Abstract
Objective
Patients diagnosed with colorectal cancer living in more deprived areas experience worse survival than those in more affluent areas. Those living in more deprived areas face barriers to accessing timely, quality healthcare. These barriers may contribute to socioeconomic inequalities in survival. We evaluated the literature for any association between socioeconomic group, hospital delay and treatments received among patients with colorectal cancer in the UK, a country with universal healthcare.
Design
MEDLINE, EMBASE, CINAHL, CENTRAL, SCIE, AMED and PsycINFO were searched from inception to January 2023. Grey literature, including HMIC, BASE and Google Advanced Search, and forward and backward citation searches were conducted. Two reviewers independently reviewed titles, abstracts and full-text articles. Observational UK-based studies were included if they reported socioeconomic measures and an association with either hospital delay or treatments received. The QUIPS tool assessed bias risk, and a narrative synthesis was conducted. The review is reported to Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020.
Results
41 of the 7209 identified references were included. 12 studies evaluated 7 different hospital intervals. There was a significant association between area-level deprivation and a longer time from first presentation in primary care to diagnosis. 32 studies evaluated treatments received. There were socioeconomic inequalities in surgery and chemotherapy but not radiotherapy.
Conclusion
Patients with colorectal cancer face inequalities across the cancer care continuum. Further research is needed to understand why and what evidence-based actions can reduce these inequalities in treatment. Qualitative research of patients and clinicians conducted across various settings would provide a rich understanding of the complex factors that drive these inequalities. Further research should also consider using a causal approach to future studies to considerably strengthen the interpretation. Clinicians can try and mitigate some potential causes of colorectal cancer inequalities, including signposting to financial advice and patient transport schemes.
PROSPERO registration number
CRD42022347652.
Keywords: SOCIAL MEDICINE, Health policy, Adult oncology, Epidemiology, PUBLIC HEALTH, Systematic Review
STRENGTHS AND LIMITATIONS OF THIS STUDY.
The searches were extensive—conducted across eight databases, supplemented with citation searching and hand-searching websites.
The search strategy was validated.
The inclusion of non-peer-reviewed literature was a key strength.
Due to heterogeneous methods, meta-analysis was not possible.
Introduction
Colorectal cancer is the second most common cause of cancer-related death in the UK.1 Survival has improved since the 1990s but lags behind comparable countries.2 There are also survival gradients within countries, including those with universal healthcare, such as the UK and Australia.3 In particular, patients living in more deprived areas experience significantly worse survival outcomes.1 3 Healthcare systems can contribute to these inequalities, as treatment differences likely compound differential outcomes across populations.2
Timely diagnosis and treatment are also essential, with delays associated with worse outcomes. The Aarhus statement suggested a framework for measuring these delays, categorising the patient journey into patient, doctor and system intervals.4 Specifically, the system interval was defined as the period from primary care-initiated investigations or referral to the commencement of treatment.4 Socioeconomic circumstances can impact this interval and yet is comparatively under-researched.
Existing inequalities have been exacerbated by the COVID-19 pandemic, with vulnerable patient groups disproportionately affected by suboptimal care.5 The evolution of precision medicine and the development of new technologies and surgical approaches will likely worsen existing inequalities, a process described as the ‘inverse equity law’.6 Worryingly, disparities in access to precision oncology are already well documented.7 Understanding where inequalities are in the pathways of care for patients with colorectal cancer is essential to inform policy and identify areas of further research to target evidence-based action.
We evaluated the literature for any association between socioeconomic group, system interval and treatment among patients with colorectal cancer in the UK. By focusing exclusively on studies conducted within a single country with a universal healthcare system, our systematic review homogenised the healthcare infrastructure, policy and patient population, ensuring a more interpretable analysis of disparities in cancer care with greater scope for policy impact.
Methods
This systematic review was registered with PROSPERO (CRD42022347652). The review is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 statement (online supplemental appendix S1).8
bmjopen-2023-080467supp001.pdf (763.3KB, pdf)
Patient and public involvement
This study was discussed with Involve Hull, a patient and public involvement group affiliated with the author’s institution. The review was considered necessary by all members of the group.
Eligibility criteria
Published and grey literature observational studies were considered for inclusion if relevant outcomes of patients with a primary diagnosis of colorectal cancer (International Classification of Diseases Tenth Revision C18-C20) in the UK were reported.
Outcomes were only included if they had been analysed by a measure of socioeconomic status (eg, an area-based measure such as the Index of Multiple Deprivation or individual measures such as occupation). The relevant outcomes were defined as follows:
The association between socioeconomic status and the length of the system interval, as defined by the Aarhus statement.4 Any part of the system interval could have been measured.
Receipt of cancer-directed treatment (defined as receipt of surgery, chemotherapy or radiotherapy). Studies evaluating palliative or supportive care only were excluded.
Information sources
The following bibliographic databases were searched from inception to 26 January 2023: MEDLINE, EMBASE, AMED, PsycINFO, CINAHL, CENTRAL and Science Citation Index Expanded.
The grey literature was searched using HMIC, BASE, NICE Evidence Search and Google Advanced Search on 26 January 2023. In addition, 12 websites were systematically hand-searched, and backward and forward citation searches were conducted on 30 March 2023 (details in online supplemental appendix S2).
Search strategy
The search strategies are listed in online supplemental appendix S3. The search strategy was developed and validated in conjunction with SG, an information specialist (details in online supplemental appendix S4). BAP-S and another reviewer (MHS or KS) independently screened all titles and abstracts against the predetermined eligibility criteria. The full texts of eligible titles and abstracts were obtained and independently screened for inclusion. Conflicts were resolved by consensus.
Data collection process
One researcher (BAP-S) extracted information from the included studies, collating the relevant data onto a data extraction form. A second author (KS) checked the extracted data, and discrepancies were reconciled by consensus. The data items and effect measures that were sought for extraction are detailed in online supplemental appendix S5.
Study risk of bias assessment
Two researchers (BAP-S and KS) independently evaluated the study risk of bias against domains adapted from the Quality in Prognosis Studies (QUIPS) tool.9 Each domain was judged to have a high, moderate or low risk of bias, with the evaluations collated onto a pre-prepared form (online supplemental appendix S6).
Risk of bias assessments informed the narrative synthesis, with greater weight given to studies with a lower risk of bias. A study’s evidence was considered ‘strong’ if there were no high risk of bias categories, ‘moderate’ if there was a high risk of bias in one category and ‘weak’ if there were two or more categories at high risk of bias. However, studies were not excluded based on this.
Synthesis methods
A narrative synthesis was conducted, according to the synthesis without meta-analysis in systematic reviews reporting guideline.10 An overall assessment of the association between socioeconomic status and each outcome was made, considering the consistency and strength of supporting evidence from each study. Coefficients were extracted based on multivariable models. Given the inherent methodological heterogeneity, diverse patient populations, varying measures of deprivation and significant statistical heterogeneity observed across the included studies, a meta-analysis was deemed inappropriate as it could yield misleading or oversimplified results. While a meta-analysis was not conducted, forest plots were generated to visually illustrate the observed outcomes in individual studies.
