Abstract
Azacitidine (AZA) has been one of the standard treatments for transplantation-ineligible patients with myelodysplastic syndrome (MDS); however, hematological toxicities frequently cause treatment interruption in the early phase of the therapy. The present study conducted a multicenter retrospective study to investigate the prognostic impacts of various factors, including factors included in the Revised International Prognostic Scoring System (IPSS-R) and severe cytopenia in the early phase of AZA monotherapy in 212 patients with MDS. Severe cytopenia was evaluated after the initiation of therapy by absolute neutrophil counts on the 29th day after AZA (ANC29) initiation, and red cell concentrates (RCC) and platelet concentrate (PC) transfusion units required within 28 days from the start of AZA, designated in the present study as RCC28 and PC28, respectively. The survival period was determined from the 29th day of AZA treatment to death from any cause as the conditional survival period after the first cycle of AZA (CS-AZA1). Multivariate analysis demonstrated that severe thrombocytopenia defined by >30 units of PC28 and very poor risk cytogenetics according to IPSS-R were independent prognostic factors for CS-AZA1. The Kyoto Conditional Survival Scoring System was subsequently developed by incorporating severe thrombocytopenia defined by PC28 and very poor risk cytogenetics, which successfully stratified the risks of the patients in CS-AZA1. In conclusion, extreme PC transfusion dependency during the first cycle of AZA and very poor risk cytogenetics are important prognostic factors in AZA monotherapy for MDS.
Keywords: myelodysplastic syndrome, azacitidine, cytopenia, transfusion, platelet concentrate, cytogenetics
Introduction
Myelodysplastic syndrome (MDS) is a bone marrow (BM) failure syndrome of hematopoietic stem cell disorder characterized by ineffective hematopoiesis, blood cell dysplasia, and a high risk of progression to acute myeloid leukemia (AML), especially in high risk (HR) patients defined by the International Prognostic Scoring System (IPSS) or the revised IPSS (IPSS-R) (1–4). Although allogeneic stem cell transplantation (ASCT) is the only curative treatment option for HR-MDS, most patients are ineligible for ASCT because of their high age and comorbidities (1–5). A hypomethylating agent azacitidine (AZA) has been one of the standards of care for patients with ASCT-ineligible HR-MDS (6) since the AZA-001 trial demonstrated its superior efficacy to conventional care regimens of physicians' choice in improving overall survival (OS) reaching 24.5 months as the median period (7). With this regard, AZA showed the potential to induce hematological improvement and delay leukemic evolution. However, the median OS in patients with MDS treated with AZA seemed shorted in real-world daily (RWD) practice around 10–17 months (8–10). Moreover, patients received a median of 9 cycles of AZA in the AZA-001 trial (7), while only 4–6 cycles in real-world practice (8–10). Therefore, it is critically important to predict the treatment outcome of patients with HR-MDS by AZA treatment in RWD practice for treatment decision-making, i.e., who will and will not be expected to benefit from AZA monotherapy.
The IPSS-R consisting of pre-treatment variables, including blood cell counts, BM blast ratio, and cytogenetics, has been widely utilized as the prognosis prediction model in various treatment situations in MDS (11). Many studies have also reported the adverse prognostic impact of cytopenia before treatment initiation in MDS (11–15). Moreover, AZA treatment potentially causes various types of adverse events that hamper treatment continuation (10,16–17), and, especially, hematologic toxicities frequently necessitate the increase of blood transfusion, trigger severe infection, and even result in the treatment cessation in the early phase of treatment (18). Although several previous studies have reported the influence of blood cell transition after the initiation of hypomethylating agents (HMA) on response and prognosis in patients with MDS (19–21), the prognostic impact of hematologic toxicities in the early clinical phase of AZA monotherapy has not been evaluated in conjunction with pre-treatment prognostic factors commonly utilized in the classical prognostic indices, such as IPSS-R.
To answer this question, we conducted a multi-institutional retrospective analysis to investigate the prognostic impact of cytopenia during the first cycle of AZA monotherapy in conjunction with other classical prognostic factors. In addition to the evaluation of neutropenia after the first cycle of AZA monotherapy for 28 days, we evaluated the degree of anemia and thrombocytopenia by the dose of blood transfusion required during the first cycle as the surrogates of anemia and thrombocytopenia, because the minimum levels of hemoglobin (Hb) level and platelet counts are masked by blood transfusion, and, therefore, are not considered to be suitable as biomarkers.
Materials and methods
Study design and patients
We retrospectively collected clinical and survival data of 212 patients with MDS who started AZA monotherapy between January 2012 and December 2021 and survived more than 29 days after the start of AZA at seven institutes belonging to the Kyoto Clinical Hematology Study Group (KOTOSG), i.e., Kyoto Prefectural University of Medicine (Kyoto, Japan), Aiseikai Yamashina Hospital (Kyoto, Japan), Japan Community Health Care Organization Kyoto Kuramaguchi Medical Center (Kyoto, Japan), Fukuchiyama City Hospital (Fukuchiyama, Japan), Japanese Red Cross Kyoto Daiichi Hospital (Kyoto, Japan), Japanese Red Cross Kyoto Daini Hospital (Kyoto, Japan) and Matsushita Memorial Hospital (Moriguchi, Japan). Diagnosis of MDS and MDS subtypes were re-evaluated based on the 2016 World Health Organization (WHO) classification (22). This study included patients with intermediate, high, or very high risk MDS according to the IPSS-R (11), while excluded patients who underwent ASCT. We evaluated the factors included in the IPSS-R, i.e., karyotype, rate of BM blasts, Hb level, platelet counts, absolute neutrophil counts (ANC), age at diagnosis, gender, ANC on the 29th day after the initiation of AZA (ANC29), and the transfusion units required within 28 days from the start of AZA with red cell concentrates, designated here as RCC28, and platelet concentrate (PC28). This study was conducted following the Declaration of Helsinki and the ethical guidelines and approved by The Ethics Committee of each institute that participated in the study.
Treatment with AZA and blood transfusion
All patients were treated with AZA monotherapy as standard clinical treatment, administered subcutaneously or intravenously at 75 mg/m2/day for seven days every 28 days. Dose reduction of AZA based on the patient's condition was allowed at the discretion of each treating physician. RCC and PC transfusions were performed at each treating physician's discretion, along with the transfusion guidelines defined by the Japanese Society of Transfusion Medicine and Cell Therapy, which recommend that the trigger Hb level for RCC transfusion is 6–7 g/dl and the trigger platelet counts for PC transfusion is 10×109/l (23,24). G-CSF was allowed at the discretion of the attending physician.
Survival and statistical analysis
The conditional survival period after the first cycle of AZA treatment (CS-AZA1) was defined as the time from the 29th day after the start of AZA to the date of death from any cause. The conditional leukemia-free survival period after the first cycle of AZA treatment (CLFS-AZA1) was defined as the time from the 29th day after the start of AZA to the date of progression to AML or death from any cause, whichever came first. CS-AZA1 and CLFS-AZA1 were analyzed using the Kaplan-Meier method and compared by log-rank test.
