Abstract
Bone pain is a well-known quality-of-life detriment for individuals with prostate cancer and is associated with survival. This study expands previous work into racial differences in multiple patient-reported dimensions of pain and the association between baseline and longitudinal pain and mortality. This is a prospective cohort study of individuals with newly diagnosed advanced prostate cancer enrolled in the International Registry for Men with Advanced Prostate Cancer (IRONMAN) from 2017 to 2023 at U.S. sites. Differences in four pain scores at study enrollment by race were investigated. Cox proportional hazards models and joint longitudinal survival models were fit for each of the scale scores to estimate HRs and 95% confidence intervals (CI) for the association with all-cause mortality. The cohort included 879 individuals (20% self-identifying as Black) enrolled at 38 U.S. sites. Black participants had worse pain at baseline compared with White participants, most notably a higher average pain rating (mean 3.1 vs. 2.2 on a 10-point scale). For each pain scale, higher pain was associated with higher mortality after adjusting for measures of disease burden, particularly for severe bone pain compared with no pain (HR, 2.47; 95% CI: 1.44–4.22). The association between pain and all-cause mortality was stronger for participants with castration-resistant prostate cancer compared with those with metastatic hormone-sensitive prostate cancer and was similar among Black and White participants. Overall, Black participants reported worse pain than White participants, and more severe pain was associated with higher mortality independent of clinical covariates for all pain scales.
Significance:
Black participants with advanced prostate cancer reported worse pain than White participants, and more pain was associated with worse survival. More holistic clinical assessments of pain in this population are needed to determine the factors upon which to intervene to improve quality of life and survivorship, particularly for Black individuals.
Introduction
In the United States, an estimated 120,000 individuals are living with advanced prostate cancer (1), defined as metastatic hormone-sensitive prostate cancer (mHSPC) or castration-resistant prostate cancer (CRPC; ref. 2). Black individuals experience the most significant advanced prostate cancer burden with over double the age-adjusted prevalence and mortality compared with White individuals (1).
An important quality-of-life detriment experienced by many individuals with advanced prostate cancer is pain, often driven by metastasis to the bone (3). In addition to bone pain, other types of pain, including neuropathic, nociceptive, osteoarthritic, and muscular pain, are frequently experienced as chronic pain with poorly-managed breakthrough episodes (4). Pain is a subjective experience shaped by cultural and individual expectations in addition to exacerbating factors such as psychosocial stressors, medical comorbidities, and ability to navigate the health system (5).
In this study, we consider race a social construct that serves as a proxy marker for a range of social experiences shaped by structural racism (6). As a result of structural racism, social determinants of health impact the lives of Black individuals in almost every domain of life (health, education, housing, employment, etc.). The subjective experience of pain described above can also be shaped by these social determinants of health, leading to differential reporting and experience of pain by race. Importantly, individual and systemic discrimination can also lead to health professionals prescribing analgesics less frequently to Black and Hispanic patients with cancer compared with White patients, further exacerbating disparities in the experience and management of pain in health settings (7).
Previous studies have shown that Black individuals with advanced prostate cancer report more severe bone pain than White individuals (8, 9). In addition, our group has previously shown that Black participants in the International Registry for Men with Advanced Prostate Cancer (IRONMAN) registry reported worse pain overall at study enrollment compared with White participants, and that pain increased throughout the first year of follow-up similarly for both racial groups (10). While these differences in bone and overall pain by race are known, it is not clear whether various aspects of pain (severity, duration, interference with daily activities, etc.) differ by race. This is important to investigate as pain is notoriously difficult to both report and interpret, particularly when interpersonal relationships and other social determinants of health affect the perception of the scale used for both the patient and the health professional (11). More holistically assessing the various dimensions of pain can better facilitate communication about the patient's experience of pain, leading to more cultural sensitivity around perceptions of pain and best methods for management (12).
Increased pain overall has been shown to be associated with worse survival in advanced prostate cancer populations (13–16); however, most studies were conducted in primarily White populations participating in randomized controlled trials of disease-directed therapies. There is a need to expand this research into a more real-world population, inclusive of non-White individuals and also individuals who have additional medical or socioeconomic considerations leading them to be unable to participate in randomized controlled trials.
This study aims to descriptively assess multidimensional pain (pain interference, average pain, worst pain, and bone pain) in Black and White individuals with newly diagnosed mHSPC or CRPC participating in the IRONMAN registry to better describe the experience of pain in this patient population, laying the groundwork for future studies to investigate potential mediators of the race-pain relationship (e.g., access to care, analgesic prescription, etc.) and interventions to improve quality of life. We also investigate the association between each baseline and longitudinal pain scale and survival in the overall study population to give insight into which aspects of pain have the largest prognostic value and should be followed up most closely during health appointments.
We hypothesize that Black participants will report worse pain across all scales since Black individuals tend to have more advanced prostate cancer at diagnosis and social determinants that more negatively impact their health compared with White individuals. We additionally hypothesize that interference of pain with daily activities and bone pain will have the greatest prognostic value for overall survival as they are most representative of the impact of pain on the patient's life and also likely of disease burden due to metastases to the bone.
Materials and Methods
Study Participants
Study participants included individuals newly diagnosed with mHSPC or CRPC who enrolled in the IRONMAN registry (NCT 03151629) between July 21, 2017 and January 23, 2023. IRONMAN is an international prospective cohort of individuals with no more than 90 days of life-prolonging therapy prior to enrollment for patients with CRPC and no more than 90 days of active therapy including androgen deprivation therapy (ADT) for patients with mHSPC. Participants are recruited through IRONMAN-affiliated clinicians in 16 countries (17). Study sites are primarily located in urban centers in regions with high prostate cancer mortality. This analysis focused on individuals enrolled in the United States. All study participants gave written informed consent prior to study enrollment and were able to withdraw from the study at any time. This study was approved by the Harvard Longwood Campus Institutional Review Board guided by the ethical principles set forth in the Belmont Report.
Exposure Measures: Pain
Pain was self-reported by study participants at enrollment and every 3–6 months throughout a follow-up period of up to 5 years (17). The European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30) contains two questions on the presence and interference of pain rated on a Likert scale of 1 (“not at all”) to 4 (“very much”). These two questions are combined and linearly transformed to a pain scale score with a range of 0 (least severe) to 100 (most severe; ref. 18). The minimally important difference (MID) for deterioration on the EORTC pain scale for individuals with prostate cancer is 5 points (19). The EORTC QLQ-C30 pain scale has shown high reliability (Cronbach alpha 0.83) in a racially diverse population over the age of 50 years (20).
The Brief Pain Inventory (BPI) contains two questions on severity of average and worst pain in the past 24 hours rated on a scale of 1 (“no pain”) to 10 (“pain as bad as you can imagine”). The MID for deterioration on the BPI scale is between 1.3 and 1.6 points (21). The BPI has shown high construct validity in a racially diverse population and high concordance with other pain scales in an advanced prostate cancer population (22, 23).
