Abstract
Antiretroviral preexposure prophylaxis (PrEP) is highly effective in preventing human immunodeficiency virus (HIV) infection, but uptake has been limited and inequitable. Although interventions to increase PrEP uptake are being evaluated in clinical trials among men who have sex with men (MSM), those trials cannot evaluate effects on HIV incidence. Estimates from observational studies of the causal effects of PrEP-uptake interventions on HIV incidence can inform decisions about intervention scale-up. We used longitudinal electronic health record data from HIV-negative MSM accessing care at Fenway Health, a community health center in Boston, Massachusetts, from January 2012 through February 2018, with 2 years of follow-up. We considered stochastic interventions that increased the chance of initiating PrEP in several high-priority subgroups. We estimated the effects of these interventions on population-level HIV incidence using a novel inverse-probability weighted estimator of the generalized g-formula, adjusting for baseline and time-varying confounders. Our results suggest that even modest increases in PrEP initiation in high-priority subgroups of MSM could meaningfully reduce HIV incidence in the overall population of MSM. Interventions tailored to Black and Latino MSM should be prioritized to maximize equity and impact.
Keywords: causal inference, HIV, inverse probability weighting, men who have sex with men, preexposure prophylaxis, stochastic interventions
Abbreviations
- CI
confidence interval
- EHR
electronic health record
- HIV
human immunodeficiency virus
- IPW
inverse probability weighting
- MSM
men who have sex with men
- PrEP
preexposure prophylaxis
- TMLE
targeted maximum likelihood estimation
Antiretroviral preexposure prophylaxis (PrEP) is highly effective in preventing human immunodeficiency virus (HIV) acquisition (1–6). However, of the 1.2 million people in the United States who are likely to benefit from PrEP, only 25% were prescribed it in 2020, ranging from 66% of White people to 9% of Black people with indications for PrEP use (7, 8). Men who have sex with men (MSM) account for 70% of new HIV infections annually and comprise the vast majority of PrEP users, but uptake remains limited and inequitable even in this population (9, 10).
Interventions to increase PrEP uptake—such as mobile applications, telehealth services, and behavioral counseling (11–15)—are being evaluated in clinical trials among MSM, with some interventions tailored specifically to subgroups placed at increased risk of HIV infection (16, 17) and underrepresented among PrEP users, such as Black and Latino MSM. These trials measure effects of interventions on the outcome of PrEP uptake, rather than HIV incidence, given limited follow-up time, small sample sizes, and the rarity of HIV infection. Few full-scale trials of PrEP-uptake interventions have been completed (18), but pilot trial results suggest that interventions will yield only modest increases in PrEP uptake (e.g., 12% over 3 months, 24% over 6 months) (14, 19–21), with unknown effects of those increases on population-level HIV incidence. To prioritize scaling up PrEP-uptake interventions, policymakers need estimates of the effects of these interventions on both cumulative PrEP uptake and HIV incidence; however, estimates of effects on HIV incidence will need to be obtained outside of the clinical trial setting.
Agent-based mathematical models have been used to project the impact of increases in PrEP uptake, overall and within high-priority subgroups, on HIV incidence among MSM (22–26). Mathematical modeling studies are useful for projecting effects of interventions on rare outcomes over longer time frames and allow for the consideration of an interference structure wherein the treatment of one individual may affect another individual's outcome (27). However, this approach to causal inference has several limitations, including reliance on numerous model assumptions for the full joint distribution of the underlying data generating process (28). Additionally, the model parameters for this joint distribution are typically not estimated from a single data source but instead derived from multiple data sources, complicating interpretation with regard to any particular target population (29).
An alternative approach to estimating effects of PrEP-uptake interventions is to conduct observational studies using empirical data, such as data from electronic health records (EHRs). However, valid causal inference from such data requires appropriately accounting for time-varying confounders that are affected by prior PrEP uptake (30). For example, rectal sexually transmitted infection (STI) testing—a proxy for condomless anal sex, a risk factor for contracting HIV—is a time-varying confounder of the effect of PrEP initiation on HIV incidence, but rectal STI testing is also affected by prior PrEP use. Methods that derive from Robins’s g-formula (31) can account for complex time-varying data structures. These methods, sometimes referred to as g-methods (32), include inverse probability weighting (IPW), parametric g-computation, targeted maximum likelihood estimation (TMLE), and related methods (33, 34).
Applications of g-methods have historically focused on effects of deterministic interventions, under which treatment can take only one possible value within levels of past measured confounders. In the PrEP context, examples of deterministic interventions include “Never initiate PrEP” and “At each time t of follow-up, if an individual has a recent STI, initiate PrEP at t; otherwise do not initiate at t.” These interventions are too strict to inform realistic policy decisions when many individuals, even those who are most likely to benefit from PrEP, will not initiate it because of barriers to access, competing priorities, or personal preference. Likewise, the impact of such unrealistic interventions may be difficult to estimate because of a lack of real-world data (35).
Several authors have recently proposed g-methods focused on effects of stochastic interventions, under which treatment is assigned based on a random draw from a distribution that may depend on an individual’s measured confounder history (36–41). These constitute methods for estimating the so-called generalized g-formula (31). Stochastic interventions have an advantage in semiparametric or nonparametric estimation of the g-formula because they provide inherent weight stabilization and can be designed to rely on weaker conditions for positivity. Particularly relevant to the current application are incremental propensity score interventions that shift the probability of observed treatment by a specified amount (40).
Here, we apply IPW to EHR data to estimate the effects of time-varying, incremental propensity score interventions to increase PrEP initiation on the cumulative incidence of HIV diagnosis in a cohort of MSM. The goals of this study were to: 1) estimate population-level effects of a range of increases in PrEP initiation on HIV incidence among MSM and 2) illustrate a computationally straightforward approach to estimating the causal effects of time-varying stochastic interventions on a survival outcome in real-world longitudinal data.
METHODS
Study population and follow-up
Using the Electronic Medical Record Support for Public Health (ESP) platform (42–44), we extracted data from EHRs to construct a cohort of HIV-negative MSM accessing primary care at Fenway Health, a community health center in Boston, Massachusetts, that specializes in care for sexual and gender minorities and is the largest PrEP provider in New England (45). People eligible for our study were MSM aged 15 years or older with a negative HIV test from January 1, 2012, through February 28, 2018, and no PrEP prescription in the prior 12 months. We defined MSM as cisgender men (male gender identity and assigned male sex at birth) who identified as gay or bisexual or were tested for rectal or pharyngeal gonorrhea or chlamydia in the past 12 months, suggesting reported anal or oral sex with other males. Follow-up was measured in weeks and started at baseline, defined as the week of the first negative HIV test during the study period. Follow-up ended at the earliest week of HIV diagnosis, end of study (104 weeks), or death, whichever occurred first. A total of n = 11,055 MSM met the eligibility criteria.