Results
Study selection
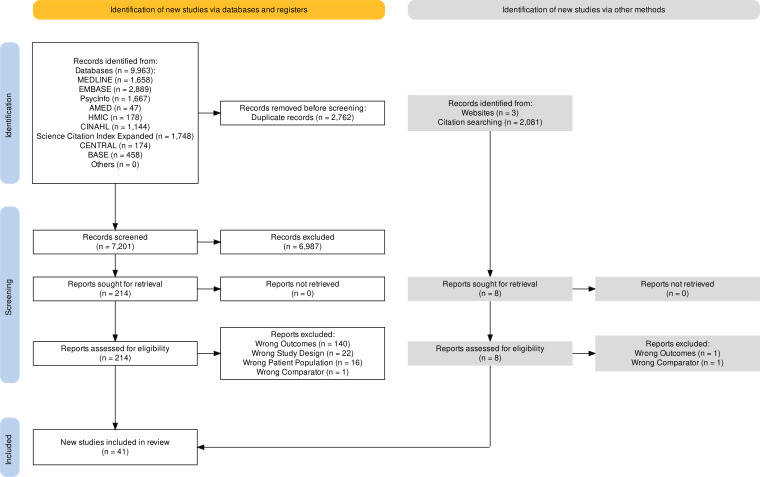
The database searches yielded 7201 studies, 214 of which were retrieved for full-text screening. An additional six studies were identified from the grey literature. Overall, 41 studies were included (figure 1).11
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram of included studies.
Study characteristics
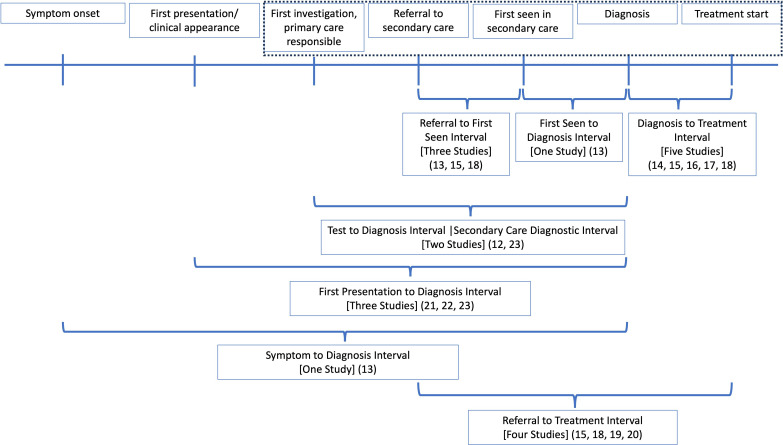
The characteristics of the included studies are summarised in online supplemental appendix S7. The system interval was examined in 12 studies, with 7 different time points evaluated, summarised in figure 2.12–23 In total, 15 studies reported the receipt of surgery,19 20 24–36 7 studies evaluated surgical variation,37–43 14 studies reported the receipt of chemotherapy,19 20 24–27 44–51 7 reported the receipt of radiotherapy19 20 25–27 43 52 and 2 reported the receipt of any treatment.17 46
Figure 2.
Time intervals evaluated in the included studies. The blue dotted line indicates the system interval defined by the Aarhus statement. Studies that included any aspect of this system interval were included, even if the interval commenced before the system interval defined here.
In total, 32 of the 41 studies adjusted or stratified for at least one other factor.12–26 32–41 44–49 51 The remaining nine studies provided unadjusted rates.27–31 42 43 50 52
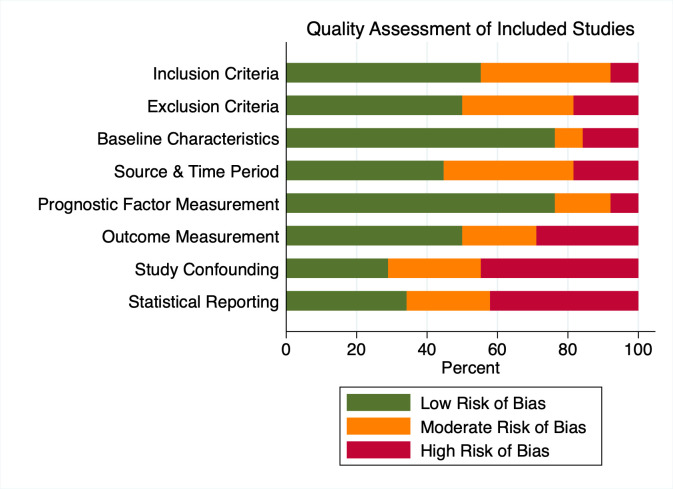
Risk of bias in studies
Assessments of the risk of bias are summarised in figure 3 and online supplemental appendix S6. The domain most at risk of bias was study confounding, with 16 studies at high risk of bias.13 27–31 39–43 47–50 52 Although some of these studies conducted adjusted analyses, important factors such as stage were unaccounted for.
Figure 3.
Risk of bias in the included studies. For each element the proportion of studies with high, moderate and low risk of bias is illustrated.
Results of studies reporting variations in the system interval
Referral to first-seen interval
Three studies evaluated the referral to first-seen interval.13 15 18 Two studies estimated the odds of being seen by a specialist within 2 weeks of referral; one demonstrated reduced unadjusted odds (OR 0.80, 95% CI 0.70 to 0.91),18 while there was no significant association in the other (OR 0.95, 95% CI 0.87 to 1.03) after adjusting for age, stage and site (colon vs rectal) (online supplemental appendix S8).15
Another study used generalised linear modelling to estimate the association between occupation and the number of days to see a specialist after referral, adjusting for age, marital status and ethnicity.13 This study reported no significant association (p>0.05).13 Overall, the evidence was inconclusive for an association between deprivation and the referral to first-seen interval (table 1 and online supplemental appendix S8).
Table 1.
