Abstract
Objective
There is limited evidence on long-term thrombosis risk in patients with obstetric antiphospholipid syndrome (OAPS). This study aimed to investigate the clinical features and risk factors associated with the first thrombosis in patients with isolated OAPS.
Methods
Data from patients with isolated OAPS were collected. All patients were followed up until the first thrombotic event during or after delivery or until the end of the study. Logistic regression analysis identified independent risk factors associated with the first thrombosis in patients with isolated OAPS.
Results
The study enrolled 186 patients with OAPS. During a mean 5.4-year follow-up, 11 (5.9%) patients experienced thrombotic events. Multivariate binary logistic regression analysis revealed that triple-positive antiphospholipid antibodies (aPLs, OR=11.662, 95% CI=2.117 to 64.243, p=0.005) and hypocomplementemia (OR=9.047, 95% CI=1.530 to 53.495, p=0.015) were identified as independent risk factors for the first thrombosis in OAPS, after adjustment for low-dose aspirin and hydroxychloroquine.
Conclusions
Triple-positive aPLs and hypocomplementemia are risk factors for the first thrombosis in patients with OAPS.
Keywords: Antiphospholipid Syndrome, Risk Factors, Treatment
WHAT IS ALREADY KNOWN ON THIS TOPIC.
Limited evidence exists regarding the long-term risk of thrombosis in patients with obstetric antiphospholipid syndrome (OAPS); previous studies investigating risk factors for thrombosis in OAPS have produced inconsistent and inconclusive findings.
WHAT THIS STUDY ADDS
Triple-positive antiphospholipid antibodies and hypocomplementemia are risk factors for the first thrombosis in patients with OAPS.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Identifying the risk factors for the first thrombosis in OAPS could facilitate more effective management of these patients.
Introduction
Antiphospholipid syndrome (APS) is an autoimmune disease characterised by thrombotic and/or obstetric morbidity in the presence of persistent antiphospholipid antibodies (aPLs).1 APS can be further classified into two subtypes, thrombotic APS (TAPS) and obstetric APS (OAPS), which share similar antibody profiles but differ in pathogenic mechanisms and clinical presentations.2 3 At the time of diagnosis, only 13.5% of patients with APS experience both thrombosis and obstetric morbidity.4 Unlike patients with TAPS, individuals with OAPS do not exhibit an elevated risk of subclinical atherosclerosis.5 Previous studies investigating risk factors for thrombosis in OAPS have produced inconsistent and inconclusive findings (online supplemental table 1).2 4 6–16 This variability can be attributed to disparities in sample sizes, geographical locations and duration of follow-up among these studies. Noteworthy risk factors identified include additional cardiovascular factors, lupus anticoagulant (LA) positivity, presence of multiple aPLs and a higher adjusted Global Antiphospholipid Syndrome Score (aGAPSS).4 7 14
lupus-2023-001044supp001.pdf (63.8KB, pdf)
Primary thromboprophylaxis following delivery remains a topic of debate due to varying annual prevalence rates of thrombosis in OAPS, ranging from less than 1% to 6.1%.2 4 6–16 The divergent prevalence rates have led to conflicting opinions regarding the benefits of primary thromboprophylaxis. Currently, the strategies for primary prevention of thrombosis in OAPS are controversial. A meta-analysis suggests that primary prevention with low-dose aspirin (LDA) in patients with OAPS is associated with a reduced risk of thrombosis.17 However, a recent systematic review concludes that there is insufficient evidence to determine the efficacy of LDA in preventing primary thrombotic events in patients with OAPS.18
Identifying the risk factors for the first thrombosis in OAPS could facilitate more effective management of these patients. Therefore, this study aims to investigate the clinical features and risk factors associated with the first thrombotic event in patients with isolated OAPS.
Materials and methods
Patients
This study included 424 consecutive patients diagnosed with APS who were admitted to Peking University People’s Hospital between June 2008 and August 2022. All patients met the 2006 Sydney and 2023 American College of Rheumatology/European Alliance of Associations for Rheumatology classification criteria for APS (including 17 patients whose aPLs transitioned from high titres to low titres upon admission).1 19 20 Male patients and patients with a history of thrombosis prior to delivery were excluded. Clinical data from 186 patients with isolated OAPS were collected. Regular follow-up was conducted every 3–6 months for all participants. Patients were advised to promptly contact the research physicians if they experienced any symptoms of thrombosis, including deep vein thrombosis (DVT), pulmonary embolism (PE), stroke, etc.
The primary outcome of this study was the incidence of thrombotic events. The diagnosis of thromboembolism was based on objective imaging techniques. All patients were followed up at the rheumatology outpatient clinic. DVT and arterial thrombosis of limb were confirmed by Doppler ultrasound examination.21 PE was diagnosed by CT angiography.22 Stroke was diagnosed by MRI. Myocardial infarction was diagnosed by raised cardiac enzymes and appropriate ECG changes.23 The follow-up period was defined as the time from the first delivery until the occurrence of the first thrombotic event or the end of the study.
LA testing
The test of LA was performed using the simplified Dilute Russell’s Viper Venom Test (dRVVT) method on the Stago STA Compact Hemostasis System. STA (USA) provided the LA1 screening reagent and LA2 confirmatory reagent, and these were used in accordance with the manufacturer’s guidelines. The presence of a positive LA activity was established based on dRVVT ratios (LA1 screen/LA2 confirmation) exceeding the threshold of 1.2. aPL positivity was confirmed 12 weeks apart.
Data collection
This is a single-centre, observational cohort study with prospective clinical follow-up. We retrospectively collected information on hospitalised patients and prospectively enrolled new patients. Baseline data, including demographics, cardiovascular risk factors (hypertension, hyperlipidaemia, arteriosclerosis, diabetes), underlying autoimmune diseases, clinical manifestations, laboratory findings (aPLs) and post-delivery treatment, were collected. Serum levels of IgM/IgG anticardiolipin antibodies (aCL) and anti-β2-glycoprotein I antibodies (aβ2GPI) were measured by an ELISA kit from EUROIMMUN (Luebeck, Germany).24 The aGAPSS was calculated for each patient.25 During follow-up, the time from the first delivery to the first thrombotic event, types of thrombosis (venous or arterial) and site of thrombosis were recorded.