Outcome, exposure, and confounders
HIV diagnosis was defined as a positive enzyme-linked immunosorbent assay, Western blot, HIV RNA viral load (>200 copies/mL), qualitative polymerase chain reaction and antigen/antibody test, or a visit record with HIV diagnosis identified by the following International Classification of Diseases (ICD) codes: 042, V08, 079.53 (ICD-9) or B20, B97.35, Z21 (ICD-10).
PrEP initiation was defined as the first PrEP prescription after baseline for tenofovir disoproxil fumarate coformulated with emtricitabine. To avoid misclassifying the use of this medication for postexposure prophylaxis (PEP) as PrEP, prescriptions for 28 or fewer days or in conjunction with other antiretroviral medications were not classified as PrEP (6).
To control confounding, we adjusted for baseline and time-varying measured covariates hypothesized a priori to be associated with subsequent PrEP initiation and incident HIV diagnosis. Baseline covariates included age, race, ethnicity, and indicators of any of the following during the 12 months prior to baseline: health-care (ambulatory) encounters, laboratory testing (HIV, STI, hepatitis B or C), prescriptions (PEP, erectile dysfunction), and diagnoses (depression, smoking or tobacco use, alcohol dependence, opioid dependence, “high-risk sexual behavior,” “exposure to venereal disease”). STIs included gonorrhea, chlamydia, and syphilis. Gonorrhea and chlamydia tests may have been conducted using samples from the rectum, pharynx, or urethra. An STI case was defined in accordance with guidelines from the Centers for Disease Control and Prevention (46) as implemented in the Electronic Medical Record Support for Public Health platform (47). Hepatitis B infection was based on a positive surface antigen, viral DNA, or immunoglobulin-M antibody test, and hepatitis C infection on a positive enzyme-linked immunosorbent assay or viral RNA test. We adjusted for an HIV risk index that provides a probability of HIV acquisition based on predictors of HIV risk from EHR data. The index was previously validated among MSM at Fenway Health (48). We divided this index into 4 categories (low, medium, high, missing). Time-varying covariates included weekly indicators of any of the following: encounter at Fenway Health, laboratory tests, positive tests, prescriptions, and diagnoses, as listed in Table 1 and Web Table 1 (available at https://doi.org/10.1093/aje/kwad097).
Table 1.
Baseline Characteristics of MSM, Stratified by PrEP Initiation During Follow-up, Fenway Health, Massachusetts, 2012–2018
|
No PrEP
(n=8,180) |
PrEP
(n=2,875) |
Overall
(n=11,055) |
||||
|---|---|---|---|---|---|---|
| Variable | No. | % | No. | % | No. | % |
| Race | ||||||
| White | 5,863 | 71.7 | 2,062 | 71.7 | 7,925 | 71.7 |
| Black | 473 | 5.8 | 163 | 5.7 | 636 | 5.8 |
| Asian | 531 | 6.5 | 191 | 6.6 | 722 | 6.5 |
| Unknown | 660 | 8.1 | 216 | 7.5 | 876 | 7.9 |
| Other | 653 | 8.0 | 243 | 8.5 | 896 | 8.1 |
| Ethnicity | ||||||
| Not Latino/Hispanic | 6,102 | 74.6 | 2,161 | 75.2 | 8,263 | 74.7 |
| Latino/Hispanic | 877 | 10.7 | 403 | 14.0 | 1,280 | 11.6 |
| Unknown | 1,201 | 14.7 | 311 | 10.8 | 1,512 | 13.7 |
| HIV risk index | ||||||
| Low | 1,884 | 23.0 | 630 | 21.9 | 2,514 | 22.7 |
| Medium | 4,712 | 57.6 | 1,671 | 58.1 | 6,383 | 57.7 |
| High | 676 | 8.3 | 354 | 12.3 | 1,030 | 9.3 |
| Missing | 908 | 11.1 | 220 | 7.7 | 1,128 | 10.2 |
| Health-care encounter | 3,149 | 38.5 | 869 | 30.2 | 4,018 | 36.3 |
| Tests | ||||||
| Hepatitis C | 349 | 4.3 | 117 | 4.1 | 466 | 4.2 |
| Hepatitis B | 189 | 2.3 | 78 | 2.7 | 267 | 2.4 |
| STI (rectal) | 317 | 3.9 | 173 | 6.0 | 490 | 4.4 |
| STI (other) | 1,008 | 12.3 | 356 | 12.4 | 1,364 | 12.3 |
| Positive tests | ||||||
| STI (rectal) | 51 | 0.6 | 32 | 1.1 | 83 | 0.8 |
| STI (other) | 136 | 1.7 | 71 | 2.5 | 207 | 1.9 |
| Prescriptions | ||||||
| STI treatment | 345 | 4.2 | 133 | 4.6 | 478 | 4.3 |
| Erectile dysfunction | 156 | 1.9 | 42 | 1.5 | 198 | 1.8 |
| Diagnoses | ||||||
| “Exposure to venereal disease” | 64 | 0.8 | 37 | 1.3 | 101 | 0.9 |
| “High-risk sexual behavior” | 176 | 2.2 | 139 | 4.8 | 315 | 2.8 |
| Depression | 563 | 6.9 | 161 | 5.6 | 724 | 6.5 |
| Smoking/tobacco use | 153 | 1.9 | 53 | 1.8 | 206 | 1.9 |
| Alcohol dependence | 96 | 1.2 | 26 | 0.9 | 122 | 1.1 |
| Opioid dependence | 18 | 0.2 | 2 | 0.1 | 20 | 0.2 |
| Age, yearsa | 34.7 (12.2) | 32.0 (9.9) | 34.0 (11.7) | |||
Abbreviations: HIV, human immunodeficiency virus; MSM, men who have sex with men; PrEP, preexposure prophylaxis; STI, sexually transmitted infection.
a Values are expressed as mean (standard deviation).