The Functional Assessment of Cancer Therapy Prostate Cancer Symptom Index (FACT-FPSI) contains one question on presence of bone pain in the past week rated on a Likert scale of 0 (“not at all”) to 4 (“very much”). While the MID and reliability/validity for the bone pain question have not specifically been investigated, these have been assessed for the overall FACT-FPSI scale in patients with prostate cancer (24, 25).
Demographic and Clinical Characteristics
Demographic information (age, highest level of education, employment status, marital status, military status, race, ethnicity) was collected through patient-reported questionnaires at study enrollment. Clinical variables (disease state at enrollment, Gleason score, first on-study PSA level, treatments received prior to study and throughout follow-up, metastatic status at study enrollment, de novo metastases at study enrollment, sites of metastases at study enrollment, and type of health center) were abstracted from patient medical records and entered by study sites at the time of enrollment. Gleason score was assessed from prostate biopsy, radical prostatectomy, transurethral resection of the prostate (TURP), or biopsy of a metastatic site at time of prostate cancer diagnosis or surgical treatment. Disease state was categorized as mHSPC (de novo metastatic disease at diagnosis or progressed to metastasis after localized prostate cancer diagnosis) and M0 or M1 CRPC (progression of disease while on ADT or with castrate level of testosterone determined by the investigator).
Statistical Analysis
We summarized demographic and clinical variables stratified by race at study enrollment. Next, we investigated racial differences in the four pain scales at enrollment by comparing average scores with t tests and χ2 tests as appropriate and creating histograms of all scores. We calculated the Pearson correlation between each of the pain scales to investigate whether the different scales measure different aspects of pain.
Second, we defined the time to all-cause mortality as the time from the date of study enrollment (referred to as “baseline” in this study) to the date of death and constructed Kaplan–Meier estimates of the survivor function to visualize differences by race, disease state, and pain scale score categories. We created three categories of pain for each pain scale corresponding to no pain, a little/light pain, and moderate-to-severe pain; significance of differences in survival by pain category were assessed using the log-rank test. We estimated 80th percentile survival and 95% confidence intervals (CI) for each category of pain at study enrollment; due to short follow-up for many participants, we were not able to estimate median overall survival.
Third, we fit separate Cox proportional hazards models using the imputed datasets to investigate the association between each of the four pain scales at enrollment and all-cause mortality. Missing individual covariates and pain scale scores on completed questionnaires were imputed using multiple imputation by chained equations (MICE; ref. 26) and sensitivity analyses were conducted to determine the impact of varying the missing indicator used. An in-depth description of missing data exploration and methods is included in Supplementary Data S1, and an overview of longitudinal missing data is shown in Supplementary Fig. S1. All Cox models were adjusted for potential confounders of disease burden including age at study enrollment (continuous), first PSA on study (continuous), Gleason score (categorical; 6 or less, 7, 8, or 9–10), disease state at enrollment (mHSPC or CRPC), de novo metastatic status at enrollment (yes/no), and site of metastases at baseline (categorical; none, lymph node only, bone and/or lymph node only, liver/lung metastases present, or other). Participants were clustered by site ID (N = 38) with robust SEs assuming there may be some correlation of pain scores within study site due to practice patterns, and the baseline hazard was stratified by year of enrollment. HRs and 95% CIs were estimated and pooled across imputed datasets using Rubin Rules (27). Analyses were additionally stratified by disease state at enrollment and race.
Finally, to investigate the association between longitudinal pain score trajectories throughout follow-up and mortality, we fit joint longitudinal survival models for each pain scale (28). Joint longitudinal survival models simultaneously fit two models: one model for the longitudinal trajectory of pain over time and one model for the multivariable-adjusted association between pain and all-cause mortality, ultimately estimating the averaged association between pain and survival given pain scores at each timepoint. For the models representing the longitudinal trajectories of pain, we fit linear mixed effects models for the pain scales with month of questionnaire as linear, quadratic, and cubic terms and with observations clustered within study participants. While we do not explicitly model the trajectories of pain in this study, our group has previously modeled the longitudinal trajectory of the EORTC pain scale in this cohort over the first year of follow-up (10).
Each linear mixed effects model was then jointly modeled with the same Cox models described above using the JM package in R, assuming a Weibull baseline hazard (29). Results were pooled across imputed datasets, and HRs and 95% CIs for the association between the time-varying pain scale scores and mortality were estimated; similar to a time-dependent Cox model, this HR represents the average of the HRs for the association of a one-point increase in pain at each timepoint with all-cause mortality. While the association between longitudinal pain interference, average pain, and worst pain with mortality could be estimated with the joint modeling procedure, the association between bone pain and mortality was not able to be assessed because of incompatibility of categorical longitudinal outcomes with joint modeling capabilities in the JM package. All analyses were completed using R version 4.1.0 with statistical significance assessed at the 0.05 level.
An advanced prostate cancer survivor is an author on this article, and a glossary of technical terms is included in Supplementary Data S1 accompanying this article for increased accessibility to non-academic audiences.
Data Availability Statement
The data analyzed in this study are available from the Prostate Cancer Clinical Trials Consortium. Restrictions apply to the availability of these data, which were used under agreement for this study. Data are available from the authors upon reasonable request with the permission of the Prostate Cancer Clinical Trials Consortium.
Results
Participant Characteristics
This analysis included 879 participants from IRONMAN self-identifying as White (N = 704, 80%) or Black (N = 175, 20%) who received care at 38 study sites across the United States (Supplementary Fig. S2; Supplementary Table S1). Demographic and clinical characteristics stratified by self-reported race are shown in Table 1. For the entire cohort, the mean age at enrollment was 69.1 (SD 8.9 years), and a larger portion of the participants (65%) had mHSPC at enrollment compared with CRPC (35%). The most commonly received therapies at any point on study were ADT (89%), androgen receptor signaling inhibitors (ARSI; 67%), and chemotherapy (24%; Supplementary Table S2).
TABLE 1.
