Abstract
Purpose
This study aimed to investigate the association between quantitative retinal vascular measurements and the risk of all-cause and premature mortality.
Methods
In this population-based cohort study using the UK Biobank data, we employed the Retina-based Microvascular Health Assessment System to assess fundus images for image quality and extracted 392 retinal vascular measurements per fundus image. These measurements encompass six categories of vascular features: caliber, density, length, tortuosity, branching angle, and complexity. Univariate Cox regression models were used to identify potential indicators of mortality risk using data on all-cause and premature mortality from death registries. Multivariate Cox regression models were then used to test these associations while controlling for confounding factors.
Results
The final analysis included 66,415 participants. After adjusting for demographic, health, and lifestyle factors and genetic risk score, 18 and 10 retinal vascular measurements were significantly associated with all-cause mortality and premature mortality, respectively. In the fully adjusted model, the following measurements of different vascular features were significantly associated with all-cause mortality and premature mortality: arterial bifurcation density (branching angle), number of arterial segments (complexity), interquartile range and median absolute deviation of arterial curve angle (tortuosity), mean and median values of mean pixel widths of all arterial segments in each image (caliber), skeleton density of arteries in macular area (density), and minimum venular arc length (length).
Conclusions
The study revealed 18 retinal vascular measurements significantly associated with all-cause mortality and 10 associated with premature mortality. Those identified parameters should be further studied for biological mechanisms connecting them to increased mortality risk.
Translational Relevance
This study identifies retinal biomarkers for increased mortality risk and provides novel targets for investigating the underlying biological mechanisms.
Keywords: retina image, microvasculature quantification, mortality, retinal vascular measurements
Introduction
In 2019, nearly half of global deaths occurred in individuals under the age of 70,1 and, in Great Britain, 22.8% of deaths in 2020 were considered avoidable.2 Identifying individuals at high risk is of great importance for the delay or prevention of mortality. A key barrier to translating preventative medicine advances to clinical practice is the accurate screening for vulnerable individuals at risk of death from preventable causes.3 To that end, several risk-scoring tools have been developed; however, most existing tools are population specific, which means that tools developed in one population may not be easily adoptable to other populations or they are associated with some cause-specific mortality.4 Further issues include low accuracy,5 information bias for questionnaire-incorporated models,6 and invasive procedures associated with increased cost and decreased feasibility.7
The retina is well known to reflect the microvascular health of the body. Previous studies have studied the associations between retinal vascular features and systemic health conditions. For example, studies have found that retinal vessel caliber is associated with hypertension, cardiovascular disease, metabolic syndrome, and mortality.8,9 Retinal vascular tortuosity10,11 and complexity10,12–15 are associated with cardiovascular outcomes, Alzheimer's disease progression, and cognitive dysfunction. In addition, an association with cognitive function, chronic obstructive pulmonary disease, and obstructive sleep apnea syndrome has been found for retinal vessel density.16–18 The association with systemic health reveals the potential for retina vascular features to serve as indicators of mortality risk. However, studies on the comprehensive association between retinal vascular features and risk of mortality are still limited.
Recent advancements in deep learning algorithms have made it possible to automatically segment and quantify retinal vascular networks. In particular, the Retina-based Microvascular Health Assessment System (RMHAS)19 is one such algorithm that completes the segmentation and quantification within 2 seconds and extracts hundreds of measurements simultaneously. RMHAS has been validated thoroughly with proven performance and accuracy across different datasets and images of varied quality. The automated nature of segmentation and the non-invasive, low-cost, and convenient nature of the retinal photography imaging modality have enabled researchers to study retinal vascular parameters in unprecedented detail in large sample sizes.
In this study, we applied RMHAS to fundus photos from the UK Biobank to investigate the associations of retinal vascular measurements with all-cause and premature mortality. Based on previous studies demonstrating the associations of retinal vascular features with systemic health conditions and mortality, we hypothesized that retinal arterial measurements obtained using RMHAS would be significantly associated with mortality risk. We propose that, by identifying parameters significantly associated with mortality risk, this study could provide novel targets for investigating the biological mechanisms underlying the increased risk of mortality and biomarkers for increased mortality risk in the population.
Methods
Study Population
We utilized data for participants of the UK Biobank, a large population-based prospective cohort that recruited over 500,000 people from England, Scotland, and Wales who were 40 to ∼69 years old and registered with the National Health Service (NHS). Details about the study are included elsewhere,20 but, in brief, participants were recruited from 2006 to 2010, and data on self-reported questionnaires, physical examinations, and blood draws were collected on all participants. From 2009 to 2010, a subgroup of participants from six assessment centers was invited to take part in the baseline eye examination, and, from 2012 to 2013, about 20,000 participants attended follow-up assessments, including eye examinations.21
In this study, we used the date of first-time image capture as the baseline measurement, and only those with fundus images of acceptable quality were included. Image quality was assessed by RMHAS,19 and mortality data were acquired through national death registries and NHS Digital. Follow-up duration was calculated from the date of first image acquisition to the date of death, or December 31, 2020 (the most recent follow-up date), whichever came first. Person-years at risk were calculated accordingly. To avoid reverse causal bias, patients who died within the first year of follow-up were excluded from final analysis.
The UK Biobank was approved by the North West Multi-Centre Research Ethics Committee (ethics application number 06/MRE08/65, protocol code 11/NW/0382), and informed consent was obtained from all participants. The current study used only unidentifiable data obtained under project application number 94372. The UK Biobank and the current study adhered to the tenets of the Declaration of Helsinki. Restrictions apply to the availability of these data. Data were obtained from UK Biobank and are available at https://www.ukbiobank.ac.uk with the permission of UK Biobank.