Statistical analysis
Analyses were conducted on all eligible patients, as well as primary APS (PAPS) and SLE-related APS (SLE-APS) to identify the risk factors for thrombosis. Descriptive analyses were performed using SPSS V.26.0. The single-sample Kolmogorov-Smirnov test was used to assess the distribution of the data. The independent sample Student’s t-test was employed to compare differences in variables with a normal distribution, while the Wilcoxon-Mann-Whitney test was used for variables that did not follow a normal distribution. Categorical variables were analysed using the Χ2 test or Fisher’s exact test, as appropriate. All significant variables associated with thrombosis in OAPS identified through univariate analysis were included in a multivariable binary logistic regression model, except for aPLs, which were included in the aGAPSS. Both unadjusted and adjusted regression models (adjusted for LDA and hydroxychloroquine (HCQ)) were presented. Kaplan-Meier survival analysis was conducted to assess the cumulative incidence of thrombosis in patients with OAPS and in the two subsets of OAPS (with/without LA). A p<0.05 was considered statistically significant.
Results
Characteristics of thrombotic events
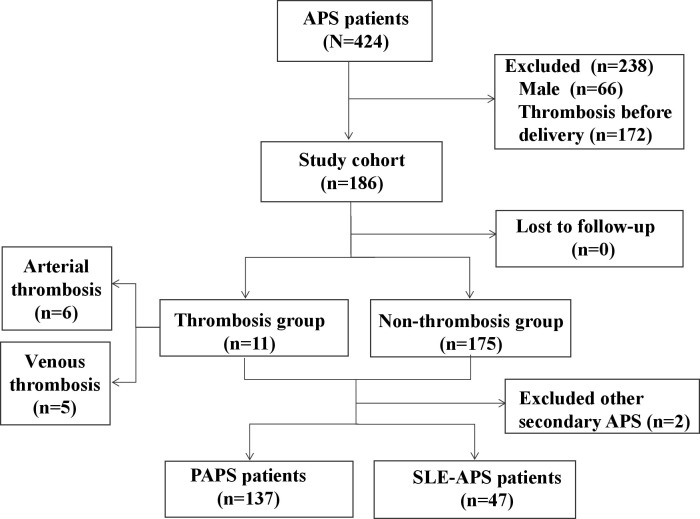
A total of 186 out of 424 patients with APS were included in this study (figure 1). Over a mean follow-up period of 5.4 years, thrombotic events occurred in 11 (5.9%) patients. Among them, six experienced arterial thromboses, including five strokes and one myocardial infarction, while five had venous thromboses, including three DVTs and two PEs. The median time from the first delivery to the first thrombosis was 4.8 (0.9–24.2) years.
Figure 1.
Flow chart of the study. APS, antiphospholipid syndrome; PAPS, primary APS.
Comparison of baseline characteristic in patients with OAPS with or without thrombosis
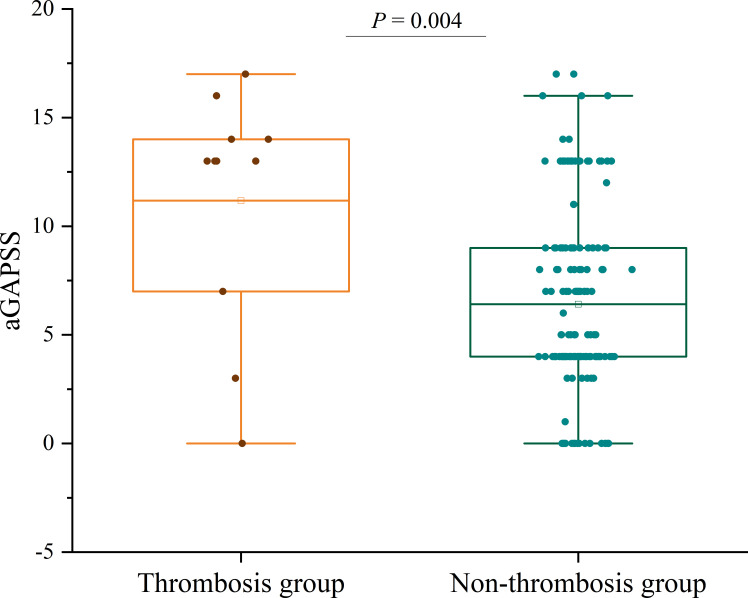
Compared with patients without thrombosis, those with thrombosis had a lower age of onset (the age at which aPL-associated obstetric complications first occurred, 27.6±4.0 years vs 31.2±4.8 years, p=0.013) and longer disease duration (5.0 (1.0–25.0) years vs 1.0 (0.3–3.0) years, p=0.049) (table 1). Thrombocytopenia was more frequent in patients with OAPS with thrombotic events (54.5% vs 16.0%, p=0.005). Furthermore, patients who experienced thrombotic events showed a higher frequency of hypocomplementemia (81.8% vs 23.4%, p<0.001) (table 1). The aGAPSS was significantly higher in patients with thrombosis compared with those without thrombosis (13.0 (7.0–14.0) vs 5.0 (4.0–9.0), p=0.004) (figure 2). The positive rate of LA was significantly higher in patients with thrombosis (81.8% vs 42.3%, p=0.025), as was the frequency of aCL positivity (72.7% vs 31.4%, p=0.013). Triple-positive aPLs were more common in patients with thrombosis (72.7% vs 17.7%, p<0.001) (table 1). In comparison with patients without thrombosis, the utilisation of LDA (27.3% vs 63.4%, p=0.043) and HCQ (45.5% vs 77.1%, p=0.045) was lower among patients with OAPS with thrombosis (table 1).