Cohort demographic and clinical characteristics by self-reported race (N = 879), 2017–2023
| White (N = 704) |
Black (N = 175) |
|
|---|---|---|
| Age at study entry, years | ||
| Mean (SD) | 69.5 (9.0) | 67.2 (8.7) |
| Disease state at enrollment | ||
| CRPC | 241 (34%) | 65 (37%) |
| mHSPC | 463 (66%) | 110 (63%) |
| De novo metastatic disease at enrollment | ||
| Yes | 294 (68%) | 70 (71%) |
| No | 137 (32%) | 28 (29%) |
| Missing | n = 273 | n = 77 |
| Location of metastases at enrollment | ||
| No metastases at baseline | 29 (7%) | 4 (3%) |
| Lymph nodes only | 36 (9%) | 15 (13%) |
| Bone ± lymph nodes only | 245 (63%) | 77 (66%) |
| Other soft-tissue metastases present | 80 (21%) | 21 (18%) |
| Missing | n = 314 | n = 58 |
| Prostatectomy or biopsy Gleason score | ||
| 6 or less | 26 (5%) | 3 (2%) |
| 7 | 163 (28%) | 45 (34%) |
| 8 | 106 (18%) | 20 (15%) |
| 9–10 | 278 (49%) | 63 (48%) |
| Missing | n = 131 | n = 44 |
| First on-study PSA (ng/mL) | ||
| Mean (SD) | 88.6 (484.8) | 156.9 (396.3) |
| Missing | n = 33 | n = 8 |
| Hispanic/Latino | ||
| No | 657 (97%) | 156 (96%) |
| Yes | 22 (3%) | 6 (4%) |
| Missing | n = 25 | n = 13 |
| Highest education level at baseline | ||
| Less than College | 29 (14%) | 18 (43%) |
| Some College or Bachelor's degree | 72 (35%) | 13 (31%) |
| Vocational School/Program | 2 (1%) | 1 (2%) |
| Graduate degree | 101 (49%) | 9 (21%) |
| Other | 3 (1%) | 1 (2%) |
| Missing | n = 497 | n = 133 |
| Marital status at baseline | ||
| Married | 545 (78%) | 88 (51%) |
| In a civil partnership | 20 (3%) | 2 (1%) |
| Widowed | 29 (4%) | 12 (7%) |
| Divorced/Separated | 76 (11%) | 43 (25%) |
| Never married | 28 (4%) | 26 (15%) |
| Missing | n = 6 | n = 4 |
| Employment status at baseline | ||
| Retired | 408 (58%) | 82 (48%) |
| Working full-time | 200 (29%) | 45 (26%) |
| Working part-time | 58 (8%) | 11 (6%) |
| Unemployed | 12 (2%) | 16 (9%) |
| Disabled | 22 (3%) | 17 (10%) |
| Missing | n = 4 | n = 4 |
| Member of national military at baseline | ||
| Yes, currently or previously | 182 (33%) | 37 (28%) |
| No, I have never served in the national military | 364 (67%) | 97 (72%) |
| Missing | n = 158 | n = 41 |
| Type of health center | ||
| Clinic | 30 (4%) | 7 (4%) |
| Hospital | 125 (18%) | 21 (12%) |
| NCI-designated | 535 (76%) | 131 (75%) |
| VA | 14 (2%) | 16 (9%) |
| Time on study (months) | ||
| Mean (SD) | 28.9 (17.4) | 24.8 (17.2) |
Abbreviations: CRPC, castration-resistant prostate cancer; mHSPC, metastatic hormone-sensitive prostate cancer; PSA, prostate-specific antigen.
Overall, clinical disease characteristics were similar by race with the exception of a higher PSA level in Black participants compared with White participants (Table 1). Regarding demographic characteristics, Black participants were younger at study enrollment, reported lower education, were less likely to be married and were less likely to be retired compared with White participants.
Baseline Differences in Pain by Self-reported Race
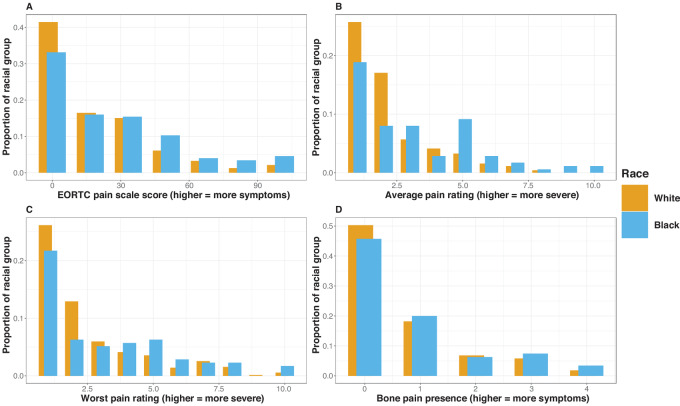
At study enrollment, Black participants reported more severe pain on average across all pain scales compared with White participants with the exception of bone pain, where no differences by race were seen (Table 2). Racial differences in each pain scale were similar for both participants with mHSPC and CRPC; for example, White participants with mHSPC had a mean EORTC pain scale score of 19.1 compared with the mean of 26.7 for Black participants with mHSPC. The scale scores for participants with CRPC were similar (mean 18.4 for White participants and 26.2 for Black participants).
TABLE 2.
Average pain at study enrollment by disease state at enrollment and self-reported race (N = 4 pain scales)
| CRPC | mHSPC | |||||
|---|---|---|---|---|---|---|
| White (N = 241) |
Black (N = 65) |
P-value | White (N = 463) |
Black (N = 110) |
P-value | |
| EORTC QLQ-C30 Pain Scale (0–100)a Minimally important difference: 5 points |
||||||
| Mean (SD) | 18.4 (23.1) | 26.2 (28.1) | 0.05 | 19.1 (25.0) | 26.7 (30.0) | 0.03 |
| Missing | n = 33 | n = 4 | n = 67 | n = 19 | ||
| Q1: How often have you had pain in the past week? | ||||||
| 1 – Not at all | 108 (51%) | 23 (38%) | 0.13 | 200 (51%) | 38 (41%) | 0.09 |
| 2 – A little | 77 (37%) | 25 (41%) | 138 (35%) | 33 (36%) | ||
| 3 – Quite a bit | 18 (9%) | 8 (13%) | 42 (11%) | 12 (13%) | ||
| 4 – Very much | 7 (3%) | 5 (8%) | 16 (4%) | 9 (10%) | ||
| Missing | n = 31 | n = 4 | n = 67 | n = 18 | ||
| Q2: How often did pain interfere with your daily activities in the past week? | ||||||
| 1 – Not at all | 132 (63%) | 34 (56%) | 0.26 | 265 (67%) | 53 (58%) | 0.11 |
| 2 – A little | 58 (28%) | 17 (28%) | 96 (24%) | 22 (24%) | ||
| 3 – Quite a bit | 15 (7%) | 7 (11%) | 20 (5%) | 8 (9%) | ||
| 4 – Very much | 3 (1%) | 3 (5%) | 16 (4%) | 8 (9%) | ||
| Missing | n = 33 | n = 4 | n = 66 | n = 19 | ||
| Brief Pain Inventory (BPI) Minimally important difference: 1.3–1.6 points |
||||||
| Rate your average pain (1–10) | ||||||
| Mean (SD) | 2.2 (1.6) | 3.1 (2.2) | 0.01 | 2.2 (1.6) | 3.2 (2.4) | <0.001 |
| Missing | n = 42 | n = 10 | n = 73 | n = 19 | ||
| Rate your pain at its worst in the last 24 hours (1–10) | ||||||
| 4 or greater | 48 (24%) | 18 (33%) | 0.27 | 85 (22%) | 37 (41%) | <0.001 |
| Less than 4 | 151 (76%) | 37 (67%) | 305 (78%) | 54 (59%) | ||
| Missing | n = 42 | n = 10 | n = 73 | n = 19 | ||
| FACT-FPSI Minimally important difference: Not established |
||||||
| How often have you had bone pain in the past week? | ||||||
| 0 – Not at all | 120 (60%) | 31 (55%) | 0.90 | 234 (61%) | 49 (55%) | 0.27 |
| 1 – A little bit | 42 (21%) | 14 (25%) | 86 (22%) | 21 (24%) | ||
| 2 – Somewhat | 21 (11%) | 6 (11%) | 27 (7%) | 5 (6%) | ||
| 3 – Quite a bit | 10 (5%) | 4 (7%) | 31 (8%) | 9 (10%) | ||
| 4 – Very much | 6 (3%) | 1 (2%) | 7 (2%) | 5 (6%) | ||
| Missing | n = 42 | n = 9 | n = 78 | n = 21 | ||
Abbreviations: CRPC, castration-resistant prostate cancer; mHSPC, metastatic hormone-sensitive prostate cancer.
