Abstract
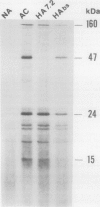
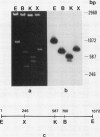
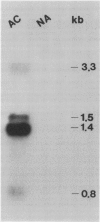
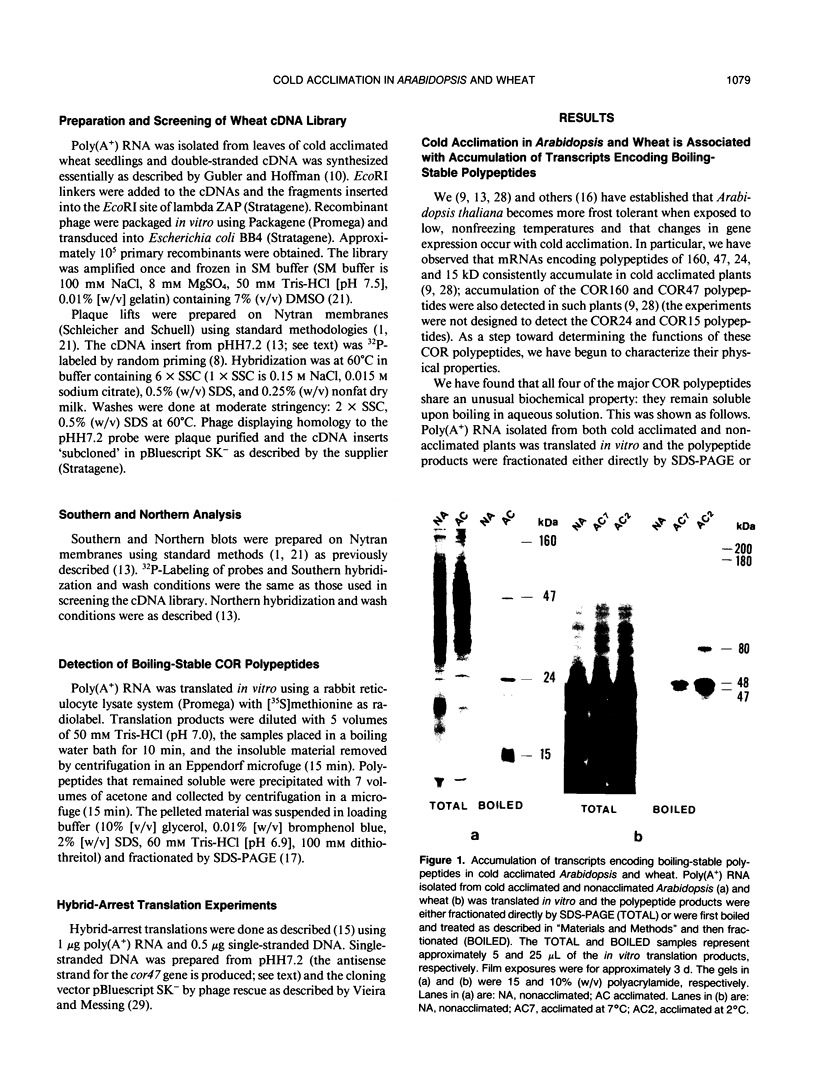
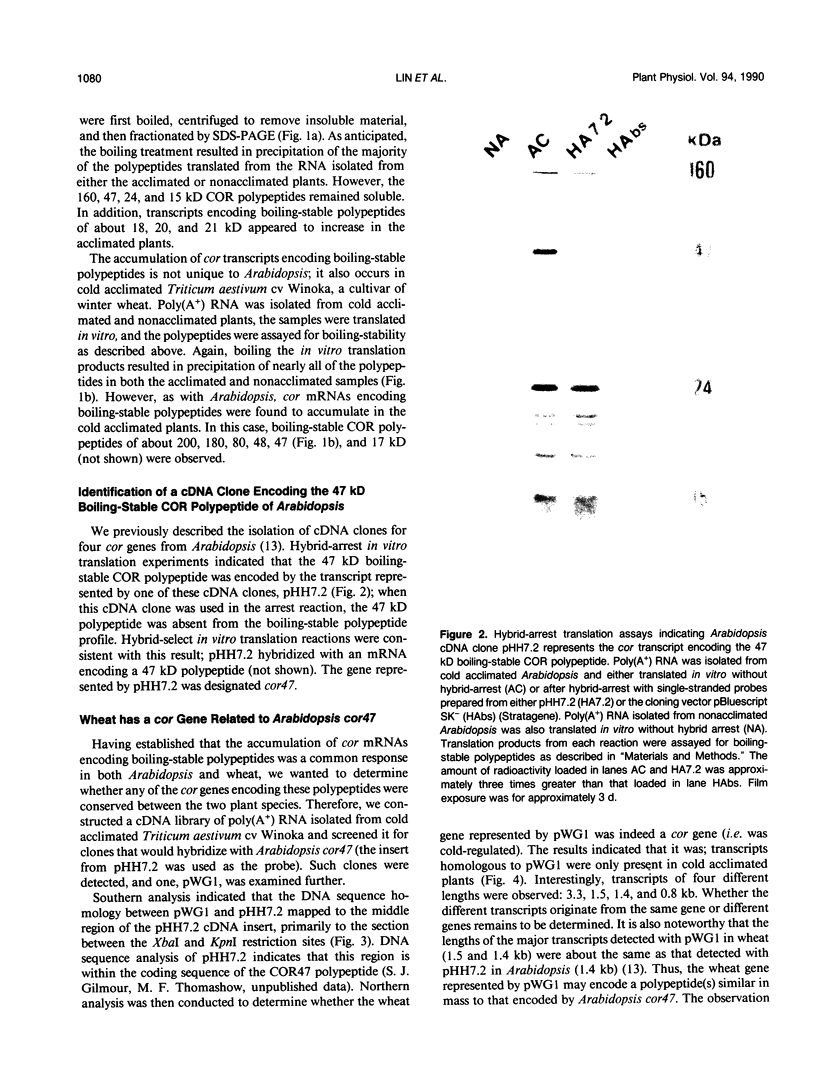
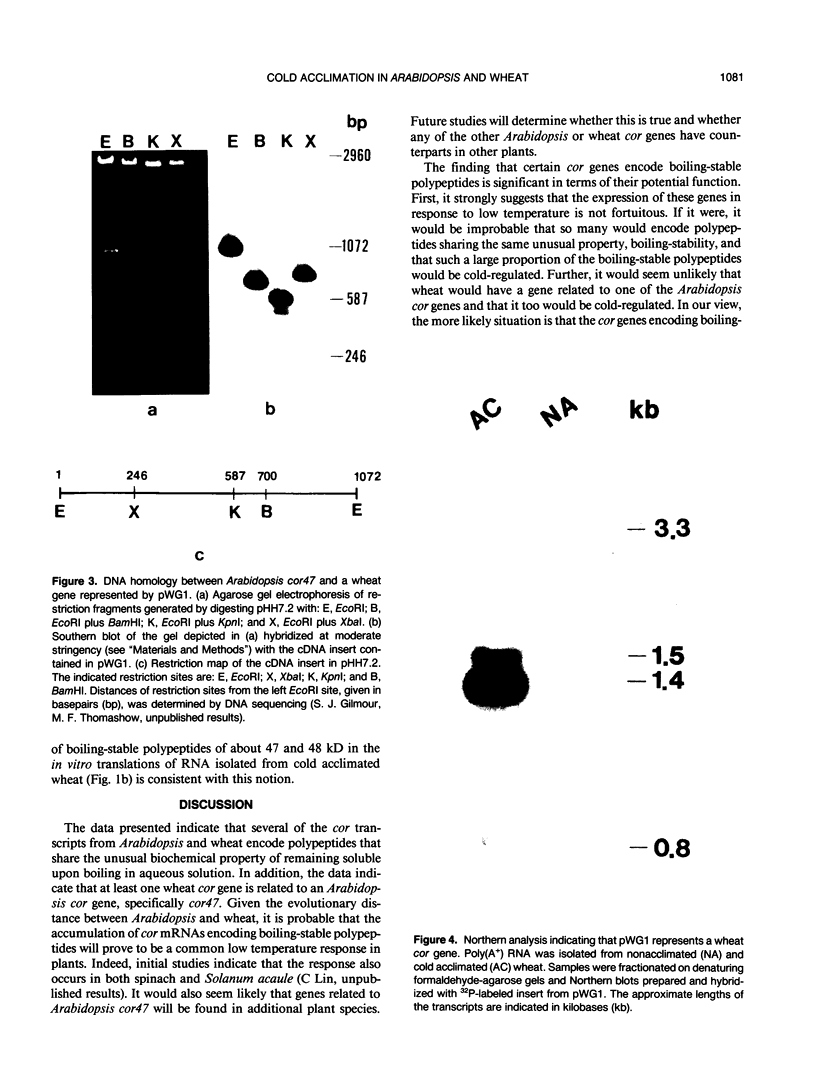
Changes in gene expression occur during cold acclimation in a wide variety of plant species. Here we show that a number of the polypeptides encoded by cold-regulated (cor) genes of Arabidopsis thaliana L. (Heyn) and wheat share the unusual biochemical property that they remain soluble upon boiling in aqueous solution. Further, cDNA cloning in conjunction with Southern and Northern analyses indicate that wheat has a cor gene that is related to Arabidopsis cor47, a gene encoding a 47 kilodalton `boiling-stable' COR polypeptide. We suggest it is likely that the boiling-stable COR polypeptides have a fundamental role in plants acclimating to cold temperatures and discuss the possibility that they may act as cryoprotectants.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carpenter J. F., Crowe J. H. The mechanism of cryoprotection of proteins by solutes. Cryobiology. 1988 Jun;25(3):244–255. doi: 10.1016/0011-2240(88)90032-6. [DOI] [PubMed] [Google Scholar]
- Close T. J., Kortt A. A., Chandler P. M. A cDNA-based comparison of dehydration-induced proteins (dehydrins) in barley and corn. Plant Mol Biol. 1989 Jul;13(1):95–108. doi: 10.1007/BF00027338. [DOI] [PubMed] [Google Scholar]