Table 1.
Comparison of baseline characteristics, clinical features and treatment between patients with OAPS with or without thrombotic events
| Variables | Thrombosis group (n=11) | Venous thrombosis group (n=5) | Arterial thrombosis group (n=6) | Non-thrombosis group (n=175) | P value* | P value† | P value‡ |
| Age at onset (years), mean±SD | 27.6±4.0 | 27.4±4.5 | 27.7±4.0 | 31.2±4.8 | 0.013 | 0.130 | 0.083 |
| Disease duration (years), IQR | 5.0 (1.0–25.0) | 2.0 (0.8–20.5) | 13.5 (0.8–27.0) | 1.0 (0.3–3.0) | 0.049 | 0.332 | 0.069 |
| BMI (kg/m2), mean±SD | 24.3±4.8 | 22.1±4.4 | 26.1±4.7 | 24.4±3.9 | 0.924 | 0.305 | 0.429 |
| Smoking, n (%) | 0 (0) | 0 (0) | 0 (0) | 3 (1.7) | 1.000 | 1.000 | 1.000 |
| Cardiovascular risk factors, n (%) | |||||||
| Hypertension | 3 (27.3) | 1 (20.0) | 2 (33.3) | 16 (9.1) | 0.158 | 0.966 | 0.110 |
| Hyperlipidaemia | 4 (36.4) | 2 (40.0) | 2 (33.3) | 36 (20.6) | 0.393 | 0.632 | 0.806 |
| Arteriosclerosis | 1 (9.1) | 1 (20.0) | 0 (0) | 0 (0) | 0.059 | 0.028 | – |
| Diabetes | 1 (9.1) | 1 (20.0) | 0 (0) | 8 (4.6) | 1.000 | 0.603 | 1.000 |
| Underlying autoimmune diseases, n (%) | |||||||
| SLE | 6 (54.5) | 2 (40) | 4 (66.7) | 41 (23.4) | 0.052 | 0.475 | 0.054 |
| RA | 1 (9.1) | 1 (20.0) | 0 (0) | 7 (4.0) | 0.392 | 0.205 | 1.000 |
| SS | 1 (9.1) | 1 (20.0) | 0 (0) | 3 (1.7) | 0.218 | 0.107 | 1.000 |
| Clinical manifestations | |||||||
| Fetal loss, n (%) | |||||||
| <10 weeks | 9 (81.8) | 4 (80.0) | 5 (83.3) | 104 (59.4) | 0.247 | 0.643 | 0.452 |
| ≥10 weeks | 4 (36.4) | 1 (20.0) | 3 (50.0) | 74 (42.3) | 0.943 | 0.943 | 1.000 |
| Premature birth <34 weeks, n (%) | 4 (36.4) | 2 (40.0) | 2 (33.3) | 27 (15.4) | 0.164 | 0.392 | 0.246 |
| Pre-eclampsia, n (%) | 4 (36.4) | 1 (20.0) | 3 (50.0) | 30 (17.1) | 0.231 | 1.000 | 0.131 |
| FGR, n (%) | 2 (18.2) | 2 (40.0) | 0 (0) | 23 (13.1) | 0.984 | 0.291 | 1.000 |
| Stillbirth, n (%) | 1 (9.1) | 0 (0) | 1 (16.7) | 6 (3.4) | 0.888 | 1.000 | 0.213 |
| Thrombocytopenia, n (%) | 6 (54.5) | 2 (40.0) | 4 (66.7) | 28 (16.0) | 0.005 | 0.417 | 0.008 |
| Hypocomplementemia, n (%) | 9 (81.8) | 4 (80.0) | 5 (83.3) | 41 (23.4) | <0.001 | 0.018 | 0.005 |
| Laboratory tests, n (%) | |||||||
| LA positive | 9 (81.8) | 3 (60.0) | 6 (100.0) | 74 (42.3) | 0.025 | 0.741 | 0.017 |
| aβ2GPI positive | 8 (72.7) | 3 (60.0) | 5 (83.3) | 106 (60.6) | 0.629 | 1.000 | 0.484 |
| aCL positive | 8 (72.7) | 3 (60.0) | 5 (83.3) | 55 (31.4) | 0.013 | 0.388 | 0.027 |
| Double-positive aPLs | 0 (0) | 0 (0) | 0 (0) | 24 (13.7) | 0.364 | 1.000 | 1.000 |
| Triple-positive aPLs | 8 (72.7) | 3 (60.0) | 5 (83.3) | 30 (17.1) | <0.001 | 0.044 | <0.001 |
| High-risk aPLs | 9 (81.8) | 3 (60.0) | 6 (100.0) | 93 (53.1) | 0.123 | 1.000 | 0.064 |
| Treatment after delivery, n (%) | |||||||
| LDA | 3 (27.3) | 2 (40.0) | 1 (16.7) | 111 (63.4) | 0.043 | 0.549 | 0.059 |
| LMWH | 5 (45.5) | 4 (80.0) | 1 (16.7) | 123 (70.3) | 0.165 | 1.000 | 0.020 |
| LDA+LMWH | 3 (27.3) | 3 (60.0) | 0 (0) | 85 (48.6) | 0.170 | 0.960 | 0.054 |
| HCQ | 5 (45.5) | 3 (60.0) | 2 (33.3) | 135 (77.1) | 0.045 | 0.721 | 0.048 |
| Azathioprine | 1 (9.1) | 0 (0) | 1 (16.7) | 2 (1.1) | 0.168 | 1.000 | 0.097 |
| Mycophenolate mofetil | 0 (0) | 0 (0) | 0 (0) | 9 (5.1) | 1.000 | 1.000 | 1.000 |
| Cyclosporin A | 2 (18.2) | 0 (0) | 2 (33.3) | 11 (6.3) | 0.173 | 1.000 | 0.061 |
| Tacrolimus | 0 (0) | 0 (0) | 0 (0) | 2 (1.1) | 1.000 | 1.000 | 1.000 |
| Cyclophosphamide | 1 (9.1) | 0 (0) | 1 (16.7) | 5 (2.9) | 0.310 | 1.000 | 0.185 |
| Statins | 0 (0) | 0 (0) | 0 (0) | 7 (4.0) | 1.000 | 1.000 | 1.000 |
Bold entries indicate statistically significant differences between the two groups.