We randomly selected 70% of all patients as a training set and the remaining 30% as a validation set. Severe neutropenia was defined as ANC29 less than the first quartile. As described, severe anemia and thrombocytopenia were surrogated by the degree of transfusion dependency in this study, and severe anemia and severe thrombocytopenia were defined by RCC28 and PC28 more than the third quartile. The relative dose intensity in the first cycle of AZA treatment (RDI-AZA1) was defined by the ratio of the dose administered in the first cycle of AZA divided by the amount determined in the AZA-001 trial, i.e., 75 mg/m2 for seven days (7). Fisher's exact test was used to compare categorical variables, and Mann-Whitney U test or Kruskal-Wallis test was used to compare continuous variables between two and more than two groups, respectively. Steel-Dwass test was used as post hoc analysis after the Kruskal-Wallis test. Because all continuous variables analyzed in this study, i.e., BM blast ratio, Hb level, platelet counts, white blood cell counts (WBC), ANC, age at diagnosis, RCC28, PC28, ANC29, and RDI-AZA1 were not found to follow a normal distribution by the Shapiro-Wilk test in the training set patients, the correlation between the two variables was analyzed using Spearman's rank correlation coefficient (25). The univariate and multivariate analyses were performed by Cox proportional hazards regression to identify significant independent prognostic factors for CS-AZA1 and CLFS-AZA1. Elements with P<0.1 in the univariate analysis were selected for evaluation in the multivariate analysis. In addition, we tried to create a new predictive model by combining independent prognostic factors extracted in the training cohort and verified it in the validation cohort. To evaluate the prognostic discriminatory ability of the new prognostic prediction score, we evaluated Harrell's c-index, which estimates the probability that out of two randomly selected patients, the patient with a lower (better) prognostic score will live longer than the patient with a higher (worse) prognostic score (26,27). The P-values of <0.05 were considered statistically significant. All statistical analyses were performed with EZR version 1.61 (28).
Results
Patient characteristics
The training and the validation sets comprised 143 and 69 patients, respectively. There were no significant differences in patients' characteristics between the training and the validation sets (Table I). In the training set, the median age of patients was 76 years old (range 52–94), and 98 (68.5%) patients were male. According to the IPSS-R, 75 patients (52.4%) were classified as very high-risk, 39 (27.3%) as high-risk, and 29 (20.3%) as intermediate-risk. Precise data about RCC28, PC28, and ANC29 in the training set are shown in Table II. As a result, the median numbers of RCC28 and PC28 were four units and 0 units, respectively, and the median number of ANC29 was 0.43×109/l. Severe anemia and severe thrombocytopenia were determined to be more than six and 30 units, respectively. Severe neutropenia was determined to be less than 0.18×109/l. G-CSF was administered in 10 patients, including those with severe neutropenic patients with infection.
Table I.
Baseline characteristics, RCC and PC units within 28 days from the start of AZA, and ANC on the 29th day after the start of AZA of patients in the training and the validation set.
| Characteristics | Training set (n=143) | Validation set (n=69) | P-value |
|---|---|---|---|
| Age, years | 76 (52–94) | 73 (31–93) | 0.192 |
| Male/female, n (%) | 98 (68.5)/45 (31.5) | 54 (78.3)/15 (21.7) | 0.148 |
| WHO 2016 classification, n (%) | 0.388 | ||
| MDS-SLD | 2 (1.4) | 1 (1.4) | |
| MDS-MLD | 22 (15.4) | 17 (24.6) | |
| MDS-RS-SLD | 1 (0.7) | 1 (1.4) | |
| MDS-RS-MLD | 6 (4.2) | 2 (2.9) | |
| MDS-EB1 | 54 (37.8) | 17 (24.6) | |
| MDS-EB2 | 55 (38.5) | 28 (40.6) | |
| MDS-U | 3 (2.0) | 3 (4.3) | |
| Hemoglobin level, g/dl | 7.8 (3.5–13.0) | 7.5 (3.2–11.4) | 0.242 |
| Platelet counts, ×109/l | 68.0 (2.0–1139.0) | 50.0 (10.0–685.0) | 0.216 |
| WBC, ×109/l | 2.5 (0.8–24.4) | 2.4 (0.2–42.4) | 0.442 |
| ANC, ×109/l | 1.1 (0.0–19.6) | 1.2 (0.0–23.3) | 0.887 |
| Blast in bone marrow, % | 7.4 (0.0–19.8) | 6.0 (0.0–18.8) | 0.700 |
| Cytogenetic risk defined by IPSS-R, n (%) | 0.515 | ||
| Very good | 2 (1.4) | 0 (0.0) | |
| Good | 34 (23.8) | 16 (23.2) | |
| Intermediate | 33 (23.1) | 16 (23.2) | |
| Poor | 15 (10.5) | 3 (4.3) | |
| Very poor | 59 (41.3) | 34 (49.3) | |
| IPSS-R risk group, n (%) | 0.107 | ||
| Intermediate | 29 (20.3) | 7 (10.1) | |
| High | 39 (27.3) | 26 (37.7) | |
| Very high | 75 (52.4) | 36 (52.2) | |
| RCC transfusion units within 28 days from the start of AZA, days | 4 (0–30) | 4 (0–12) | 0.153 |
| PC transfusion units within 28 days from the start of AZA, days | 0 (0–170) | 0 (0–120) | 0.682 |
| ANC on the 29th day after the start of AZA, ×109/l | 0.4 (0.0–9.3) | 0.5 (0.0–9.8) | 0.793 |
Data presented as median (range) unless otherwise shown. ANC, absolute neutrophil counts; AZA, azacitidine; EB, excess blasts; IPSS-R, Revised International Prognostic Scoring System; MDS, myelodysplastic syndrome; MLD, multilineage dysplasia; PC, platelet concentrate; RCC, red cell concentrates; RS, ring sideroblasts; SLD, single lineage dysplasia; WBC, white blood cells.
Table II.
RCC28, PC28, and ANC29 in the training set.
| Variable | Minimum | 1st quartile | Median | 3rd quartile | Maximum |
|---|---|---|---|---|---|
| RCC28, unit | 0 | 0 | 4 | 6a | 30 |
| PC28, unit | 0 | 0 | 0 | 30a | 170 |
| ANC29, ×109/l | 0.02 | 0.18a | 0.43 | 1.32 | 9.31 |
Threshold of severe anemia, severe thrombocytopenia, and severe neutropenia. RCC, red cell concentrates; PC, platelet concentrate; ANC, absolute neutrophil counts.
Survival data and prognostic factors for CS-AZA1 and CLFS-AZA1 in the training set
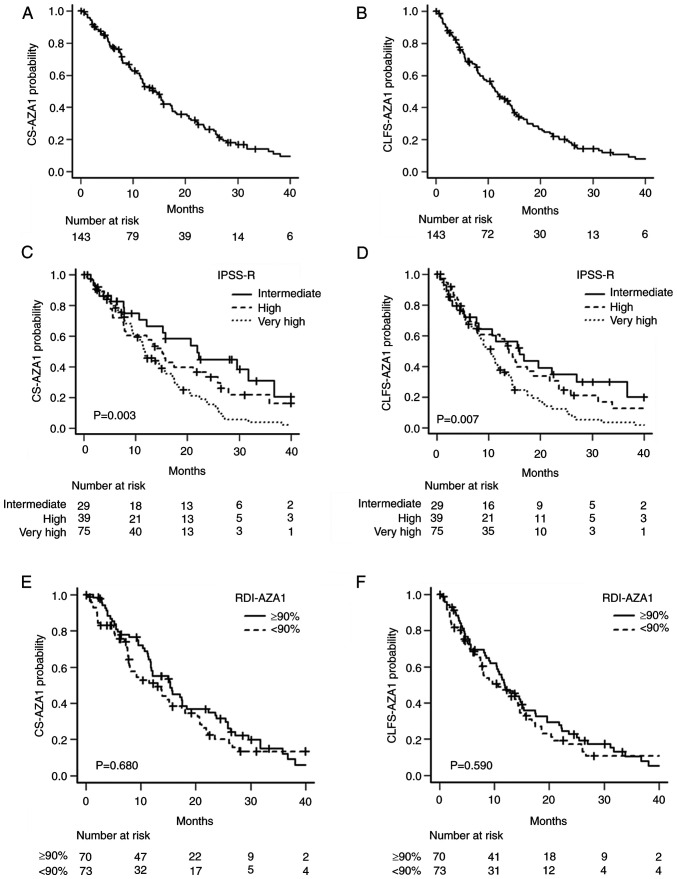
In the training set, with the median follow-up period calculated from the 29th day after the start of the first-cycle AZA was 11.7 months (range, 0.1–60.8), the median CS-AZA1 was 13.9 months (95% confidence interval (CI), 11.5–17.1) (Fig. 1A), and the median CLFS-AZA1 was 11.4 months (95% CI, 9.0–14.3) (Fig. 1B). Both CS-AZA1 and CLFS-AZA1 showed significant differences in the stratification with IPSS-R-defined groups. Namely, the median CS-AZA1 was 11.8 (95% CI, 9.5–15.4), 15.1 (95% CI, 7.7–23.6), and 21.9 (95% CI, 10.7–36.8) months (P=0.003), in the very high-, high-, and intermediate-risk groups, respectively (Fig. 1C), whereas the median CLFS-AZA1 was 10.4 (95% CI, 7.9–11.9), 14.0 (95% CI, 7.5–18.7), and 16.0 (95% CI, 7.7–27.1) (P=0.007) (Fig. 1D), respectively. The median RDI-AZA1 in the training set was 90% (interquartile range (IQR), 66–97), and we determined the cutoff of RDI-AZA1 was set to 90%. RDI-AZA1 had no prognostic impact on either CS-AZA1 or CLFS-AZA1 (Fig. 1E, F).