aThe EORTC pain scale score is created through a linear transformation of Q1 and Q2 below.
Though the largest proportion of participants reported no pain at baseline across each of the four pain scales, the majority of study participants reported at least some pain on each of the scales, with Black participants tending to report more severe pain (Fig. 1). The four pain scales have moderate-to-high correlation with each other (correlation coefficient between 0.56 and 0.87; Supplementary Table S3).
FIGURE 1.
Distributions of pain at study enrollment by race. A, Distributions of scores on the EORTC pain scale by race. B, Distributions of scores on the average pain scale by race. C, Distributions of scores on the worst pain scale by race. D, Distributions of scores on the bone pain scale by race.
Differences in Survival Time by Self-reported Race, Disease State, and Baseline Pain
The median follow-up time for White participants in the study was 2.24 years compared with 1.73 years for Black participants with 137 (19.5%) White participants and 37 (21.4%) Black participants dying throughout follow-up (Supplementary Table S4). The 80th percentile survival for White participants was 2.57 years (95% CI: 2.34–2.95) and for Black participants was 2.09 years (95% CI: 1.68–2.98; Supplementary Fig. S3). The 80th percentile survival for participants with mHSPC was 2.84 years (95% CI: 2.57–3.83) and for participants with CRPC was 2.08 years (95% CI: 1.75–2.40; Supplementary Fig. S4).
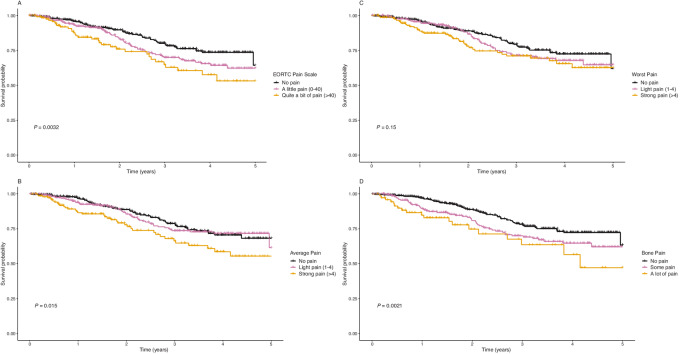
The 80th percentile survival for participants experiencing the highest amounts of pain at enrollment was substantially lower than the 80th percentile survival for participants experiencing no pain at enrollment for all four pain scales (Fig. 2; Supplementary Table S5). For example, the 80th percentile survival for participants reporting a score of more than 40 on the EORTC pain scale at baseline was 1.60 years (95% CI: 0.99–2.66) compared with 3.01 years (95% CI: 2.50–3.83) for participants with no pain on the EORTC scale at baseline.
FIGURE 2.
Kaplan–Meier curves for overall survival by pain categories at study enrollment, IRONMAN Registry 2017–2023. A, Kaplan–Meier curve for the EORTC pain scale. B, Kaplan–Meier curve for the average pain scale. C, Kaplan–Meier curve for the worst pain scale. D, Kaplan–Meier curve for the bone pain scale.
Association Between Pain and All-cause Mortality
For all four pain scales, more frequent or severe pain at study enrollment was associated with an increased risk of all-cause mortality (Table 3). Most notably, compared with participants with no bone pain, participants with a lot of bone pain at baseline had an adjusted HR for death of 2.47 (95% CI: 1.44–4.22). The association between pain and all-cause mortality was stronger for participants with CRPC compared with those with mHSPC and was similar among Black and White participants (Table 4). Results were robust to varying missing data assumptions in the MICE procedure (Supplementary Tables S6–S9).
TABLE 3.
HRs and 95% CIs for the association between baseline and longitudinal pain scales and death, IRONMAN Registry 2017–2023 (N = 879; 137 deaths in White participants, 37 deaths in Black participants)
| Pain at enrollment | Longitudinal pain | ||||
|---|---|---|---|---|---|
| Pain scale | Comparison | Age-only model HR (95% CI) | Fully-adjusted modela HR (95% CI) |
Age-only model HR (95% CI) | Fully-adjusted modela HR (95% CI) |
| EORTC scale | 10 points on 0–100 scale | 1.12 (1.06–1.17) | 1.10 (1.03–1.17) | 1.30 (1.20–1.42) | 1.29 (1.19–1.40) |
| Average pain | 1 point on 1–10 scale | 1.22 (1.10–1.35) | 1.19 (1.08–1.32) | 1.34 (1.21–1.48) | 1.32 (1.20–1.46) |
| Worst pain | 1 point on 1–10 scale | 1.17 (1.08–1.26) | 1.16 (1.08–1.25) | 1.31 (0.90–1.91)b | 1.31 (1.20–1.43) |
| Bone pain | Some vs. none | 1.62 (1.12–2.33) | 1.61 (1.10–2.37) | —c | —c |
| Bone pain | A lot vs. none | 2.70 (1.65–4.42) | 2.47 (1.44–4.22) | —c | —c |
aCox model for pain and death adjusted for potential confounders of disease burden including age at enrollment, first PSA level on-study, Gleason score, disease state at enrollment (mHSPC vs. CRPC), de novo metastatic disease at baseline, and sites of metastases at baseline.
bSE is inflated as all imputed datasets led to Hessian matrices that were not positive definite for the worst pain scale.
cLongitudinal models for bone pain not fit because categorical outcomes are currently incompatible with joint longitudinal survival model capabilities in JM R package.
TABLE 4.