*Baseline comparison between thrombosis and non-thrombosis groups.
†Baseline comparison between venous thrombosis and non-thrombosis groups.
‡Baseline comparison between arterial thrombosis and non-thrombosis groups.
aCL, anticardiolipin antibodies; aPLs, antiphospholipid antibodies; aβ2GPI, anti-β2-glycoprotein I antibodies; BMI, body mass index; FGR, fetal growth restriction; HCQ, hydroxychloroquine; LA, lupus anticoagulant; LDA, low-dose aspirin; LMWH, low-molecular-weight heparin; OAPS, obstetric antiphospholipid syndrome; RA, rheumatoid arthritis; SS, Sjögren's syndrome.
Figure 2.
Comparison of aGAPSS between patients with or without thrombosis. aGAPSS, adjusted Global Antiphospholipid Syndrome Score.
Risk factors of first thrombosis in patients with OAPS
Multivariate binary logistic regression analysis revealed that triple-positive aPLs (OR=11.662, 95% CI=2.117 to 64.243, p=0.005) and hypocomplementemia (OR=9.047, 95% CI=1.530 to 53.495, p=0.015) were risk factors for the first thrombosis in patients with OAPS, after adjustment for LDA and HCQ (table 2).
Table 2.
Multivariate logistic regression of first thrombosis in patients with OAPS
| OR | 95% CI | P value | aOR* | 95% CI | P value | |
| All patients | ||||||
| Disease duration, years | 1.072 | 0.999 to 1.149 | 0.052 | |||
| Triple-positive aPLs | 4.758 | 1.016 to 22.276 | 0.048 | 11.662 | 2.117 to 64.243 | 0.005 |
| Hypocomplementemia | 7.682 | 1.444 to 40.875 | 0.017 | 9.047 | 1.530 to 53.495 | 0.015 |
| Arterial thrombosis subgroup | ||||||
| Triple-positive aPLs | 24.167 | 2.724 to 214.369 | 0.004 | NA | ||
| Venous thrombosis subgroup | ||||||
| Hypocomplementemia | 13.073 | 1.421 to 120.255 | 0.023 | 7.966 | 0.788 to 80.552 | 0.079 |
| PAPS subgroup | ||||||
| Premature birth <34 weeks | 21.599 | 1.247 to 374.179 | 0.035 | NA | ||
| Triple-positive aPLs | 36.195 | 2.037 to 643.120 | 0.015 | NA | ||
| Hypocomplementemia | 25.738 | 1.725 to 383.955 | 0.018 | NA |
Data on IQR or n (%).
*aOR (adjustment for low-dose aspirin and hydroxychloroquine).
aOR, adjusted OR; aPLs, antiphospholipid antibodies; NA, not applicable; OAPS, obstetric antiphospholipid syndrome; PAPS, primary antiphospholipid syndrome.
Subgroup analysis
The comparison between patients with APS with arterial or venous thrombosis and patients without thrombosis was shown in table 1. In the unadjusted analysis, multivariable analysis revealed that the presence of triple-positive aPLs (OR=24.167, 95% CI=2.724 to 214.369, p=0.004) was identified as an independent risk factor for arterial thrombosis, while hypocomplementemia (OR=13.073, 95% CI=1.421 to 120.255, p=0.023) was an independent risk factor for venous thrombosis (table 2). The comparison of thrombotic and non-thrombotic data among patients with PAPS and those with SLE-APS was presented in table 3. In the unadjusted model without considering medication, preterm birth <34 weeks (OR=21.599, 95% CI=1.247 to 374.179, p=0.035), triple-positive aPLs (OR=36.195, 95% CI=2.037 to 643.120, p=0.015) and hypocomplementemia (OR=25.738, 95% CI=1.725 to 383.955, p=0.018) were defined as independent risk factors for the first thrombosis in PAPS. However, this association was not observed in patients with SLE-APS (table 2). No significant thrombotic risk factors were identified in subgroups after adjustment for LDA and HCQ (table 2).
Table 3.