Ascertainment of Retinal Vascular Measurements
The fundus photographs with a field angle of 45° were obtained without mydriasis.21 For extraction of vascular measurements, images of the right eye were used; images of the left eye were utilized only when those of the right eye were not available. Retinal vascular measurements were extracted using RMHAS.19 In brief, images with a vessel area density of <0.08 obtained with RMHAS were considered of poor quality and excluded. The selection of this threshold was based on a manual assessment of both the original image and the segmented vessels. Eligible images were segmented for arteries, veins, and the optic disc, followed by measurement extraction (Fig. 1). Measurements of the following six types were obtained: caliber, density, length, tortuosity, branching angle, and complexity (see Supplementary Material). Considering there are hundreds of small vessel segments, the central tendency, variability, and shape of the distribution of their measurements were generated as a summarization of their general features (Supplementary Material).
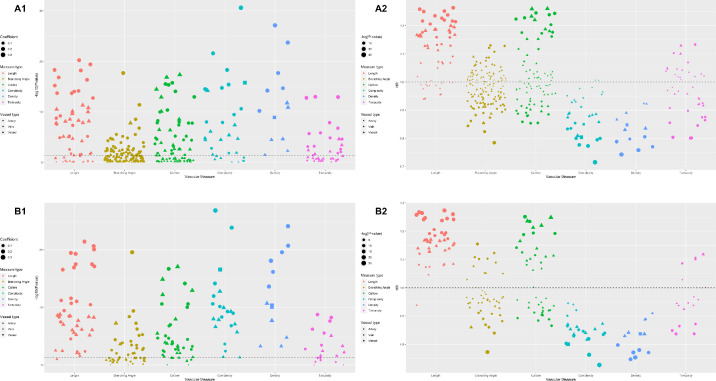
Figure 1.
HRs and FDR-adjusted P values of retinal vascular measurements for all-cause mortality. (A1) FDR-adjusted P values of retinal vascular measurements in the training set. (A2) HRs of retinal vascular measurements in the training set. (B1) FDR-adjusted P values of retinal vascular measurements in the validation set. (B2) HRs of retinal vascular measurements in the validation set. All P values in the figure were FDR adjusted.
Ascertainment of Mortality Data
Mortality data of participants who died were linked through national death registries, and those without death records were considered alive. Our primary outcome was all death events based on death registry data from NHS Digital for participants in England and Wales; mortality data for participants in Scotland were linked from the NHS Central Register and part of the National Records of Scotland. The mortality data were last updated on December 31, 2020. The secondary outcome was premature mortality, which refers to death events that occurred before the age of 75 years.15 The age of death was calculated based on the age at recruitment and date of death (details can be found at https://biobank.ndph.ox.ac.uk/showcase/refer.cgi?id=115559).
Covariates
We collected data on potential confounders to control for their effects. Demographic factors included age, sex, ethnicity, Townsend index, and education. Education level was classified as high, medium, or low. Lifestyle and health factors included body mass index (BMI), self-reported overall health rating, number of treatments/medications taken, diagnosis of heart disease and diabetes, smoking status, alcohol consumption, and physical activity. In addition, the genetic risk score (GRS) for longevity was also included,22 as previous studies have revealed a genetic influence on retinal vessel morphology (Supplementary Material).23–25
Statistical Analysis
Data were described as the mean ± standard deviation (SD) for continuous variables or the percentage for categorical variables. For numerical values, a t-test was performed to test the inter-group differences of data with normal distributions, and the Wilcoxon rank–sum test was used for data with non-normal distributions. The χ2 test was applied for categorical variables.
Before the regression analyses, we excluded sex-specific extreme outliers, normalized the distribution of retinal vascular measurements (Supplementary Material), and rescaled them to SD units. To identify the potential indicators of all-cause and premature mortality risk, we randomly split the dataset in half into training and validation sets, and P values were further adjusted by the false discovery rate (FDR) method. Afterward, measurements that showed significant association in both training and validation datasets were considered potential indicators of mortality risk and included in further analyses.
Previously identified covariates26,27 were incrementally added to further validate the robustness of the associations by four Cox proportional hazard regression models: unadjusted (Model 1), adjusted for demographic factors (Model 2), further adjusted for health and lifestyle factors (Model 3), and including additional GRS on top of Model 3 (Model 4). Additionally, we investigated the potential nonlinear associations (Supplementary Table S1) and performed subgroup analyses (Supplementary Table S2). Two-tailed P < 0.05 was considered statistically significant. All statistical analyses were performed using R 4.2.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline Demographic Characteristics
The study population was comprised of 81,107 participants who had baseline fundus images. Of these, 14,649 participants with poor-quality images and 43 participants who died within the first year of follow-up were excluded, leaving a total of 66,415 participants (Supplementary Fig. S1). The participants had an average age of 56.7 ± 8.24 years, and 91.4% of them identified as white. The median BMI was 26.6 (range, 12.6∼66.0), and 49,002 participants (73.8%) reported an overall health rating of good/excellent (Table).
Table.
