol reagents N-ethylmaleimide, p-chloromercuriphenylsulfonic acid, and 5,5′ -dithio-bis-(2-nitrobenzoic acid) were also potent inhibitors, but the enzyme was protected from covalent modification by β-mercaptoethanol. The kinetics of desulfobenzylglucosinolate sulfation were consistent with a rapid equilibrium ordered mechanism with desulfobenzylglucosinolate binding first and PAPS second.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brandan E., Hirschberg C. B. Purification of rat liver N-heparan-sulfate sulfotransferase. J Biol Chem. 1988 Feb 15;263(5):2417–2422. [PubMed] [Google Scholar]
- Burnell J. N., Anderson J. W. Adenosine 5'-sulphatophosphate kinase activity in spinach leaf tissue. Biochem J. 1973 Jun;134(2):565–579. doi: 10.1042/bj1340565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- Durham P. L., Poulton J. E. Effect of Castanospermine and Related Polyhydroxyalkaloids on Purified Myrosinase from Lepidium sativum Seedlings. Plant Physiol. 1989 May;90(1):48–52. doi: 10.1104/pp.90.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis S., Conway T. W. Initial velocity kinetic analysis of 30 S initiation complex formation in an in vitro translation system derived from Escherichia coli. J Biol Chem. 1984 Jun 25;259(12):7607–7614. [PubMed] [Google Scholar]
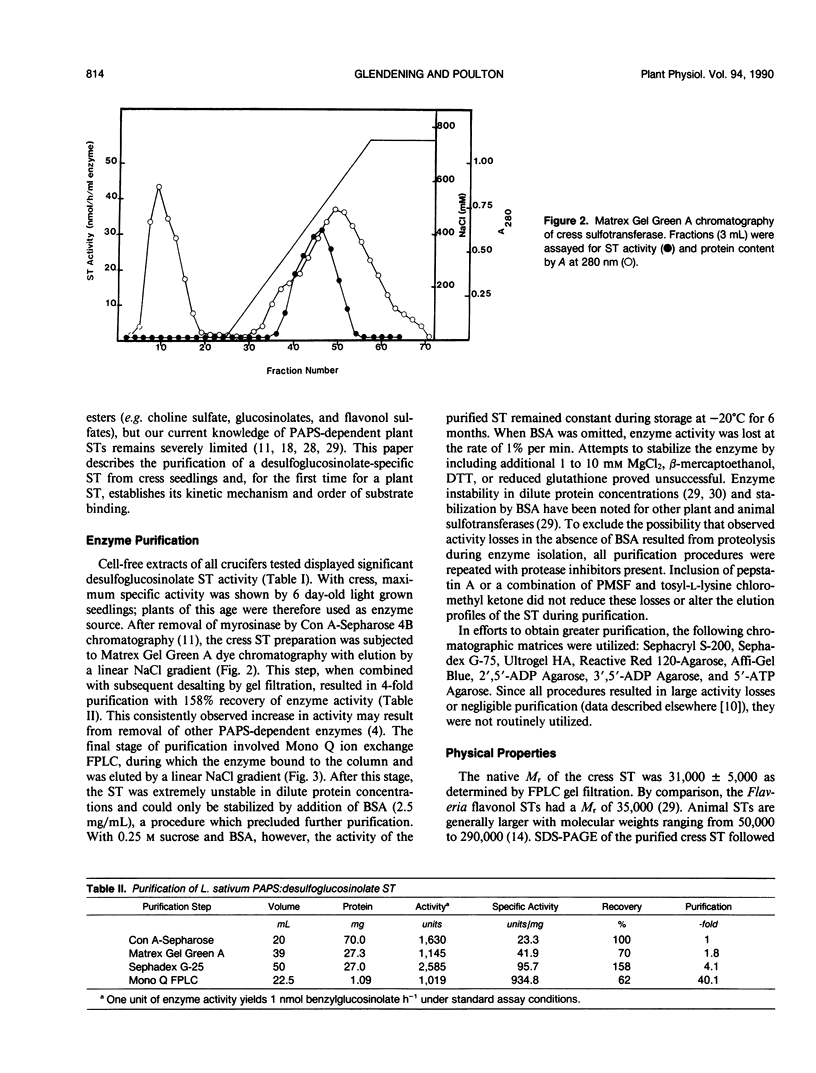
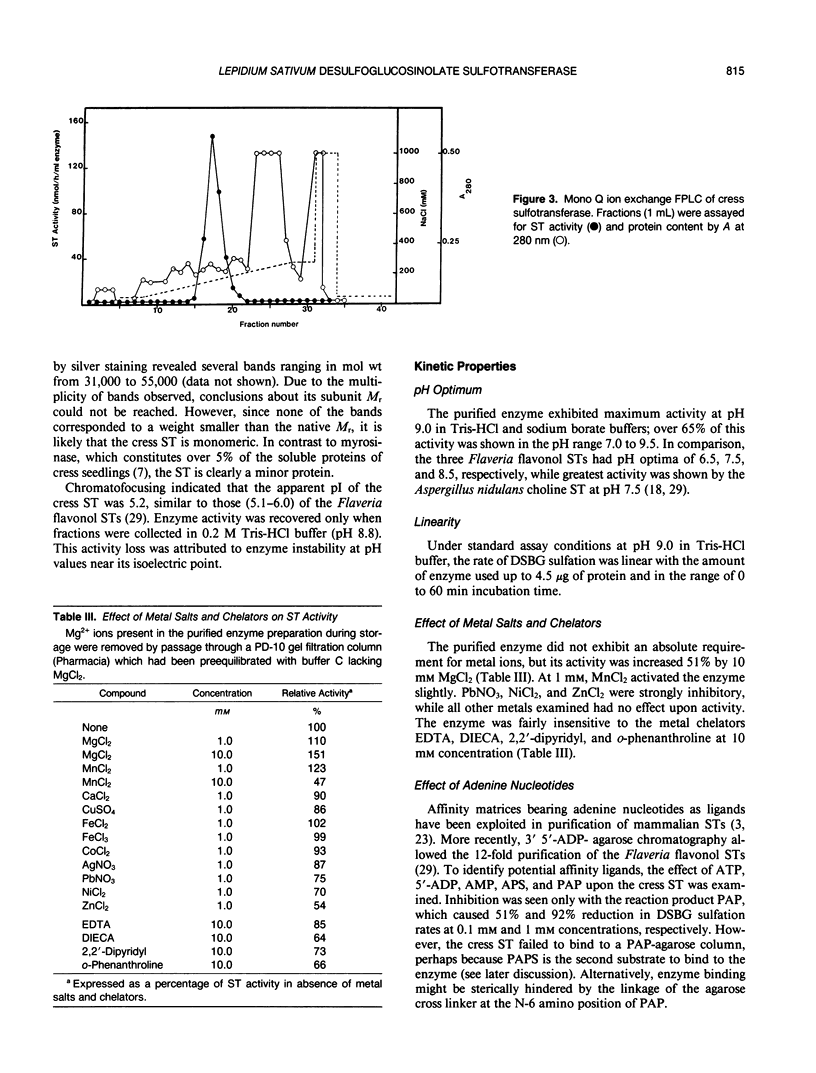
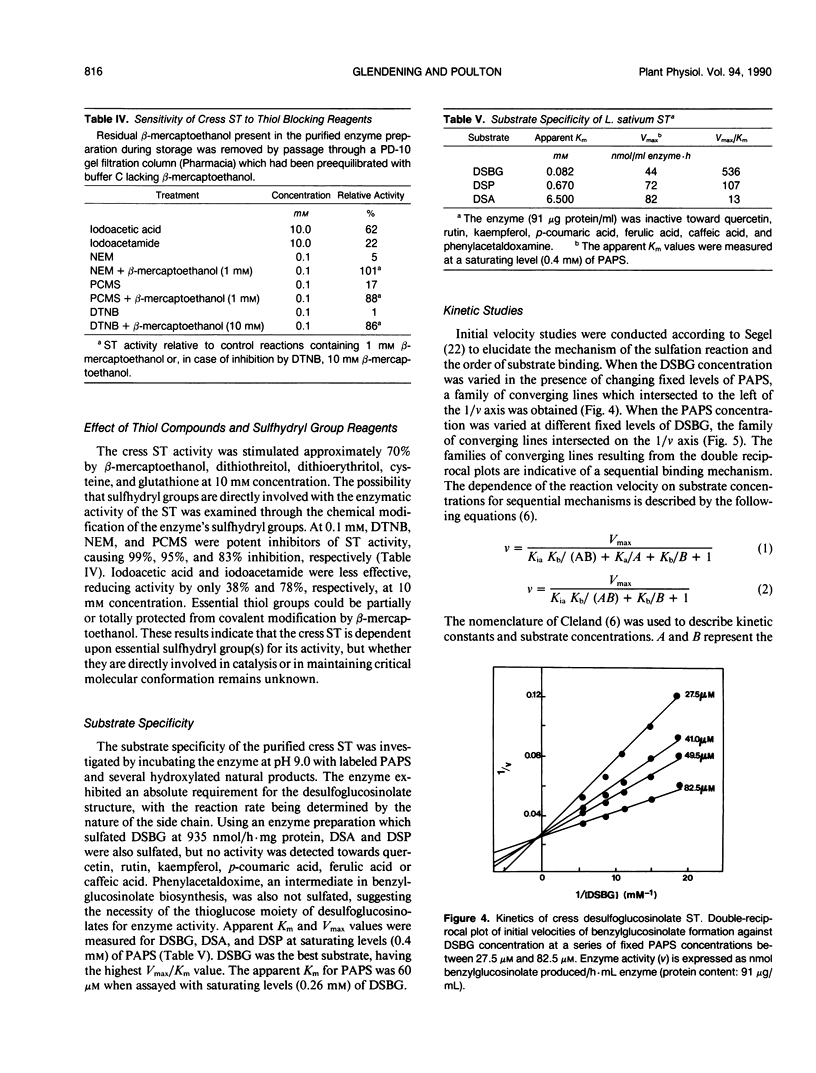
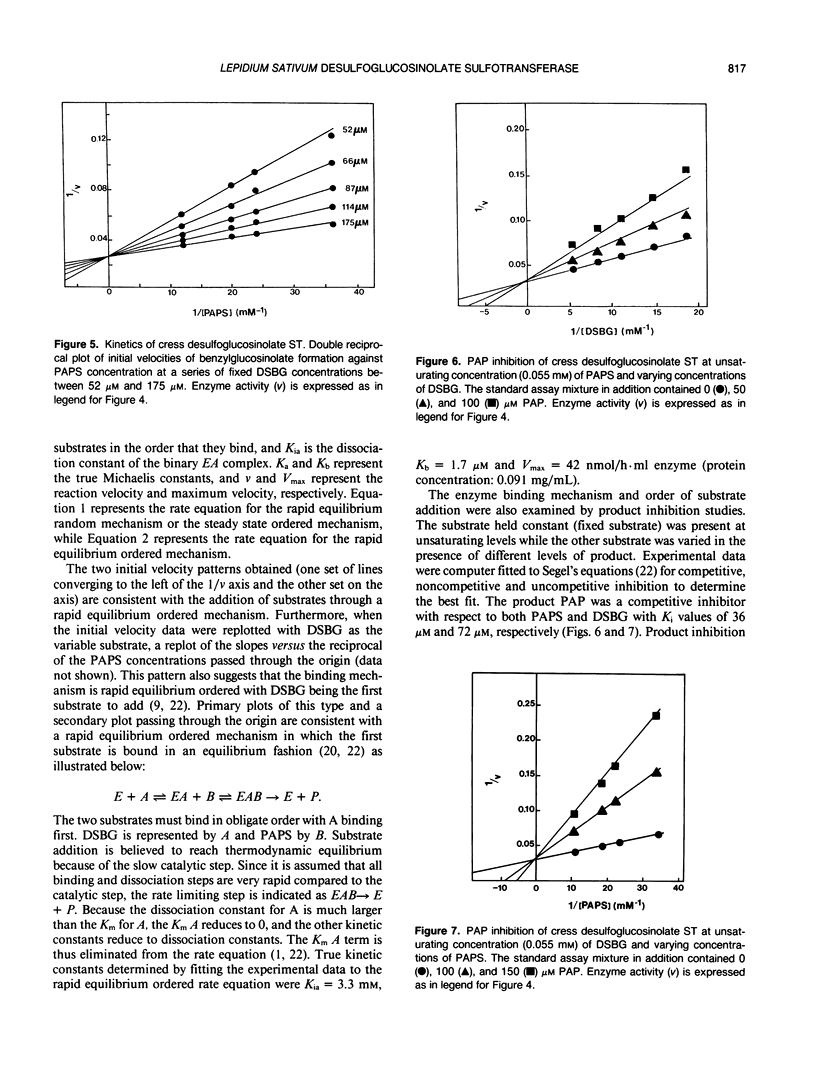
- Glendening T. M., Poulton J. E. Glucosinolate Biosynthesis: Sulfation of Desulfobenzylglucosinolate by Cell-Free Extracts of Cress (Lepidium sativum L.) Seedlings. Plant Physiol. 1988 Feb;86(2):319–321. doi: 10.1104/pp.86.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain J. C., Reed D. W., GrootWassink J. W., Underhill E. W. A radioassay of enzymes catalyzing the glucosylation and sulfation steps of glucosinolate biosynthesis in Brassica species. Anal Biochem. 