Comparison of baseline characteristics, clinical features and treatment among patients with PAPS and those with SLE-APS
| Variables | PAPS | SLE-APS | ||||
| Thrombosis group (n=5) | Non-thrombosis group (n=132) | P value | Thrombosis group (n=6) | Non-thrombosis group (n=41) | P value | |
| Age at onset (years), mean±SD | 29.6±3.5 | 31.5±4.1 | 0.287 | 25.8±3.9 | 30.7±6.4 | 0.028 |
| Disease duration (years), IQR | 6.0 (0.8–30.0) | 1.0 (0.4–3.0) | 0.111 | 3.5 (0.8–24.8) | 2.0 (0.1–5.0) | 0.369 |
| BMI (kg/m2), mean±SD | 23.0±5.2 | 24.3±3.8 | 0.603 | 25.4±4.5 | 24.8±3.9 | 0.772 |
| Smoking, n (%) | 0 (0) | 0 (0) | – | 0 (0) | 2 (4.9) | 1.000 |
| Cardiovascular risk factors, n (%) | ||||||
| Hypertension | 2 (40.0) | 10 (7.6) | 0.087 | 1 (16.7) | 6 (14.6) | 1.000 |
| Hyperlipidaemia | 3 (60.0) | 28 (21.5) | 0.143 | 1 (16.7) | 7 (17.1) | 1.000 |
| Arteriosclerosis | 1 (20.0) | 0 (0) | 0.036 | 0 (0) | 0 (0) | – |
| Diabetes | 1 (20.0) | 4 (3.0) | 0.440 | 0 (0) | 4 (9.8) | 1.000 |
| Clinical manifestations | ||||||
| Fetal loss, n (%) | ||||||
| <10 weeks | 4 (80.0) | 84 (63.6) | 0.784 | 5 (83.3) | 20 (48.8) | 0.252 |
| ≥10 weeks | 2 (40.0) | 44 (33.3) | 1.000 | 2 (33.3) | 28 (68.3) | 0.226 |
| Premature birth <34 weeks, n (%) | 3 (60.0) | 16 (12.1) | 0.017 | 1 (16.7) | 11 (26.8) | 0.974 |
| Pre-eclampsia, n (%) | 1 (20.0) | 22 (16.7) | 1.000 | 3 (50.0) | 8 (19.5) | 0.258 |
| FGR, n (%) | 2 (18.2) | 23 (13.1) | 0.984 | 1 (16.7) | 5 (12.2) | 1.000 |
| Stillbirth, n (%) | 1 (20.0) | 18 (13.6) | 1.000 | 0 (0) | 2 (4.9) | 1.000 |
| Thrombocytopenia, n (%) | 3 (60.0) | 15 (11.4) | 0.013 | 3 (50.0) | 13 (31.7) | 0.673 |
| Hypocomplementemia, n (%) | 4 (80.0) | 16 (12.1) | <0.001 | 5 (83.3) | 23 (56.1) | 0.410 |
| Laboratory tests, n (%) | ||||||
| LA positive | 3 (60.0) | 47 (35.6) | 0.523 | 6 (100.0) | 26 (63.4) | 0.185 |
| aβ2GPI positive | 3 (60.0) | 71 (53.8) | 1.000 | 5 (83.3) | 35 (85.4) | 1.000 |
| aCL positive | 3 (60.0) | 27 (20.5) | 0.122 | 5 (83.3) | 28 (68.3) | 0.784 |
| Double-positive aPLs | 0 (0) | 16 (12.1) | 1.000 | 0 (0) | 9 (22.0) | 0.579 |
| Triple-positive aPLs | 3 (60.0) | 9 (6.8) | 0.001 | 5 (83.3) | 21 (51.2) | 0.299 |
| High-risk aPLs | 3 (60.0) | 61 (46.2) | 0.881 | 6 (100.0) | 32 (78.0) | 0.471 |
| Treatment after delivery, n (%) | ||||||
| LDA | 1 (20.0) | 89 (67.4) | 0.087 | 2 (33.3) | 33 (80.5) | 0.049 |
| LMWH | 4 (20.0) | 104 (78.8) | 0.136 | 3 (50.0) | 19 (46.3) | 1.000 |
| LDA+LMWH | 1 (20.0) | 74 (56.1) | 0.257 | 2 (33.3) | 12 (29.3) | 1.000 |
| HCQ | 3 (60.0) | 104 (78.8) | 0.655 | 2 (33.3) | 34 (82.9) | 0.030 |
| Azathioprine | 0 (0) | 0 (0) | – | 1 (16.7) | 0 (0) | 0.128 |
| Mycophenolate mofetil | 0 (0) | 1 (0.8) | 1.000 | 0 (0) | 8 (19.5) | 0.544 |
| Cyclosporin A | 1 (20) | 2 (1.5) | 0.106 | 2 (33.3) | 9 (22.0) | 0.921 |
| Tacrolimus | 0 (0) | 1 (0.8) | 1.000 | 0 (0) | 1 (2.4) | 1.000 |
| Cyclophosphamide | 0 (0) | 1 (0.8) | 1.000 | 0 (0) | 2 (4.9) | 1.000 |
| Statins | 0 (0) | 5 (3.8) | 1.000 | 0 (0) | 0 (0) | – |
| Follow-up | ||||||
| Arterial thrombosis, n (%) | 2 (20.0) | – | – | 4 (66.7) | – | – |
| Venous thrombosis, n (%) | 3 (60.0) | – | – | 2 (33.3) | – | – |
Bold entries indicate statistically significant differences between the two groups.
aCL, anticardiolipin antibodies; aPLs, antiphospholipid antibodies; aβ2GPI, anti-β2-glycoprotein I antibodies; BMI, body mass index; FGR, fetal growth restriction; HCQ, hydroxychloroquine; LA, lupus anticoagulant; LDA, low-dose aspirin; LMWH, low-molecular-weight heparin; PAPS, primary antiphospholipid syndrome; SLE-APS, SLE-related antiphospholipid syndrome.
Survival analysis
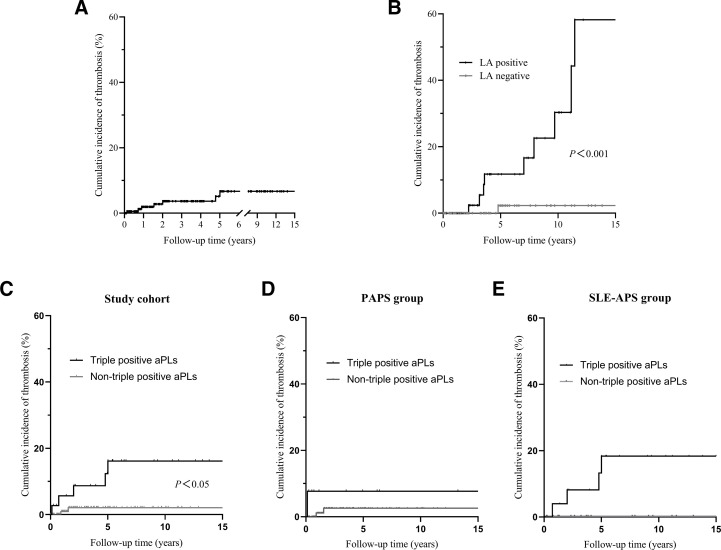
Kaplan-Meier survival analysis demonstrated a 15-year cumulative thrombosis rate of 6.7% in patients with OAPS, with a significantly higher cumulative incidence of first thrombosis in patients positive for LA compared with those negative for LA (58.2% vs 2.3%, p<0.001) (figure 3A,B). In the study cohort, the 15-year cumulative thrombosis rate was significantly higher in triple-positive aPLs patients with OAPS compared with non-triple-positive individuals (16.2% vs 2.1%, p=0.014) (figure 3C). However, this trend was no longer significant in patients with PAPS (7.7% vs 2.6%, p=0.065) and those with SLE-APS (18.4% vs 0%, p=0.139) (figure 3D,E).
