Abstract
The effective prognostic factors for primary mediastinal large B-cell lymphoma (PMLBCL) vary among published studies. The aim of the present study was to explore the factors influencing the overall survival (OS) and progression-free survival (PFS) of patients with PMLBCL at a single institute in Taiwan. This retrospective study was conducted to analyze the prognostic impact of age, sex, disease stage, International Prognostic Index (IPI) score, treatment modality and initial response. A total of 72 patients with a median age of 28 years were included in the study. The mean OS and PFS were 171.40 and 159.77 months, respectively. Female sex, age ≤60 years, receiving radiotherapy (RT) and achieving a complete response were found to be associated with a significantly improved OS and PFS. In addition, high-intensity chemotherapy and an IPI score ≤1 were associated with longer OS, and early-stage disease was associated with a PFS superior to that of advanced-stage disease. The predictive value of IPI is limited in PMLBCL. Therefore, it is necessary to develop a novel prognostic system. The present study revealed the impact of sex on prognosis and, therefore, this factor should be considered in future prognostic evaluations. Since a complete post-treatment response was found to be important, high-intensity chemotherapy is recommended. However, low-intensity treatment followed by RT consolidation appears to be a feasible approach in elderly patients.
Keywords: primary mediastinal large B cell lymphoma, age, sex, prognosis
Introduction
Primary mediastinal large B cell lymphoma (PMLBCL) is a distinct subtype of aggressive B cell lymphoma, accounting for 2–3% of all non-Hodgkin's lymphomas, and predominantly occurring in women and 30–39-year-old patients (1,2). PMLBCL was originally classified as a subtype of diffuse large B cell lymphoma. However, in 2008, PMLBCL was identified as a distinctive entity by the World Health Organization owing to its unique clinical and biological features (3). PMLBCL originates in the mediastinal region, and usually forms a bulky mass that leads to local compression and infiltration of the lungs, pleura and pericardium. Common symptoms of PMLBCL include cough, dyspnea, dysphagia, airway compromise, great vessel compromise and superior vena cava syndrome (1,4).
The standard first-line therapy for patients with PMLBCL is unclear because of the lack of randomized trials. The recommended standard treatments of PMLBCL are currently: Rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone (R-CHOP); rituximab, etoposide, doxorubicin, cyclophosphamide, vincristine, prednisolone and bleomycin (R-VACOP-B); rituximab, methotrexate, doxorubicin, cyclophosphamide, vincristine, prednisolone and bleomycin (R-MACOP-B); dose-dense CHOP; and dose-adjusted etoposide, prednisone, vincristine cyclophosphamide, doxorubicin and rituximab (DA-EPOCH-R). Consolidative radiotherapy (RT) is recommended for patients receiving R-CHOP and R-V/MACOP-B regimens. For patients achieving a complete metabolic response after more intensive regimens, such as DA-EPOCH-R, consolidative RT may be excluded (5,6). However, as this recommendation is based on a small phase II study, the omission of consolidative RT requires investigation in large randomized trials (7).
At present, there is no consensus on prognostic models in PMLBCL. A retrospective study of patients in the British Columbia Cancer Agency lymphoma database showed that poor performance status was the only factor predicting poor survival in patients treated with CHOP or V/MACOP-B regimens (8). However, a multicenter retrospective study conducted by Todeschini et al (9) showed that the achievement of complete remission and treatment with a V/MACOP-B regimen contributed to improved survival. In the rituximab era, a retrospective study conducted by Yang et al (10) found that the inclusion of rituximab in the induction chemotherapy regimen was independently associated with superior overall survival (OS), and age >60 years was independently associated with poor OS. A multicenter retrospective study conducted by Aoki et al (11) showed that for patients receiving R-CHOP without consolidative RT, stage III/IV disease and the presence of pleural or pericardial effusion were associated with inferior progression-free survival (PFS). In addition, patients without pleural or pericardial effusion and with low International Prognostic Index (IPI) scores had higher OS and PFS rates compared with those with pleural or pericardial effusion and high IPI scores. In another multicenter retrospective study, conducted by Zhou et al (12), it was reported that patients with IPI >1, stage III–IV disease, Ki-67 expression ≥70% and maximum standardized uptake values of positron emission tomography imaging at diagnosis of >11.6 had significantly poorer survival. By contrast, patients with higher lymphocyte/monocyte ratios and multiple myeloma 1 protein expression had significantly improved survival. The factors identified to affect survival and treatment response vary among studies. Therefore, the present study was designed to assess the factors that affect OS and PFS in patients with PMLBCL at a single institute in Taiwan.
Materials and methods
Patients
The lymphoma registry at Linkou Chang Gung Memorial Hospital (Taipei, Taiwan) between January 2004 and June 2020 was screened. All patients with a diagnosis of PMLBCL were included and patients with a diagnosis of other types of lymphoma were excluded. The medical records of patients newly diagnosed with PMLBCL were retrospectively reviewed. The study was conducted in accordance with the principles of the Declaration of Helsinki. The Chang Gung Medical Foundation Institutional Review Board approved the study protocol (ref. no. 202100653B0).