Characteristics of Participants by Survival Status and Death Age
| Alive vs. Dead From All-Cause Mortality | Age at Death (y) | ||||||
|---|---|---|---|---|---|---|---|
| Demographic Factors | Total | Alive | Dead | P | <75 (Premature) | ≥75 | P |
| Number of participants | 66,415 | 63,603 | 2812 | — | 2285 | 527 | |
| Age (y), mean ± SD | 56.7 ± 8.24 | 56.5 ± 8.23 | 61.9 ± 6.46 | <0.001 | 60.4 ± 6.23 | 68.2 ± 2.09 | <0.001 |
| Sex, n (%) | <0.001 | 0.263 | |||||
| Female | 36,327 (54.7) | 35,132 (55.2) | 1195 (42.5) | 983 (43.0) | 212 (40.2) | ||
| Male | 30,088 (45.3) | 28,471 (44.8) | 1617 (57.5) | 1302 (57.0) | 315 (59.8) | ||
| Ethnicity, n (%) | <0.001 | 0.189 | |||||
| White | 60,707 (91.4) | 58,093 (91.3) | 2614 (93.0) | 2110 (92.3) | 504 (95.6) | ||
| Mixed | 535 (0.8) | 524 (0.8) | 11 (0.4) | 10 (0.4) | 1 (0.2) | ||
| Asian | 2032 (3.1) | 1976 (3.1) | 56 (2.0) | 51 (2.2) | 5 (0.9) | ||
| Black | 1816 (2.7) | 1745 (2.7) | 71 (2.5) | 61 (2.7) | 10 (1.9) | ||
| Other | 920 (1.4) | 888 (1.4) | 32 (1.1) | 28 (1.2) | 4 (0.8) | ||
| Townsend index, mean ± SD | –1.18 ± 2.97 | –1.19 ± 2.96 | –0.858 ± 3.13 | <0.001 | –0.804 ± 3.15 | –1.09 ± 3.02 | 0.053 |
| Education, n (%) | <0.001 | <0.001 | |||||
| High | 24,815 (37.4) | 24,042 (37.8) | 773 (27.5) | 639 (28.0) | 134 (25.4) | ||
| Intermediate | 32640 (49.1) | 31286 (49.2) | 1354 (48.2) | 1155 (50.5) | 199 (37.8) | ||
| Low | 8209 (12.4) | 7571 (11.9) | 638 (22.7) | 455 (19.9) | 183 (34.7) | ||
| BMI (kg/m2), mean ± SD | 27.2 (4.73) | 27.2 (4.71) | 28.0 (5.18) | <0.001 | 28.1 (5.25) | 27.9 (4.87) | 0.564 |
| Self-reported overall health rating, n (%) | <0.001 | 0.002 | |||||
| Good/excellent | 49,002 (73.8) | 47,400 (74.5) | 1602 (57.0) | 1275 (55.8) | 327 (62.0) | ||
| Fair | 14,241 (21.4) | 13391 (21.1) | 850 (30.2) | 697 (30.5) | 153 (29.0) | ||
| Poor | 2789 (4.2) | 2456 (3.9) | 333 (11.8) | 292 (12.8) | 41 (7.8) | ||
| Treatments/medications taken, n (%) | 2.24 (2.49) | 2.17 (2.42) | 3.62 (3.40) | <0.001 | 3.50 (3.38) | 4.17 (3.42) | <0.001 |
| CVD diagnosis, n (%) | <0.001 | <0.001 | |||||
| Yes | 18,463 (27.8) | 17,232 (27.1) | 1231 (43.8) | 963 (42.1) | 268 (50.9) | ||
| No | 47,649 (71.7) | 46,087 (72.5) | 1562 (55.5) | 1307 (57.2) | 255 (48.4) | ||
| Diabetes, n (%) | <0.001 | 0.005 | |||||
| No | 62,740 (94.5) | 60,278 (94.8) | 2462 (87.6) | 2020 (88.4) | 442 (83.9) | ||
| Yes | 3309 (5.0) | 2983 (4.7) | 326 (11.6) | 246 (10.8) | 80 (15.2) | ||
| Cancer, n (%) | <0.001 | 0.170 | |||||
| No | 60,739 (91.5) | 58,505 (92.0) | 2234 (79.4) | 1804 (78.9) | 430 (81.6) | ||
| Yes | 5311 (8.0) | 4751 (7.5) | 560 (19.9) | 467 (20.4) | 93 (17.6) | ||
| Smoke, n (%) | <0.001 | 0.002 | |||||
| Never | 37,741 (56.8) | 36,550 (57.5) | 1191 (42.4) | 956 (41.8) | 235 (44.6) | ||
| Previous | 22,472 (33.8) | 21314 (33.5) | 1158 (41.2) | 926 (40.5) | 232 (44.0) | ||
| Current | 5837 (8.8) | 5396 (8.5) | 441 (15.7) | 385 (16.8) | 56 (10.6) | ||
| Drink alcohol, n (%) | <0.001 | 0.403 | |||||
| Never | 3051 (4.6) | 2921 (4.6) | 130 (4.6) | 100 (4.4) | 30 (5.7) | ||
| Previous | 2295 (3.5) | 2123 (3.3) | 172 (6.1) | 142 (6.2) | 30 (5.7) | ||
| Current | 60,845 (91.6) | 58,348 (91.7) | 2497 (88.8) | 2032 (88.9) | 465 (88.2) | ||
| Physical activities, n (%) | <0.001 | 0.150 | |||||
| Low | 9911 (14.9) | 9382 (14.8) | 529 (18.8) | 438 (19.2) | 91 (17.3) | ||
| Moderate | 22527 (33.9) | 21646 (34.0) | 881 (31.3) | 728 (31.9) | 153 (29.0) | ||
| High | 22147 (33.3) | 21348 (33.6) | 799 (28.4) | 634 (27.7) | 165 (31.3) | ||
| Survival time (y), median (min, max) | 10.6 (1.10, 11.7) | 10.6 (7.57, 11.1) | 7.20 (1.10, 11.7) | <0.001 | 6.50 (1.10, 11.7) | 9.40 (2.30, 11.6) | <0.001 |
For differences between groups, t-tests were performed for numerical data with normal distributions and Wilcoxon rank–sum tests for numerical data with non-normal distributions. The χ2 test was used for categorical variables. CVD, cardiovascular disease; min, minimum; max, maximum.