Figure 3.
Kaplan-Meier survival analysis. Cumulative incidence of thrombosis in patients with OAPS (A). Cumulative incidence of thrombosis in the LA-negative and LA-positive groups (B). Cumulative incidence of thrombosis in the triple-positive aPL and non-triple-positive aPL patients in the entire cohort (C), PAPS group (D) and SLE-APS group (E). aPLs, antiphospholipid antibodies; LA, lupus anticoagulant; OAPS, obstetric antiphospholipid syndrome; PAPS, primary antiphospholipid syndrome; SLE-APS, SLE-related antiphospholipid syndrome.
Discussion
The 15-year cumulative thrombosis rate among patients with OAPS in this study was 6.7%. We found that triple-positive aPLs and hypocomplementemia were risk factors for the first thrombosis in OAPS.
Consistent with previous studies,11 26–28 our findings revealed higher rates of LA positivity and triple-positive aPLs in patients with OAPS with thrombosis. Furthermore, the cumulative thrombosis rates were higher in LA-positive patients over time. The RATIO Study also demonstrated a significant association between LA positivity and an elevated risk of thrombosis.29
The utility of GAPSS in predicting thrombosis in patients with APS has been demonstrated in prior studies and validated in the APS ACTION Study.4 30 31 Although aGAPSS is a simplified version of GAPSS, it does not include anti-phosphatidylserine/prothrombin complex. This makes testing more convenient, and in recent years, aGAPSS has also been reported for its role in thrombosis prediction.32 33 Similarly, our study observed a relatively higher aGAPSS in patients with OAPS with thrombosis compared with those without. However, this effect was attenuated when adjusted for relevant confounding factors.
Our results suggest that hypocomplementemia might be a biomarker of thrombotic risk in APS. Complement activation-induced thromboinflammation plays an important role in thrombosis.34 Recent research has shown an association between complement activation induced by aPLs and thrombotic events.35 Nevertheless, further investigation is needed to elucidate the role of the complement system in thrombosis among patients with OAPS.
Maternal hypercoagulability can persist until approximately 12 weeks after delivery. To mitigate the risk of postpartum thrombotic events in patients with OAPS, it is recommended continuing prophylactic doses of heparin for 6 weeks.36 37 However, strategies for primary thromboprophylaxis against long-term thrombosis in patients with isolated OAPS remain uncertain. A previous study demonstrated that the combination of LDA and low-molecular-weight heparin reduces the risk of maternal thrombosis in OAPS.38 A meta-analysis indicated that LDA is associated with a lower risk of thrombosis.17 The benefits of LDA in thrombosis prevention in patients with OAPS have not been fully confirmed, yet, it is essential to take into account traditional cardiovascular risk factors and other clinical factors for individualised interventions in patients.
The beneficial effects of HCQ in OAPS have been primarily reported in relation to the prevention of obstetric complications,39 40 with limited evidence regarding its role in postpartum thromboprophylaxis in OAPS. HCQ can disrupt aPL IgG–β2GPI complexes, diminishing the affinity of both individual proteins and complexes to phospholipid bilayers.41 Additionally, HCQ has the capacity to safeguard the anticoagulant shield of annexin A5 against disruption by aPLs on phospholipid bilayers, on the apical membranes of cultured human umbilical vein endothelial cells and syncytialised trophoblast cells, or in samples of plasma of patients with APS.42 This action effectively reverses the thrombogenic properties of aPLs. Our study revealed a lower thrombosis rate among patients with OAPS using HCQ, likely due to its inhibition of inflammatory cytokines, platelet aggregation and adhesion.42 43
Our study has certain limitations. It is important to note that this was not a multicentre study, which may limit the generalisability of our findings to diverse populations. However, the utilisation of the same medical centre and a single laboratory ensured a controlled quality of research.
Conclusion
In conclusion, our study highlights that triple-positive aPLs and hypocomplementemia are risk factors for the first thrombosis in OAPS.
Acknowledgments
We are thankful to all the participants for their generous contributions to this study.
Footnotes
Presented at: The results derived from this study were partially presented in the American College of Rheumatology Convergence 2023 conference.
Contributors: LL and QC performed the data collection and wrote the manuscript. CL conceived the study and made substantial contributions to the interpretation of data for the work, revising it critically for important intellectual content. XL and YH contributed to statistical analysis. All authors read and approved the final manuscript. CL is responsible for the overall content as the guarantor.
Funding: This work was supported by the Natural Science Foundation of China (32141004), Peking University Clinical Scientist Training Program (BMU2023PYJH010), Beijing Natural Science Foundation (no. 7192211) and the People's Hospital of Yubei District of Chongqing City Clinical Research Project (ybyk2023-06).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study involves human participants and was approved by the Ethics Committee of Peking University People’s Hospital (2019PHB252). Informed consent was obtained from all participants.