Demographic and clinical data
Patients diagnosed with PMLBCL based on biopsy specimens of lymph nodes or mediastinal masses were included (13). The data collected included age, sex, Eastern Cooperative Oncology Group (ECOG) performance status (14), disease stage, the presence of B symptoms (fever, weight loss and sweats), serum lactate dehydrogenase (LDH) levels, mediastinal mass size, IPI (15), first-line treatment modality, treatment response and survival status. Disease stage was determined using the Ann Arbor system, and a maximum mediastinal mass diameter larger than one-third of the thoracic diameter or >10 cm was defined as bulky disease (16). Intensive chemotherapy was defined as DA-EPOCH-R, R-MACOP-B or similar regimens, whereas less intensive chemotherapy was defined as R-CHOP or similar regimens. OS was defined as the time from diagnosis to death from any cause. PFS was defined as the time from diagnosis to disease progression or death, whichever occurred first. Treatment response was assessed according to the Lugano Classification (17).
Statistical analysis
Descriptive statistics are used for baseline characteristics, which are expressed as count (percentage) or median (range). Survival curves were estimated using the Kaplan-Meier method and compared using the log-rank test based on sex (male vs. female), disease stage (stage I/II vs. III/IV), age (≤60 vs. >60 years), IPI score (≤1 vs. >1), treatment modality (low- vs. high-intensity chemotherapy; with vs. without consolidative RT) and treatment response (complete response achieved vs. not achieved). P<0.05 was considered to indicate a statistically significant difference. Data analyses were performed using IBM SPSS Statistics 26 software (IBM Corp.).
Results
Patient characteristics
A total of 72 patients were included in the analysis. The median age of the patients was 28 years (range, 17–78 years), and most of the patients (90.3%) were ≤60 years old. The study included 34 female and 38 male patients (female-to-male ratio, 0.89). The majority of the patients had an ECOG performance status 0–1 (88.9%), early disease (stages I–II, 75%), elevated levels of serum LDH (69.4%), bulky disease (79.2%) and low-risk disease (IPI scores 0–1; 87.5%). There were 23 (31.9%) patients with B symptoms. Regarding initial therapy, 47 (66.2%) patients received intensive chemotherapy (DA-EPOCH-R, R-MACOP-B or similar regimens) and 24 (33.8%) received less intensive chemotherapy (R-CHOP or similar regimens). In addition, more than half of the patients (56.9%) received consolidative RT. After initial therapy, 57 (83.8%) patients achieved a complete response (Table I). A total of 15 patients had relapsed or refractory disease.
Table I.
Demographic and clinical characteristics of the 72 patients.
| Characteristic | n (%)a |
|---|---|
| Median (range) age, years | 28 (17–78) |
| Age, years | |
| ≤60 | 65 (90.3) |
| >60 | 7 (9.7) |
| Sex | |
| Female | 34 (47.2) |
| Male | 38 (52.8) |
| ECOG performance status | |
| 0-1 | 64 (88.9) |
| 2-4 | 8 (11.1) |
| Stage | |
| I–II | 54 (75) |
| III–IV | 18 (25) |
| B symptoms | 23 (31.9) |
| Elevated LDH | 50 (69.4) |
| Bulky disease | 57 (79.2) |
| IPI | |
| 0-1 | 63 (87.5) |
| 2 | 8 (11.1) |
| 3-5 | 1 (1.4) |
| Chemotherapyb | |
| High intensity | 47 (66.2) |
| Low intensity | 24 (33.8) |
| Radiotherapy | |
| Yes | 41 (56.9) |
| No | 31 (43.1) |
| Complete responsec | 57 (83.8) |
For all variables except median age;
n=71, as one patient did not undergo chemotherapy;
n=68, as four patients did not have a post-treatment image study. ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; IPI, International Prognostic Index.