1989 Apr;178(1):137–140. doi: 10.1016/0003-2697(89)90369-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mahadevan S., Stowe B. B. An intermediate in the synthesis of glucobrassicins from 3-indoleacetaldoxime by woad leaves. Plant Physiol. 1972 Jul;50(1):43–50. doi: 10.1104/pp.50.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORSI B. A., SPENCER B. CHOLINE SULPHOKINASE (SULPHOTRANSFERASE). J Biochem. 1964 Jul;56:81–91. doi: 10.1093/oxfordjournals.jbchem.a127963. [DOI] [PubMed] [Google Scholar]
- Perrella F. W. EZ-FIT: a practical curve-fitting microcomputer program for the analysis of enzyme kinetic data on IBM-PC compatible computers. Anal Biochem. 1988 Nov 1;174(2):437–447. doi: 10.1016/0003-2697(88)90042-5. [DOI] [PubMed] [Google Scholar]
- Schimerlik M. I., Cleland W. W. Inhibition of creatine kinase by chromium nucleotides. J Biol Chem. 1973 Dec 25;248(24):8418–8423. [PubMed] [Google Scholar]
- Sekura R. D., Jakoby W. B. Phenol sulfotransferases. J Biol Chem. 1979 Jul 10;254(13):5658–5663. [PubMed] [Google Scholar]