Incidence of All-Cause Mortality and Premature Mortality
During a median follow-up duration of 10.6 years (interquartile range [IQR], 10.50–10.79), there were 2812 cases of all-cause mortality and 2285 cases of premature mortality. The mean age of death was 68.5 ± 7.20 years for females and 69.1 ± 6.94 years for males. Male participants were more likely to die or die prematurely during follow-up (57.5% vs. 42.5% and 59.8% vs. 40.2%, respectively), although the difference was only significant for all-cause mortality (P < 0.001 for all-cause mortality and P = 0.263 for premature mortality). Of the demographic factors, only age and education level were significantly different between all-cause mortality and premature mortality cases (both P < 0.001). Regarding lifestyle factors, only smoking status showed a statistically significant difference between the mortality groups, whereas all health factors except BMI and cancer were significantly different (Table).
Retinal Vascular Measurements and All-Cause Mortality
A total of 373 retinal vascular measurements from six major categories of measures were included in the training set after excluding variables with 99% identical values. Subsequently, 191 retinal vascular measurements with FDR-adjusted P < 0.05 were tested in the validation set. Of these, 149 retinal vascular measurements remained significant following FDR adjustment in validation. The hazard ratios (HRs) and FDR-adjusted P values of these retinal vascular measurements in the two datasets are presented in Figure 1.
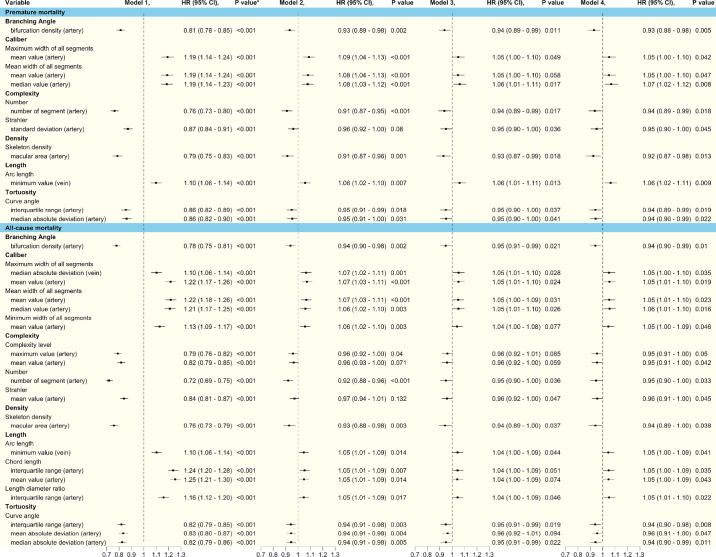
We fitted four models to examine the associations between the 149 retinal vascular measurements and all-cause mortality in the full dataset. Model 1 showed that all 149 retinal vascular measurements were significantly related to all-cause mortality after FDR adjustment. After adjusting for demographic factors (Model 2), 49 retinal vascular measurements remained significant. Following adjustment for demographic, lifestyle, and health factors (Model 3), only 14 retinal vascular measurements remained significantly associated with all-cause mortality. After further adjusting for GRS (Model 4), 18 retinal vascular measurements showed significant associations. The features of these 18 retinal vascular measurements and their association with all-cause mortality across the four models are presented in Figure 2.
Figure 2.
HRs for the association of retinal parameters with premature and all-cause mortality across all models. Model 1 included retinal vascular measurements only. Model 2 included both retinal vascular measurements and demographic factors, including age, sex, ethnicity, Townsend index (social deprivation), and education. Model 3 used covariates from Model 2 and added health factors, including BMI, self-reported overall health rating, number of treatments/medications taken, and comorbidities (diabetes mellitus, history of cardiovascular diseases, and cancer), as well as lifestyle factors (smoking, alcohol, and physical activity). Model 4 was adjusted for Model 3 plus genetic risk score. *FDR-adjusted P value.
Retinal Vascular Measurements and Premature Mortality
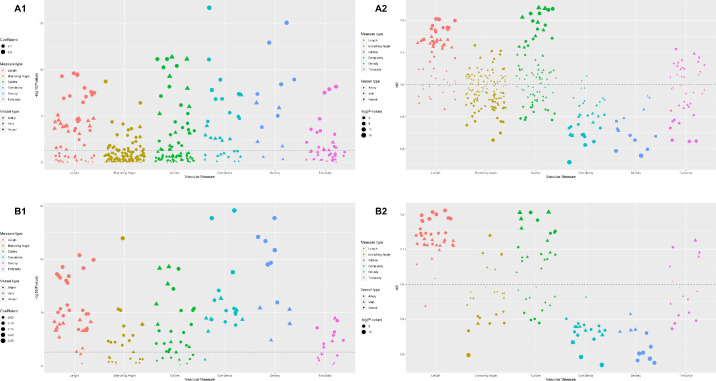
Following Cox proportional hazard regression in the training set, 141 out of 373 retinal vascular measurements were significantly associated with premature mortality after FDR adjustment. This narrowed to 111 retinal vascular measurements with FDR-adjusted P < 0.05 in the validation set. Figure 3 shows the HRs and FDR-adjusted P values of retinal vascular measurements in the two datasets. After fitting four models to the full dataset, all 111 retinal vascular measurements were significantly related to premature mortality after FDR adjustment (Model 1). After adjusting for demographic factors (Model 2), only 37 retinal vascular measurements remained significant. In the full model, 10 measurements remained significant (Fig. 2).
Figure 3.
HRs and FDR-adjusted P values of retinal vascular measurements for premature mortality. (A1) FDR-adjusted P values of retinal vascular measurements in the training set. (A2) HRs of retinal vascular measurements in the training set. (B1) FDR-adjusted P values of retinal vascular measurements in the validation set. (B2) HRs of retinal vascular measurements in the validation set. All P values in the figure were FDR adjusted.