Overall survival
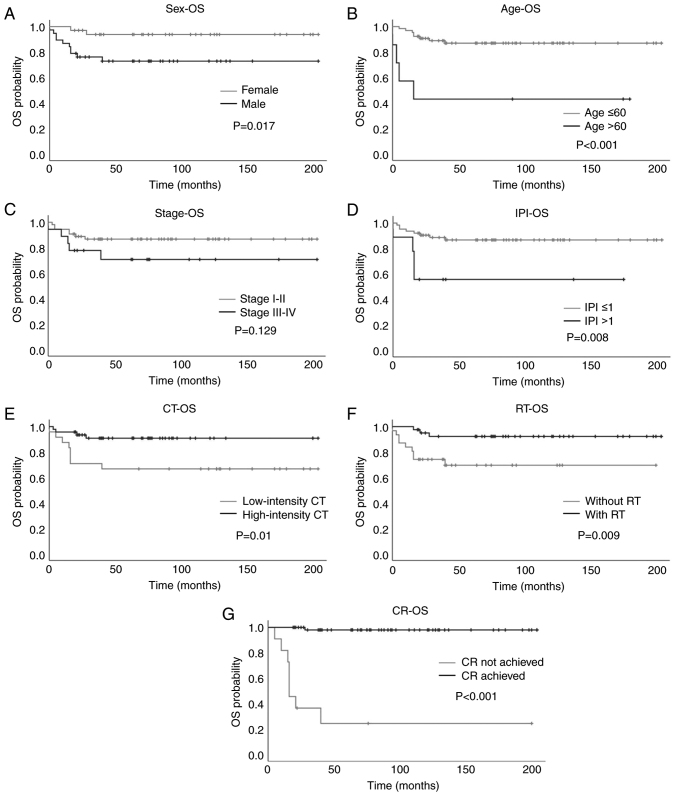
The mean OS for the entire cohort was 171.40 months [95% confidence interval (CI): 154.56, 188.23]. Regarding sex, female patients had a mean OS of 192.78 months (95% CI: 177.70, 207.86) and 5-year OS rate of 93.8%, while male patients had a mean OS of 152.31 months (95% CI: 124.82, 179.80) and 5-year OS rate of 72.8% (P=0.017; Fig. 1A). Regarding age, patients aged ≤60 years had a mean OS of 180.00 months (95% CI: 164.43, 195.56) and 5-year OS rate of 87.0%. However, patients aged >60 years had a mean OS of only 80.57 months (95% CI: 16.69, 144.45) and a 5-year OS rate of 42.9%. These results indicate that younger patients had significantly improved OS compared with older patients (P<0.001; Fig. 1B). Regarding disease stage, patients with stage I–II disease had a mean OS of 178.78 months (95% CI: 161.34, 196.21) and 5-year OS rate of 86.7%, while patients with stage III–IV disease had a mean OS of 149.35 months (95% CI: 108.71, 189.99) and 5-year OS rate of 70.7%. The difference between the early and advanced disease groups was not found to be significant (P=0.129; Fig. 1C). When patients were analyzed according to their IPI scores, patients with an IPI score ≤1 had a mean OS of 179.08 months (95% CI: 162.95, 195.20) and 5-year OS rate of 86.7%. By contrast, patients with an IPI score >1 had a mean OS of 102.44 months (95% CI: 49.36, 155.53) and 5-year OS rate of 55.6%. A significant difference was identified between the two groups (P=0.008; Fig. 1D).
Figure 1.
Kaplan-Meier plots of OS in patients with primary mediastinal large B-cell lymphoma based on various prognostic factors. (A) Sex, (B) age, (C) stage, (D) IPI, (E) intensity of CT, (F) with or without RT and (G) achievement of CR. OS, overall survival; IPI, International Prognostic Index; CT, chemotherapy; RT, radiotherapy; CR, complete response.
With regard to treatment modality, patients treated with high-intensity chemotherapy had significantly longer OS than those treated with low-intensity chemotherapy [mean OS, 186.75 months (95% CI: 170.59, 202.91) vs. 140.92 months (95% CI: 105.13, 176.70); 5-year OS rate, 90.9 vs. 66.7%, respectively; P=0.01; Fig. 1E]. Patients who received consolidative RT had significantly longer OS than those who did not [mean OS, 189.94 months (95% CI: 174.64, 205.24) vs. 143.23 months (95% CI: 111.94, 174.51); 5-year OS rate, 92.3 vs. 69.6%, respectively; P=0.009, Fig. 1F]. Patients who achieved a complete response had significantly longer OS than those who did not [mean OS, 200.48 months (95% CI: 193.65, 207.31) vs. 62.33 months (95% CI: 13.47, 111.20); 5-year OS rate, 98.0 vs. 24.2%, respectively; P<0.001; Fig. 1G]. These results indicated that female sex, age ≤60 years, IPI score ≤1, treatment with high-intensity chemotherapy and consolidative RT, and achievement of a complete response were significantly associated with improved OS (Table II).
Table II.
Prognostic factors for progression-free survival and overall survival.
| First author/s, year | Progression/event-free survival | Overall survival | (Refs.) |
|---|---|---|---|
| Present study | Female, age ≤60 years, stage I–II disease, with radiotherapy, CR achievement | Female, age ≤60 years, IPI ≤1, high-intensity chemotherapy, with radiotherapy, CR achievement | - |
| Savage et al, 2006 | - | ECOG ≤1 | (8) |
| Todeschini et al, 2004 | CR achievement, V/MACOP-B chemotherapy | CR achievement, V/MACOP-B chemotherapy | (9) |
| Yang et al, 2015 | - | Rituximab induction, age ≤60 years | (10) |
| Aoki et al, 2014 | Without pleural or pericardial effusion, IPI <3 | Without pleural or pericardial effusion, IPI <3 | (11) |
| Zhou et al, 2020 | IPI ≤1, stage I–II disease, Ki-67 expression <70%, higher lymphocyte/monocyte ratios, MUM1 expression | IPI ≤1, Ki-67 expression <70%, age ≤60 years, maximum standardized uptake values of positron emission tomography imaging ≤11.6, higher lymphocyte/monocyte ratios, MUM1 expression | (12) |
IPI, International Prognostic Index; CR, complete response; ECOG, Eastern Cooperative Oncology Group; V/MACOP-B, etoposide/methotrexate, doxorubicin, cyclophosphamide, vincristine, prednisolone and bleomycin; MUM1, multiple myeloma 1.