Notation, intervention definitions, and causal effects
Let 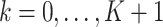 denote weekly follow-up intervals, with
denote weekly follow-up intervals, with 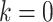 corresponding to baseline and the interval
corresponding to baseline and the interval 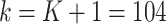 the end of the study follow-up. Let
the end of the study follow-up. Let  denote an individual’s observed indicator of whether PrEP was initiated by week
denote an individual’s observed indicator of whether PrEP was initiated by week  and
and 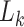 the measured confounders in week
the measured confounders in week  , with
, with  additionally including the baseline confounders. Let
additionally including the baseline confounders. Let  and
and 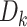 denote indicators of incident HIV diagnosis and death due to any cause by week
denote indicators of incident HIV diagnosis and death due to any cause by week 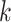 , respectively. By definition,
, respectively. By definition, 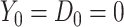 because the study population is restricted to surviving HIV-negative MSM. During any given interval
because the study population is restricted to surviving HIV-negative MSM. During any given interval  , we assume the temporal ordering
, we assume the temporal ordering  . We denote the history of a random variable up to and including interval
. We denote the history of a random variable up to and including interval  using overbars; for example
using overbars; for example  is the observed treatment history through the end of week
is the observed treatment history through the end of week 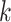 .
.
Denote the propensity score (49) as:
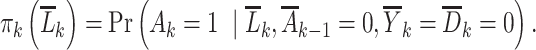 |
(1) |
This is the probability of initiating PrEP in week  among surviving individuals who have not yet been diagnosed with HIV or initiated PrEP, conditional on measured confounder history.
among surviving individuals who have not yet been diagnosed with HIV or initiated PrEP, conditional on measured confounder history. 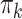 can also be understood as a (discrete) hazard of initiating PrEP at
can also be understood as a (discrete) hazard of initiating PrEP at  . In turn, we will define
. In turn, we will define
 |
(2) |
as the intervention propensity score, which is the analogous probability of initiating PrEP under a stochastic intervention, with PrEP initiation status at 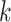 assigned by a random draw among those who have not initiated by week
assigned by a random draw among those who have not initiated by week  given their particular confounder history. This intervention propensity score is guaranteed to be larger than or equal to the propensity score. In equation 2,
given their particular confounder history. This intervention propensity score is guaranteed to be larger than or equal to the propensity score. In equation 2, 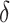 is a positive, known function of the measured confounder history. Throughout, we will refer to the propensity score in equation 1 as the natural propensity score to distinguish it from the intervention propensity score.
is a positive, known function of the measured confounder history. Throughout, we will refer to the propensity score in equation 1 as the natural propensity score to distinguish it from the intervention propensity score.
Interventions characterized by equation 2 are similar to the incremental propensity score interventions posed by Kennedy (40). Both ensure that, for any measured confounder history, the chance of receiving treatment under intervention will be at least as large as under no intervention or the natural course (50). However, Kennedy (40) defined these interventions such that the natural and intervention propensity scores relate through a fixed shift on an odds scale, ranging in 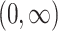 . We consider the class of interventions specified by equation 2 because they conveniently constrain possible choices of
. We consider the class of interventions specified by equation 2 because they conveniently constrain possible choices of  , capturing deterministic rules when we select
, capturing deterministic rules when we select  at an extreme. For example, when we select
at an extreme. For example, when we select  for all levels of
for all levels of 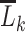 , then equation 2 coincides with the (static) deterministic rule “always initiate.” Alternatively, when we select
, then equation 2 coincides with the (static) deterministic rule “always initiate.” Alternatively, when we select 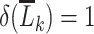 only under the condition that
only under the condition that 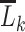 includes a recent rectal STI test, and
includes a recent rectal STI test, and 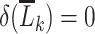 otherwise, then equation 2 coincides with the (dynamic) deterministic rule “initiate PrEP for all individuals at
otherwise, then equation 2 coincides with the (dynamic) deterministic rule “initiate PrEP for all individuals at 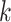 with a recent rectal STI test; otherwise, do not intervene.” An interesting property of the interventions posed by Kennedy (40) is that they ensure the required positivity condition cannot be violated no matter the properties of the natural propensity score in a given study (see further discussion in Web Appendix 1).
with a recent rectal STI test; otherwise, do not intervene.” An interesting property of the interventions posed by Kennedy (40) is that they ensure the required positivity condition cannot be violated no matter the properties of the natural propensity score in a given study (see further discussion in Web Appendix 1).
We conceptualized 5 interventions characterized by equation 2 that would be implemented at each time k among MSM meeting the following conditions, respectively: 1) MSM with a recent rectal STI test (suggesting condomless receptive anal sex), 2) MSM with a recent health-care encounter, 3) MSM with a recent positive STI test, 4) non-Hispanic Black MSM with a recent health-care encounter, and 5) Black or Latino MSM with a recent health-care encounter. For all interventions 1–5, we defined “recent” as any time within the 6 weeks prior to 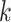 . Intervention 3 would increase the hazard of receiving PrEP in more individuals than 1 or 2 but would include many who would not benefit from PrEP. Given the national racial and ethnic disparities in PrEP uptake (51, 52), we considered interventions 4 and 5 to evaluate equitable approaches that focus on populations with the greatest unmet need. A summary of the conditions defining interventions 1–5 can be found in Table 2. Formally, an individual meeting the conditions for intervention at
. Intervention 3 would increase the hazard of receiving PrEP in more individuals than 1 or 2 but would include many who would not benefit from PrEP. Given the national racial and ethnic disparities in PrEP uptake (51, 52), we considered interventions 4 and 5 to evaluate equitable approaches that focus on populations with the greatest unmet need. A summary of the conditions defining interventions 1–5 can be found in Table 2. Formally, an individual meeting the conditions for intervention at  would be assigned treatment from the distribution in equation 2 for a choice of
would be assigned treatment from the distribution in equation 2 for a choice of  . By contrast, an individual not meeting the conditions at
. By contrast, an individual not meeting the conditions at  would be assigned treatment from this distribution but for
would be assigned treatment from this distribution but for  .
.
Table 2.
Description of Interventions of Interest to Increase Initiation of Preexposure HIV Prophylaxis
| Intervention | Label | Baseline Intervention Condition | Time-Varying Intervention Condition |
|---|---|---|---|
| 1 | Rectal STI test | All MSM | Rectal gonorrhea or chlamydia test in the current week or prior 6 weeks |
| 2 | Any health-care encounter | All MSM | Any health-care encounter in the current week or prior 6 weeks |
| 3 | Positive STI test | All MSM | Any positive gonorrhea or chlamydia test (rectal, pharyngeal, or urogenital), or a positive syphilis serology test |
| 4 | Non-Hispanic Black | Non-Hispanic Black MSM | Any health-care encounter in the current week or prior 6 weeks |
| 5 | Black or Latino | Black or Latino MSM | Any health-care encounter in the current week or prior 6 weeks |
Abbreviations: HIV, human immunodeficiency virus; MSM, men who have sex with men; STI, sexually transmitted infection.