Figure 1.
Survival curves of CS-AZA1 and CLFS-AZA1 stratified according to disease risk and treatment intensity. (A) CS-AZA1 and (B) CLFS-AZA1 in the entire training set. (C) CS-AZA1 and (D) CLFS-AZA1 were stratified by the Revised International Prognostic Scoring System in the training set. RDI-AZA1 had no prognostic impact on (E) CS-AZA1 and (F) CLFS-AZA1. CS-AZA1, conditional survival period after the first cycle of azacitidine treatment; CLFS-AZA1, conditional leukemia-free survival period after the first cycle of azacitidine treatment; RDI-AZA1, relative dose intensity in the first cycle of azacitidine treatment.
The univariate analysis for CS-AZA1 found that the male gender, the very poor cytogenetic risk, the low Hb level of <8.0 g/dl at diagnosis, the low platelet counts of <50×109/l at diagnosis, the low ANC of <0.8×109/l at diagnosis, severe anemia defined by RCC28, and severe thrombocytopenia defined by PC28, and severe neutropenia defined by ANC29 were associated with short CS-AZA1 (Table III). Then, the multivariate analysis for CS-AZA1 identified that the very poor risk cytogenetics (HR, 2.18; 95% CI, 1.42–3.33; P<0.001) and severe thrombocytopenia defined by the more than 30 units of PC28 (HR, 2.90; 95% CI, 1.56–5.37; P<0.001) were independent poor prognostic factors (Table III). The multivariate analysis for CLFS-AZA1 also showed that the very poor risk cytogenetics (HR, 1.80; 95% CI, 1.20–2.71; P=0.004) and severe thrombocytopenia defined by the more than 30 units of PC28 (HR, 2.05; 95% CI, 1.13–3.73; P=0.018) were independent poor prognostic factors (Table III).
Table III.
Uni- and multivariate analyses for CS-AZA1 and CLFS-AZA1.
| CS-AZA1 | CLFS-AZA1 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||||
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | ||||||||||
|
|
|
|
|
||||||||||
| Factors | Variables | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Age | <75 | Reference | Reference | ||||||||||
| ≥75 | 1.10 | 0.76–1.62 | 0.609 | 1.26 | 0.87–1.83 | 0.214 | |||||||
| Sex | Female | Reference | Reference | Reference | |||||||||
| Male | 1.42 | 0.94–2.13 | 0.095 | 1.45 | 0.98–2.29 | 0.062 | 1.26 | 0.85–1.86 | 0.255 | ||||
| Cytogenetic | Very good, good, | Reference | Reference | Reference | Reference | ||||||||
| risk according | intermediate, | ||||||||||||
| to IPSS-R | and poor | ||||||||||||
| classification | Very poor | 2.12 | 1.44–3.13 | <0.001 | 2.18 | 1.42–3.33 | <0.001 | 1.80 | 1.24–2.61 | 0.002 | 1.80 | 1.20–2.71 | 0.004 |
| Blast in bone | <10% | Reference | Reference | ||||||||||
| marrow | ≥10% | 0.94 | 0.63–1.41 | 0.782 | 1.06 | 0.72–1.56 | 0.764 | ||||||
| Hemoglobin | ≥8 g/dl | Reference | Reference | Reference | Reference | ||||||||
| level | <8 g/dl | 1.42 | 0.97–2.08 | 0.070 | 1.33 | 0.88–1.99 | 0.175 | 1.49 | 1.03–2.15 | 0.034 | 1.39 | 0.94–2.06 | 0.098 |
| Platelet counts | ≥50×109/l | Reference | Reference | Reference | Reference | ||||||||
| <50×109/l | 1.60 | 1.08–2.36 | 0.018 | 0.73 | 0.44–1.22 | 0.227 | 1.47 | 1.01–2.15 | 0.047 | 0.89 | 0.56–1.43 | 0.639 | |
| ANC | ≥0.8×109/l | Reference | Reference | Reference | Reference | ||||||||
| <0.8×109/l | 1.55 | 1.05–2.29 | 0.028 | 1.40 | 0.89–2.22 | 0.146 | 1,40 | 0.95–2.05 | 0.088 | 1.23 | 0.78–1.91 | 0.372 | |
| RCC28 | ≤6 units | Reference | Reference | Reference | Reference | ||||||||
| >6 units | 2.98 | 1.89–4.70 | <0.001 | 1.57 | 0.90–2.73 | 0.114 | 2.60 | 1.66–4.09 | <0.001 | 1.50 | 0.87–2.61 | 0.148 | |
| PC28 | ≤30 units/month | Reference | Reference | Reference | Reference | ||||||||
| >30 units/month | 2.74 | 1.68–4.47 | <0.001 | 2.90 | 1.56–5.37 | <0.001 | 2.36 | 1.45–3.83 | 0.001 | 2.05 | 1.13–3.73 | 0.018 | |
| ANC29 | ≥0.18×109/l | Reference | Reference | Reference | Reference | ||||||||
| <0.18×109/l | 1.71 | 1.11–2.64 | 0.016 | 1.09 | 0.65–1.83 | 0.739 | 1.58 | 1.04–2.46 | 0.032 | 1.13 | 0.68–1.87 | 0.643 | |
ANC, absolute neutrophil counts; AZA, azacitidine; CS-AZA1, conditional survival period after the first cycle of AZA treatment; CLFS-AZA1, conditional leukemia-free survival period after the first cycle of AZA treatment; IPSS-R, revised international prognostic scoring system; RCC28, Unit of red cell concentrates transfused within the first 28 days of AZA treatment; PC28, Unit of platelet concentrate transfused within the first 28 days of AZA treatment; ANC29, absolute neutrophil counts on day 29 after the start of AZA treatment.