Stratified analyses for the association between pain scales at enrollment and death, IRONMAN Registry 2017–2023
| mHSPC (N = 573) | CRPC (N = 306) | ||||
|---|---|---|---|---|---|
| Pain scale | Comparison | Age-only model HR (95% CI) | Fully-adjusted modela HR (95% CI) |
Age-only model HR (95% CI) |
Fully-adjusted modela HR (95% CI) |
| EORTC scale | 10-points on 0–100 scale | 1.08 (1.00–1.17) | 1.08 (0.99–1.18) | 1.15 (1.06–1.24) | 1.12 (1.03–1.22) |
| Average pain | 1 point on 1–10 scale | 1.14 (0.99–1.30) | 1.12 (0.97–1.30) | 1.31 (1.11–1.54) | 1.25 (1.06–1.47) |
| Worst pain | 1 point on 1–10 scale | 1.12 (1.02–1.24) | 1.13 (1.01–1.26) | 1.21 (1.07–1.38) | 1.18 (1.05–1.32) |
| Bone pain | Some vs. none | 1.02 (0.57–1.83) | 0.98 (0.54–1.79) | 2.54 (1.69–3.81) | 2.43 (1.56–3.79) |
| Bone pain | A lot vs. none | 2.48 (1.11–5.52) | 2.22 (0.99–4.98) | 3.19 (1.22–8.32) | 3.01 (1.00–9.06) |
| White (N = 704 total, 137 deaths) | Black (N = 175 total, 37 deaths) | ||||
| Pain scale | Comparison |
Age-only model
HR (95% CI) |
Fully-adjusted model
b
HR (95% CI) |
Age-only model
HR (95% CI) |
Fully-adjusted model
b
HR (95% CI) |
| EORTC scale | 10 points on 0–100 scale | 1.11 (1.04–1.18) | 1.10 (1.02–1.18) | 1.11 (1.04–1.19) | 1.09 (0.98–1.21) |
| Average pain | 1 point on 1–10 scale | 1.21 (1.07–1.37) | 1.20 (1.04–1.37) | 1.25 (1.11–1.40) | 1.24 (1.06–1.44) |
| Worst pain | 1 point on 1–10 scale | 1.14 (1.04–1.25) | 1.14 (1.04–1.25) | 1.26 (1.11–1.42) | 1.27 (1.05–1.53) |
| Bone pain | Some vs. none | 1.56 (1.05–2.32) | 1.53 (1.02–2.29) | 1.64 (0.59–4.50) | 2.85 (0.73–11.15) |
| Bone pain | A lot vs. none | 2.76 (1.64–4.64) | 2.56 (1.47–4.46) | 2.61 (0.88–7.80) | 3.57 (1.02–12.55) |
aCox model for pain and death adjusted for age at enrollment, first PSA level on-study, Gleason score, metastatic status at baseline, de novo metastases at baseline, and sites of metastases at baseline.
bCox model for pain and death adjusted for age at enrollment, first PSA level on-study, Gleason score, disease state at enrollment (mHSPC vs. CRPC), metastatic status at baseline, de novo metastases at baseline, and sites of metastases at baseline.
For the EORTC pain scale, average pain rating, and worst pain rating, more frequent or severe pain longitudinally was associated with an increased risk of mortality (Table 3). A one-point increase in average pain severity at each timepoint longitudinally was associated with an average 34% higher hazard of mortality (95% CI: 1.21–1.48). In the longitudinal survival models, the association between more frequent or severe pain and risk of death was larger than in the respective survival models for baseline pain.
Discussion
We found a high prevalence of pain at the time of enrollment in the IRONMAN registry across multiple aspects of pain, including the interference of pain with daily life, average and worst pain ratings, and presence of bone pain. While the majority of participants reported at least some pain on any scale at study enrollment, the mean pain interference level as reported on the EORTC scale in our population was less burdensome than that of the EORTC reference population in 2008 by approximately 10 points on a 100-point scale. (30) This is likely due to the EORTC population including a more representative advanced prostate cancer population in addition to only including individuals who have not yet begun cancer treatment.
In our study population, Black participants reported more pain than White participants across all four pain scales at study enrollment. Though not described in this study, our group previously explored longitudinal pain in this population, finding that pain increased over the first year of follow-up similarly for both Black and White participants (10). As race is a social construct, we believe that the racial disparities we see in pain in our study are a result of structural racism and social determinants of health impacting the experience, reporting, and management of pain. We believe that these factors (including variables like education, employment status, disease characteristics, and type of health center at which the participant receives care) are all mediators of the association between race and pain. Given the incompleteness of data for variables representing social determinants of health in IRONMAN and the lack of collection of information on income and insurance status, we chose to descriptively assess the association between race and pain in this study population. We hope that this study lays the groundwork for future studies that are better equipped for targeted mediation analyses with more complete data on social factors impacting reporting of pain to disentangle mechanisms and potential points for intervention.
One potential mechanism underlying the racial disparities in pain that we see at enrollment could be whether disease characteristics were more severe in Black participants compared with White participants (as is typically the case in the general population of individuals with prostate cancer), where greater disease burden leads to increased pain. Interestingly, we found that Black and White participants had a similar prevalence of high-grade tumors, CRPC, and de novo metastatic cancer in our study; only PSA levels at enrollment were significantly elevated in Black participants. It will be important for future studies to investigate the different dimensions of pain in this population to identify additional mechanisms upon which to intervene to improve survivorship, including how patients experience the different dimensions of pain, racial bias in provider acknowledgment of pain and analgesic prescribing patterns, and access to prostate cancer therapies.
Regarding the association between pain and survival, we found that higher pain on all four pain scales at study enrollment was associated with higher mortality after controlling for measures of disease burden and adjusting SEs to account for potential correlation within study site. We also found that worse pain interference, average pain, and worst pain scales longitudinally was associated with higher mortality independent of clinical factors associated with increased disease burden. Our results show similar associations between pain interference and survival compared with previous randomized controlled trials of disease-directed therapies for CRPC or mHSPC in which participants are largely White, well resourced, and without other major medical comorbidities (13–15). As an observational study with eligibility criteria only requiring being diagnosed with mHSPC or CRPC and receiving no more than 90 days of treatment at the time of enrollment, IRONMAN is more inclusive of individuals from different races and socioeconomic backgrounds compared with traditional clinical trials. Though there is still a need to make observational studies like IRONMAN more accessible to more representative patient populations, our study validates these previous clinical trial results in a more real-world population of individuals with advanced prostate cancer in the United States compared with these previous studies.
The biologic mechanisms causing pain are complex and poorly understood, involving the interplay between tumor, bone, inflammatory, and nerve cells. In addition, pain can be caused by the cancer itself or specific cancer therapies and is also influenced by structural racism and individual-level factors (31–36). Because of this, it is challenging to ascertain the mechanism by which pain is associated with a worse prognosis in our study. The IRONMAN registry collects a number of biologic samples that will soon allow a deeper understanding of the biologic drivers of cancer-associated pain, potentially leading to more effective palliative therapies.