The target causal effects of interest are contrasts in risk of incident HIV diagnosis under an intervention relative to the natural course (no intervention). For each intervention we considered various magnitudes of the shift in propensity score, 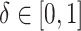 , for those meeting the intervention conditions. Death from other causes is a competing event for HIV, and the risk in this case coincides with the cause-specific cumulative incidence (53) of HIV. Contrasts in counterfactual cause-specific cumulative incidence functions under different interventions capture the total effect of treatment on the outcome of interest in that they may capture treatment effects on the outcome of interest via mechanisms that include the treatment’s effects on death (54). In this case, there is no evidence to suggest that PrEP affects mortality beyond its effects on the outcome of interest, HIV. In a setting with interference, such that one individual’s treatment may affect another individual’s outcome in the study population, our target effects best align with so-called overall effects as defined by Halloran and Struchiner (55).
, for those meeting the intervention conditions. Death from other causes is a competing event for HIV, and the risk in this case coincides with the cause-specific cumulative incidence (53) of HIV. Contrasts in counterfactual cause-specific cumulative incidence functions under different interventions capture the total effect of treatment on the outcome of interest in that they may capture treatment effects on the outcome of interest via mechanisms that include the treatment’s effects on death (54). In this case, there is no evidence to suggest that PrEP affects mortality beyond its effects on the outcome of interest, HIV. In a setting with interference, such that one individual’s treatment may affect another individual’s outcome in the study population, our target effects best align with so-called overall effects as defined by Halloran and Struchiner (55).
To understand the efficiency of an intervention, researchers and policymakers may be interested in effects on not only HIV incidence but also cumulative PrEP initiation. Despite the mathematical convenience of the shift parameter 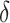 , it does not directly translate into a population-level summary metric of PrEP initiation. Thus, we also developed an IPW estimator for effects on cumulative incidence of PrEP initiation under each intervention relative to the natural course. This metric can be compared with results from analogous trials of PrEP-uptake interventions and inform their corresponding expected reductions in HIV incidence.
, it does not directly translate into a population-level summary metric of PrEP initiation. Thus, we also developed an IPW estimator for effects on cumulative incidence of PrEP initiation under each intervention relative to the natural course. This metric can be compared with results from analogous trials of PrEP-uptake interventions and inform their corresponding expected reductions in HIV incidence.
Identification and statistical methods
A counterfactual cause-specific cumulative incidence under a time-varying stochastic treatment rule that depends at most on measured variables in an observational study can be identified by the generalized g-formula, a function of only measured variables in that study (31). This identification result depends on several assumptions that are reviewed in Web Appendix 1. Importantly, this classical result requires no interference, presenting a limitation of the proposed methodology in the current application, as interference may be present in this context.
We implemented an IPW estimator derived from a weighted representation of the generalized g-formula indexed by equation 2 (see Web Appendixes 1 and 2) according to the following algorithm applied to a person-time data set containing baseline and follow-up data for each of the 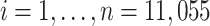 MSM meeting eligibility criteria:
MSM meeting eligibility criteria:
Step 1.
Fit a pooled over time logistic regression restricted to records with  (those still alive and HIV-negative at
(those still alive and HIV-negative at  , and who did not previously initiate PrEP). The dependent variable in this regression is
, and who did not previously initiate PrEP). The dependent variable in this regression is  and independent variables a function
and independent variables a function  of the measured confounder history
of the measured confounder history 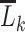 and time
and time  , parameterized by coefficients
, parameterized by coefficients 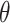 . From this model, for each individual
. From this model, for each individual  , we can estimate the time-varying propensity score by
, we can estimate the time-varying propensity score by
 |
(3) |
with 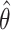 the maximum likelihood estimator of
the maximum likelihood estimator of 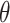 . See Web Table 2 for the form of
. See Web Table 2 for the form of 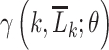 assumed in our analyses.
assumed in our analyses.
Step 2.
For each individual 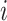 and time
and time  , calculate a weight
, calculate a weight 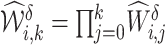 based on the estimated propensity scores from step 1 such that, for all times
based on the estimated propensity scores from step 1 such that, for all times  where
where 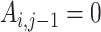 (the individual has not yet started treatment), set
(the individual has not yet started treatment), set
 |
(4) |
for a given choice of shift  . Otherwise, for all times
. Otherwise, for all times 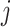 where
where 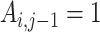 (the individual has previously started treatment) set
(the individual has previously started treatment) set  .
.
Step 3.
Given the weights from step 2, for  , estimate the intervention cause-specific hazard of HIV,
, estimate the intervention cause-specific hazard of HIV,
 |
(5) |
and death,
 |
(6) |
Step 4.
Estimate the intervention cause-specific cumulative HIV incidence by  by plugging in the weighted time-varying hazard estimates
by plugging in the weighted time-varying hazard estimates
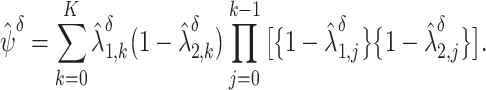 |
(7) |
By arguments in Web Appendix 3, we also implemented the following IPW estimator of the intervention cumulative incidence of PrEP initiation by  :
:
 |
(8) |
Both (equations 7 and 8) rely on a correctly specified propensity score model and ensure that cumulative incidence estimates are monotonically increasing over time and bounded by 1.
For a particular intervention with a specified 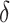 , we estimated the relative risk reduction by computing
, we estimated the relative risk reduction by computing 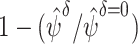 and the risk difference by
and the risk difference by  . Ninety-five percent confidence intervals (CIs) were obtained using nonparametric bootstrap percentiles with 1,000 bootstrap samples. Given the weight in equation 4, a small enough choice of shift
. Ninety-five percent confidence intervals (CIs) were obtained using nonparametric bootstrap percentiles with 1,000 bootstrap samples. Given the weight in equation 4, a small enough choice of shift 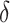 will ensure the numerator does not become much larger than the denominator, thus avoiding extreme weights regardless of the data (also see Web Appendix 1).
will ensure the numerator does not become much larger than the denominator, thus avoiding extreme weights regardless of the data (also see Web Appendix 1).
We used R, version 4.1.3 (R Foundation for Statistical Computing, Vienna, Austria), for the analyses.
RESULTS
Table 1 summarizes baseline characteristics for the 11,055 MSM included in the study, overall and by PrEP initiation status by the end of the 2-year follow-up period. Mean age was 34.0 years, 71.7% were White, 5.8% were Black and 11.6% were Latino/Hispanic. Overall, 2,875 (26.0%) initiated PrEP during follow-up. MSM who initiated PrEP had a lower incidence of HIV diagnosis compared with those who did not initiate (0.5% vs 0.8%). On average, MSM who initiated PrEP had a similar distribution of age, race, and ethnicity relative to noninitiators.