Characteristics and outcomes of patients with severe thrombocytopenia defined by PC28
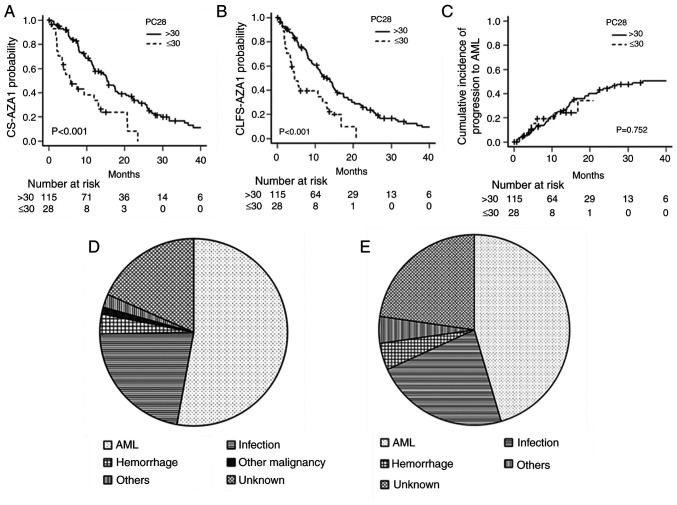
We next analyzed the correlation between PC28 and other variables, characteristics, and treatment outcomes of patients with severe thrombocytopenia defined by more than 30 units of PC28 in the training set. We investigated whether PC28 correlated with age, gender, Hb level, platelet counts, WBC, ANC, blast ratio in bone marrow, cytogenetic risk, RCC28, ANC29, and RDI-AZA1 by Spearman's rank correlation coefficient. Hb level (r=−0.258, P=0.002), platelet counts (r=−0.623, P<0.001), RCC28 (r=0.583, P<0.001), and ANC29 (r=−0.281, P=0.001) had a correlation with PC28, and other variables did not have a correlation with PC28 (Table SI). The baseline characteristics, RCC28, PC28, ANC29, and RDI-AZA1 of patients with severe thrombocytopenia and those without severe thrombocytopenia were summarized in Table IV. Patients with severe thrombocytopenia had lower baseline Hb levels, lower baseline platelet counts, higher RCC28, and lower ANC29. RDI-AZA1 in patients with severe thrombocytopenia tended to be lower, although not statistically significant. The median CS-AZA1 was 5.8 months (95% CI, 2.5–13.1) in patients with severe thrombocytopenia and 15.4 months (95% CI, 11.8–18.1) in patients without severe thrombocytopenia (P<0.001) (Fig. 2A). The median CLFS-AZA1 was 4.5 months (95% CI, 2.5–11.9) in patients with severe thrombocytopenia and 12.3 months (95% CI, 10.4–15.1) in patients without severe thrombocytopenia (P<0.001) (Fig. 2B). The cumulative incidence of progression to AML was similar between patients with and without severe thrombocytopenia (P=0.752) (Fig. 2C).
Table IV.
Baseline clinical characteristics, RCC28, PC28 and ANC29 of patients with/without severe thrombocytopenia defined by >30 units of PC28.
| Variable | No severe thrombocytopenia (n=115) | Severe thrombocytopenia (n=28) | P-value |
|---|---|---|---|
| Age, years | 76 (52–94) | 74 (52–90) | 0.178 |
| Male/Female, n (%) | 79 (68.7)/36 (31.3) | 19 (67.9)/9 (32.1) | 1.000 |
| Hemoglobin level, g/dl | 8.1 (3.5–13.0) | 7.0 (5.5–10.2) | 0.004 |
| Platelet counts, ×109/l | 76.0 (14.0–1139.0) | 24.5 (2.0–270.0) | <0.001 |
| WBC, ×109/l | 2.5 (0.8–19.0) | 2.2 (0.8–24.4) | 0.344 |
| ANC, ×109/l | 1.1 (0.0–13.1) | 0.9 (0.1–19.6) | 0.692 |
| Blast in bone marrow, % | 6.8 (0.4–19.8) | 8.3 (0.3–18.4) | 0.557 |
| Cytogenetic risk according to IPSS-R, n (%) | 0.598 | ||
| Very good | 2 (1.7) | 0 (0.0) | |
| Good | 25 (21.7) | 9 (32.1) | |
| Intermediate | 27 (23.5) | 6 (21.4) | |
| Poor | 14 (12.2) | 1 (3.6) | |
| Very poor | 47 (40.9) | 12 (42.9) | |
| RCC28, units/month | 2 (0–14) | 8 (0–30) | <0.001 |
| PC28, units/month | 0 (0–30) | 60 (35–170) | <0.001 |
| ANC29, ×109/l | 0.5 (0.0–9.0) | 0.3 (0.0–9.3) | 0.039 |
| RDI-AZA1, % | 91 (68–97) | 81 (62–95) | 0.059 |
Data presented as median (range) unless otherwise shown. RDI-AZA1, relative dose intensity in the first cycle of azacitidine treatment; RCC, red cell concentrates; PC, platelet concentrate; ANC, absolute neutrophil counts; WBC, white blood cells.
Figure 2.
Prognostic impact of the transfusion units required within 28 days from the start of AZA with PC28. (A) CS-AZA1, (B) CLFS-AZA-1 and (C) cumulative incidence of progression to AML based on severe thrombocytopenia defined by PC28 or not. The causes of death in patients with (D) severe thrombocytopenia and (E) those without who died within the follow-up period. CS-AZA1, conditional survival period after the first cycle of azacitidine treatment; CLFS-AZA1, conditional leukemia-free survival period after the first cycle of azacitidine treatment; AML, acute myeloid leukemia; PC28, platelet concentrate transfusion units required within 28 days from the start of AZA.
Since severe thrombocytopenia defined by PC28 was significantly worse in the CS-AZA1 but was not linked to the cumulative incidence of progression to AML, we compared the causes of death. In twenty-two patients with severe thrombocytopenia died within the follow-up period, the causatives of death were AML in 10 (45.5%), infection in 5 (22.7%), hemorrhagic event in 1 (4.5%), and other disease in 1 (4.5%), and unknown in 5 patients (22.7%) (Fig. 2D). In eighty-seven patients without severe thrombocytopenia died within the follow-up period, the causatives of death were AML in 46 (52.9%), infection in 19 (21.8%), hemorrhagic event in 3 (3.4%), other malignancy in 1 (1.1%), other diseases in 2 (2.3%), and unknown in 16 patients (18.4%) (Fig. 2E). Thus, the causatives of death were not significantly different between patients with severe thrombocytopenia and those without (P=0.862).
Establishment of a new prognostic model for CS-AZA1 and CLFS-AZA1
Because most of the variables utilized in IPSS-R, except cytogenetics, were not shown to have prognostic impacts on CS-AZA1 and CLFS-AZA1 in our cohort, we next tried to develop a new prognostic model, here designated as the Kyoto Conditional Survival Scoring System (KCSS), for patients with MDS treated by AZA monotherapy, by incorporating the very poor risk cytogenetics according to the IPSS-R and severe thrombocytopenia defined by more than 30 units of PC28 as prognostic variables. We classified patients into three risk groups by the number of risk factors, i.e., the good-risk group without any risk factor, the intermediate-risk group with one risk factor, and the poor-risk group with two risk factors.