Progression-free survival
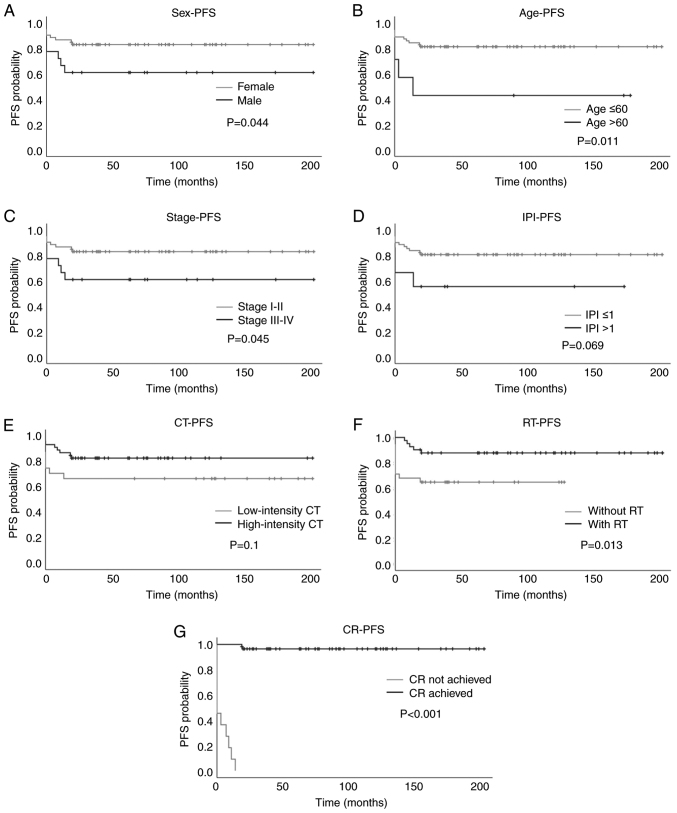
The mean PFS for the entire cohort was 159.77 months (95% CI: 140.65, 178.90). Regarding sex, female patients had a mean PFS of 180.82 months (95% CI: 159.67, 202.18) and 5-year PFS rate of 88.2%, whereas male patients had a mean PFS of 141.03 months (95% CI: 111.53, 170.52) and 5-year PFS rate of 68.4% (P=0.044; Fig. 2A). Regarding age, patients aged ≤60 years had a mean PFS time of 167.30 months (95% CI: 148.5, 186.06) and 5-year PFS rate of 81.5%. By contrast, patients aged >60 years had a mean PFS of 79.57 months (95% CI: 15.06, 144.08) and 5-year PFS rate of 42.9%. A significant difference in PFS was identified between patients younger and older than 60 years (P=0.011; Fig. 2B). Regarding disease stage, patients with stage I–II disease had a mean PFS of 170.83 months (95% CI: 151.04, 190.63) and 5-year PFS rate of 83.3%, while patients with stage III–IV disease had a mean PFS of 126.56 months (95% CI: 81.68, 171.44) and 5-year PFS rate of 61.1%. The patients with early disease had a significantly longer PFS than those with advanced disease (P=0.045; Fig. 2C). In terms of IPI score, patients with an IPI score ≤1 had a mean PFS of 166.18 months (95% CI: 146.92, 185.45) and 5-year PFS rate of 80.9%, while those with an IPI score >1 had a mean PFS of 98.78 months (95% CI: 43.04, 154.52) and 5-year PFS rate of 55.6%. No significant difference in PFS was detected between the two groups (P=0.069; Fig. 2D).
Figure 2.
Kaplan-Meier plots of PFS in patients with primary mediastinal large B-cell lymphoma based on various prognostic factors. (A) Sex, (B) age, (C) stage, (D) IPI, (E) intensity of CT, (F) with or without RT and (G) achievement of CR. PFS, progression-free survival; IPI, International Prognostic Index; CT, chemotherapy; RT, radiotherapy; CR, complete response.
With regards to treatment modality, no significant difference in PFS was detected between patients treated with high-intensity chemotherapy and those treated with low-intensity chemotherapy [mean PFS, 170.58 months (95% CI: 149.47, 191.69) vs. 136.71 months (95% CI: 98.62, 174.80); 5-year PFS rate, 82.9 vs. 66.7%; P=0.1, respectively; Fig. 2E]. Patients who received consolidative RT had longer PFS than those who did not [mean PFS, 180.49 months (95% CI: 161.17, 199.80) vs. 83.94 months (95% CI: 62.51, 105.36); 5-year PFS rate, 87.7 vs. 64.5%, respectively; P=0.013; Fig. 2F]. Patients who achieved a complete response had longer PFS than those who did not [mean PFS, 197.47 months (95% CI: 188.58, 206.36) vs. 4.0 months (95% CI: 0.87, 7.13); 5-year PFS rate, 96.5 vs. 0%, respectively; P<0.001; Fig. 2G]. In summary, female sex, early-stage disease, age ≤60 years, treatment with consolidative RT and achievement of a complete response were found to have a significant association with longer PFS (Table II).