- Cox W. Interrelations between Environmental Factors and Freezing Resistance of Cabbage Leaves. Plant Physiol. 1976 Apr;57(4):553–555. doi: 10.1104/pp.57.4.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gilmour S. J., Hajela R. K., Thomashow M. F. Cold Acclimation in Arabidopsis thaliana. Plant Physiol. 1988 Jul;87(3):745–750. doi: 10.1104/pp.87.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hahn M., Walbot V. Effects of cold-treatment on protein synthesis and mRNA levels in rice leaves. Plant Physiol. 1989 Nov;91(3):930–938. doi: 10.1104/pp.91.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajela R. K., Horvath D. P., Gilmour S. J., Thomashow M. F. Molecular Cloning and Expression of cor (Cold-Regulated) Genes in Arabidopsis thaliana. Plant Physiol. 1990 Jul;93(3):1246–1252. doi: 10.1104/pp.93.3.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen J. V., Shaw D. C. Heat-stable proteins and abscisic Acid action in barley aleurone cells. Plant Physiol. 1989 Dec;91(4):1520–1526. doi: 10.1104/pp.91.4.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagus R. Hybrid selection of mRNA and hybrid arrest of translation. Methods Enzymol. 1987;152:567–572. doi: 10.1016/0076-6879(87)52063-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mundy J., Chua N. H. Abscisic acid and water-stress induce the expression of a novel rice gene. EMBO J. 1988 Aug;7(8):2279–2286. doi: 10.1002/j.1460-2075.1988.tb03070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siminovitch D., Cloutier Y. Drought and freezing tolerance and adaptation in plants: some evidence of near equivalences. Cryobiology. 