References
- 1.Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006;4:295–306. 10.1111/j.1538-7836.2006.01753.x [DOI] [PubMed] [Google Scholar]
- 2.Lefèvre G, Lambert M, Bacri J-L, et al. Thrombotic events during long-term follow-up of obstetric antiphospholipid syndrome patients. Lupus 2011;20:861–5. 10.1177/0961203310397080 [DOI] [PubMed] [Google Scholar]
- 3.Taraborelli M, Reggia R, Dall’Ara F, et al. Longterm outcome of patients with primary antiphospholipid syndrome: a retrospective multicenter study. J Rheumatol 2017;44:1165–72. 10.3899/jrheum.161364 [DOI] [PubMed] [Google Scholar]
- 4.de Jesús GR, Sciascia S, Andrade D, et al. Factors associated with first thrombosis in patients presenting with obstetric antiphospholipid syndrome (APS) in the APS alliance for clinical trials and international networking clinical database and repository: a retrospective study. BJOG 2019;126:656–61. 10.1111/1471-0528.15469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bettiol A, Emmi G, Finocchi M, et al. Obstetric antiphospholipid syndrome is not associated with an increased risk of subclinical atherosclerosis. Rheumatology (Oxford) 2020;59:3709–16. 10.1093/rheumatology/keaa116 [DOI] [PubMed] [Google Scholar]
- 6.Erkan D, Merrill JT, Yazici Y, et al. High thrombosis rate after fetal loss in antiphospholipid syndrome: effective prophylaxis with aspirin. Arthritis Rheum 2001;44:1466–7. [DOI] [PubMed] [Google Scholar]
- 7.Gris J-C, Bouvier S, Molinari N, et al. Comparative incidence of a first thrombotic event in purely obstetric antiphospholipid syndrome with pregnancy loss: the NOH-APS observational study. Blood 2012;119:2624–32. 10.1182/blood-2011-09-381913 [DOI] [PubMed] [Google Scholar]
- 8.Martinez-Zamora MA, Peralta S, Creus M, et al. Risk of thromboembolic events after recurrent spontaneous abortion in antiphospholipid syndrome: a case-control study. Ann Rheum Dis 2012;71:61–6. 10.1136/ard.2011.153817 [DOI] [PubMed] [Google Scholar]
- 9.Alijotas-Reig J, Ferrer-Oliveras R, Ruffatti A, et al. The European registry on obstetric antiphospholipid syndrome (EUROAPS): a survey of 247 consecutive cases. Autoimmun Rev 2015;14:387–95. 10.1016/j.autrev.2014.12.010 [DOI] [PubMed] [Google Scholar]
- 10.Drozdinsky G, Hadar E, Shmueli A, et al. Obstetric antiphospholipid syndrome and long term arterial thrombosis risk. J Thromb Thrombolysis 2017;44:371–5. 10.1007/s11239-017-1538-5 [DOI] [PubMed] [Google Scholar]
- 11.Rottenstreich A, Arad A, Terespolsky H, et al. Antiphospholipid antibody profile-based outcome of purely vascular and purely obstetric antiphospholipid syndrome. J Thromb Thrombolysis 2018;46:166–73. 10.1007/s11239-018-1672-8 [DOI] [PubMed] [Google Scholar]
- 12.Udry S, Latino JO, Belizna C, et al. A high-risk laboratory profile of antiphospholipid antibodies and thrombosis is associated with a large number of extra-criteria manifestations in obstetric antiphospholipid syndrome. Immunol Res 2019;67:478–85. 10.1007/s12026-019-09110-x [DOI] [PubMed] [Google Scholar]
- 13.Alijotas-Reig J, Esteve-Valverde E, Ferrer-Oliveras R, et al. The European registry on obstetric antiphospholipid syndrome (EUROAPS): a survey of 1000 consecutive cases. Autoimmun Rev 2019;18:406–14. 10.1016/j.autrev.2018.12.006 [DOI] [PubMed] [Google Scholar]
- 14.Tonello M, Calligaro A, Favaro M, et al. The first thrombotic event in purely obstetric antiphospholipid syndrome patients and in antiphospholipid antibody carriers: comparison of incidence and characteristics. Arch Gynecol Obstet 2021;303:455–61. 10.1007/s00404-020-05766-1 [DOI] [PubMed] [Google Scholar]
- 15.Jiang H, Wang C-H, Jiang N, et al. Clinical characteristics and prognosis of patients with isolated thrombotic vs. obstetric antiphospholipid syndrome: a prospective cohort study. Arthritis Res Ther 2021;23:138. 10.1186/s13075-021-02515-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niznik S, Rapoport MJ, Avnery O, et al. Long term follow up of patients with primary obstetric antiphospholipid syndrome. Front Pharmacol 2022;13:824775. 10.3389/fphar.2022.824775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arnaud L, Mathian A, Devilliers H, et al. Efficacy of aspirin for the primary prevention of thrombosis in patients with antiphospholipid antibodies: an international and collaborative meta-analysis. Autoimmun Rev 2015;14:192–200. 10.1016/j.autrev.2014.10.019 [DOI] [PubMed] [Google Scholar]
- 18.Bala MM, Paszek E, Lesniak W, et al. Antiplatelet and anticoagulant agents for primary prevention of thrombosis in individuals with antiphospholipid antibodies. Cochrane Database Syst Rev 2018;7:CD012534. 10.1002/14651858.CD012534.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barbhaiya M, Zuily S, Naden R, et al. ACR/EULAR antiphospholipid syndrome classification criteria. Ann Rheum Dis 2023;82:1258–70. 10.1136/ard-2023-224609 [DOI] [PubMed] [Google Scholar]
- 20.Barbhaiya M, Zuily S, Naden R, et al. The 2023 ACR/EULAR antiphospholipid syndrome classification criteria. Arthritis Rheumatol 2023;75:1687–702. 10.1002/art.42624 [DOI] [PubMed] [Google Scholar]
- 21.Streiff MB, Agnelli G, Connors JM, et al. Guidance for the treatment of deep vein thrombosis and pulmonary embolism. J Thromb Thrombolysis 2016;41:32–67. 10.1007/s11239-015-1317-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Konstantinides SV, Meyer G, Becattini C, et al. ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European respiratory society (ERS): the task force for the diagnosis and management of acute pulmonary embolism of the European society of cardiology (ESC). Eur Respir J 2019;54:1901647. 10.1183/13993003.01647-2019 [DOI] [PubMed] [Google Scholar]