Narrative synthesis—assessment of the relationship between deprivation, the system interval and treatment received
| Specific outcome reported | Overall assessment/conclusion | Studies, n (subjects, n) | Studies demonstrating adverse effect of deprivation | Studies demonstrating protective effect of deprivation | Studies demonstrating no impact of deprivation | Further information |
| Referral to first-seen interval | Inconclusive impact of deprivation on the length of the referral to first-seen interval | 3 (86 644) | 1 strong18 | – | 1 strong15 1 weak13 |
Online supplemental appendix S8: Results of studies reporting variations in the system interval |
| First-seen to diagnosis interval | Inconclusive impact of deprivation on the length of the first-seen to diagnosis interval | 1 (15 891) | – | 1 weak13 | – | |
| Referral to treatment interval | Inconclusive impact of deprivation on the length of the referral to treatment interval | 4 (69 892) | 1 strong15 | – | 1 strong18 2 weak19 20 |
|
| Diagnosis to treatment interval | Inconclusive impact of deprivation on the length of the diagnosis to treatment interval | 5 (292 502) | 1 strong15 1 moderate17 |
1 strong18 | 2 strong14 16 | |
| Test to diagnosis/secondary care diagnostic interval (SCDI) | No impact of deprivation on the length of the test to diagnosis/SCDI | 2 (68 794) | – | – | 2 strong12 23 | |
| First presentation to diagnosis interval | Deprivation associated with increased length of the first presentation to diagnosis interval | 3 (at least 6951) |
3 strong*21–23 | – | 1 strong*23 | |
| Symptom to diagnosis interval | Inconclusive impact of deprivation on the length of the symptom to diagnosis interval | 1 (15 891) | – | – | 1 weak13 | |
| Likelihood of receipt of surgery | Strong evidence for reduced surgery with increasing deprivation | 11 (374 869) | 2 strong*24 36 1 moderate27 4 weak26 28 30 31 |
1 strong25 | 1 strong*36 3 weak19 20 29 |
Online supplemental appendix S9: Results—likelihood of receipt of surgery |
| Likelihood of receipt of liver resection | Strong evidence for reduced liver resection with increasing deprivation | 3 (285 194) | 3 strong32–34 | – | – | Online supplemental appendix S9: Results—likelihood of receipt of surgery |
| Likelihood of receipt of pulmonary resection | No impact of deprivation on likelihood of pulmonary resection | 1 (80 869) | – | – | 1 strong35 | Online supplemental appendix S9: Results—likelihood of receipt of surgery |
| Likelihood of receipt of APER | Strong evidence for increased likelihood of APER versus AR with increasing deprivation | 6 (128 946) | 1 strong37 4 weak39–42 |
– | 1 weak38 | Online supplemental appendix S11: Results—likelihood of surgical variation |
| Likelihood of receipt of TPE | No impact of deprivation on likelihood of TPE versus PPE with increasing deprivation | 1 (120) | – | – | 1 weak43 | Online supplemental appendix S11: Results—likelihood of surgical variation |
| Likelihood of receipt of chemotherapy | Strong evidence for reduced chemotherapy with increasing deprivation | 13 (251 862) | 4 strong24 25 44 45 2 moderate*27 47 5 weak*19 26 46 48 50 |
– | 1 moderate*27 3 weak*20 46 49 |
Online supplemental appendix S13: Results—likelihood of receipt of chemotherapy |
| Likelihood of receipt of combination chemotherapy | Strong evidence for reduced use of combination chemotherapy with increasing deprivation | 1 (8750) | 1 strong51 | – | – | Online supplemental appendix S13: Results—likelihood of receipt of chemotherapy |
| Likelihood of receipt of radiotherapy | No impact of deprivation on likelihood of radiotherapy | 7 (79 053) | – | 1 moderate27 1 weak52 |
1 strong25 4 weak19 20 26 43 |
Online supplemental appendix S15: Results—likelihood of receipt of radiotherapy |
| Likelihood of receipt of any treatment | Moderate evidence for reduced any treatment with increasing deprivation | 2 (90 138) | 1 moderate17 1 weak46 |
– | – | Online supplemental appendix S16: Results—likelihood of receipt of any treatment |
*Studies represented in more than one column due to different conclusions depending on the underlying cancer type (colon vs rectal cancer).23 27 36 46
APER, abdominoperineal resection; AR, anterior resection; TPE, total pelvic exenteration.
First-seen to diagnosis interval
One study estimated the association between occupation and the number of days from the first hospital appointment to communication of diagnosis.13 A significant association was demonstrated (p=0.028), but no magnitude or direction of effect was provided. The evidence was, therefore, inconclusive (table 1 and online supplemental appendix S8).
Diagnosis to treatment interval
Five studies evaluated the diagnosis to treatment interval.14–18 Two estimated the number of days from diagnosis to major surgery, adjusting for stage, sex, age, grade and morphology.14 16 There was no significant impact of deprivation on the length of the diagnosis to treatment interval demonstrated in these two studies (coefficient 0.99, 95% CI 0.97 to 1.02)14 (coefficient 0.21, 95% CI −0.55 to 0.98) (online supplemental appendix S8).16
Two studies evaluated the likelihood of commencing treatment within 31 days from the date a treatment plan was agreed on.15 18 One study demonstrated increased unadjusted odds (OR 1.28, 95% CI 1.14 to 1.44),18 while the other presented reduced adjusted odds of patients from the most deprived areas commencing treatment within 31 days (OR 0.91, 95% CI 0.84 to 0.98) (online supplemental appendix S8).15
Another study calculated the likelihood of treatment for the most deprived quintile across several time points. They demonstrated reduced adjusted odds of treatment within 1 week (OR 0.78, 95% CI 0.72 to 0.84), 1 month (OR 0.84, 95% CI 0.78 to 0.90) and 2–3 months (OR 0.91, 95% CI 0.85 to 0.98) but non-reduced odds at 4–6 months (OR 1.07, 95% CI 0.96 to 1.18) after the first contact with the health system (online supplemental appendix S8).17
Overall, the evidence for an association between deprivation and length of the diagnosis to treatment interval was inconclusive (table 1 and online supplemental appendix S8).
Test to diagnosis interval/secondary care diagnostic interval (SCDI)
One study evaluated the SCDI, defined as the period between the date of the first interaction with secondary care and the date of diagnosis.12 This study evaluated the factors associated with an interval greater than the median, adjusting for sex, age, stage, comorbidities, ethnicity, route to diagnosis and additional diagnostic tests.12 The odds of a longer interval were not significantly increased for patients from the most deprived quintile (OR 1.07, 95% CI 1.00 to 1.13) (online supplemental appendix S8).
Another study evaluated the time from the first investigation to cancer diagnosis.23 The authors conducted quantile regression, adjusting for age, comorbidities, sex, test type and symptom category, focusing on the median and 75th centiles.23 There was no significant association between deprivation and interval length (coefficient 0.7, 95% CI −2.7 to 4.1) (online supplemental appendix S8).
Overall, there was no evidence of a prolonged SCDI or test-to-diagnosis interval for patients from the most deprived background (table 1 and online supplemental appendix S8).
First presentation to diagnosis interval
Three studies evaluated the time from the first symptom or feature of colorectal cancer in primary care records to diagnosis.21–23 One study demonstrated an association between deprivation and a longer interval in two of three econometric analyses (pre-to-post difference-in-differences 95% CI −0.03 to 0.2 and p=0.147 or event-study difference-in-differences 95% CI 0.002 to 0.136 and p=0.043 or semiparametric varying-coefficient analysis significance stated but not reported).21 The other two studies conducted quantile regression, focusing on the median and 75th centiles, adjusting for age, comorbidities, sex and type of symptom.22 23 Both studies demonstrated an association between the most deprived quintile and a longer first presentation to diagnosis interval for patients with colon cancer (eg, adjusted median interval of 204 vs 126 days, p=0.04).22 Meanwhile, there was no such association among patients with rectal cancer,23 possibly reflecting that patients with rectal cancer are more likely to present with localising symptoms (online supplemental appendix S8).