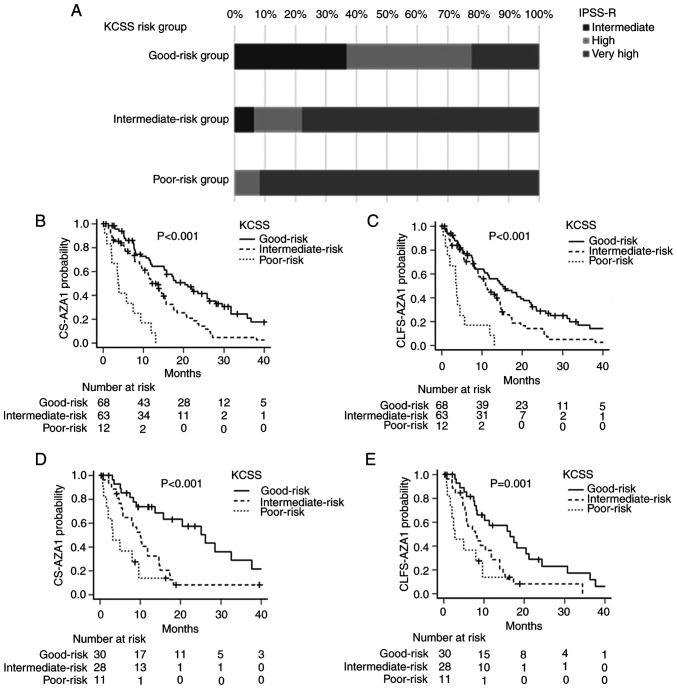
The baseline characteristics, RCC28, PC28, ANC29, and RDI-AZA1 of patients in the training set according to the risk groups by the KCSS were summarized in Table V. There were significant differences in platelet counts at diagnosis, RCC28, and ANC29, in addition to IPSS-R-defined cytogenetic features and PC28, among different risk groups. When analyzing the relationship between the disease risks evaluated by IPSS-R and KCSS, the good-risk group by KCSS consisted of 36.8, 41.2, and 22.1% of the intermediate-, high-, and very high-risk patients according to the IPSS-R, respectively. The KCSS-defined intermediate-risk group consisted of 6.3, 15.9, and 77.8% of intermediate-, high-, and very high-risk patients, according to the IPSS-R. The poor-risk group defined by KCSS consisted of 8.3 and 91.7% of high- and very high-risk patients, according to the IPSS-R (Fig. 3A).
Table V.
Baseline characteristics, RCC28, PC28, ANC29 and RDI-AZA1 according to the risk group defined by KCSS in the training set.
| Parameter | Good-risk group (n=68) | Intermediate-risk group (n=63) | Poor-risk group (n=12) | P-value |
|---|---|---|---|---|
| Age, years | 76 (56–94) | 76 (52–90) | 68 (52–84) | 0.079 |
| Male/Female, n (%) | 45 (66.2)/23 (33.8) | 47 (74.6)/16 (25.4) | 6 (50.0)/6 (50.0) | 0.206 |
| Hemoglobin level, g/dl | 8.1 (5.7–13.0) | 7.7 (3.5–11.2) | 7.3 (6.1–10.0) | 0.174 |
| Platelet counts, ×109/l | 88.5 (17.0–1139.0) | 46.0 (3.0–470.0) | 23.5 (2.0–270.0) | <0.001 |
| WBC, ×109/l | 2.6 (0.8–19.0) | 2.4 (1.0–24.4) | 2.4 (0.8–22.1) | 0.888 |
| ANC, ×109/l | 1.1 (0.0–13.1) | 1.0 (0.1–10.6) | 0.8 (0.1–19.6) | 0.703 |
| Blast in bone marrow, % | 7.8 (0.4–19.4) | 6.8 (0.0–19.8) | 8.0 (0.3–18.4) | 0.959 |
| Cytogenetic risk according to IPSS-R, n (%) | <0.001 | |||
| Very good | 2 (2.9) | 0 (0.0) | 0 (0.0) | |
| Good | 25 (36.8) | 9 (14.3) | 0 (0.0) | |
| Intermediate | 27 (39.7) | 6 (9.5) | 0 (0.0) | |
| Poor | 14 (20.6) | 1 (1.6) | 0 (0.0) | |
| Very poor | 0 (0.0) | 47 (74.6) | 12 (100.0) | |
| RCC28, unit | 2 (0–10) | 4 (0–24) | 8 (2–30) | <0.001 |
| PC28, unit | 0 (0–30) | 10 (0–170) | 60 (40–120) | <0.001 |
| ANC29, ×109/l | 0.7 (0.0–9.0) | 0.4 (0.0–9.3) | 0.2 (0.1–5.6) | 0.003 |
| RDI-AZA1, %, median (IQR) | 90 (69–98) | 89 (63–96) | 92 (66–96) | 0.553 |
Data presented as median (range) unless otherwise shown. KCSS, Kyoto Conditional Survival Scoring System; RCC, red cell concentrates; PC, platelet concentrate; ANC, absolute neutrophil counts; RDI-AZA1, relative dose intensity in the first cycle of azacitidine treatment.
Figure 3.
CS-AZA1 and CLFS-AZA1 stratified according to the KCSS. (A) Patient distribution of IPSS-R and KCSS. (B-E) Survival curves based on KCSS. KCSS stratified (B) CS-AZA1 and (C) CLFS-AZA1 in the training set, and KCSS stratified (D) CS-AZA-1 and (E) CLFS-AZA-1 in the validation set. CS-AZA1, conditional survival period after the first cycle of azacitidine treatment; CLFS-AZA1, conditional leukemia-free survival period after the first cycle of azacitidine treatment; IPSS-R, Revised International Prognostic Scoring System; KCSS, Kyoto Conditional Survival Scoring System.
The CS-AZA1 and CLFS-AZA1 of the three risk groups defined by KCCS were significantly different in the training set. The median CS-AZA1 was 3.8 (95% CI, 0.9–9.3), 12.2 (95% CI, 9.5–15.4), 20.4 (95% CI, 15.1–25.9) months in patients with the poor-risk group, intermediate-risk group, and good-risk group defined by KCSS, respectively (P<0.001) (Fig. 3B), while the median CLFS-AZA1 were 3.6 (95% CI, 0.9–5.7), 11.2 (95% CI, 8.5–14.0), and 15.1 (95% CI, 10.5–20.4) months in patients with poor-risk group, intermediate-risk group, and good-risk group defined by KCSS, respectively (P<0.001) (Fig. 3C).
KCSS also successfully stratified the patients' risks in CS-AZA1 and CLFS-AZA1 in the validation set. The median CS-AZA1 was 3.2 (95% CI, 0.9–9.7), 10.0 (95% CI, 5.6–14.7), and 26.2 (95% CI, 13.7–37.7) months in patients with poor-risk group, intermediate-risk group, and good-risk group defined by KCSS, respectively (P<0.001) (Fig. 3D), and the median CLFS-AZA-1 was 2.8 (95% CI, 0.9–9.7), 7.9 (95% CI, 5.3–11.9), 16.5 (95% CI, 8.0–21.2) months in patients with poor-risk group, intermediate-risk group, and good-risk group defined by KCSS, respectively (P<0.001) (Fig. 3E).
Assessing the predictive value and treatment cycles of KCSS
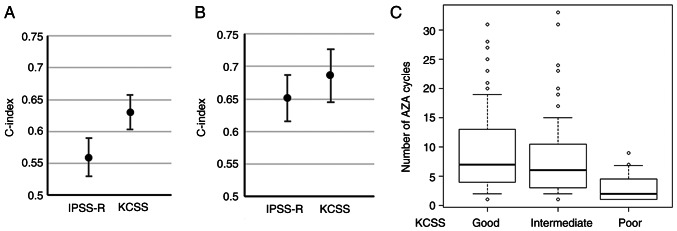
To evaluate the predictive value of KCSS, we evaluated the c-index of KCSS and the treatment cycles of each risk group concerning the comparison with IPSS-R. In the training set, c-indices for CS-AZA1 of IPSS-R and KCSS were 0.559 (standard error (SE), 0.030) and 0.630 (SE, 0.027), respectively (Fig. 4A). In the validation set, c-indices for CS-AZA1 of IPSS-R and KCSS were 0.651 (SE, 0.036) and 0.686 (SE, 0.041), respectively (Fig. 4B). Finally, we analyzed the cycle numbers of AZA treatment in the entire cohort. The median cycle number of AZA in the whole cohort was 6 (interquartile range (IQR), 3–11) cycles. According to IPSS-R, the median cycle numbers of AZA were 6 (IQR, 3–10), 6 (IQR, 4–11), and 6 (IQR, 3–11) in the very high-, high-, intermediate-risk groups, respectively (P=0.372). According to KCSS, the median cycle numbers of AZA were 2 (IQR, 1–5), 6 (IQR, 3–11), and 7 (IQR, 4–13) in patients with the poor-risk group, intermediate-risk group, and good-risk group, respectively (P<0.001), thus, were significantly different among risk groups (Fig. 4C). In post hoc analysis, the number of AZA cycles of poor-risk patients was lower than that of intermediate-(P<0.001) and good-risk (P<0.001) patients.