There are several potential limitations of this analysis. First, potential unmeasured confounding by prostate cancer therapy could bias our results because lines of therapy in IRONMAN are currently in the process of being extracted. While ADT and ARSIs (the most commonly received therapies in this population) can alleviate pain due to cancer in untreated disease, they have also been associated with exacerbation of osteoarthritic and back pain (37, 38), leading to a downward bias in our HRs. Second, we did not have access to information on prescription and use of analgesics in the IRONMAN population, which is important given their impact on the experience and reporting of pain as well as racial differences in prescriptions of analgesics in patients with cancer (7). As the association between pain and survival is similar for both Black and White participants in our study population, we do not think that racial differences in prescribing patterns of analgesics is greatly impacting our results; however, the overall use of analgesics and their mediating role of the pain-survival relationship in the study population as a whole remains unclear. Third, survivor bias may be inflating our survival estimates as IRONMAN eligibility criteria allows for any diagnosis of advanced prostate cancer as long as there has been no longer than 90 days of systemic cancer-directed treatment at the time of enrollment. As such, it is possible that there are individuals whose pain at diagnosis increases their likelihood to miss follow-up visits or die prior to enrolling in IRONMAN, leading to a stronger true association between pain and all-cause mortality in the overall population of individuals diagnosed with advanced prostate cancer compared with the estimates we see here. Finally, these results may not be generalizable to individuals who choose not to participate in IRONMAN or individuals receiving care at other health centers within or outside of the United States. Centers participating in IRONMAN tend to be highly resourced and located in urban environments; individuals living in more rural areas of the United States or receiving care at urban centers with less clinical trial infrastructure could have different distributions and trajectories of pain and survival.
Our analysis deepens the understanding of racial disparities in pain and survival in individuals with advanced prostate cancer. Black participants experienced substantially higher pain at study enrollment compared with White participants, and more pain is associated with higher mortality independent of covariates related to disease burden. Our analysis highlights the need for further investigation of the experience and management of pain in this population in addition to the biologic drivers of the association between pain and mortality to improve survivorship, particularly for Black individuals experiencing the most severe pain and highest prostate cancer mortality.
Supplementary Material
Study sites for IRONMAN participants by race (N = 38 sites)
Number of participants receiving each therapy category between 3 months prior to enrollment and any time after enrollment stratified by disease state and self-reported race (N=19 therapy categories), N (%)
Correlation coefficients between baseline pain scale scores
Reasons for being off-study by self-reported race
80th percentile survival and 95% confidence intervals for each category of pain at study enrollment
Baseline EORTC pain scale Cox model results from sensitivity analysis for missing indicator values during MICE procedure
Baseline average pain scale Cox model results from sensitivity analysis for missing indicator values during MICE procedure
Baseline worst pain scale Cox model results from sensitivity analysis for missing indicator values during MICE procedure
Baseline bone pain scale Cox model results from sensitivity analysis for missing indicator values during MICE procedure
Longitudinal observation status of questionnaires and reasons for being off-study throughout follow-up
Selection of study participants exclusion flowchart
Kaplan-Meier survival curve by self-reported race
Kaplan-Meier survival curve by disease state at study enrollment
Further information guiding the choice of methods and a glossary of technical terms
Acknowledgments
E.M. Rencsok was supported by the NCI (F30CA264965) and the National Institute of General Medical Sciences (T32GM007753, T32GM144273). L.A. Mucci is a Prostate Cancer Foundation young investigator. D.J. George and L.A. Mucci are recipients of the Prostate Cancer Foundation Challenge Award. The International Registry for Men with Advanced Prostate Cancer (IRONMAN, NCT 03151629) is funded by Amgen, AstraZeneca, Astellas, Bayer, Janssen, Merck, and Sanofi. This project is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the NIH.
Footnotes
Note: Supplementary data for this article are available at Cancer Research Communications Online (https://aacrjournals.org/cancerrescommun/).
Authors’ Disclosures
K.A. Autio reports grants from Prostate Cancer Foundation during the conduct of the study; grants from Pfizer, Amgen, AstraZeneca, Parker Institute of Cancer Immunotherapy, and Janssen outside the submitted work. A.K. Morgans reports personal fees from Astellas, AstraZeneca, Janssen, Exelixis, Lantheus, Novartis, Telix, and Sanofi; grants and personal fees from Bayer, Myovant, Pfizer outside the submitted work. P. Barata reports personal fees from AVEO Oncology, Exelixis, Caris Life Sciences, UroToday, AstraZeneca, Targeted Oncology, Bristol Myers Squibb, MJH Life Sciences, Seagen, Bayer, and Eisai; grants and personal fees from Pfizer, Myovant, Merck outside the submitted work. H.H. Cheng reports grants from Clovis Oncology, Color Genomics, Janssen, Medivation, Promontory Pharmaceutics; personal fees from AstraZeneca and Sanofi; and other from UpToDate during the conduct of the study. R. Green reports other from Movember Foundation, Amgen, Inc, AstraZeneca Pharmaceuticals LP, Astellas Pharma Global Development, Inc, Bayer HealthCare Pharmaceuticals, Genzyme Corporation, Janssen Scientific Affairs, LLC, and Merck Sharp & Dohme Corp. during the conduct of the study. E.I. Heath reports other from Astellas Pharma, Arvinas, AstraZeneca, Bayer, BioXcel Therapeutics, Bristol Myers Squibb, Calibr, Calithera Biosciences Inc., Caris Life Sciences, Corcept Therapeutics, Corvis Pharmaceuticals, Daiichi Sankyo Inc., Eisai Inc., Exelixis, Five Prime Therapeutics, Fortis Therapeutics, GlaxoSmithKline, Gilead Sciences Inc., Harpoon Therapeutics, Hoffman-La Roche, Infinity Pharmaceuticals, iTeos Therapeutics, Janssen Research & Development LLC, Merck Sharp & Dohme, Merck, Mirati Therapeutics, Modra Pharmaceuticals, Novartis, Oncolys BioPharma, Peloton Therapeutics Inc., Pfizer, Pharmacyclics LLC, POINT Biopharma, Sanofi, and Seattle Genetics outside the submitted work. R.R. McKay reports consultant: AstraZeneca, Aveo, Bayer, Blue Earth Diagnostics, Bristol-Myers Squibb, Calithera, Caris, Dendreon, Eisai, Exelixis, Johnson & Johnson, Lilly, Merck, Myovant, Novartis, Pfizer, Sanofi, SeaGen, Sorrento Therapeutics, Telix, Tempus; institutional research support: Artera, AstraZeneca, Bayer, Bristol Myers Squibb, Exelixis, Oncternal, Tempus. D.E. Rathkopf reports grants from DOD PCCTC during the conduct of the study. S.T. Tagawa reports grants from Department of Defense during the conduct of the study; grants and personal fees from Sanofi, Astellas, Pfizer, Janssen, Gilead, Novartis, Ambrx, Seagen, POINT Biopharma, Clarity, Bayer, Merck; grants from Amgen; personal fees from Convergent Therapeutics, Telix, and Blue Earth outside the submitted work. Y.E. Whang reports grants from Alliance Foundation Trials, Clovis Oncology, Novartis, and Arvinas during the conduct of the study; personal fees from Janssen Pharmaceutical outside the submitted work. C. Ragin reports grants from Prostate cancer clinical trials Consortium and NCI during the conduct of the study. L.A. Mucci reports grants from Prostate Cancer Foundation during the conduct of the study; grants from AstraZeneca, Janssen; grants and non-financial support from Veracyte; personal fees from Bayer and Convergent Therapeutics outside the submitted work. No disclosures were reported by the other authors.