Discussion
In the present study, several factors were found to be associated with PMLBCL prognosis, including age, sex, disease stage, IPI, chemotherapy intensity and initial response. While other risk factors are well known, male sex has not been reported as a poor prognostic factor in the literature. Notably, no female predominance of PMLBCL was observed in the present study, unlike in other patient populations (2). There was a slightly higher number of male patients than female patients in the present study. Interestingly, sex was found to create a difference in patient outcomes; male patients had significantly worse OS and PFS than female patients. In addition, among the 15 patients with relapsed or refractory disease, 11 were males. As other prognostic factors such as age, stage, IPI, and treatment intensity were balanced between male and female patients in the present study (data not shown), this indicates that the poor prognosis for male patients did not result from common clinical or demographic factors. Although a scientific explanation for such sex differences is lacking, sex differences in cancer prognosis are not a novel issue. For example, male sex has been considered a poor risk factor for Hodgkin's lymphoma for decades without proper pathophysiological explanations (18). Several hypotheses can be proposed for sex as a prognostic factor for PMLBCL. Firstly, drug metabolism may differ between male and female patients; for example, the pharmacokinetic differences in rituximab between male and female patients are well known (19). Additionally, androgen receptor expression in lymphomas and the effects of hormones on lymphoma growth have been demonstrated in previous studies (20,21). These findings highlight the biological nature of sex differences in lymphomas. However, further studies are required to clarify the underlying pathophysiology. In clinical practice, based on the above findings, it is suggested that sex should be considered when designing a prognostic system specific for PMLBCL.
Age is a common prognostic factor for lymphomas. Compared with other types of diffuse large B-cell lymphoma (DLBCL), PMLBCL predominantly occurs in young individuals. The age distribution observed in the present study was consistent with this. Age was demonstrated to be a major prognostic factor, as the OS and PFS durations were significantly shorter in patients aged >60 years than in younger patients. This may be due to elderly patients being unable to tolerate intensive chemotherapy. Indeed, in the present study, only one of the seven elderly patients who received R-MACOP-B had progressive disease and succumbed before completing the protocol. The remaining elderly patients were treated with R-CHOP or less-intensive regimens. A large population-based study of the Surveillance, Epidemiology and End Results (SEER) database analyzed 426 cases of PMLBCL, and the results of multivariate analysis showed that the OS of patients >60 years was significantly reduced compared with that of patients aged 18–39 years [hazard ratio (HR)=3.568 (95% CI: 2.653, 4.798); P=0.005] (2). Another single-center retrospective study in which 48 cases of PMLBCL were analyzed also showed that age >60 years was an independent prognostic factor for OS, with an HR of 16.697 (95% CI: 1.106, 252.022; P=0.042) (10). As the predominant age of patients with PMLBCL is <60 years, a cutoff age of 60 years may not be a proper predictor of survival. In a retrospective study of 153 patients with PMLBCL, univariate analysis showed that age >40 years was associated with poor survival; however, this association was not observed by multivariate analysis (8). Another study based on the SEER database analyzed 474 patients with PMLBCL who were aged <60 years, and univariate analysis showed that age (18–39 vs. 40–59 years) was not significantly associated with OS (22). Therefore, whether age is an appropriate prognostic factor for survival requires further investigation. Despite the risk of an unfavorable prognosis in elderly patients treated with high-intensity chemotherapy, a cure can be achieved in some of such patients. Among the elderly patients who received low-intensity treatment, three had relapse-free survival. The two elderly long-term survivors both underwent RT. While the number of patients was small, such an experience suggests that low-intensity treatment and consolidative RT are tolerable for elderly patients, resulting in potentially more favorable outcomes.
The present study showed that patients with stage III/IV PMLBCL had worse PFS than those with stage I/II disease, but no significant difference in OS. This indicates that patients with advanced disease are prone to relapse and that salvage therapies are beneficial for OS. As relapse is not common, there is no consensus regarding the standard treatment for refractory/relapsed PMLBCL (rrPMLBCL). However, a systematic review investigated published guidelines and real-world treatment patterns for rrPMLBCL. It revealed that only four guidelines for the treatment of rrPMLBCL recommend rituximab plus chemotherapy protocols with or without consolidative RT followed by high-dose chemotherapy and autologous stem cell transplantation. Regarding real-world treatment strategies, chemotherapy alone or in combination with rituximab followed by high-dose treatment and stem cell transplantation has been used in the majority of published studies (23). Owing to the frequent expression of programmed cell death-1 (PD-1) ligand 1 and 2 in PMLBCL, it has been proposed that PMLBCL may be susceptible to PD-1 inhibition. In the phase IB KEYNOTE-013 and phase II KEYNOTE-170 trials, the objective response rate of adults with rrPMLBCL receiving pembrolizumab, a PD-1 inhibitor, was 48 and 45%, respectively, and the incidence of treatment-related adverse events was 24 and 23%, respectively (24). Several multicenter trials evaluating pembrolizumab for the treatment of rrPMLBCL are ongoing (25).