Overall, three robust studies provided evidence that patients from the most deprived quintile experienced a longer first presentation to diagnosis interval (table 1 and online supplemental appendix S8).
Symptom to diagnosis interval
One study estimated the effect of occupation on the time between a patient’s first symptom and diagnosis.13 No significant effect was demonstrated, adjusting for ethnicity, age, marital status and sex (p>0.05) (table 1 and online supplemental appendix S8).13
Referral to treatment interval
Four studies evaluated the time from referral to treatment.15 18–20 Two studies demonstrated no significant association between deprivation and the likelihood of commencing treatment within 62 days of referral (range of ORs 1.02–1.07).18 19 Another study demonstrated reduced odds of patients commencing treatment within 62 days of referral, adjusted for age, stage, referral interval and first treatment received (OR 0.82, 95% CI 0.74 to 0.91) (online supplemental appendix S8).15
Meanwhile, one study estimated HRs for the time between referral and first treatment, adjusting for stage, distance and presentation.20 There was no significant association between deprivation and time to treatment (HR 1.24, 95% CI 0.93 to 1.67) (online supplemental appendix S8).
Overall, the association between deprivation and this interval was inconclusive (table 1 and online supplemental appendix S8).
Results of studies reporting treatment inequalities
Results of studies reporting likelihood of receipt of primary surgery
The outcome of interest was primary surgery in 11 studies, here defined as resection of the tumour.19 20 24–31 36 Five studies clearly defined the outcome as a tumour resection,25 27–29 36 while the received surgical procedure was not identified in the other six studies (online supplemental appendix S9).19 20 24 26 30 31
Across seven studies, adjustment was made for different factors: age,19 20 24–26 29 36 stage,19 20 24–26 36 sex,19 24–26 29 36 comorbidity,24 25 36 site (colon vs rectum),19 25 36 distance or time to hospital,20 26 year of diagnosis,24 36 region19 and histology, grade and presentation.36 Meanwhile, four studies provided only rates of patients receiving surgery (online supplemental appendix S9).27 28 30 31
Six studies presented reduced odds of surgery for patients from the most deprived background (range of ORs 0.32–0.99).24 26–28 30 31 One study presented increased odds of not receiving surgery among the most deprived patients with rectal cancer (OR 1.35, 95% CI 1.22 to 1.49) but no significant association among patients with colon cancer (OR 0.96, 95% CI 0.87 to 1.07).36 Meanwhile, three studies demonstrated no association (range of ORs 0.52–0.88).19 20 29
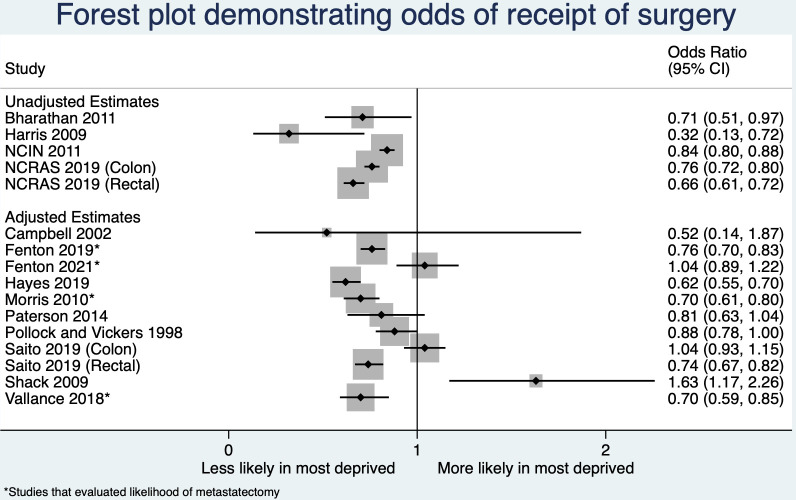
One study revealed a higher likelihood of surgery for patients from the most deprived background (OR 1.63, 95% CI 1.17 to 2.26).25 Additionally, the study reported increased odds of surgery in older age groups. These findings, which were unexpected, were confirmed by consulting the author. However, it is important to note that this analysis was based on regional data from a historical cohort of colorectal cancers diagnosed between 1997 and 2004. While the reported methodology appears robust, the results of this small study are opposed to other studies (see figure 4) and cautious interpretation is required.
Figure 4.
Forest plot demonstrating the odds of receipt of surgery in the most deprived versus the least deprived patient group.
Figure 4 displays a forest plot, which provides an overview of the findings from multiple studies investigating the likelihood of undergoing surgery for colorectal cancer. The plot reveals that a majority of studies considering primary surgery (10/12) indicate a decrease in the likelihood of surgical intervention among patients belonging to the most deprived group. Overall, the evidence strongly supports the hypothesis that patients from the most deprived group are less likely to receive surgery (table 1 and online supplemental appendix S9).
Results of studies reporting likelihood of receipt of surgery for oligometastatic disease
Four studies examined the receipt of surgery in presumed oligometastatic disease, all adjusted for age, stage, comorbidity and site (colon vs rectal).32–35 Three studies examined the receipt of liver resection, demonstrating significantly reduced odds of resection for patients from the most deprived group (range of ORs 0.70–0.76).32–34 One study examined the receipt of pulmonary resection, with no significant association demonstrated between deprivation and the likelihood of resection (OR 1.04, 95% CI 0.89 to 1.22) (table 1 and online supplemental appendix S9).35 and Figure 4 displays a forest plot, providing an overview of the findings from these studies, each highlighted with an asterisk.
Results of studies reporting likelihood of surgical variation
Seven studies evaluated variations in surgery.37–43 Six reported rates or odds of abdominoperineal resection (APER) or anterior resection (AR).37–42 Five studies adjusted for variables, including age,37–40 sex,37–41 stage,37 38 year of diagnosis or resection,37–41 surgeon workload37 38 and admission type.37–40 Online supplemental appendix S10 displays a forest plot, providing an overview of the findings from these studies. Five of the seven studies demonstrated that APER was significantly more likely than AR for patients from the most deprived areas (range of ORs 1.37–1.64) (table 1 and online supplemental appendix S11).37 39–42
Meanwhile, one study of 120 patients presented unadjusted rates of total pelvic exenteration (TPE) compared with partial pelvic exenteration.43 There was a non-significant association between deprivation and the unadjusted odds of TPE (OR 1.75, 95% CI 0.55 to 5.68) (table 1 and online supplemental appendix S11).