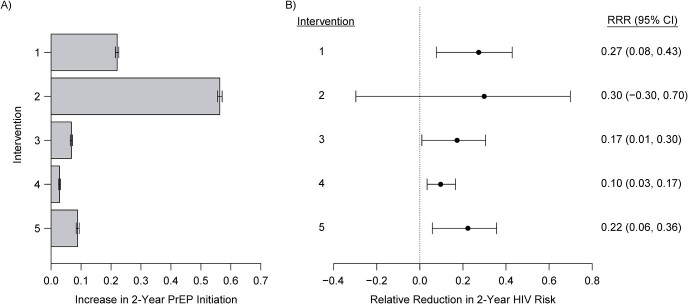
Figure 1 displays effect estimates of the 5 examined interventions versus the natural course on the increase in PrEP initiation and the risk of incident HIV diagnosis over 2 years for a fixed shift of  . We focus on
. We focus on  as an illustration of a modest increase in the probability of PrEP initiation. Results for a range of
as an illustration of a modest increase in the probability of PrEP initiation. Results for a range of 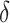 values are presented in Web Tables 3–7. We estimated that the intervention increasing the chance of initiating PrEP in individuals with a recent rectal STI test (intervention 1) would result in an increase in the proportion initiating PrEP of 22.0% (95% CI: 21.3%, 22.8%)—i.e., from the observed 26.0% to 48.0%—and a relative reduction in 2-year HIV risk of 27.3% (95% CI: 7.6%, 42.9%) compared with the natural course. The intervention increasing the hazard of PrEP initiation among Black or Latino MSM with a recent health-care encounter (intervention 5) would result in an increase in the proportion initiating PrEP of only 8.9% (95% CI: 8.0%, 9.7%) and a relative reduction in 2-year HIV risk of 22.3% (95% CI: 5.8% to 35.6%). The intervention increasing the probability of initiating PrEP in MSM with a recent health-care encounter (intervention 2) would result in a similar reduction in HIV risk (i.e., 29.9% (95% CI: −29.7% to 69.8%), while resulting in a much larger increase in the proportion initiating PrEP (56.3%, 95% CI: 55.8% to 56.8%). Among the 5 hypothetical interventions, the intervention among Black or Latino MSM with a recent health-care encounter would be the most efficient, yielding the largest reduction in HIV risk relative to the increase in the total proportion initiating PrEP.
values are presented in Web Tables 3–7. We estimated that the intervention increasing the chance of initiating PrEP in individuals with a recent rectal STI test (intervention 1) would result in an increase in the proportion initiating PrEP of 22.0% (95% CI: 21.3%, 22.8%)—i.e., from the observed 26.0% to 48.0%—and a relative reduction in 2-year HIV risk of 27.3% (95% CI: 7.6%, 42.9%) compared with the natural course. The intervention increasing the hazard of PrEP initiation among Black or Latino MSM with a recent health-care encounter (intervention 5) would result in an increase in the proportion initiating PrEP of only 8.9% (95% CI: 8.0%, 9.7%) and a relative reduction in 2-year HIV risk of 22.3% (95% CI: 5.8% to 35.6%). The intervention increasing the probability of initiating PrEP in MSM with a recent health-care encounter (intervention 2) would result in a similar reduction in HIV risk (i.e., 29.9% (95% CI: −29.7% to 69.8%), while resulting in a much larger increase in the proportion initiating PrEP (56.3%, 95% CI: 55.8% to 56.8%). Among the 5 hypothetical interventions, the intervention among Black or Latino MSM with a recent health-care encounter would be the most efficient, yielding the largest reduction in HIV risk relative to the increase in the total proportion initiating PrEP.
Figure 1.
Estimated effects of interventions that increase the propensity score (hazard) for initiating preexposure prophylaxis (PrEP) by 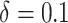 in high-priority subgroups of men who have sex with men (MSM) relative to the natural course, Fenway Health, Massachusetts, 2012–2018. A) Estimated increase in the 2-year cumulative proportion of the population initiating PrEP; B) estimated relative reduction in 2-year risk of incident human immunodeficiency virus (HIV) diagnosis. The error bars show the 95% nonparametric bootstrap confidence intervals (CIs) of the estimates. RRR, relative risk reduction.
in high-priority subgroups of men who have sex with men (MSM) relative to the natural course, Fenway Health, Massachusetts, 2012–2018. A) Estimated increase in the 2-year cumulative proportion of the population initiating PrEP; B) estimated relative reduction in 2-year risk of incident human immunodeficiency virus (HIV) diagnosis. The error bars show the 95% nonparametric bootstrap confidence intervals (CIs) of the estimates. RRR, relative risk reduction.
We estimated the 2-year cumulative incidence of HIV diagnosis for increasing values of  for the intervention implemented among Black or Latino MSM with a recent health-care encounter (intervention 5), ranging from
for the intervention implemented among Black or Latino MSM with a recent health-care encounter (intervention 5), ranging from 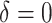 (i.e., the natural course) to
(i.e., the natural course) to 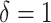 (i.e., “always-treat”), shown in Figure 2. A propensity score shift of
(i.e., “always-treat”), shown in Figure 2. A propensity score shift of 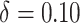 results in a 22.3% reduction in HIV incidence over the 2-year follow-up, as also shown in Figure 1. Similarly, we estimate that HIV risk would be reduced by 25.8% (95% CI: 6.3% to 40.4%) for a shift of
results in a 22.3% reduction in HIV incidence over the 2-year follow-up, as also shown in Figure 1. Similarly, we estimate that HIV risk would be reduced by 25.8% (95% CI: 6.3% to 40.4%) for a shift of 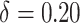 , which would increase the total proportion of individuals initiating PrEP by the end of follow-up by 10.4% (95% CI: 9.5% to 11.3%) relative to the natural course. Web Figure 1 analogously displays the estimated relative reduction in HIV risk against cumulative PrEP initiation by varying
, which would increase the total proportion of individuals initiating PrEP by the end of follow-up by 10.4% (95% CI: 9.5% to 11.3%) relative to the natural course. Web Figure 1 analogously displays the estimated relative reduction in HIV risk against cumulative PrEP initiation by varying 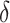 under an intervention that increases the propensity of initiating PrEP among MSM with a recent rectal STI test (intervention 1) relative to the natural course.
under an intervention that increases the propensity of initiating PrEP among MSM with a recent rectal STI test (intervention 1) relative to the natural course.
Figure 2.