The IPI utilizes five clinicopathological parameters, namely age, stage, pretreatment serum concentrations of LDH, ECOG performance status and the involvement of extranodal sites, and has been shown to predict prognosis in patients with newly diagnosed DLBCL (15). Since the age, stage and possibility of extranodal involvement of PMLBCL are very different from those of DLBCL, the prognostic value of IPI in PMLBCL is not as powerful as that in DLBCL (26,27). Although the present study showed that an IPI score >1 was associated with inferior OS, the number of cases in the high- and low-IPI groups was not well balanced, as there were only nine patients in the high-IPI group. Therefore, it is not recommended that the IPI should be a standard prognostic tool for PMLBCL, as it is in other types of DLBCL. However, a novel prognostic system for PMLBCL, based on the risk factors identified in the present and previous studies, may be constructed to determine the prognosis of patients more accurately. This system should be evaluated and validated in subsequent large-scale cohorts or prospective studies.
The role of RT in the treatment of PMLBCL remains controversial. There are concerns regarding the late toxicity of mediastinal irradiation, including cardiovascular diseases and secondary malignancies. However, RT was shown to be significantly associated with survival benefits in two retrospective population-based studies performed using the SEER and National Cancer Databases. However, these studies also found that in the USA, approximately half of the patients in the post-rituximab era did not receive RT (28,29). The UNFOLDER trial included 134 patients with PMLBCL receiving R-CHOP who were randomized to receive R-CHOP-14 or R-CHOP-21 with or without RT for bulky or extranodal involvement. The results showed that 3-year event-free survival was superior in patients receiving RT (94 vs. 78%; P=0.007). No significant differences in the OS and PFS rates were detected between the RT and no-RT groups. The authors concluded that the benefits of RT were observed only in patients who showed a partial response to R-CHOP (30). Based on these findings and clinical experience, it is proposed that the cornerstone of successful treatment is the achievement of a complete response. Therefore, the administration of high-intensity chemotherapy is recommended after the initial diagnosis if the patient is able to tolerate the regimen. A complete response can often be achieved, and RT may not be necessary. By contrast, when patients receive only low-intensity chemotherapy, a complete response is not achieved or is equivocal. It is recommended that RT should be administered immediately since disease progression is often rapid in patients receiving low-intensity chemotherapy.
The current study has several limitations. Firstly, this was a single-center retrospective study with a small sample size, which might have been susceptible to selection bias. Secondly, the patients were mostly <60 years old, and with disease stage I/II and IPI score ≤1. Therefore, the number of patients was not balanced between the groups being compared. Finally, a multivariate analysis was not performed because of the small number of patients. Therefore, whether the analyzed variables are independent prognostic factors for survival requires further investigation. In conclusion, age, sex, stage, IPI and type of chemotherapy were identified as prognostic factors for PMLBCL. The achievement of a CR after frontline treatment appears to be the key to success; therefore, high-intensity chemotherapy is recommended. Male patients had significantly worse outcomes than female patients, for reasons that are not yet clear. The IPI is not an ideal tool for the prognostication of PMLBCL due to the unique clinical features of young age, early stage and rare extranodal lesions. It is proposed that a novel prognostic tool specific for PMLBCL should be designed, in which the impact of sex is considered.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- PMLBCL
primary mediastinal large B-cell lymphoma
- OS
overall survival
- PFS
progression-free survival
- IPI
International Prognostic Index
- RT
radiotherapy
- ECOG
Eastern Cooperative Oncology Group
- R-CHOP
rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone
- R-VACOP-B
rituximab, etoposide, doxorubicin, cyclophosphamide, vincristine, prednisolone and bleomycin
- R-MACOP-B
rituximab, methotrexate, doxorubicin, cyclophosphamide, vincristine, prednisolone and bleomycin
- DA-EPOCH-R
dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin and rituximab
- LDH
lactate dehydrogenase
- rrPMLBCL
refractory/relapsed PMLBCL
- PD-1
programmed cell death-1
- DLBCL
diffuse large B-cell lymphoma
Funding Statement