Results of studies reporting likelihood of receipt of chemotherapy
In total, 13 studies examined whether patients received any chemotherapy,19 20 24–27 44–50 11 of which conducted adjusted analyses.19 20 24–26 44–49 Six studies evaluated the use of adjuvant chemotherapy.24 44 45 49–51 Two studies evaluated the use of palliative chemotherapy.24 46 Meanwhile, the intent of chemotherapy was unknown in the remaining seven studies.19 20 25–27 47 48
Online supplemental appendix S12 displays a forest plot, providing an overview of the findings from the studies. Eight studies demonstrated reduced adjusted odds of chemotherapy for patients from the most deprived group (range of ORs 0.44–0.99).19 24–26 44 45 47 48 One study demonstrated reduced adjusted odds for patients from the most deprived group with colon (OR 0.45, 95% CI 0.27 to 0.77) but not rectal cancer (OR 0.73, 95% CI 0.36 to 1.50).46 Two studies did not show a significant association between deprivation and receipt of chemotherapy (range of ORs 0.49–2.13) (online supplemental appendix S13).20 49
Meanwhile, two studies presented unadjusted rates.27 50 One demonstrated reduced odds of chemotherapy for the most deprived patients with colorectal cancer (OR 0.31, 95% CI 0.09 to 0.91).50 The other demonstrated reduced odds of chemotherapy for the most deprived patients with colon (OR 0.85, 95% CI 0.81 to 0.89) but not rectal cancer (OR 1.03, 95% CI 0.95 to 1.11) (online supplemental appendix S13).27
One study examined the receipt of combination versus single-agent chemotherapy, adjusting for age, sex, ethnicity, tumour size, lymph node yield and year of diagnosis.51 However, no adjustment was made for comorbidity. Patients from the most deprived area had significantly reduced odds of receiving combination chemotherapy (OR 0.50, 95% CI 0.42 to 0.59) (online supplemental appendix S13).51
Five of the six studies evaluating the use of adjuvant chemotherapy demonstrated inequalities.24 44 45 50 51 Meanwhile, both studies evaluating the use of palliative chemotherapy demonstrated similar inequalities.24 46 Overall, the evidence strongly supports the hypothesis that patients from the most deprived group are less likely to receive chemotherapy or combination adjuvant chemotherapy (table 1 and online supplemental appendix S13).
Results of studies reporting likelihood of receipt of radiotherapy
Seven studies reported receipt of radiotherapy by socioeconomic group.19 20 25–27 43 52 Two studies evaluated the use of neoadjuvant radiotherapy.19 43 One study evaluated patterns of preoperative and postoperative radiotherapy.52 The intent of radiotherapy was unknown in four studies.20 25–27
Three studies conducted analyses that adjusted for important factors, including age,20 25 26 stage,20 25 26 sex,25 26 distance or journey time,20 26 tumour site (colon vs rectum)20 and comorbidity.25 None of these studies demonstrated a significant association between deprivation group and radiotherapy (range of ORs 0.85–0.99). Online supplemental appendix S14 presents a forest plot, providing an overview of the findings from these studies. The remaining four studies reported unadjusted rates of radiotherapy.19 27 43 52 Two of these studies demonstrated increased odds of radiotherapy for patients from the most deprived group (range of ORs 1.33–1.39).27 52 The other two studies looked at rates of neoadjuvant radiotherapy specifically and did not show a significant association between deprivation and odds of treatment (range of ORs 1.00–1.15) (online supplemental appendix S15).19 43
Overall, there was no evidence to support an association between socioeconomic status and receipt of radiotherapy (table 1 and online supplemental appendix S15). This conclusion may depend on the intent of radiotherapy and would, therefore, have been stronger if all outcomes were differentiated by intent (eg, neoadjuvant or palliative).
Results of studies reporting receipt of any treatment
Two studies evaluated the likelihood of any treatment by deprivation quintile, adjusting for age,17 46 sex46 and stage.17 46 It was assumed this meant receiving surgery, radiotherapy or chemotherapy. However, these outcomes needed to be more clearly defined. For the most socioeconomically deprived quintile, both studies reported significantly reduced odds of any treatment within 6 months of diagnosis46 or 6 months of the first contact with the NHS (range of ORs 0.54–0.87) (table 1 and online supplemental appendix S16).17
Discussion
Main findings
This is the first systematic review to evaluate what is already known about the relationship between socioeconomic status, the system interval and the treatment that patients with colorectal cancer receive.
Diagnostic and treatment delays
There were seven intervals evaluated. The evidence for system delays was generally inconclusive, given substantial heterogeneity in methods and outcomes. However, there was substantial evidence that the first presentation to diagnosis interval was longer for patients from the most deprived background, depending on the underlying site. The underlying reasons require further elucidation using qualitative studies. This would help us understand the extent to which these delays are driven by patient or healthcare factors and how these can be addressed. Possible causes include missed appointments due to competing demands such as employment or care responsibilities.53 54 Other reasons might include complex transport and travel arrangements causing difficulties in attending appointments.53 54
Surgery in the management of colorectal cancer
There was strong evidence for inequalities in primary surgery. However, most studies had limitations; few adjusted for stage, most combined colon and rectal cancers and many included patients diagnosed before 2010.
There was also strong and consistent evidence that patients from the most deprived areas were less likely to undergo a liver resection and were more likely to undergo an APER than AR. APER is associated with a worse quality of life and is generally considered less preferable if a less deforming surgery is possible.
Despite adjustment, socioeconomic inequalities were frequently observed. This suggests the presence of uncaptured factors such as comorbidity or frailty. There may also have been variations in access to specialist care, financial and employment factors, patient choice, health-seeking behaviours and health literacy, all of which warrant further investigation.55–57
Chemotherapy in the management of colorectal cancer
There was strong evidence that patients from more deprived areas were less likely to receive chemotherapy or combination adjuvant chemotherapy. Trust in clinicians, financial and employment factors, social support, adequate communication and provision of information are critical in influencing the use of chemotherapy.58–61 These, among other uncaptured factors such as comorbidity or frailty, could be responsible for the observed inequalities.
Radiotherapy in the management of rectal cancer
There was no evidence that patients from more deprived areas were less likely to receive radiotherapy. The absence of observed inequalities could reflect the nature of this outpatient treatment and the availability of patient transport. This is compared with, for example, surgery, which necessitates hospital admission and prolonged time away from work and social support. A lung cancer study similarly demonstrated a greater likelihood of radiotherapy but a reduced likelihood of surgery among less affluent patients.62
Strengths and weaknesses
This systematic review identified many studies and employed a robust methodology. The process of identifying search terms was thorough, and the search was validated. The searches were extensive, conducted across eight databases, supplemented with citation searching and a thorough examination of the grey literature. These additional search methods identified six studies.27 28 35 36 44 52 Inclusion of non-peer-reviewed literature was also a key strength of this review.25 27 28 36
The included studies were, however, heterogeneous in the methodology and populations studied. Out of 41 studies, only 15 included patients diagnosed after 2010.12 14 18 21–23 27 32 33 35 36 43–45 51 Of the six studies evaluating the system interval in patients diagnosed since 2010, four demonstrated some inequalities.18 21–23 Meanwhile, seven out of the nine studies that evaluated inequalities in treatments among patients diagnosed after 2010 demonstrated the presence of inequalities.27 32 33 36 44 45 51 Therefore, although most studies included patients from over a decade ago, inequalities persisted in recent cohorts despite a national focus on reducing inequalities.
