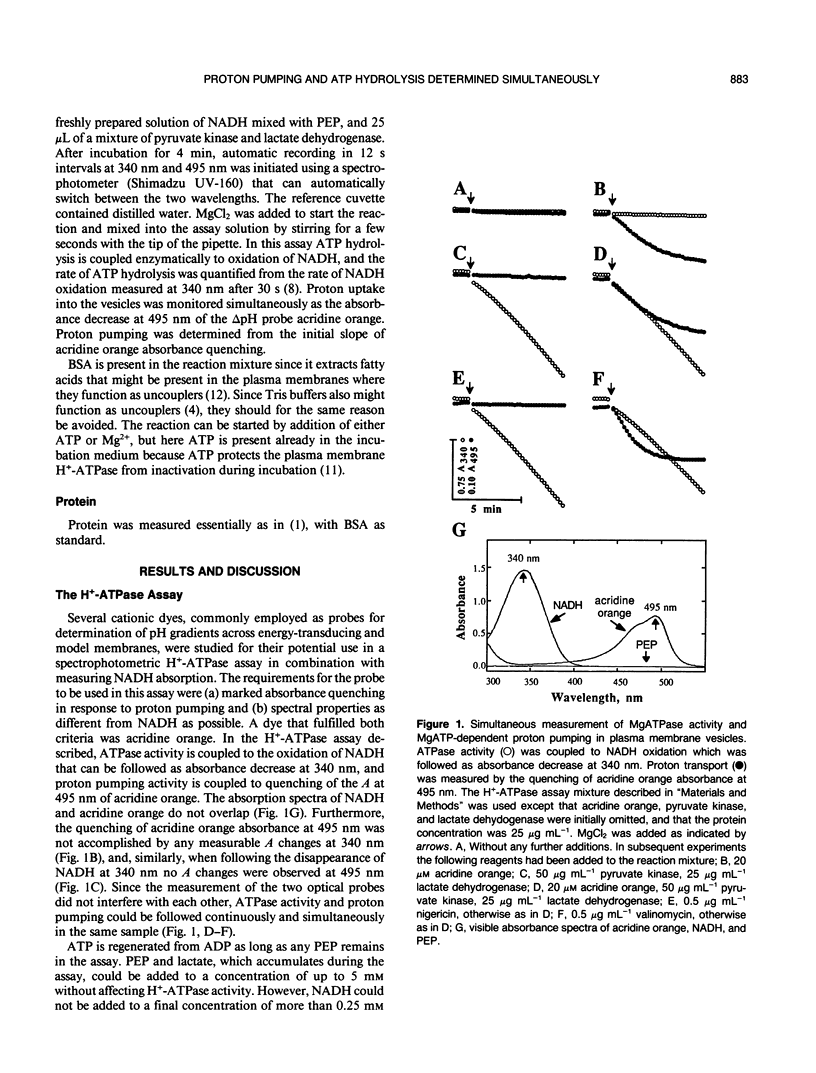
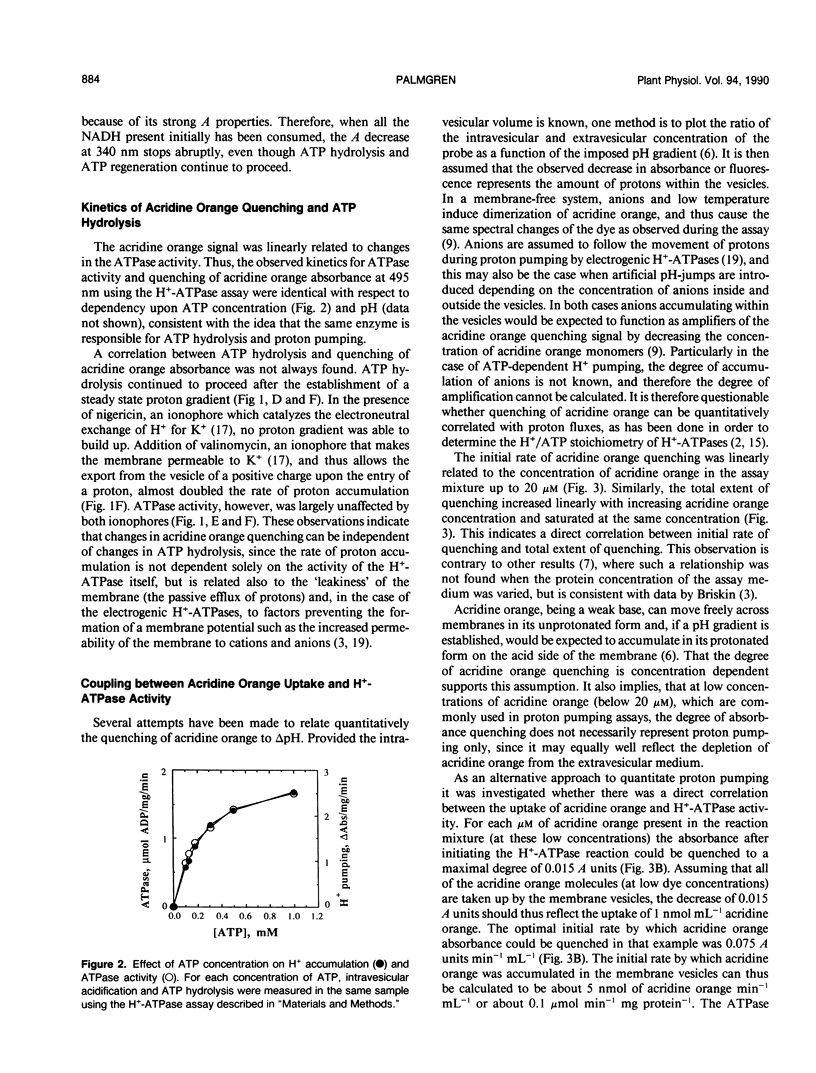
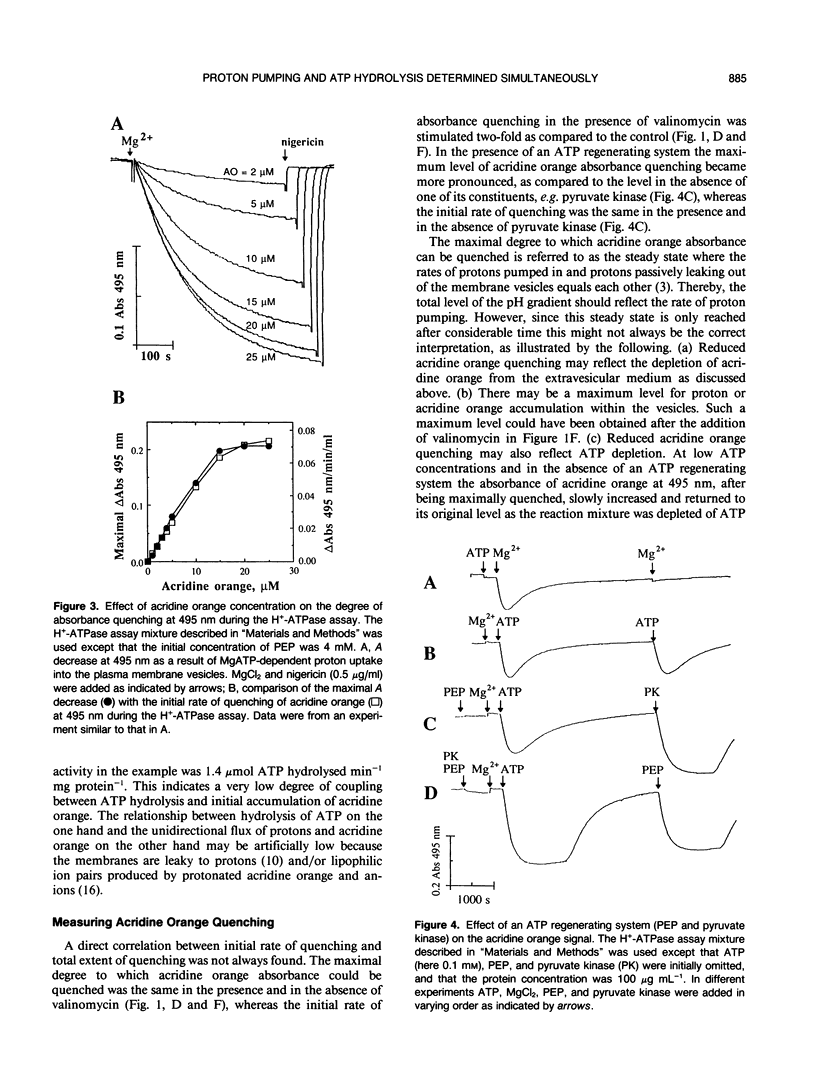
Abstract
A continuous spectrophotometric assay of H+-ATPase activity was developed by combining two well-known methods for measuring proton pumping and ATPase activity. Proton uptake into plasma membrane vesicles from Avena sativa L. (cv Rhiannon) was monitored as the absorbance decrease at 495 nm of the ΔpH probe acridine orange. Simultaneously, ATPase activity was measured by following the absorbance decrease at 340 nanometers by coupling ATP hydrolysis enzymatically to the oxidation of NADH. This H+-ATPase assay is convenient for determining the relative relationship between ATP hydrolysis and proton pumping.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bearden J. C., Jr Quantitation of submicrogram quantities of protein by an improved protein-dye binding assay. Biochim Biophys Acta. 1978 Apr 26;533(2):525–529. doi: 10.1016/0005-2795(78)90398-7. [DOI] [PubMed] [Google Scholar]