Branching Angle and Mortality
Among all branching angle–related measurements, arterial bifurcation density was the only factor that was significantly associated with all-cause mortality (HR = 0.94; 95% confidence interval [CI], 0.90–0.99; P = 0.01) and premature mortality (HR = 0.93; 95% CI, 0.88–0.98; P < 0.01) in the fully adjusted model.
Complexity and Mortality
After full adjustment, the maximum value (HR = 0.95; 95% CI, 0.91–1.00; P = 0.05) and mean value (HR = 0.95; 95% CI, 0.91–1.00; P = 0.04) of arterial complexity (the number of arterial segments passing by a certain point), number of arterial segments (HR = 0.95; 95% CI, 0.90–1.00; P = 0.03), and mean Strahler number (a numerical measure of artery branching complexity; HR = 0.96; 95% CI, 0.91–1.00; P = 0.05) were associated with all-cause mortality. For premature mortality, the number of arterial segments (HR = 0.94, 95% CI, 0.89–0.99; P = 0.02) still showed significant association after full adjustment. In addition, the standard deviation of the Strahler number of arteries (HR = 0.95; 95% CI, 0.90–1.00; P = 0.05) was also associated with premature mortality in Model 4.
Density and Mortality
Among all density-related measurements, only the skeleton density of arteries in the macular area was significantly associated with all-cause mortality risk (HR = 0.94; 95% CI, 0.89–1.00; P = 0.04) and premature mortality (HR = 0.92; 95% CI, 0.87–0.98; P = 0.01) after full adjustment.
Length and Mortality
For all-cause mortality, four length-related measurements showed significant association: minimum venular arc length (HR = 1.05; 95% CI, 1.00–1.09; P = 0.04), IQR (HR = 1.05; 95% CI, 1.00–1.09; P = 0.03), and mean value (HR = 1.05; 95% CI, 1.00–1.09; P = 0.04) of the chord length of arteries, and IQR of the artery length-to-diameter ratio (HR = 1.05; 95% CI, 1.01–1.10; P = 0.02) in the fully adjusted model. In the analysis of association with premature mortality in the full model, only minimum venular arc length (HR = 1.06; 95% CI, 1.02–1.11; P < 0.01) remained significant.
Tortuosity and Mortality
After full model adjustment, IQR (HR = 0.94; 95% CI, 0.90–0.98; P < 0.01), mean absolute deviation (HR = 0.96; 95% CI, 0.91–1.00; P = 0.05), and median absolute deviation (HR = 0.94; 95% CI, 0.90–0.99; P = 0.01) of arterial curve angle were significantly associated with all-cause mortality after full adjustment. For premature mortality, only IQR (HR = 0.94; 95% CI, 0.89–0.99; P = 0.02) and median absolute deviation (HR = 0.94; 95% CI, 0.90–0.99; P = 0.02) of the arterial curve angle had a significant association in Model 4.
Caliber and Mortality
In the fully adjusted model, the central tendency of all vessel segment caliber showed a significant association with all-cause mortality. The mean and median values of mean pixel widths of all arterial segments (mean: HR = 1.05; 95% CI, 1.01–1.10; P = 0.02; median: HR = 1.06; 95% CI, 1.01–1.10; P = 0.02), mean values of minimum and maximum widths of all arterial segments (minimum: HR = 1.05; 95% CI, 1.00–1.09; P = 0.05; maximum: HR = 1.05; 95% CI, 1.01–1.10; P = 0.02), and median absolute deviation of maximum venular width (HR = 1.05; 95% CI, 1.00–1.10; P = 0.03) were significantly associated with all-cause mortality after full adjustment. For premature mortality, the mean and median values of mean pixel widths of all arterial segments (mean: HR = 1.05; 95% CI, 1.00–1.10; P = 0.05; median: HR = 1.07; 95% CI, 1.02–1.12; P < 0.01) and the mean values of maximum widths of all arterial segments (HR = 1.05; 95% CI, 1.00–1.09; P = 0.05) had significant associations in Model 4.
Discussion
We used over 60,000 images to explore the association between retinal microvasculature measurements and all-cause and premature mortality. After accounting for demographic, health, and lifestyle factors and GRS, we found that 18 retinal vascular measurements were significantly linked to all-cause mortality, with 16 originating from arteries. Similarly, 10 retinal vascular measurements exhibited significant associations with premature mortality, with nine being arterial parameters.
In Model 4, lower arterial bifurcation density was significantly associated with both all-cause mortality and premature mortality risk. Previous studies on bifurcation and mortality or life-threatening events have yielded no association; however, these studies focused on bifurcation angle and branching asymmetry ratio rather than bifurcation density, with cardiovascular and cerebrovascular events being endpoints.28,29 Pathologically, lower arterial bifurcation density may correspond to less oxygen and nutrient supply,30 likely causing hypoxia and vessel remodeling with the outcome of a higher risk for mortality. As a snapshot of the microvasculature, end-organ damage of the retina can correspond to that of the kidney, and larger blood vessels if advanced. Hence, this vascular damage is representative of whole-body cardiovascular insult. Given its pathological indication, it will be of interest to explore its association with fatal vascular diseases and vascular disease-related mortality.