1983 Aug;20(4):487–503. doi: 10.1016/0011-2240(83)90037-8. [DOI] [PubMed] [Google Scholar]
- Steponkus P. L., Lynch D. V. Freeze/thaw-induced destabilization of the plasma membrane and the effects of cold acclimation. J Bioenerg Biomembr. 1989 Feb;21(1):21–41. doi: 10.1007/BF00762210. [DOI] [PubMed] [Google Scholar]
- Steponkus P. L., Uemura M., Balsamo R. A., Arvinte T., Lynch D. V. Transformation of the cryobehavior of rye protoplasts by modification of the plasma membrane lipid composition. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9026–9030. doi: 10.1073/pnas.85.23.9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss G., Hauser H. Stabilization of lipid bilayer vesicles by sucrose during freezing. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2422–2426. doi: 10.1073/pnas.83.8.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Volger H. G., Heber U. Cryoprotective leaf proteins. Biochim Biophys Acta. 1975 Dec 15;412(2):335–349. doi: 10.1016/0005-2795(75)90048-3. [DOI] [PubMed] [Google Scholar]
- Weiser C. J. Cold Resistance and Injury in Woody Plants: Knowledge of hardy plant adaptations to freezing stress may help us to reduce winter damage. Science. 1970 Sep 25;169(3952):1269–1278. doi: 10.1126/science.169.3952.1269. [DOI] [PubMed] [Google Scholar]