Figure 4.
Prognostic value of KCSS. C-indices of Revised International Prognostic Scoring System and KCSS in the (A) training set and the (B) validation set. (C) Box plot of the number of AZA cycles based on KCSS. KCSS, Kyoto Conditional Survival Scoring System; AZA, azacytidine.
Discussion
Many studies have supported the adverse prognostic impact of cytopenia before treatment initiation in MDS (11–15). Although AZA improved the prognosis of patients with HR-MDS, cytopenia after starting AZA often hampers treatment continuation, resulting in severe infection and intolerance (10,16–17). Moreover, it has been reported that cytopenia after starting AZA is particularly severe in the first cycle (18). Although several previous studies have reported the influence of blood cell transition after initiating HMA on response and prognosis in patients with MDS (19–21), the prognostic impact of severe cytopenia in the early phase of treatment has not been evaluated. Therefore, we in this study retrospectively investigated the prognostic significance of severe cytopenia occurring during the first cycle of AZA monotherapy in the setting of real-world practice.
This study showed that the very poor risk cytogenetics according to the IPSS-R and the higher requirements of PLT transfusion units during the first AZA cycle (PC28) had independent adverse prognostic impacts in patients with MDS treated by AZA monotherapy. In contrast, the IPSS-R factors other than cytogenetics, i.e., blast ratio in BM, Hb level, platelet counts, and ANC before AZA treatment, were not significantly associated with CS-AZA1 and CLFS-AZA1. This provoked us to develop a new prognostic model of KCSS consisting of two independent prognostic factors described above, and, importantly, KCSS successfully discriminated risk groups in CS-AZA1 and CLFS-AZA1 in our cohort. Considering that the pre-treatment findings define IPSS-R, while KCSS utilizes the post-treatment information, comparing the predictive values of IPSS-R and KCSS is unreasonable. However, our study highlighted the prognostic significance of hematologic status during the first cycle of AZA in MDS.
In previous studies, hematologic recovery after starting HMA in MDS patients has been reported to be associated with treatment outcomes. Lieke et al. and Ping et al showed that platelet count changes after HMA initiation correlate with prognosis (19,20). Nathan et al. created a machine learning model to predict favorable treatment responses in 424 MDS patients treated with AZA for over four months. They found that the post-treatment response of Hb level and platelet counts was crucial in predicting treatment outcomes (21). Our study demonstrated that the number of transfusions representing platelet changes was related to prognosis, which validates these previous findings. Unlike previous studies that predicted a favorable prognosis in patients whose platelet counts increased after starting HMA (19,20) or predicted the response rate based on blood cell changes after starting HMA (21), our study has a notable strength in identifying patients with a very inferior prognosis as evidenced by the median survival of approximately four months in the poor-risk group according to KCSS, which was not included in previous studies.
It has been reported that 91% of the first responses by AZA were achieved within six cycles in the AZA-001 trial (29) and that responders obtained a better prognosis (30,31). It has also been recommended to continue AZA monotherapy for at least 4–6 cycles to assess response, even in cases with progressive cytopenia after starting AZA (32,33). However, this has not been easily reproduced in real-world practice (8–10). With this regard, it was intriguing that the treatment cycle was more closely associated with KCSS than IPSS-R in our cohort. Indeed, the median cycle of AZA was only two cycles in patients judged as poor-risk by KCSS in our cohort, again reflecting the difficulty of treatment continuation in this particular population. Most of the poor-risk patients defined by KCSS had severe anemia and neutropenia in addition to severe thrombocytopenia after the initiation of AZA, and these might collectively make treatment continuation difficult by causing various kinds of adverse events.
KCSS showed superior abilities in discriminating risk groups in CS-AZA1 and CLFS-AZA1 to IPSS-R in our cohort and identified patients who did not benefit from AZA treatment and could not continue AZA for a sufficient period. Approximately 50% of patients had less than 90% of RDI-AZA1, and the RDI-AZA1 of the oldest patient, a 93-year-old, was 75%. Patients with severe thrombocytopenia defined by PC28 were found to have higher RCC28 and lower ANC29 despite a lower tendency in RDI-AZA1, which suggested the difficulty of administrating full doses of AZA to some patients, especially those with severe thrombocytopenia, in clinical practice. Although a previous study showed that reducing the dosage of AZA before achieving the objective response was associated with poor prognosis and that dose reduction of AZA should be decided with caution (34), based on the results of this study, the dose reduction of AZA in consideration of hematologic toxicity is unavoidable in actual clinical practice. Considering the severe hematologic toxicity of AZA in some patients, new therapeutic agents may be needed to improve the prognosis of this group of patients. For example, magrolimab, an anti-CD47 antibody (35), and sabatolimab, which inhibits T-cell immunoglobulin and mucin domain 3 (36), are expected to be new therapeutic agents for MDS.
This study had several limitations. First, there was the possibility that the threshold for transfusion varied among attending physicians. However, most researchers belonging to KOTOSG adhered to the established guidelines for RCC and PC transfusions (23,24), and, therefore, we consider that the bias was minimal. Second, genetic information about the patients was not obtained. It is well recognized that genetic and epigenetic mutations are closely linked to therapeutic response and prognosis (37–43). Future studies should, therefore, integrate the genetic information to establish a more accurate predictive model. Third, due to the retrospective observational design of this study, information regarding the cause of death was often unknown. Therefore, we could not thoroughly analyze the etiology underlying the poor prognosis of patients with severe thrombocytopenia defined by PC28. Additionally, the prognostic significance of performance status (PS) was not assessed in this study due to the limited number of patients with a PS of >2 because most patients analyzed had a PS of 0 or 1 based on the Eastern Cooperative Oncology Group criteria (44).
In conclusion, severe PC transfusion dependency during the first cycle of AZA and very poor risk cytogenetics are the critically important prognostic indicators in AZA monotherapy for MDS, and KCSS may be a useful predictive prediction model of CS-AZA1 and CLFS-AZA1.
Supplementary Material
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
YI, HO and JK designed the study. AM, YKK, SC, TF, TT, YS, SM, HK, SKO, SF, DN, KH, HU, EK, NU and JK acquired and provided data. YI and HO confirm the authenticity of all the raw data and performed statistical analyses. YI, HO and JK wrote the manuscript. JK supervised the study. All authors interpreted data and read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by The Ethics Committee of each institute that participated in the study. The Ethics Committee of each institute waived the need to obtain documented informed consent from the study subjects under the setting of an opt-out, and we disclosed information about the study to the patients. The name, location, and approval number of each institute participating in this study are: Division of Hematology and Oncology, Department of Medicine, Kyoto Prefectural University of Medicine (Kyoto, Japan), approval no ERB-C-1335-1; Department of Hematology, Aiseikai Yamashina Hospital (Kyoto, Japan), approval no. FY2016 No. 8.; Department of Hematology, Japan Community Health Care Organization Kyoto Kuramaguchi Medical Center (Kyoto, Japan), approval no. 2023022101; Department of Hematology, Fukuchiyama City Hospital (Kyoto, Japan), approval no. 4-18; Department of Hematology, Japanese Red Cross Kyoto Daiichi Hospital (Kyoto, Japan), approval no. 1490; Department of Hematology, Japanese Red Cross Kyoto Daini Hospital (Kyoto, Japan), approval no. S2022-19; Department of Hematology, Matsushita Memorial Hospital (Osaka, Japan), approval no. 22014.