Lower blood vessel complexity was associated with an increased risk of all-cause mortality and premature mortality, consistent with previous studies showcasing an independent association with cardiovascular and cerebrovascular risk factors, events, and deaths.10,14,31,32 Previously, risk factors for mortality such as aging and increased blood pressure have been related to reduced retinal vascular branching complexity.33,34 This lower complexity may indicate the presence of endothelium dysfunction, causing dysregulation of vascular bifurcation35 and consequently reducing the efficiency of vascular networks.30
A similar outcome was found for reduced arterial skeleton density. Previous studies10,14,15,34 adopting fractal dimensions as a proxy for the complexity and density of retinal microvasculature have reported similar findings. Retinal vessel density is also linked to vascular diseases with higher death rates.14,29 In addition, our study demonstrated that, compared with the peripheral area, the density of arteries in the macula had stronger associations with mortality risk. One possible explanation is that age-related changes are more prominent in the macular region, as with age-related macular degeneration, and it may indicate a variety of pathological changes such as chronic inflammation, atherosclerosis, oxidative stress, and abnormal lipid metabolism.36–38
The current study found that the IQR and mean value of the chord length of arteries, IQR of the length-to-diameter ratio of arteries, and the minimum arc length of arteries were associated with all-cause mortality. As the inception of artificial intelligence algorithms for automated quantification of retinal vasculature is relatively recent, previously it has not been possible to investigate arc length and chord length in extensive detail. Nonetheless, previous studies have associated a higher arteriolar length-to-diameter ratio with higher plaque burden and higher total cholesterol levels, indicating one possible mechanism for increased mortality risk.11,39 In addition, when examining for premature mortality, only the venular length parameter has shown a significant association. The underlying mechanism may be related to the different roles of arteries and veins. Although changes in both veins and arteries are related to higher mortality risk, a previous study showed that varicose veins are associated with a higher risk of atherosclerotic cardiovascular disease,40 and venous insufficiency is linked to increased mortality risk after multiple adjustments.41 Furthermore, pathological changes in veins and compromised function of clearing toxins and metabolites are related to complications such as blood clots,42 which would lead to an increased risk of fatal events,43 contributing to higher premature mortality risk.
Lower tortuosity was significantly associated with all-cause mortality and premature mortality. Previously, greater arteriolar and venular tortuosity was associated with cerebrovascular events and risk factors for mortality.10,34 However, the study by Sandoval-Garcia et al.10 was carried out in people with type 2 diabetes, and diabetes itself was associated with retinal vascular changes,44 and the other studies focused on high blood pressure. Our study controlled for these confounding factors and found that decreased features of tortuosity corresponded to mortality risk, consistent with previous studies.12,28,29 Witt et al.29 reported that arteriolar tortuosity was significantly associated with mortality even after adjusting for demographic factors, history of cardiovascular disease, and other confounding factors. Both Witt et al.29 and Wang et al.12 suggested that endothelial dysfunction of the microvasculature led to lower tortuosity via atherosclerotic plaques straightening arteries, which interrupted the course of flow, thereby slowing and impairing oxygenation.29
Most retinal measurements of calibers that were associated with all-cause mortality were arterial in origin, and only arterial caliber-related measurements had significant associations with premature mortality. Previously, findings on associations between arterial and venular calibers with mortality or risk factors for mortality have been inconclusive,8,28,29,45,46 thus making interpretation of the current study difficult. For example, previous studies have reported that venular dilatation signifies vascular damage from hyperglycemia and hypoxia47 and wider venular caliber is related to the risk of stroke,48 but narrowed venular caliber has been associated with the risk of dementia.49 These inconsistencies may indicate that caliber itself may not be a robust indicator for all-cause mortality, and its relationship with mortality could vary depending on the cause. In addition, we also generated the mean and median values of all vessel segments in each image as a summarization of central tendency and found that the central tendency had a strong relevance to the risk of both all-cause and premature mortality.
We used RMHAS19 to simultaneously extract nearly 400 retinal microvascular measurements and controlled for traditional risk factors in our analysis. The ability to identify individuals at high risk of death, independent of traditional risk factors, opens up new avenues for intervention. In many cases, the features analyzed had not yet been examined for their association with mortality or risk factors—for example, arc length and chord length of vessels. Therefore, by identifying these retinal measurements that may be used to assess an individual's mortality risk, our study provides novel targets for critical exploration of the pathological mechanisms for a higher risk of mortality. Our findings have important implications for longevity research, as they shed light on retinal biomarkers for increased mortality risk and offer new targets for investigations aimed at understanding the underlying biological mechanisms.
The large number of retinal vascular measurements may increase the risk of identifying associations by chance. To lower this probability, we tested the associations in training and validation sets and used the FDR method to lower the number of measurements included in the regression analysis. However, some residual findings from chance may exist. Also, the participants at recruitment were relatively young (40–69 years) compared with the cut-off age of premature mortality (75 years). Considering the age range at baseline and the follow-up period of 10 years, the likelihood of observing deaths over the age of 75 becomes smaller, and the proportion of premature mortality in this study would be higher than that in the general UK population. In the future, a dataset with a greater number of elderly people > 75 years old would be useful to study for a more balanced analysis. Finally, this study consisted of participants who were mainly white and from small geographical regions. In the future, a similar study should be carried out to explore these associations across different ethnic groups and geographical regions.
Conclusions
Our study revealed 18 retinal vascular measurements significantly associated with all-cause mortality and 10 associated with premature mortality. This study identified retinal measurements that may be used to assess an individual's mortality risk, thus offering guidance for further investigations aimed at understanding the underlying biological mechanisms that link these changes to a higher risk of mortality.
Supplementary Material
Acknowledgments
This project received grant funding from the Australian Government: the National Critical Research Infrastructure Initiative, Medical Research Future Fund (MRFAI00035) and the NHMRC Investigator Grant (APP1175405). The contents of the published material are solely the responsibility of the Administering Institution, a participating institution or individual authors and do not reflect the views of the NHMRC. This work was supported by grants from the Fundamental Research Funds of the State Key Laboratory of Ophthalmology, Project of Investigation on Health Status of Employees in Financial Industry in Guangzhou, China (Z012014075); and Global STEM Professorship Scheme (P0046113). The Centre for Eye Research Australia receives Operational Infrastructure Support from the Victorian State Government. MY is supported by a Melbourne Research Scholarship established by the University of Melbourne. The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Disclosure: M. Yusufu, None; Y. Chen, None; A. Dayimu, None; G. Bulloch, None; S. Jin, None; A.J. Vingrys, None; L. Zhang, None; X. Shang, None; D. Shi, None; M. He, None
References
- 1. Institute for Health Metrics and Evaluation. Global Burden of Disease Study 2019. Available at: https://www.healthdata.org/research-analysis/gbd. Accessed December 7, 2023.