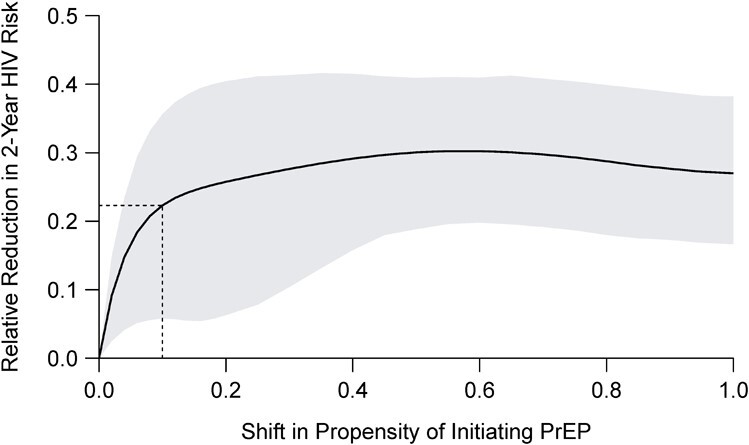
Estimated relative reduction in 2-year human immunodeficiency virus (HIV) risk under a stochastic intervention that increases the propensity (hazard) of initiating preexposure prophylaxis (PrEP) by  among Black or Latino men who have sex with men (MSM) with a recent health-care encounter at Fenway Health compared with the natural course, Massachusetts, 2012–2018. The shaded area represents 95% pointwise nonparametric bootstrap confidence bands of the estimates. The dashed lines relate to the intervention with the shift of
among Black or Latino men who have sex with men (MSM) with a recent health-care encounter at Fenway Health compared with the natural course, Massachusetts, 2012–2018. The shaded area represents 95% pointwise nonparametric bootstrap confidence bands of the estimates. The dashed lines relate to the intervention with the shift of  specified in Figure 1.
specified in Figure 1.
Figure 3 displays the estimated HIV risk difference per 1,000 individuals over time comparing the risk under the natural course with the risk under intervention 5 for  . Similarly, Web Figure 2 displays HIV risk difference estimates over time for intervention 1. Corresponding effect estimates for all interventions are summarized in Web Tables 3–7, with complete details on the range of
. Similarly, Web Figure 2 displays HIV risk difference estimates over time for intervention 1. Corresponding effect estimates for all interventions are summarized in Web Tables 3–7, with complete details on the range of  examined.
examined.
Figure 3.
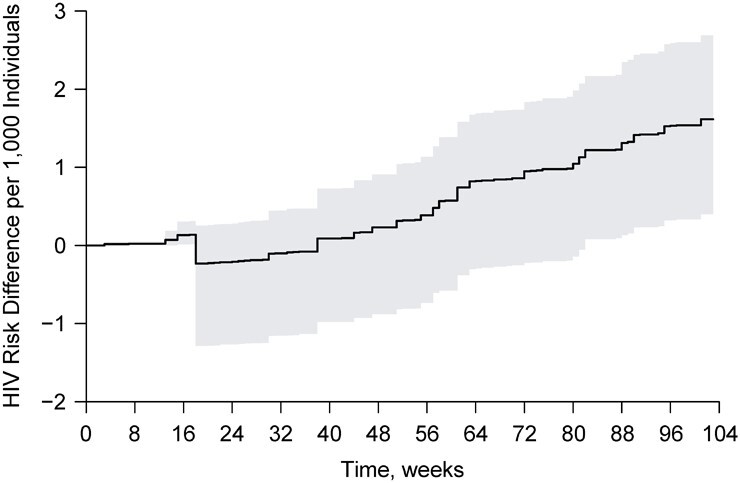
Estimated 2-year human immunodeficiency virus (HIV) risk difference per 1,000 individuals over time under a stochastic intervention that increases the propensity of initiating preexposure prophylaxis (PrEP) by  among Black or Latino men who have sex with men (MSM) with a recent health-care encounter at Fenway Health versus under observed PrEP initiation, or the natural course, Massachusetts, 2012–2018. The shaded area represents a 95% pointwise nonparametric bootstrap confidence band of the estimates.
among Black or Latino men who have sex with men (MSM) with a recent health-care encounter at Fenway Health versus under observed PrEP initiation, or the natural course, Massachusetts, 2012–2018. The shaded area represents a 95% pointwise nonparametric bootstrap confidence band of the estimates.
DISCUSSION
This study applied IPW methods to longitudinal EHR data to estimate effects of hypothetical interventions to increase PrEP initiation on the cumulative incidence of HIV diagnosis among MSM. Our results suggest that interventions resulting in only modest increases in PrEP initiation in high-priority subgroups of MSM could meaningfully reduce the 2-year incidence of HIV acquisition in the overall population of MSM. We found that for particular interventions and choices of 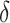 , estimates of increases in PrEP uptake aligned with published results of pilot trials. For example, intervention 1 with a choice of
, estimates of increases in PrEP uptake aligned with published results of pilot trials. For example, intervention 1 with a choice of 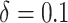 provided a stochastic intervention analogue for a behavioral intervention to promote PrEP uptake among MSM and resulted in a similar increase in PrEP initiation (22.0% vs. 24.4%) (19).
provided a stochastic intervention analogue for a behavioral intervention to promote PrEP uptake among MSM and resulted in a similar increase in PrEP initiation (22.0% vs. 24.4%) (19).
In particular, our findings demonstrate that PrEP-uptake interventions tailored to Black and Latino MSM, who are placed at increased risk of HIV infection and have been underserved by PrEP programs to date, may reduce not only inequities in PrEP uptake but also population-level HIV incidence among MSM. Our results support prioritizing the scale-up of such interventions as part of the federal Ending the HIV Epidemic initiative (56). Given that Black MSM have been deterred from PrEP use by historical and ongoing mistreatment in health-care settings, interventions focused on Black MSM must be undertaken with recognition of medical mistrust, cultural humility, and engagement of community stakeholders (57).
The Ending the HIV Epidemic initiative aims to decrease HIV incidence in the United States by 75% by 2025, and by 90% by 2030. Our study suggests that these goals may be approachable among MSM if there is substantial PrEP uptake in high-priority groups. Under the various interventions we considered, the largest estimated HIV relative risk reduction was 66% for the intervention among MSM with a recent rectal STI test, corresponding to 62% of all MSM initiating PrEP (Web Table 3). This estimated reduction in HIV risk does not meet the goal of a 75% reduction by 2025, and does not even approach the 90% goal for 2030. However, it is likely that some MSM in our cohort who were prescribed PrEP did not initiate the medication, and that some who initiated did not continue after the first prescription. Thus, our results may represent conservative estimates of the effects of PrEP-uptake interventions on HIV incidence.