This study was supported by Chang Gung Memorial Hospital (grant no. CORPG3G0821).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
HJS and HC designed the study. HJS wrote the manuscript. MCK, TLL, HWK, JHW, YSH, CWO and YJS contributed to the acquisition and analysis of clinical data. MCK supervised the conduction of the clinical study and performed the data analysis. HJS and HC confirm the authenticity of all the raw data. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
This study was reviewed and approved by the Chang Gung Medical Foundation Institutional Review Board (approval no. 202100653B0). The study was exempt from the requirement for written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Martelli M, Ferreri A, Di Rocco A, Ansuinelli M, Johnson PWML. Primary mediastinal large B-cell lymphoma. Crit Rev Oncol Hematol. 2017;113:318–327. doi: 10.1016/j.critrevonc.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Liu PP, Wang KF, Xia Y, Bi XW, Sun P, Wang Y, Li ZM, Jiang WQ. Racial patterns of patients with primary mediastinal large B-cell lymphoma: SEER analysis. Medicine (Baltimore) 2016;95:e4054. doi: 10.1097/MD.0000000000004054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaffe ES, Harris NL, Stein H, Isaacson PG. Classification of lymphoid neoplasms: The microscope as a tool for disease discovery. Blood. 2008;112:4384–4399. doi: 10.1182/blood-2008-07-077982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu Y, Dong X, Tu M, Wang H. Primary mediastinal large B cell lymphoma. Thorac Cancer. 2021;12:2831–2837. doi: 10.1111/1759-7714.14155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zelenetz AD, Gordon LI, Wierda WG, Abramson JS, Advani RH, Andreadis CB, Bartlett N, Byrd JC, Fayad LE, Fisher RI, et al. Diffuse Large B-cell lymphoma version 1.2016. J Natl Compr Canc Netw. 2016;14:196–231. doi: 10.6004/jnccn.2016.0023. [DOI] [PubMed] [Google Scholar]
- 6.Vitolo U, Seymour JF, Martelli M, Illerhaus G, Illidge T, Zucca E, Campo E, Ladetto M, ESMO Guidelines Committee Extranodal diffuse large B-cell lymphoma (DLBCL) and primary mediastinal B-cell lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27((Suppl 5)):v91–v102. doi: 10.1093/annonc/mdw175. [DOI] [PubMed] [Google Scholar]
- 7.Dunleavy K, Pittaluga S, Maeda LS, Advani R, Chen CC, Hessler J, Steinberg SM, Grant C, Wright G, Varma G, et al. Dose-adjusted EPOCH-rituximab therapy in primary mediastinal B-cell lymphoma. N Engl J Med. 2013;368:1408–1416. doi: 10.1056/NEJMoa1214561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Savage KJ, Al-Rajhi N, Voss N, Paltiel C, Klasa R, Gascoyne RD, Connors JM. Favorable outcome of primary mediastinal large B-cell lymphoma in a single institution: The British Columbia experience. Ann Oncol. 2006;17:123–130. doi: 10.1093/annonc/mdj030. [DOI] [PubMed] [Google Scholar]
- 9.Todeschini G, Secchi S, Morra E, Vitolo U, Orlandi E, Pasini F, Gallo E, Ambrosetti A, Tecchio C, Tarella C, et al. Primary mediastinal large B-cell lymphoma (PMLBCL): Long-term results from a retrospective multicentre Italian experience in 138 patients treated with CHOP or MACOP-B/VACOP-B. Br J Cancer. 2004;90:372–376. doi: 10.1038/sj.bjc.6601460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang SH, Hsiao LT, Chiou TJ, Yang CF, Yu YB, Liu CY, Gau JP, Liu JH, Chen PM, Tzeng CH. Rituximab induction therapy, survival benefits, and the increasing selection of radiotherapy as the postinduction treatment in patients with primary mediastinal large B-cell lymphoma. J Chin Med Assoc. 2015;78:400–407. doi: 10.1016/j.jcma.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Aoki T, Izutsu K, Suzuki R, Nakaseko C, Arima H, Shimada K, Tomita A, Sasaki M, Takizawa J, Mitani K, et al. Prognostic significance of pleural or pericardial effusion and the implication of optimal treatment in primary mediastinal large B-cell lymphoma: A multicenter retrospective study in Japan. Haematologica. 2014;99:1817–1825. doi: 10.3324/haematol.2014.111203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou H, Xu-Monette ZY, Xiao L, Strati P, Hagemeister FB, He Y, Chen H, Li Y, Manyam GC, Li Y, et al. Prognostic factors, therapeutic approaches, and distinct immunobiologic features in patients with primary mediastinal large B-cell lymphoma on long-term follow-up. Blood Cancer J. 2020;10:49. doi: 10.1038/s41408-020-0312-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris NL, Jaffe ES, Stein H, Banks PM, Chan JK, Cleary ML, Delsol G, De Wolf-Peeters C, Falini B, Gatter KC, et al. A revised European-American classification of lymphoid neoplasms: A proposal from the International Lymphoma Study Group. Blood. 1994;84:1361–1392. doi: 10.1182/blood.V84.5.1361.bloodjournal8451361. [DOI] [PubMed] [Google Scholar]