- 2. Office for National Statistics. Avoidable mortality in Great Britain: 2020. Available at: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/causesofdeath/bulletins/avoidablemortalityinenglandandwales/2020. Accessed September 30, 2022.
- 3. Brown LTL, Nepal B. The Cost of Inaction on the Social Determinants of Health. Canberra: National Centre for Social and Economic Modelling; 2012. [Google Scholar]
- 4. Oliver JM, Gallego P, Gonzalez AE, et al.. Predicting sudden cardiac death in adults with congenital heart disease. Heart. 2021; 107(1): 67–75. [DOI] [PubMed] [Google Scholar]
- 5. Loprinzi PD, Addoh O.. Predictive validity of the American College of Cardiology/American Heart Association pooled cohort equations in predicting all-cause and cardiovascular disease-specific mortality in a national prospective cohort study of adults in the United States. Mayo Clin Proc. 2016; 91(6): 763–769. [DOI] [PubMed] [Google Scholar]
- 6. Ganna A, Ingelsson E.. 5 year mortality predictors in 498,103 UK Biobank participants: a prospective population-based study. Lancet. 2015; 386(9993): 533–540. [DOI] [PubMed] [Google Scholar]
- 7. Bérard E, Bongard V, Arveiler D, et al.. Ten-year risk of all-cause mortality: assessment of a risk prediction algorithm in a French general population. Eur J Epidemiol. 2011; 26(5): 359–368. [DOI] [PubMed] [Google Scholar]
- 8. Mutlu U, Ikram MK, Wolters FJ, Hofman A, Klaver CCW, Ikram MA. Retinal microvasculature is associated with long-term survival in the general adult Dutch population. Hypertension. 2016; 67(2): 281–287. [DOI] [PubMed] [Google Scholar]
- 9. Wong TY, Klein R, Klein BE, Tielsch JM, Hubbard L, Nieto FJ.. Retinal microvascular abnormalities and their relationship with hypertension, cardiovascular disease, and mortality. Surv Ophthalmol. 2001; 46(1): 59–80. [DOI] [PubMed] [Google Scholar]
- 10. Sandoval-Garcia E, McLachlan S, Price AH, et al.. Retinal arteriolar tortuosity and fractal dimension are associated with long-term cardiovascular outcomes in people with type 2 diabetes. Diabetologia. 2021; 64(10): 2215–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Frost S, Kanagasingam Y, Sohrabi H, et al.. Retinal vascular biomarkers for early detection and monitoring of Alzheimer's disease. Transl Psychiatry. 2013; 3(2): e233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang SB, Mitchell P, Liew G, et al.. A spectrum of retinal vasculature measures and coronary artery disease. Atherosclerosis. 2018; 268: 215–224. [DOI] [PubMed] [Google Scholar]
- 13. Cheung CY, Ong S, Ikram MK, et al.. Retinal vascular fractal dimension is associated with cognitive dysfunction. J Stroke Cerebrovasc Dis. 2014; 23(1): 43–50. [DOI] [PubMed] [Google Scholar]
- 14. Liew G, Gopinath B, White AJ, Burlutsky G, Yin Wong T, Mitchell P. Retinal vasculature fractal and stroke mortality. Stroke. 2021; 52(4): 1276–1282. [DOI] [PubMed] [Google Scholar]
- 15. Liew G, Mitchell P, Rochtchina E, et al.. Fractal analysis of retinal microvasculature and coronary heart disease mortality. Eur Heart J. 2011; 32(4): 422–429. [DOI] [PubMed] [Google Scholar]
- 16. Fang M, Strand K, Zhang J, et al.. Retinal vessel density correlates with cognitive function in older adults. Exp Gerontol. 2021; 152: 111433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alkan AA, Duzgun E, Karapapak M, et al.. Retinal vascular changes in patients with chronic obstructive pulmonary disease: an optical coherence tomography angiography study. Sisli Etfal Hastan Tip Bul. 2021; 55(2): 210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yu J, Xiao K, Huang J, Sun X, Jiang C.. Reduced retinal vessel density in obstructive sleep apnea syndrome patients: an optical coherence tomography angiography study. Invest Ophthalmol Vis Sci. 2017; 58(9): 3506–3512. [DOI] [PubMed] [Google Scholar]
- 19. Shi D, Lin Z, Wang W, et al.. A deep learning system for fully automated retinal vessel measurement in high throughput image analysis. Front Cardiovasc Med. 2022; 9: 823436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sudlow C, Gallacher J, Allen N, et al.. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015; 12(3): e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chua SYL, Thomas D, Allen N, et al.. Cohort profile: design and methods in the eye and vision consortium of UK Biobank. BMJ Open. 2019; 9(2): e025077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Timmers P, Wilson JF, Joshi PK, Deelen J.. Multivariate genomic scan implicates novel loci and haem metabolism in human ageing. Nat Commun. 2020; 11(1): 3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sim X, Jensen RA, Ikram MK, et al.. Genetic loci for retinal arteriolar microcirculation. PLoS One. 2013; 8(6): e65804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee KE, Klein BE, Klein R, Knudtson MD.. Familial aggregation of retinal vessel caliber in the beaver dam eye study. Invest Ophthalmol Vis Sci. 2004; 45(11): 3929–3933. [DOI] [PubMed] [Google Scholar]