Another limitation was that studies frequently analysed colorectal cancer as a single disease despite differences in presentation and management. Significantly, no study used causal inference approaches, exemplified by an absence of reported directed acyclic graphs.63 The methods used could have introduced a bias known as the ‘table 2 fallacy’, whereby estimates from regression models are mistakenly interpreted.63 Using a causal approach to future studies would considerably strengthen the interpretation and, thus, meaningfully impact policy.64
Implications for policy and practice
Due to significant heterogeneity across studies, we could not firmly conclude whether patients from more deprived backgrounds systematically experience longer system intervals. However, COVID-19 detrimentally impacted cancer diagnostic activity for most patients, especially those in deprived areas.5 It is important to ensure measures are in place to monitor the system interval for patients most at risk of delays.5
There was strong evidence of socioeconomic inequalities in surgery and chemotherapy. Some inequalities may partly be due to wording in clinical guidelines. For example, the National Institute for Health and Care Excellence advises that primary surgery for colorectal cancer is ‘offered’ (a strong recommendation); the same guideline advises liver resection be ‘considered’ (less certain benefit).65 Similarly, adjuvant chemotherapy can be estimated to reduce the risk of death in stage III disease by 10%–15%. However, there is a significant risk of long-term toxicity. Patients must carefully weigh the potential harms and benefits of these less strongly recommended treatments. Shared decision-making is vital. Inequalities will result when some patients experience better shared decision-making and can cover the costs of additional treatment, such as time off work.66
Clinicians can mitigate some of the effects of deprivation. Such strategies may include referring patients for pre rehabilitation, tailored communication and ensuring patients are aware of appropriate financial support and transport schemes.66
Further studies are needed to evaluate for inequalities in novel treatments. In the era of precision oncology and an ever-increasing armamentarium of novel treatments, the marginal benefits of new therapies must not just be experienced by the most affluent. A prostate cancer study exemplified this, demonstrating that patients from more deprived backgrounds living at greater distances from specialist centres were significantly less likely to receive robotic prostatectomy.67 If we accept the benefit of newer surgical technology and techniques, such as robotic surgery, these should be available for all patients no matter where they live.
Future research
Further research evaluating the whole of the system interval is needed. Further research should also aim to understand why deprivation is associated with a reduced likelihood of chemotherapy and surgery. In particular, observational research of recent cohorts should use causal inference. Beyond this, qualitative research will be of great value in gaining a richer insight into the causes and drivers of these inequalities.
Conclusions
Despite a healthcare system that provides free healthcare at the point of access, there were unexplained socioeconomic inequalities in surgery, chemotherapy and aspects of the system interval. Further research is needed to understand the variations in treatment between socioeconomic groups.
Differences in patient selection for treatment have been linked with worse colorectal cancer survival within and between countries, with evidence of improved outcomes when care is aligned with optimal pathways.68 Eliminating inequalities could narrow survival gaps within and between countries. These findings will interest policymakers, clinicians and researchers worldwide, as inequalities in cancer care and outcomes of different socioeconomic groups have been recognised across healthcare jurisdictions.
Supplementary Material
Footnotes
Twitter: @bapickwellsmith
Contributors: BAP-S: conceptualisation, developed search strategy, screening, data curation and formal analysis, project administration and writing—original draft. KS: conceptualisation, screening, data curation and formal analysis and review of the manuscript. MHS: screening and review of the manuscript. SG: developed the search strategy and manuscript review. ML: conceptualisation, supervision and review of the manuscript. UM: conceptualisation, developed search strategy, screening, data curation and formal analysis, supervision and manuscript review. BAP-S has overall responsibility as guarantor.
Funding: This work was funded in whole by Yorkshire Cancer Research (award reference number HEND405). Yorkshire Cancer Research has not been involved in any other aspect of the project, such as the design, data collection, analysis or interpretation.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information. This published article and its supplementary information files include all data generated or analysed during this study.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1.Cancer Research UK . Bowel cancer Statistics: bowel cancer mortality. n.d. Available: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/bowel-cancer#heading-Zero
- 2.Araghi M, Arnold M, Rutherford MJ, et al. Colon and rectal cancer survival in seven high-income countries 2010–2014: variation by age and stage at diagnosis (the ICBP SURVMARK-2 project). Gut 2021;70:114–26. 10.1136/gutjnl-2020-320625 [DOI] [PubMed] [Google Scholar]
- 3.Afshar N, English DR, Blakely T, et al. Differences in cancer survival by area-level socio-economic disadvantage: a population-based study using cancer registry data. PLOS ONE 2020;15:e0228551. 10.1371/journal.pone.0228551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weller D, Vedsted P, Rubin G, et al. The aarhus statement: improving design and reporting of studies on early cancer diagnosis. Br J Cancer 2012;106:1262–7. 10.1038/bjc.2012.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watt T, Sullivan R, Aggarwal A. Primary care and cancer: an analysis of the impact and inequalities of the COVID-19 pandemic on patient pathways. BMJ Open 2022;12:e059374. 10.1136/bmjopen-2021-059374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lyratzopoulos G, Barbiere JM, Rachet B, et al. Changes over time in socioeconomic inequalities in breast and rectal cancer survival in England and Wales during a 32-year period (1973–2004): the potential role of health care. Ann Oncol 2011;22:1661–6. 10.1093/annonc/mdq647 [DOI] [PubMed] [Google Scholar]
- 7.Martin AR, Kanai M, Kamatani Y, et al. Clinical use of current polygenic risk scores may exacerbate health disparities. Nat Genet 2019;51:584–91. 10.1038/s41588-019-0379-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayden JA, van der Windt DA, Cartwright JL, et al. Assessing bias in studies of prognostic factors. Ann Intern Med 2013;158:280–6. 10.7326/0003-4819-158-4-201302190-00009 [DOI] [PubMed] [Google Scholar]