- 23.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Glob Heart 2012;7:275–95. 10.1016/j.gheart.2012.08.001 [DOI] [PubMed] [Google Scholar]
- 24.Li C, Zuo Y, Zhang S, et al. Additional risk factors associated with thrombosis and pregnancy morbidity in a unique cohort of antiphospholipid antibody-positive patients. Chin Med J 2022;135:658–64. 10.1097/CM9.0000000000001964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Radin M, Schreiber K, Costanzo P, et al. The adjusted global antiphospholipid syndrome score (aGAPSS) for risk stratification in young APS patients with acute myocardial infarction. Int J Cardiol 2017;240:72–7. 10.1016/j.ijcard.2017.02.155 [DOI] [PubMed] [Google Scholar]
- 26.Reynaud Q, Lega J-C, Mismetti P, et al. Risk of venous and arterial thrombosis according to type of antiphospholipid antibodies in adults without systemic lupus erythematosus: a systematic review and meta-analysis. Autoimmun Rev 2014;13:595–608. 10.1016/j.autrev.2013.11.004 [DOI] [PubMed] [Google Scholar]
- 27.Gebhart J, Posch F, Koder S, et al. Increased mortality in patients with the lupus anticoagulant: the Vienna lupus anticoagulant and thrombosis study (LATS). Blood 2015;125:3477–83. 10.1182/blood-2014-11-611129 [DOI] [PubMed] [Google Scholar]
- 28.Pengo V, Ruffatti A, Legnani C, et al. Incidence of a first thromboembolic event in asymptomatic carriers of high-risk antiphospholipid antibody profile: a multicenter prospective study. Blood 2011;118:4714–8. 10.1182/blood-2011-03-340232 [DOI] [PubMed] [Google Scholar]
- 29.Urbanus RT, Siegerink B, Roest M, et al. Antiphospholipid antibodies and risk of myocardial infarction and ischaemic stroke in young women in the RATIO study: a case-control study. Lancet Neurol 2009;8:998–1005. 10.1016/S1474-4422(09)70239-X [DOI] [PubMed] [Google Scholar]
- 30.Chighizola CB, Andreoli L, Gerosa M, et al. The treatment of anti-phospholipid syndrome: a comprehensive clinical approach. J Autoimmun 2018;90:1–27. 10.1016/j.jaut.2018.02.003 [DOI] [PubMed] [Google Scholar]
- 31.Garcia D, Erkan D. Diagnosis and management of the antiphospholipid syndrome. N Engl J Med 2018;379:2010–21. 10.1056/NEJMc1808253 [DOI] [PubMed] [Google Scholar]
- 32.Song X, Fan Y, Jia Y, et al. A novel aGAPSS-based nomogram for the prediction of ischemic stroke in patients with antiphospholipid syndrome. Front Immunol 2022;13:930087. 10.3389/fimmu.2022.930087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barilaro G, Esteves A, Della Rocca C, et al. Predictive value of the adjusted global anti-phospholipid syndrome score on clinical recurrence in APS patients: a longitudinal study. Rheumatology (Oxford) 2023;62:1576–85. 10.1093/rheumatology/keac485 [DOI] [PubMed] [Google Scholar]
- 34.Thomas AM, Gerogianni A, McAdam MB, et al. Complement component C5 and TLR molecule CD14 mediate Heme-induced thromboinflammation in human blood. J Immunol 2019;203:1571–8. 10.4049/jimmunol.1900047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chaturvedi S, Braunstein EM, Yuan X, et al. Complement activity and complement regulatory gene mutations are associated with thrombosis in APS and CAPS. Blood 2020;135:239–51. 10.1182/blood.2019003863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sammaritano LR, Bermas BL, Chakravarty EE, et al. American college of rheumatology guideline for the management of reproductive health in rheumatic and musculoskeletal diseases. Arthritis Rheumatol 2020;72:529–56. 10.1002/art.41191 [DOI] [PubMed] [Google Scholar]
- 37.Tektonidou MG, Andreoli L, Limper M, et al. EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann Rheum Dis 2019;78:1296–304. 10.1136/annrheumdis-2019-215213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alijotas-Reig J, Ferrer-Oliveras R, Esteve-Valverde E, et al. Inherited thrombophilia in women with poor aPL-related obstetric history: prevalence and outcomes. survey of 208 cases from the European registry on obstetric antiphospholipid syndrome cohort. Am J Reprod Immunol 2016;76:164–71. 10.1111/aji.12534 [DOI] [PubMed] [Google Scholar]
- 39.Mekinian A, Lazzaroni MG, Kuzenko A, et al. The efficacy of hydroxychloroquine for obstetrical outcome in anti-phospholipid syndrome: data from a European multicenter retrospective study. Autoimmunity Reviews 2015;14:498–502. 10.1016/j.autrev.2015.01.012 [DOI] [PubMed] [Google Scholar]
- 40.Mekinian A, Costedoat-Chalumeau N, Masseau A, et al. Obstetrical APS: is there a place for hydroxychloroquine to improve the pregnancy outcome. Autoimmun Rev 2015;14:23–9. 10.1016/j.autrev.2014.08.040 [DOI] [PubMed] [Google Scholar]
- 41.Rand JH, Wu X-X, Quinn AS, et al. Hydroxychloroquine directly reduces the binding of antiphospholipid antibody-beta2-glycoprotein I complexes to phospholipid bilayers. Blood 2008;112:1687–95. 10.1182/blood-2008-03-144204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rand JH, Wu X-X, Quinn AS, et al. Hydroxychloroquine protects the annexin A5 anticoagulant shield from disruption by antiphospholipid antibodies: evidence for a novel effect for an old antimalarial drug. Blood 2010;115:2292–9. 10.1182/blood-2009-04-213520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaiser R, Cleveland CM, Criswell LA. Risk and protective factors for thrombosis in systemic lupus erythematosus: results from a large, multi-ethnic cohort. Ann Rheum Dis 2009;68:238–41. 10.1136/ard.2008.093013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
lupus-2023-001044supp001.pdf (63.8KB, pdf)
Data Availability Statement
Data are available upon reasonable request.