Authors’ Contributions
E.M. Rencsok: Conceptualization, data curation, software, formal analysis, funding acquisition, validation, investigation, visualization, methodology, writing-original draft, project administration, writing-review and editing. N. Slopen: Conceptualization, formal analysis, visualization, writing-review and editing. H.D. McManus: Writing-review and editing. K.A. Autio: Data curation, methodology, writing-review and editing. A.K. Morgans: Data curation, methodology, writing-review and editing. L. McSwain: Conceptualization, methodology, writing-review and editing. P. Barata: Data curation, methodology, writing-review and editing. H.H. Cheng: Data curation, methodology, writing-review and editing. R. Dreicer: Data curation, methodology, writing-review and editing. T. Gerke: Data curation, software, formal analysis, methodology, writing-review and editing. R. Green: Data curation, software, formal analysis, methodology, project administration, writing-review and editing. E.I. Heath: Data curation, methodology, writing-review and editing. L.E. Howard: Data curation, software, formal analysis, methodology, writing-review and editing. R.R. McKay: Data curation, methodology, writing-review and editing. J. Nowak: Data curation, methodology, writing-review and editing. S. Pileggi: Data curation, software, formal analysis, methodology, writing-review and editing. M.M. Pomerantz: Data curation, methodology, writing-review and editing. D.E. Rathkopf: Data curation, methodology, writing-review and editing. S.T. Tagawa: Data curation, methodology, writing-review and editing. Y.E. Whang: Data curation, methodology, writing-review and editing. C. Ragin: Data curation, methodology, writing-review and editing. F.T. Odedina: Conceptualization, resources, methodology, writing-review and editing. P.W. Kantoff: Conceptualization, data curation, supervision, methodology, writing-review and editing. J. Vinson: Conceptualization, resources, data curation, supervision, methodology, writing-review and editing. P. Villanti: Conceptualization, data curation, supervision, methodology, writing-review and editing. S. Haneuse: Conceptualization, resources, software, formal analysis, supervision, visualization, methodology, writing-original draft, writing-review and editing. L.A. Mucci: Conceptualization, resources, data curation, formal analysis, supervision, funding acquisition, investigation, visualization, methodology, writing-original draft, writing-review and editing. D.J. George: Conceptualization, data curation, formal analysis, supervision, methodology, writing-review and editing.
References
- 1. Devasia TP, Mariotto AB, Nyame YA, Etzioni R. Estimating the number of men living with metastatic prostate cancer in the United States. Cancer Epidemiol Biomarkers Prev 2023;32:659–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aggarwal RR, Feng FY, Small EJ. Emerging categories of disease in advanced prostate cancer and their therapeutic implications. Oncology 2017;31:467–74. [PubMed] [Google Scholar]
- 3. Drudge-Coates L, Oh WK, Tombal B, Delacruz A, Tomlinson B, Ripley AV, et al. Recognizing symptom burden in advanced prostate cancer: a global patient and caregiver survey. Clin Genitourin Cancer 2018;16:e411–9. [DOI] [PubMed] [Google Scholar]
- 4. Gater A, Abetz-Webb L, Battersby C, Parasuraman B, McIntosh S, Nathan F, et al. Pain in castration-resistant prostate cancer with bone metastases: a qualitative study. Health Qual Life Outcomes 2011;9:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Coghill RC. Individual differences in the subjective experience of pain: new insights into mechanisms and models. Headache 2010;50:1531–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guess TJ. The social construction of whiteness: racism by intent, racism by consequence. Crit Sociol 2006;32:649–73. [Google Scholar]
- 7. Enzinger AC, Ghosh K, Keating NL, Cutler DM, Clark CR, Florez N, et al. Racial and ethnic disparities in opioid access and urine drug screening among older patients with poor-prognosis cancer near the end of life. J Clin Oncol 2023;41:2511–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thompson IM, Tangen CM, Tolcher A, Crawford ED, Eisenberger M, Moinpour CM. Association of African-American ethnic background with survival in men with metastatic prostate cancer. J Natl Cancer Inst 2001;93:219–25. [DOI] [PubMed] [Google Scholar]
- 9. Lubeck DP, Kim H, Grossfeld G, Ray P, Penson DF, Flanders SC, et al. Health related quality of life differences between black and white men with prostate cancer: data from the cancer of the prostate strategic urologic research endeavor. J Urol 2001;166:2281–5. [PubMed] [Google Scholar]
- 10. Rencsok EM, Slopen N, Autio K, Morgans A, McSwain L, Barata P, et al. Quality of life in the year after new diagnosis with advanced prostate cancer for Black and White individuals living in the US. Qual Life Res 2023;32:3209–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fink R. Pain assessment: the cornerstone to optimal pain management. Proc (Bayl Univ Med Cent) 2000;13:236–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boring BL, Walsh KT, Nanavaty N, Ng BW, Mathur VA. How and why patient concerns influence pain reporting: a qualitative analysis of personal accounts and perceptions of others’ use of numerical pain scales. Front Psychol 2021;12:663890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Halabi S, Vogelzang NJ, Kornblith AB, Ou SS, Kantoff PW, Dawson NA, et al. Pain predicts overall survival in men with metastatic castration-refractory prostate cancer. J Clin Oncol 2008;26:2544–9. [DOI] [PubMed] [Google Scholar]
- 14. Roy S, Morgan SC, Wallis CJD, Sun Y, Spratt DE, Malone J, et al. Association of dynamic change in patient-reported pain with survival in metastatic castrate sensitive prostate cancer-exploratory analysis of LATITUDE study. Prostate Cancer Prostatic Dis 2023;26:96–104. [DOI] [PubMed] [Google Scholar]
- 15. Roviello G, Gallicchio R, Bozza G, Rodriquenz MG, Aieta M, Storto G. Pain predicts overall survival in men with metastatic castration-resistant prostate cancer treated with radium-223. Onco Targets Ther 2019;12:9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Oudard S, Banu E, Medioni J, Scotte F, Banu A, Levy E, et al. What is the real impact of bone pain on survival in patients with metastatic hormone-refractory prostate cancer treated with docetaxel? BJU Int 2009;103:1641–6. [DOI] [PubMed] [Google Scholar]