- 25. Jiang X, Hysi PG, Khawaja AP, et al.. GWAS on retinal vasculometry phenotypes. PLoS Genet. 2023; 19(2): e1010583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ma H, Xue Q, Wang X, et al.. Adding salt to foods and hazard of premature mortality. Eur Heart J. 2022; 43(30): 2878–2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhu Z, Shi D, Guankai P, et al.. Retinal age gap as a predictive biomarker for mortality risk. Br J Ophthalmol. 2023; 107(4): 547–554. [DOI] [PubMed] [Google Scholar]
- 28. Cheung CY, Tay WT, Ikram MK, et al.. Retinal microvascular changes and risk of stroke: the Singapore Malay Eye Study. Stroke. 2013; 44(9): 2402–2408. [DOI] [PubMed] [Google Scholar]
- 29. Witt N, Wong TY, Hughes AD, et al.. Abnormalities of retinal microvascular structure and risk of mortality from ischemic heart disease and stroke. Hypertension. 2006; 47(5): 975–981. [DOI] [PubMed] [Google Scholar]
- 30. Murray CD. The physiological principle of minimum work applied to the angle of branching of arteries. J Gen Physiol. 1926; 9(6): 835–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huang Y, Li C, Shi D, et al.. Integrating oculomics with genomics reveals imaging biomarkers for preventive and personalized prediction of arterial aneurysms. EPMA J. 2023; 14(1): 73–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fu Y, Yusufu M, Wang Y, He M, Shi D, Wang R.. Association of retinal microvascular density and complexity with incident coronary heart disease. Atherosclerosis. 2023; 380: 117196. [DOI] [PubMed] [Google Scholar]
- 33. Stanton AV, Wasan B, Cerutti A, et al.. Vascular network changes in the retina with age and hypertension. J Hypertens. 1995; 13(12 pt 2): 1724–1728. [PubMed] [Google Scholar]
- 34. Cheung CY, Tay WT, Mitchell P, et al.. Quantitative and qualitative retinal microvascular characteristics and blood pressure. J Hypertens. 2011; 29(7): 1380–1391. [DOI] [PubMed] [Google Scholar]
- 35. Griffith TM, Edwards DH.. Basal EDRF activity helps to keep the geometrical configuration of arterial bifurcations close to the Murray optimum. J Theor Biol. 1990; 146(4): 545–573. [DOI] [PubMed] [Google Scholar]
- 36. Snow KK, Seddon JM.. Do age-related macular degeneration and cardiovascular disease share common antecedents? Ophthalmic Epidemiol. 1999; 6(2): 125–143. [DOI] [PubMed] [Google Scholar]
- 37. Wong TY, Klein R, Sun C, et al.. Age-related macular degeneration and risk for stroke. Ann Intern Med. 2006; 145(2): 98–106. [DOI] [PubMed] [Google Scholar]
- 38. Kishan AU, Modjtahedi BS, Martins EN, Modjtahedi SP, Morse LS.. Lipids and age-related macular degeneration. Surv Ophthalmol. 2011; 56(3): 195–213. [DOI] [PubMed] [Google Scholar]
- 39. Sasongko MB, Wang JJ, Donaghue KC, et al.. Alterations in retinal microvascular geometry in young type 1 diabetes. Diabetes Care. 2010; 33(6): 1331–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brand FN, Dannenberg AL, Abbott RD, Kannel WB.. The epidemiology of varicose veins: the Framingham Study. Am J Prev Med. 1988; 4(2): 96–101. [PubMed] [Google Scholar]
- 41. Prochaska JH, Arnold N, Falcke A, et al.. Chronic venous insufficiency, cardiovascular disease, and mortality: a population study. Eur Heart J. 2021; 42(40): 4157–4165. [DOI] [PubMed] [Google Scholar]
- 42. Shalhoub J, Lawton R, Hudson J, et al.. Compression stockings in addition to low-molecular-weight heparin to prevent venous thromboembolism in surgical inpatients requiring pharmacoprophylaxis: the GAPS non-inferiority RCT. Health Technol Assess. 2020; 24(69): 1–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li Q, Khatibi N, Zhang JH.. Vascular neural network: the importance of vein drainage in stroke. Transl Stroke Res. 2014; 5(2): 163–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cheung CY, Ikram MK, Klein R, Wong TY.. The clinical implications of recent studies on the structure and function of the retinal microvasculature in diabetes. Diabetologia. 2015; 58(5): 871–885. [DOI] [PubMed] [Google Scholar]
- 45. Wang JJ, Lew G, Klein R, et al.. Retinal vessel diameter and cardiovascular mortality: pooled data analysis from two older populations. Eur Heart J. 2007; 28(16): 1984–1992. [DOI] [PubMed] [Google Scholar]
- 46. Wong TY, Knudtson MD, Klein R, Klein BEK, Hubbard LD. A prospective cohort study of retinal arteriolar narrowing and mortality. Am J Epidemiol. 2004; 159(9): 819–825. [DOI] [PubMed] [Google Scholar]
- 47. Klijn CJ, Kappelle LJ, van Schooneveld MJ, et al.. Venous stasis retinopathy in symptomatic carotid artery occlusion: prevalence, cause, and outcome. Stroke. 2002; 33(3): 695–701. [DOI] [PubMed] [Google Scholar]
- 48. Ong YT, De Silva DA, Cheung CY, et al.. Microvascular structure and network in the retina of patients with ischemic stroke. Stroke. 2013; 44(8): 2121–2127. [DOI] [PubMed] [Google Scholar]
- 49. Cheung CY, Ong YT, Ikram MK, et al.. Microvascular network alterations in the retina of patients with Alzheimer's disease. Alzheimers Dement. 2014; 10(2): 135–142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.