- 14.Oken MM. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. doi: 10.1097/00000421-198212000-00014. [DOI] [PubMed] [Google Scholar]
- 15.International Non-Hodgkin's Lymphoma Prognostic Factors Project, corp-author. A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 16.Olweny CL. Cotswolds modification of the Ann Arbor staging system for Hodgkin's disease. J Clin Oncol. 1990;8:1598. [PubMed] [Google Scholar]
- 17.Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, Lister TA, Alliance Australasian Leukaemia and Lymphoma Group; Eastern Cooperative Oncology Group; European Mantle Cell Lymphoma Consortium et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J Clin Oncol. 2014;32:3059–3068. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin's disease. International prognostic factors project on advanced Hodgkin's disease. N Engl J Med. 1998;339:1506–1514. doi: 10.1056/NEJM199811193392104. [DOI] [PubMed] [Google Scholar]
- 19.Pfreundschuh M, Muller C, Zeynalova S, Kuhnt E, Wiesen MH, Held G, Rixecker T, Poeschel V, Zwick C, Reiser M, et al. Suboptimal dosing of rituximab in male and female patients with DLBCL. Blood. 2014;123:640–646. doi: 10.1182/blood-2013-07-517037. [DOI] [PubMed] [Google Scholar]
- 20.Mostaghel EA, Martin PS, Mongovin S, Frayo S, Zhang A, Edlefsen KL, Press OW, Gopal AK. Androgen receptor expression in mantle cell lymphoma: Potential novel therapeutic implications. Exp Hematol. 2017;49:34–38.e32. doi: 10.1016/j.exphem.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Talaber G, Yakimchuk K, Guan J, Inzunza J, Okret S. Inhibition of estrogen biosynthesis enhances lymphoma growth in mice. Oncotarget. 2016;7:20718–20727. doi: 10.18632/oncotarget.7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang S, Zhen H, Jiang H. Role of radiation therapy in younger and older adults with primary mediastinal large B cell lymphoma in rituximab Era: A U.S. Population-based analysis. J Adolesc Young Adult Oncol. 2019;8:623–627. doi: 10.1089/jayao.2019.0018. [DOI] [PubMed] [Google Scholar]
- 23.Takyar J, Raut M, Borse R, Balakumaran A, Sehgal M. Relapsed/refractory primary mediastinal large B-cell lymphoma: A structured review of epidemiology, treatment guidelines and real-world treatment practices. Expert Rev Hematol. 2020;13:275–287. doi: 10.1080/17474086.2020.1716725. [DOI] [PubMed] [Google Scholar]
- 24.Armand P, Rodig S, Melnichenko V, Thieblemont C, Bouabdallah K, Tumyan G, Özcan M, Portino S, Fogliatto L, Caballero MD, et al. Pembrolizumab in relapsed or refractory primary mediastinal large B-cell lymphoma. J Clin Oncol. 2019;37:3291–3299. doi: 10.1200/JCO.19.01389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomassetti S, Chen R, Dandapani S. The role of pembrolizumab in relapsed/refractory primary mediastinal large B-cell lymphoma. Ther Adv Hematol. 2019;10:2040620719841591. doi: 10.1177/2040620719841591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamlin PA, Portlock CS, Straus DJ, Noy A, Singer A, Horwitz SM, Oconnor OA, Yahalom J, Zelenetz AD, Moskowitz CH. Primary mediastinal large B-cell lymphoma: Optimal therapy and prognostic factor analysis in 141 consecutive patients treated at Memorial Sloan Kettering from 1980 to 1999. Br J Haematol. 2005;130:691–699. doi: 10.1111/j.1365-2141.2005.05661.x. [DOI] [PubMed] [Google Scholar]
- 27.Romaguera JE, Rodriguez Diaz-Pavon J, Carias L, Hagemeister FB, McLaughlin P, Rodriguez MA, Sarris AH, Younes A, Preti A, Bachier C, et al. Use of the international prognostic index and the tumor score to detect poor-risk patients with primary mediastinal large B-cell lymphoma: A study of 37 previously untreated patients. Leuk Lymphoma. 1998;28:295–306. doi: 10.3109/10428199809092685. [DOI] [PubMed] [Google Scholar]
- 28.Jackson MW, Rusthoven CG, Jones BL, Kamdar M, Rabinovitch R. Improved survival with radiation therapy in stage I–II primary mediastinal B cell lymphoma: A surveillance, epidemiology, and end results database analysis. Int J Radiat Oncol Biol Phys. 2016;94:126–132. doi: 10.1016/j.ijrobp.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 29.Jackson MW, Rusthoven CG, Jones BL, Kamdar M, Rabinovitch R. Improved survival with combined modality therapy in the modern era for primary mediastinal B-cell lymphoma. Am J Hematol. 2016;91:476–480. doi: 10.1002/ajh.24325. [DOI] [PubMed] [Google Scholar]
- 30.Held G, Thurner L, Poeschel V, Berdel C, Ott G, Schmidt C, Viardot A, Borchmann P, Shpilberg O, Nickelsen M, et al. Role of radiotherapy and dose-densification of R-CHOP in primary mediastinal B-cell lymphoma: A subgroup analysis of the unfolder trial of the German Lymphoma Alliance (GLA) J Clin Oncol. 2020;38((Suppl 15)):S8041. doi: 10.1200/JCO.2020.38.15_suppl.8041. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.