- 17. Mucci LA, Vinson J, Gold T, Gerke T, Filipenko J, Green RM, et al. IRONMAN: a novel international registry of men with advanced prostate cancer. JCO Glob Oncol 2022;8:e2200154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fayers PM, Aaronson NK, Bjordal K, Groenvold M, Curran D, Bottomley A. The EORTC QLQ-C30 scoring manual. 3rd ed. Brussels: European Organisation for Research and Treatment of Cancer; 2001. [Google Scholar]
- 19. Gamper EM, Musoro JZ, Coens C, Stelmes JJ, Falato C, Groenvold M, et al. Minimally important differences for the EORTC QLQ-C30 in prostate cancer clinical trials. BMC Cancer 2021;21:1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ford ME, Havstad SL, Kart CS. Assessing the reliability of the EORTC QLQ-C30 in a sample of older African American and Caucasian adults. Qual Life Res 2001;10:533–41. [DOI] [PubMed] [Google Scholar]
- 21. Mathias SD, Crosby RD, Qian Y, Jiang Q, Dansey R, Chung K. Estimating minimally important differences for the worst pain rating of the brief pain inventory-short form. J Support Oncol 2011;9:72–8. [DOI] [PubMed] [Google Scholar]
- 22. Atkinson TM, Rosenfeld BD, Sit L, Mendoza TR, Fruscione M, Lavene D, et al. Using confirmatory factor analysis to evaluate construct validity of the Brief Pain Inventory (BPI). J Pain Symptom Manage 2011;41:558–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Robinson DW, Zhao N, Dawkins F, Qi M, Revicki D. Pain questionnaire performance in advanced prostate cancer: comparative results from two international clinical trials. Qual Life Res 2013;22:2777–86. [DOI] [PubMed] [Google Scholar]
- 24. Beaumont JL, Butt Z, Li R, Cella D. Meaningful differences and validity for the NCCN/FACT-P symptom index: an analysis of the ALSYMPCA data. Cancer 2019;125:1877–85. [DOI] [PubMed] [Google Scholar]
- 25. Esper P, Mo F, Chodak G, Sinner M, Cella D, Pienta KJ. Measuring quality of life in men with prostate cancer using the functional assessment of cancer therapy-prostate instrument. Urology 1997;50:920–8. [DOI] [PubMed] [Google Scholar]
- 26. Azur MJ, Stuart EA, Frangakis C, Leaf PJ. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res 2011;20:40–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Little RJ, Rubin DB. Statistical analysis with missing data. 2nd ed. Hoboken: John Wiley & Sons; 2014. [Google Scholar]
- 28. Ibrahim JG, Chu H, Chen LM. Basic concepts and methods for joint models of longitudinal and survival data. J Clin Oncol 2010;28:2796–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rizopoulos D. JM: an R package for the joint modelling of longitudinal and time-to-event data. J Stat Softw 2010;35:1–33.21603108 [Google Scholar]
- 30. Scott NW, Fayers PM, Aaronson NK, Bottomley A, de Graeff A, Groenvold M, et al. EORTC QLQ-C30 reference values. Brussels: European Organisation for Research and Treatment of Cancer; 2008. [Google Scholar]
- 31. White K, Haas JS, Williams DR. Elucidating the role of place in health care disparities: the example of racial/ethnic residential segregation. Health Serv Res 2012;47:1278–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kirby JB, Kaneda T. Neighborhood socioeconomic disadvantage and access to health care. J Health Soc Behav 2005;46:15–31. [DOI] [PubMed] [Google Scholar]
- 33. Mahal BA, Chen YW, Muralidhar V, Mahal AR, Choueiri TK, Hoffman KE, et al. National sociodemographic disparities in the treatment of high-risk prostate cancer: Do academic cancer centers perform better than community cancer centers? Cancer 2016;122:3371–7. [DOI] [PubMed] [Google Scholar]
- 34. Darcey E, Pereira G, Salter A, Fritschi L, Leavy J, Ambrosini GL, et al. The impact of lifestyle-related factors on survival after a prostate cancer diagnosis. Eur Urol 2019;75:884–5. [DOI] [PubMed] [Google Scholar]
- 35. Helgstrand JT, Røder MA, Klemann N, Toft BG, Brasso K, Vainer B, et al. Diagnostic characteristics of lethal prostate cancer. Eur J Cancer 2017;84:18–26. [DOI] [PubMed] [Google Scholar]
- 36. Smith ZL, Eggener SE, Murphy AB. African-American prostate cancer disparities. Curr Urol Rep 2017;18:81. [DOI] [PubMed] [Google Scholar]
- 37. Drevinskaite M, Dadoniene J, Miltiniene D, Patasius A, Smailyte G. Clinical medicine association between androgen deprivation therapy and the risk of inflammatory rheumatic diseases in men with prostate cancer: nationwide cohort study in lithuania. J Clin Med 2022;11:2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zobniw CM, Causebrook A, Fong MK. Clinical use of abiraterone in the treatment of metastatic castration-resistant prostate cancer. Res Rep Urol 2014;6:97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study sites for IRONMAN participants by race (N = 38 sites)
Number of participants receiving each therapy category between 3 months prior to enrollment and any time after enrollment stratified by disease state and self-reported race (N=19 therapy categories), N (%)
Correlation coefficients between baseline pain scale scores
Reasons for being off-study by self-reported race
80th percentile survival and 95% confidence intervals for each category of pain at study enrollment
Baseline EORTC pain scale Cox model results from sensitivity analysis for missing indicator values during MICE procedure
Baseline average pain scale Cox model results from sensitivity analysis for missing indicator values during MICE procedure
Baseline worst pain scale Cox model results from sensitivity analysis for missing indicator values during MICE procedure
Baseline bone pain scale Cox model results from sensitivity analysis for missing indicator values during MICE procedure
Longitudinal observation status of questionnaires and reasons for being off-study throughout follow-up
Selection of study participants exclusion flowchart
Kaplan-Meier survival curve by self-reported race
Kaplan-Meier survival curve by disease state at study enrollment
Further information guiding the choice of methods and a glossary of technical terms
Data Availability Statement
The data analyzed in this study are available from the Prostate Cancer Clinical Trials Consortium. Restrictions apply to the availability of these data, which were used under agreement for this study. Data are available from the authors upon reasonable request with the permission of the Prostate Cancer Clinical Trials Consortium.