- Truscott R. J., Minchinton I., Sang J. The isolation and purification of indole glucosinolates from Brassica species. J Sci Food Agric. 1983 Mar;34(3):247–254. doi: 10.1002/jsfa.2740340308. [DOI] [PubMed] [Google Scholar]
- Underhill E. W. Biosynthesis of mustard oil glucosides: conversion of phenylacetaldehyde oxime and 3-phenylpropionaldehyde oxime to glucotropaeolin and gluconasturtiin. Eur J Biochem. 1967 Jul;2(1):61–63. doi: 10.1111/j.1432-1033.1967.tb00106.x. [DOI] [PubMed] [Google Scholar]
- Underhill E. W., Wetter L. R., Chisholm M. D. Biosynthesis of glucosinolates. Biochem Soc Symp. 1973;(38):303–326. [PubMed] [Google Scholar]
- Varin L., Ibrahim R. K. Partial Purification and Characterization of Three Flavonol-Specific Sulfotransferases from Flaveria chloraefolia. Plant Physiol. 1989 Jul;90(3):977–981. doi: 10.1104/pp.90.3.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S. G., Straub K. D. Purification and characterization of N-hydroxy-2-acetylaminofluorene sulfotransferase from rat liver. J Biol Chem. 1976 Nov 10;251(21):6529–6536. [PubMed] [Google Scholar]