Our study has several strengths. We illustrated a novel application of IPW to estimate effects of incremental propensity score interventions to increase PrEP initiation on cumulative incidence of HIV diagnosis in a large observational cohort. This approach is particularly suited to settings where the most policy-relevant questions are about the effects of small-to-moderate increases in use of a treatment on population health outcomes. Our methods appropriately adjust for time-varying confounding and competing risk events. We also developed an IPW estimator of cumulative treatment uptake associated with such interventions, which provides estimates of PrEP uptake on a more comparable scale to those of ongoing pilot trials and may be more intuitive to subject-matter experts than hazard-based measures.
Our study also has limitations. First, while we adjusted for measured proxies of condomless anal sex, these do not perfectly capture recent or planned sexual activity. Thus, exchangeability (no unmeasured confounding), at best, approximately holds. Second, we did not include prescriptions for tenofovir alafenamide coformulated with emtricitabine, which was approved for use as PrEP in October 2019. However, our cohort was only followed for 4 months after approval, and we previously observed a low frequency of switching to the new formulation among male patients at Fenway Health (58). Third, some patients may have transferred to a different care setting before the end of follow-up, and these patients may have differed from those who remained at Fenway Health. One approach to mitigating bias from loss to follow-up is artificially censoring after a certain amount of time without a health-care encounter (59), but we chose not to do this given the limited duration of follow-up, rarity of the outcome, and precision/bias tradeoff.
MSM receiving care at Fenway Health may not be representative of all MSM, limiting generalizability. Most MSM in our study were non-Hispanic White, and in contrast to the racial and ethnic disparities in PrEP uptake observed in other health-care systems (60) and nationally (51, 52), we found no evidence of these disparities at Fenway Health. Nevertheless, disparities in PrEP use are compounding racial and ethnic inequities in HIV incidence in the broader population of MSM (61–63). Thus, PrEP-uptake interventions tailored to Black and Latino MSM, such as the hypothetical interventions we considered, remain a high priority to maximize equity in PrEP use and population-level impact on HIV incidence.
Our approach does not address interference, including that the covariates of connected individuals may need to be measured to ensure exchangeability (64, 65). Interference is difficult to address in EHR-based studies because of a lack of data on sexual contacts, making agent-based models a useful complementary approach to causal inference in studies of the population impact of PrEP. Future work for optimizing g-methods with EHR data might include the development of bounds for IPW estimates and related methods that consider working assumptions related to the structure of interference, particularly when the outcome is rare and treatment is highly effective, as in the PrEP context.
Finally, our analysis used simple IPW estimators, requiring correct specification of a parametric propensity score model. This is in contrast to TMLE, AIPW, and related methods based on the efficient influence function, which have been developed for estimating effects of incremental propensity score interventions (40, 66, 67). These approaches have the advantage of maintaining 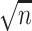 consistency when the propensity score and other nuisance functions are estimated at slower rates through machine learning methods (68–70). Our IPW approach has the advantage of guaranteeing monotonic cumulative incidence estimates over time in the survival setting. To our knowledge, this is not guaranteed by existing TMLE methods outside of the time-fixed treatment setting (71, 72). Further, advanced computing resources are necessary to implement these methods to full theoretical advantage. Our simpler approach may encourage accessibility and understanding of methods for causal inference in complex longitudinal data for a more flexible class of causal questions while computing options become more advanced and accessible.
consistency when the propensity score and other nuisance functions are estimated at slower rates through machine learning methods (68–70). Our IPW approach has the advantage of guaranteeing monotonic cumulative incidence estimates over time in the survival setting. To our knowledge, this is not guaranteed by existing TMLE methods outside of the time-fixed treatment setting (71, 72). Further, advanced computing resources are necessary to implement these methods to full theoretical advantage. Our simpler approach may encourage accessibility and understanding of methods for causal inference in complex longitudinal data for a more flexible class of causal questions while computing options become more advanced and accessible.
As PrEP uptake increases, g-methods for stochastic interventions could be applied in studies of other populations, such as Black women, who are underrepresented among PrEP users and for whom PrEP-uptake interventions are a high priority. These approaches could also be extended to geographic regions with greater unmet need for PrEP, including Southern states, and to other PrEP delivery settings. Finally, approaches for targeting causal effects in longitudinal EHR data are ideally positioned to evaluate interventions tailored to the uptake of long-acting injectable PrEP, which received regulatory approval in December 2021, and to other long-acting PrEP modalities (e.g., monthly pills) as they emerge. With this work, we intend to move the PrEP implementation field forward, with the goal of maximizing impact on the HIV epidemic.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Zurich, Switzerland (Ainesh Sewak); Department of Biostatistics, Boston University School of Public Health, Boston, Massachusetts, United States (Sara Lodi); Department of Population Medicine, Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston, Massachusetts, United States (Xiaojuan Li, Douglas S. Krakower, Jessica G. Young, Julia L. Marcus); Department of Biostatistics, Epidemiology and Informatics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania, United States (Di Shu); Department of Statistics and Actuarial Science, University of Waterloo, Ontario, Canada (Lan Wen); CAUSALab, Harvard T. H. Chan School of Public Health, Boston, Massachusetts, United States (Lan Wen, Jessica G. Young); The Fenway Institute, Fenway Health, Boston, Massachusetts, United States (Kenneth H. Mayer, Douglas S. Krakower, Julia L. Marcus); Division of Infectious Diseases, Beth Israel Deaconess Medical Center, Boston, Massachusetts, United States (Kenneth H. Mayer, Douglas S. Krakower); and Department of Epidemiology, Harvard T. H. Chan School of Public Health, Boston, Massachusetts, United States (Jessica G. Young).
J.G.Y. and J.L.M. contributed equally to this work.
This work was supported by the National Institute of Allergy and Infectious Diseases (grant R21 AI143386), awarded to both J.G.Y. and J.L.M.
Confidentiality concerns prevent us from uploading our data to a public repository. We provide an example of a simulated data set with similar properties to the study population on GitHub (https://github.com/asewak/stoch-int). Implementation of our inverse probability weighting code and associated plotting procedures to generate the results of this paper can be found in the same repository.
K.H.M. has received unrestricted research grants from Gilead Sciences and served on a scientific advisory board for them. D.S.K. has conducted research at Fenway Health funded by grants from Merck and has received personal fees for developing medical education content for DKBMed, Virology Education, and UpToDate, Inc. The other authors report no conflicts.