Patient consent for publication
The Ethics Committee of each institute waived the need to obtain documented informed consent from the study subjects under the setting of an opt-out, and we disclosed information about the study to the patients.
Competing interests
TF has received honoraria from Takeda Pharmaceutical; TT has received honoraria from Bristol Myers Squibb (BMS), Janssen Pharmaceutical, Sanofi, Kyowa Kirin and Chugai Pharmaceutical; YS has received honoraria from Ono Pharmaceutical, BMS, Janssen Pharmaceutical, Sanofi, Kyowa Kirin, Takeda Pharmaceutical and Chugai Pharmaceutical; SM has received honoraria from Sanofi and Ono Pharmaceutical; and NU has received honoraria from BMS and Takeda. JK is a consultant for Janssen Pharmaceutical, Abbvie and BMS; has received research funding from Kyowa Kirin, Chugai Pharmaceutical, Ono Pharmaceutical, Shionogi and BMS; and has received honoraria from Janssen Pharmaceutical, Kyowa Kirin, Chugai Pharmaceutical, Ono Pharmaceutical, Sanofi and BMS. None of the pharmaceutical companies aforementioned benefited from the present research or played any role in our study. All other authors declare that they have no competing interests.
References
- 1.Fenaux P, Haase D, Santini V, Sanz GF, Platzbecker U, Mey U. Myelodysplastic syndromes: ESMO clinical practice guidelines for diagnosis, treatment, and follow-up. Ann Oncol. 2021;32:142–156. doi: 10.1016/j.annonc.2020.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Sekeres MA, Cutler C. How we treat higher-risk myelodysplastic syndromes. Blood. 2014;123:829–836. doi: 10.1182/blood-2013-08-496935. [DOI] [PubMed] [Google Scholar]
- 3.Aubrey BJ, Brunner AM. SOHO state of the art and next questions: Treatment of higher-risk myelodysplastic syndromes. Clin Lymphoma Myeloma Leuk. 2022;22:869–877. doi: 10.1016/j.clml.2022.07.012. [DOI] [PubMed] [Google Scholar]
- 4.Adès L, Itzykson R, Fenaux P. Myelodysplastic syndromes. Lancet. 2014;383:2239–2252. doi: 10.1016/S0140-6736(13)61901-7. [DOI] [PubMed] [Google Scholar]
- 5.Neukirchen J, Schoonen WM, Strupp C, Gattermann N, Aul C, Haas R, Germing U. Incidence and prevalence of myelodysplastic syndromes: Data from the Düsseldorf MDS-registry. Leuk Res. 2011;35:1591–1596. doi: 10.1016/j.leukres.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Bewersdorf JP, Carraway H, Prebet T. Emerging treatment options for patients with high-risk myelodysplastic syndrome. Ther Adv Hematol. 2020;11:2040620720955006. doi: 10.1177/2040620720955006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, Schoch R, Gattermann N, Sanz G, List A, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: A randomised, open-label, phase III study. Lancet Oncol. 2009;10:223–232. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeidan AM, Sekeres MA, Garcia-Manero G, Steensma DP, Zell K, Barnard J, Ali NA, Zimmerman C, Roboz G, DeZern A, et al. Comparison of risk stratification tools in predicting outcomes of patients with higher-risk myelodysplastic syndromes treated with azanucleosides. Leukemia. 2016;30:649–657. doi: 10.1038/leu.2015.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernal T, Martínez-Camblor P, Sánchez-García J, de Paz R, Luño E, Nomdedeu B, Ardanaz MT, Pedro C, Amigo ML, Xicoy B, et al. Effectiveness of azacitidine in unselected high-risk myelodysplastic syndromes: Results from the Spanish registry. Leukemia. 2015;29:1875–1881. doi: 10.1038/leu.2015.115. [DOI] [PubMed] [Google Scholar]
- 10.Zeidan AM, Hu X, Zhu W, Stahl M, Wang R, Huntington SF, Giri S, Bewersdorf JP, Podoltsev NA, Gore SD, et al. Association of provider experience and clinical outcomes in patients with myelodysplastic syndromes receiving hypomethylating agents. Leuk Lymphoma. 2020;61:397–408. doi: 10.1080/10428194.2019.1663423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Solé F, Bennett JM, Bowen D, Fenaux P, Dreyfus F, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120:2454–2465. doi: 10.1182/blood-2012-03-420489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, Sanz M, Vallespi T, Hamblin T, Oscier D, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–2088. doi: 10.1182/blood.V89.6.2079. [DOI] [PubMed] [Google Scholar]
- 13.Kantarjian H, O'Brien S, Ravandi F, Cortes J, Shan J, Bennett JM, List A, Fenaux P, Sanz G, Issa JP, et al. Proposal for a new risk model in myelodysplastic syndrome that accounts for events not considered in the original international prognostic scoring system. Cancer. 2008;113:1351–1361. doi: 10.1002/cncr.23697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mozessohn L, Cheung MC, Fallahpour S, Gill T, Maloul A, Zhang L, Lau O, Buckstein R. Azacitidine in the ‘real-world’: An evaluation of 1101 higher-risk myelodysplastic syndrome/low blast count acute myeloid leukaemia patients in Ontario, Canada. Br J Haematol. 2018;181:803–815. doi: 10.1111/bjh.15273. [DOI] [PubMed] [Google Scholar]
- 15.Diamantopoulos PT, Viniou NA. Factors affecting response to 5-azacytidine and prognosis of myelodysplastic syndrome. Is long-term survival a realistic goal? Leuk Res. 2021;103:106543. doi: 10.1016/j.leukres.2021.106543. [DOI] [PubMed] [Google Scholar]
- 16.Diamantopoulos PT, Symeonidis A, Pappa V, Kotsianidis I, Galanopoulos A, Pontikoglou C, Anagnostopoulos A, Vassilopoulos G, Zikos P, Hatzimichael E, et al. The effect of 5-azacytidine treatment delays and dose reductions on the prognosis of patients with myelodysplastic syndrome: How to optimize treatment results and outcomes. Br J Haematol. 2011;192:978–987. doi: 10.1111/bjh.17062. [DOI] [PubMed] [Google Scholar]
- 17.Derissen EJB, Beijnen JH, Schellens JHM. Concise drug review: Azacitidine and decitabine. Oncologist. 2013;18:619–624. doi: 10.1634/theoncologist.2012-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uchida T, Ogawa Y, Kobayashi Y, Ishikawa T, Ohashi H, Hata T, Usui N, Taniwaki M, Ohnishi K, Akiyama H, et al. Phase I and II study of azacitidine in Japanese patients with myelodysplastic syndromes. Cancer Sci. 2011;102:1680–1686. doi: 10.1111/j.1349-7006.2011.01993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Helm LH, Alhan C, Wijermans PW, van Marwijk Kooy M, Schaafsma R, Biemond BJ, Beeker A, Hoogendoorn M, van Rees BP, de Weerdt O, et al. Platelet doubling after the first azacitidine cycle is a promising predictor for response in myelodysplastic syndromes (MDS), chronic myelomonocytic leukaemia (CMML) and acute myeloid leukaemia (AML) patients in the Dutch azacitidine compassionate named patient programme. Br J Haematol. 2011;155:599–606. doi: 10.1111/j.1365-2141.2011.08893.x. [DOI] [PubMed] [Google Scholar]