- 10.Campbell M, McKenzie JE, Sowden A, et al. Synthesis without meta-analysis (swim) in systematic reviews: reporting guideline. BMJ 2020;368:l6890. 10.1136/bmj.l6890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haddaway NR, Page MJ, Pritchard CC, et al. PRISMA2020: an R package and shiny app for producing PRISMA 2020-compliant flow diagrams, with Interactivity for optimised digital transparency and open synthesis. Campbell Syst Rev 2022;18:e1230. 10.1002/cl2.1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pearson C, Fraser J, Peake M, et al. Establishing population-based surveillance of diagnostic timeliness using linked cancer registry and administrative data for patients with colorectal and lung cancer. Cancer Epidemiol 2019;61:111–8. 10.1016/j.canep.2019.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neal RD, Allgar VL. Sociodemographic factors and delays in the diagnosis of six cancers: analysis of data from the ‘national survey of NHS patients: cancer Br J Cancer 2005;92:1971–5. 10.1038/sj.bjc.6602623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saito MK, Quaresma M, Fowler H, et al. Exploring socioeconomic differences in surgery and in time to elective surgery for colon cancer in England: population-based study. Cancer Epidemiol 2021;71:101896. 10.1016/j.canep.2021.101896 [DOI] [PubMed] [Google Scholar]
- 15.Hayes L, Adams J, McCallum I, et al. Age-related and socioeconomic inequalities in timeliness of referral and start of treatment in colorectal cancer: a population-based analysis. J Epidemiol Community Health 2021;75:1–9. 10.1136/jech-2020-214232 [DOI] [PubMed] [Google Scholar]
- 16.Redaniel MT, Martin RM, Blazeby JM, et al. The association of time between diagnosis and major resection with poorer colorectal cancer survival: a retrospective cohort study. BMC Cancer 2014;14:642. 10.1186/1471-2407-14-642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lejeune C, Sassi F, Ellis L, et al. Socio-economic disparities in access to treatment and their impact on colorectal cancer survival. Int J Epidemiol 2010;39:710–7. 10.1093/ije/dyq048 [DOI] [PubMed] [Google Scholar]
- 18.Di Girolamo C, Walters S, Gildea C, et al. Can we assess cancer waiting time targets with cancer survival? A population-based study of individually linked data from the national cancer waiting times monitoring dataset in England, 2009-2013. PLOS ONE 2018;13:e0201288. 10.1371/journal.pone.0201288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paterson HM, Mander BJ, Muir P, et al. Deprivation and access to treatment for colorectal cancer in Southeast Scotland 2003–2009. Colorectal Dis 2014;16:51–7. 10.1111/codi.12442 [DOI] [PubMed] [Google Scholar]
- 20.Campbell NC, Elliott AM, Sharp L, et al. Impact of deprivation and rural residence on treatment of colorectal and lung cancer. Br J Cancer 2002;87:585–90. 10.1038/sj.bjc.6600515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Price S, Zhang X, Spencer A. Measuring the impact of national guidelines: what methods can be used to uncover time-varying effects for healthcare evaluations Social Science & Medicine 2020;258:113021. 10.1016/j.socscimed.2020.113021 [DOI] [PubMed] [Google Scholar]
- 22.Benitez Majano S, Lyratzopoulos G, de Wit NJ, et al. Mental health morbidities and time to cancer diagnosis among adults with colon cancer in England. JAMA Netw Open 2022;5:e2238569. 10.1001/jamanetworkopen.2022.38569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Majano SB, Lyratzopoulos G, Rachet B, et al. Do presenting symptoms, use of pre-diagnostic endoscopy and risk of emergency cancer diagnosis vary by comorbidity burden and type in patients with colorectal cancer Br J Cancer 2022;126:652–63. 10.1038/s41416-021-01603-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayes L, Forrest L, Adams J, et al. Age-related inequalities in colon cancer treatment persist over time: a population-based analysis. J Epidemiol Community Health 2019;73:34–41. 10.1136/jech-2018-210842 [DOI] [PubMed] [Google Scholar]
- 25.Shack L. What factors influence socioeconomic inequalities in colorectal cancer survival? Phd thesis. London School of Hygiene and Tropical Medicine; 2009. Available: https://researchonline.lshtm.ac.uk [Google Scholar]
- 26.Jones AP, Haynes R, Sauerzapf V, et al. Travel time to hospital and treatment for breast, colon, rectum, lung, ovary and prostate cancer. Eur J Cancer 2008;44:992–9. 10.1016/j.ejca.2008.02.001 [DOI] [PubMed] [Google Scholar]
- 27.National Cancer Registration and Analysis Service . “Chemotherapy, radiotherapy and surgical tumour Resections in England. Workbook 1: "chemotherapy, radiotherapy and tumour resection by tumour and patient characteristics in England, 2013-2015"” National Cancer Registration and Analysis Service Website; 2018. Available: http://www.ncin.org.uk/cancer_type_and_topic_specific_work/topic_specific_work/main_cancer_treatments [Accessed Oct 2022]. [Google Scholar]
- 28.National Cancer Intelligence Network . Major surgical Resections England, 2004-2006. National Cancer Registration and Analysis Service Website; 2011. Available: http://www.ncin.org.uk/publications/reports/reports_archive [Accessed Oct 2022]. [Google Scholar]
- 29.Pollock AM, Vickers N. Deprivation and emergency admissions for cancers of colorectum, lung, and breast in South East England: ecological study. BMJ 1998;317:245–52. 10.1136/bmj.317.7153.245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bharathan B, Welfare M, Borowski DW, et al. Impact of deprivation on short- and long-term outcomes after colorectal cancer surgery. Br J Surg 2011;98:854–65. 10.1002/bjs.7427 [DOI] [PubMed] [Google Scholar]
- 31.Harris AR, Bowley DM, Stannard A, et al. Socioeconomic deprivation adversely affects survival of patients with Rectal cancer. Br J Surg 2009;96:763–768. 10.1002/bjs.6621 [DOI] [PubMed] [Google Scholar]
- 32.Vallance AE, van der Meulen J, Kuryba A, et al. Socioeconomic differences in selection for liver resection in metastatic colorectal cancer and the impact on survival. Eur J Surg Oncol 2018;44:1588–94. 10.1016/j.ejso.2018.05.024 [DOI] [PubMed] [Google Scholar]
- 33.Fenton HM, Taylor JC, Lodge JPA, et al. Variation in the use of resection for colorectal cancer liver metastases. Ann Surg 2019;270:892–8. 10.1097/SLA.0000000000003534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morris EJA, Forman D, Thomas JD, et al. Surgical management and outcomes of colorectal cancer liver metastases. Br J Surg 2010;97:1110–8. 10.1002/bjs.7032 [DOI] [PubMed] [Google Scholar]
- 35.Fenton HM, Finan PJ, Milton R, et al. National variation in pulmonary metastasectomy for colorectal cancer. Colorectal Dis 2021;23:1306–16. 10.1111/codi.15506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saito MK. Socioeconomic inequalities in colorectal cancer survival in England and Japan [Phd]. London School of Hygiene & Tropical Medicine; 2019. [Google Scholar]
- 37.Morris E, Quirke P, Thomas JD, et al. Unacceptable variation in abdominoperineal excision rates for rectal cancer: time to intervene. Gut 2008;57:1690–7. 10.1136/gut.2007.137877 [DOI] [PubMed] [Google Scholar]
- 38.Nicholson GA, Morrison DS, Finlay IG, et al. Quality of care in rectal cancer surgery. exploring influencing factors in the west of Scotland. Colorectal Dis 2012;14:731–9. 10.1111/j.1463-1318.2011.02754.x [DOI] [PubMed] [Google Scholar]
- 39.Tilney HS, Heriot AG, Purkayastha S, et al. A national perspective on the decline of abdominoperineal resection for rectal cancer. Ann Surg 2008;247:77–84. 10.1097/SLA.0b013e31816076c3 [DOI] [PubMed] [Google Scholar]