- Brauer D., Tu S. L., Hsu A. F., Thomas C. E. Kinetic analysis of proton transport by the vanadate-sensitive ATPase from maize root microsomes. Plant Physiol. 1989 Feb;89(2):464–471. doi: 10.1104/pp.89.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good N. E., Winget G. D., Winter W., Connolly T. N., Izawa S., Singh R. M. Hydrogen ion buffers for biological research. Biochemistry. 1966 Feb;5(2):467–477. doi: 10.1021/bi00866a011. [DOI] [PubMed] [Google Scholar]
- Lew R. R., Spanswick R. M. Characterization of Anion Effects on the Nitrate-Sensitive ATP-Dependent Proton Pumping Activity of Soybean (Glycine max L.) Seedling Root Microsomes. Plant Physiol. 1985 Feb;77(2):352–357. doi: 10.1104/pp.77.2.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nørby J. G. Coupled assay of Na+,K+-ATPase activity. Methods Enzymol. 1988;156:116–119. doi: 10.1016/0076-6879(88)56014-7. [DOI] [PubMed] [Google Scholar]
- Palmgren M. G., Sommarin M. Lysophosphatidylcholine stimulates ATP dependent proton accumulation in isolated oat root plasma membrane vesicles. Plant Physiol. 1989 Jul;90(3):1009–1014. doi: 10.1104/pp.90.3.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmgren M. G., Sommarin M., Ulvskov P., Larsson C. Effect of detergents on the H(+)-ATPase activity of inside-out and right-side-out plant plasma membrane vesicles. Biochim Biophys Acta. 1990 Jan 29;1021(2):133–140. doi: 10.1016/0005-2736(90)90025-j. [DOI] [PubMed] [Google Scholar]
- Perlin D. S., San Francisco M. J., Slayman C. W., Rosen B. P. H+/ATP stoichiometry of proton pumps from Neurospora crassa and Escherichia coli. Arch Biochem Biophys. 1986 Jul;248(1):53–61. doi: 10.1016/0003-9861(86)90400-5. [DOI] [PubMed] [Google Scholar]
- Pope A. J., Leigh R. A. Dissipation of pH Gradients in Tonoplast Vesicles and Liposomes by Mixtures of Acridine Orange and Anions: Implications for the Use of Acridine Orange as a pH Probe. Plant Physiol. 1988 Apr;86(4):1315–1322. doi: 10.1104/pp.86.4.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressman B. C. Biological applications of ionophores. Annu Rev Biochem. 1976;45:501–530. doi: 10.1146/annurev.bi.45.070176.002441. [DOI] [PubMed] [Google Scholar]