- 20.Lu PF, Deng LN, Meng FK, Wang Y, Xiao M, Li DJ. platelet doubling after first decitabine cycle predicts response and survival of myelodysplastic syndrome patients. Curr Med Sci. 2022;42:77–84. doi: 10.1007/s11596-022-2533-4. [DOI] [PubMed] [Google Scholar]
- 21.Radakovich N, Sallman DA, Buckstein R, Brunner A, Dezern A, Mukerjee S, Komrokji R, Al-Ali N, Shreve J, Rouphail Y, et al. A machine learning model of response to hypomethylating agents in myelodysplastic syndromes. IScience. 2022;25:104931. doi: 10.1016/j.isci.2022.104931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Beau MM, Bloomfield CD, Cazzola M, Vardiman JW. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–2405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 23.Yonemura Y, Matsumoto M, Inada E, Ueda Y, Ohishi K, Kubo T, Kumakawa M, Sueoka E, Sonoki T, Nagai K, et al. Guideline for the use of red blood cell products based on scientific evidence (Revision 2nd edition) Jpn J Transf Cell Ther. 2018;64:688–699. (In Japanese) [Google Scholar]
- 24.Takami A, Matsushita T, Ogata M, Fujii N, Kubuki Y, Fujiwara S, Matsumoto M, Tomiyama Y. Guideline for the use of platelet transfusion concentrates based on scientific evidence: Update 2019. Jpn J Transf Cell Ther. 2019;65:544–561. (In Japanese) [Google Scholar]
- 25.Schober P, Boer C, Schwarte LA. Correlation coefficients: Appropriate use and interpretation. Anesth Analg. 2018;126:1763–1768. doi: 10.1213/ANE.0000000000002864. [DOI] [PubMed] [Google Scholar]
- 26.Harrell FE, Jr, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. JAMA. 1982;247:2543–2546. doi: 10.1001/jama.1982.03320430047030. [DOI] [PubMed] [Google Scholar]
- 27.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 28.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silverman LR, Fenaux P, Mufti GJ, Santini V, Hellström-Lindberg E, Gattermann N, Sanz G, List AF, Gore SD, Seymour JF. Continued azacitidine therapy beyond time of first response improves quality of response in patients with higher-risk myelodysplastic syndromes. Cancer. 2011;117:2697–2702. doi: 10.1002/cncr.25774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gore SD, Fenaux P, Santini V, Bennett JM, Silverman LR, Seymour JF, Hellström-Lindberg E, Swern AS, Beach CL, List AF. A multivariate analysis of the relationship between response and survival among patients with higher-risk myelodysplastic syndromes treated within azacitidine or conventional care regimens in the randomized AZA-001 trial. Haematologica. 2013;98:1067–1072. doi: 10.3324/haematol.2012.074831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diamantopoulos PT, Pappa V, Symeonidis A, Kotsianidis I, Galanopoulos A, Papadaki H, Anagnostopoulos A, Vassilopoulos G, Zikos P, Hatzimichael E, et al. Characteristics of Long-term survival in patients with myelodysplastic syndrome treated with 5-Azacyditine: Results From the Hellenic 5-Azacytidine registry. Clin Lymphoma Myeloma Leuk. 2020;20:114–121. doi: 10.1016/j.clml.2019.09.614. [DOI] [PubMed] [Google Scholar]
- 32.Greenberg PL, Stone RM, Al-Kali A, Bennett JM, Borate U, Brunner AM, Chai-Ho W, Curtin P, de Castro CM, Deeg HJ, DeZem AE. NCCN Guidelines® Insights: Myelodysplastic Syndromes, Version 3.2022. Featured Updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2022;20:106–117. doi: 10.6004/jnccn.2022.0009. [DOI] [PubMed] [Google Scholar]
- 33.Palacios-Berraquero ML, Alfonso-Piérola A. Current therapy of the patients with MDS: Walking towards personalized therapy. J Clin Med. 2021;10:2107. doi: 10.3390/jcm10102107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laribi K, Bolle D, Alani M, Ghnaya H, Besançon A, Farhi J, Mheidly K, Denizon N, de Materre AB. Impact of the relative dose intensity on survival of patients with high-risk myelodysplastic syndromes treated with Azacitidine. Cancer Med. 2019;8:2188–2195. doi: 10.1002/cam4.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eladl E, Tremblay-LeMay R, Rastgoo N, Musani R, Chen W, Liu A, Chang H. Role of CD47 in hematological malignancies. J Hematol Oncol. 2020;13:96. doi: 10.1186/s13045-020-00930-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeidan AM, Komrokji RS, Brunner AM. TIM-3 pathway dysregulation and targeting in cancer. Expert Rev Anticancer Ther. 2021;21:523–534. doi: 10.1080/14737140.2021.1865814. [DOI] [PubMed] [Google Scholar]
- 37.Bersanelli M, Travaglino E, Meggendorfer M, Matteuzzi T, Sala C, Mosca E, Chiereghin C, Nanni ND, Gnocchi M, Zampini M, et al. Classification and Personalized prognostic assessment on the basis of clinical and genomic features in Myelodysplastic Syndromes. J Clin Oncol. 2021;39:1223–1233. doi: 10.1200/JCO.20.01659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nazha A, Komrokji R, Meggendorfer M, Jia X, Radakovich N, Shreve J, Hilton CB, Nagata Y, Hamilton BK, Mukherjee S, et al. Personalized prediction model to risk stratify patients with Myelodysplastic Syndromes. J Clin Oncol. 2021;39:3737–3746. doi: 10.1200/JCO.20.02810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu YC, Kwon J, Fabiani E, Xiao Z, Liu YV, Follo MY, Liu J, Huang H, Gao C, Liu J, et al. Demethylation and up-regulation of an oncogene after Hypomethylating therapy. N Engl J Med. 2022;386:1998–2010. doi: 10.1056/NEJMoa2119771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Papaemmanuil E, Gerstung M, Malcovati L, Tauro S, Gundem G, Van Loo P, Yoon CJ, Ellis P, Wedge DC, Pellagatti A, et al. Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood. 2013;122:3616–3627. doi: 10.1182/blood-2013-08-518886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haferlach T, Nagata Y, Grossmann V, Okuno Y, Bacher U, Nagae G, Schnittger S, Sanada M, Kon A, Alpermann T, et al. Landscape of genetic lesions in 944 patients with myelodysplastic syndromes. Leukemia. 2014;28:241–247. doi: 10.1038/leu.2013.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bejar R, Stevenson K, Abdel-Wahab O, Galili N, Nilsson B, Garcia-Manero G, Kantarjian H, Raza A, Levine RL, Neuberg D, Ebert BL. Clinical effect of point mutations in myelodysplastic syndromes. N Engl J Med. 2011;364:2496–2506. doi: 10.1056/NEJMoa1013343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoshida K, Sanada M, Shiraishi Y, Nowak D, Nagata Y, Yamamoto R, Sato Y, Sato-Otsubo A, Kon A, Nagasaki M, et al. Frequent pathway mutations of splicing machinery in myelodysplasia. Nature. 2011;478:64–69. doi: 10.1038/nature10496. [DOI] [PubMed] [Google Scholar]
- 44.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern cooperative oncology group. Am J Clin Oncol. 1982;5:649–655. doi: 10.1097/00000421-198212000-00014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.