- 40.Raine R, Wong W, Scholes S, et al. Social variations in access to hospital care for patients with colorectal, breast, and lung cancer between 1999 and 2006: retrospective analysis of hospital episode statistics. BMJ 2010;340:b5479. 10.1136/bmj.b5479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tilney H, Lovegrove RE, Smith JJ, et al. The national bowel cancer project: social deprivation is an independent predictor of nonrestorative rectal cancer surgery. Dis Colon Rectum 2009;52:1046–53. 10.1007/DCR.0b013e3181a65f41 [DOI] [PubMed] [Google Scholar]
- 42.Smith JJ, Tilney HS, Heriot AG, et al. Social deprivation and outcomes in colorectal cancer. Br J Surg 2006;93:1123–31. 10.1002/bjs.5357 [DOI] [PubMed] [Google Scholar]
- 43.Radwan RW, Coyne PE, Jones HG, et al. Social deprivation in patients requiring pelvic exenterative surgery. Colorectal Dis 2016;18:684–7. 10.1111/codi.13274 [DOI] [PubMed] [Google Scholar]
- 44.Taylor JC, Swinson D, Seligmann JF, et al. Addressing the variation in adjuvant chemotherapy treatment for colorectal cancer: can a regional intervention promote national change Int J Cancer 2021;148:845–56. 10.1002/ijc.33261 [DOI] [PubMed] [Google Scholar]
- 45.Boyle JM, Kuryba A, Cowling TE, et al. Determinants of variation in the use of adjuvant chemotherapy for stage III colon cancer in England. Clinical Oncology 2020;32:e135–44. 10.1016/j.clon.2019.12.008 [DOI] [PubMed] [Google Scholar]
- 46.Crawford SM, Sauerzapf V, Haynes R, et al. Social and geographical factors affecting access to treatment of colorectal cancer: a cancer registry study. BMJ Open 2012;2:e000410. 10.1136/bmjopen-2011-000410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pitchforth E, Russell E, Van der Pol M. Access to specialist cancer care: is it equitable Br J Cancer 2002;87:1221–6. 10.1038/sj.bjc.6600640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McLeod A. Variation in the provision of chemotherapy for colorectal cancer. J Epidemiol Community Health 1999;53:775–81. 10.1136/jech.53.12.775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bailey C, Corner J, Addington-Hall J, et al. Treatment decisions in older patients with colorectal cancer: the role of age and multidimensional function. Eur J Cancer Care (Engl) 2003;12:257–62. 10.1046/j.1365-2354.2003.00409.x [DOI] [PubMed] [Google Scholar]
- 50.Hole DJ, McArdle CS. Impact of socioeconomic deprivation on outcome after surgery for colorectal cancer. Br J Surg 2002;89:586–90. 10.1046/j.1365-2168.2002.02073.x [DOI] [PubMed] [Google Scholar]
- 51.Hassan S, Miles A, Rachet B, et al. Variations in the type of adjuvant chemotherapy among stage III colon cancer patients in England. J Gastrointest Cancer January 5, 2023. 10.1007/s12029-022-00899-9 [DOI] [PubMed] [Google Scholar]
- 52.Morris EJA, Finan PJ, Spencer K, et al. Wide variation in the use of radiotherapy in the management of surgically treated rectal cancer across the English national health service. Clinical Oncology 2016;28:522–31. 10.1016/j.clon.2016.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jefferson L, Atkin K, Sheridan R, et al. Non-attendance at urgent referral appointments for suspected cancer: a qualitative study to gain understanding from patients and Gps. Br J Gen Pract 2019;69:e850–9. 10.3399/bjgp19X706625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilson R, Winnard Y. Causes, impacts and possible mitigation of non-attendance of appointments within the national health service: a literature review. J Health Organ Manag 2022;ahead-of-print:892–911. 10.1108/JHOM-11-2021-0425 [DOI] [PubMed] [Google Scholar]
- 55.Azzani M, Roslani AC, Su TT. The perceived cancer-related financial hardship among patients and their families: a systematic review. Support Care Cancer 2015;23:889–98. 10.1007/s00520-014-2474-y [DOI] [PubMed] [Google Scholar]
- 56.Ngan TT, Tien TH, Donnelly M, et al. Financial toxicity among cancer patients, survivors and their families in the United kingdom: a scoping review. J Public Health (Oxf) 2023;45:e702–13. 10.1093/pubmed/fdad143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Holden CE, Wheelwright S, Harle A, et al. The role of health literacy in cancer care: a mixed studies systematic review. PLOS ONE 2021;16:e0259815. 10.1371/journal.pone.0259815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Andrews C, Childers TC, Wiseman KD, et al. Facilitators and barriers to reducing chemotherapy for early-stage breast cancer: a qualitative analysis of interviews with patients and patient advocates. BMC Cancer 2022;22:141. 10.1186/s12885-022-09189-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cavalli-Björkman N, Glimelius B, Strang P. Equal cancer treatment regardless of education level and family support? A qualitative study of oncologists’ decision-making. BMJ Open 2012;2:e001248. 10.1136/bmjopen-2012-001248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Amalraj S, Starkweather C, Nguyen C, et al. Health literacy, communication, and treatment decision-making in older cancer patients. Oncology (Williston Park) 2009;23:369–75. [PubMed] [Google Scholar]
- 61.Busch EL, Martin C, DeWalt DA, et al. Functional health literacy, chemotherapy decisions, and outcomes among a colorectal cancer cohort. Cancer Control 2015;22:95–101. 10.1177/107327481502200112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tataru D, Spencer K, Bates A, et al. Variation in geographical treatment intensity affects survival of non-small cell lung cancer patients in England. Cancer Epidemiol 2018;57:13–23. 10.1016/j.canep.2018.09.001 [DOI] [PubMed] [Google Scholar]
- 63.Tennant PWG, Murray EJ, Arnold KF, et al. Use of directed Acyclic graphs (Dags) to identify confounders in applied health research: review and recommendations. Int J Epidemiol 2021;50:620–32. 10.1093/ije/dyaa213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Petticrew M, Whitehead M, Macintyre SJ, et al. Evidence for public health policy on inequalities: 1: the reality according to policymakers. J Epidemiol Community Health 2004;58:811–6. 10.1136/jech.2003.015289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.National Institute for Health and Care Excellence (NICE) . Colorectal cancer. NICE guideline [NG151]; 2020. [PubMed]
- 66.Fitch MI, Sharp L, Hanly P, et al. Experiencing financial toxicity associated with cancer in publicly funded healthcare systems: a systematic review of qualitative studies. J Cancer Surviv 2022;16:314–28. 10.1007/s11764-021-01025-7 [DOI] [PubMed] [Google Scholar]
- 67.Aggarwal A, Han L, Tree A, et al. Impact of centralization of prostate cancer services on the choice of radical treatment. BJU Int 2023;131:53–62. 10.1111/bju.15830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Te Marvelde L, McNair P, Whitfield K, et al. Alignment with indices of a care pathway is associated with improved survival: an observational population-based study in colon cancer patients. EClinicalMedicine 2019;15:42–50. 10.1016/j.eclinm.2019.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-080467supp001.pdf (763.3KB, pdf)
Data Availability Statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information. This published article and its supplementary information files include all data generated or analysed during this study.