REFERENCES
- 1. Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363(27):2587–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McCormack S, Dunn DT, Desai M, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2016;387(10013):53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anderson PL, Glidden DV, Liu A, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med. 2012;4(151):151ra125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Molina J-M, Capitant C, Spire B, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med. 2015;373(23):2237–2246. [DOI] [PubMed] [Google Scholar]
- 5. Marcus JL, Hurley LB, Nguyen DP, et al. Redefining human immunodeficiency virus (HIV) preexposure prophylaxis failures. Clin Infect Dis. 2017;65(10):1768–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Volk JE, Marcus JL, Phengrasamy T, et al. No new HIV infections with increasing use of HIV preexposure prophylaxis in a clinical practice setting. Clin Infect Dis. 2015;61(10):1601–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Centers for Disease Control and Prevention (CDC) . PrEP coverage in the U.S. by race/ethnicity. 2020. https://www.cdc.gov/nchhstp/newsroom/fact-sheets/images/hiv/PrEP-Coverage-Race-Ethnicity-2020.jpg. Accessed May 20, 2022.
- 8. Smith DK, Van Handel M, Grey J. Estimates of adults with indications for HIV pre-exposure prophylaxis by jurisdiction, transmission risk group, and race/ethnicity, United States, 2015. Ann Epidemiol. 2018;28(12):850–857. [DOI] [PubMed] [Google Scholar]
- 9. Siegler AJ, Mouhanna F, Giler RM, et al. The prevalence of pre-exposure prophylaxis use and the pre-exposure prophylaxis–to-need ratio in the fourth quarter of 2017, United States. Ann Epidemiol. 2018;28(12):841–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mayer KH, Agwu A, Malebranche D. Barriers to the wider use of pre-exposure prophylaxis in the United States: a narrative review. Adv Ther. 2020;37(5):1778–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jones J, Dominguez K, Stephenson R, et al. A theoretically based mobile app to increase pre-exposure prophylaxis uptake among men who have sex with men: protocol for a randomized controlled trial. JMIR Res Protoc. 2020;9(2):e16231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rouffiac A-E, Whiteley L, Brown L, et al. A mobile intervention to improve uptake of pre-exposure prophylaxis for southern Black men who have sex with men: protocol for intervention development and pilot randomized controlled trial. JMIR Res Protoc. 2020;9(2):e15781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Siegler AJ, Brock JB, Hurt CB, et al. An electronic pre-exposure prophylaxis initiation and maintenance home care system for nonurban young men who have sex with men: protocol for a randomized controlled trial. JMIR Res Protoc. 2019;8(6):e13982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Desrosiers A, Levy M, Dright A, et al. A randomized controlled pilot study of a culturally-tailored counseling intervention to increase uptake of HIV pre-exposure prophylaxis among young Black men who have sex with men in Washington, DC. AIDS Behav. 2019;23(1):105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Biello KB, Hill-Rorie J, Valente PK, et al. Development and evaluation of a mobile app designed to increase HIV testing and pre-exposure prophylaxis use among young men who have sex with men in the United States: open pilot trial. J Med Internet Res. 2021;23(3):e25107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guinness RR, Volk JE, Hurley LB, et al. Low-intensity outreach to increase uptake of HIV preexposure prophylaxis among patients with sexually transmitted infections. AIDS Behav. 2019;23(2):544–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bonner R, Stewart J, Upadhyay A, et al. A primary care intervention to increase HIV pre-exposure prophylaxis (PrEP) uptake in patients with syphilis. J Int Assoc Provid AIDS Care. 2022;21. 10.1177/23259582211073393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sullivan PS, Stephenson R, Hirshfield S, et al. Behavioral efficacy of a sexual health mobile app for men who have sex with men: the M-cubed randomized controlled trial. J Med Internet Res. 2022;24(2):e34574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chan PA, Nunn A, Van Den Berg JJ, et al. A randomized trial of a brief behavioral intervention for PrEP uptake among men who have sex with men at increased risk for HIV infection. J Acquir Immune Defic Syndr. 2021;87(3):937–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bonacci RA, Smith DK, Ojikutu BO. Toward greater pre-exposure prophylaxis equity: increasing provision and uptake for Black and Hispanic/Latino individuals in the US. Am J Prev Med. 2021;61(5):S60–S72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Garrison LE, Haberer JE. Pre-exposure prophylaxis uptake, adherence, and persistence: a narrative review of interventions in the U.S. Am J Prev Med. 2021;61(5):S73–S86. [DOI] [PubMed] [Google Scholar]
- 22. Jenness SM, Goodreau SM, Rosenberg E, et al. Impact of the Centers for Disease Control’s HIV preexposure prophylaxis guidelines for men who have sex with men in the United States. J Infect Dis. 2016;214(12):1800–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kasaie P, Pennington J, Shah MS, et al. The impact of pre-exposure prophylaxis among men who have sex with men: an individual-based model. J Acquir Immune Defic Syndr. 2017;75(2):175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rozhnova G, Heijne J, Bezemer D, et al. Elimination prospects of the Dutch HIV epidemic among men who have sex with men in the era of preexposure prophylaxis. AIDS. 2018;32(17):2615–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goedel WC, King MRF, Lurie MN, et al. Effect of racial inequities in pre-exposure prophylaxis use on racial disparities in HIV incidence among men who have sex with men: a modeling study. J Acquir Immune Defic Syndr. 2018;79(3):323–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jenness SM, Maloney KM, Smith DK, et al. Addressing gaps in HIV preexposure prophylaxis care to reduce racial disparities in HIV incidence in the United States. Am J Epidemiol. 2019;188(4):743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hudgens MG, Halloran ME. Toward causal inference with interference. J Am Stat Assoc. 2008;103(482):832–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Robins JM, Wasserman LA. Estimation of effects of sequential treatments by reparameterizing directed acyclic graphs [preprint]. arXiv. 2013. https://arxiv.org/abs/1302.1566. Accessed January 4, 2023. [Google Scholar]
- 29. Murray EJ, Robins JM, Seage GR, et al. A comparison of agent-based models and the parametric g-formula for causal inference. Am J Epidemiol. 2017;186(2):131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Robins JM. Causal inference from complex longitudinal data. In: Berkane M, ed. Latent Variable Modeling and Applications to Causality. New York, NY: Springer New York; 1997:69–117. [Google Scholar]
- 31. Robins J. A new approach to causal inference in mortality studies with a sustained exposure period—application to control of the healthy worker survivor effect. Math Model. 1986;7(9–12):1393–1512. [Google Scholar]
- 32. Hernán MA, Robins JM. Causal Inference: What If. Boca Raton, FL: CRC Press; 2020. [Google Scholar]
- 33. Van der Laan MJ, Robins JM. Unified Methods for Censored Longitudinal Data and Causality. 1st ed. New York, NY: Springer New York; 2003. [Google Scholar]
- 34. Van der Laan MJ, Rose S, et al. Targeted Learning: Causal Inference for Observational and Experimental Data. 1st ed. New York, NY: Springer New York; 2011. [Google Scholar]
- 35. Petersen ML, Porter KE, Gruber S, et al. Diagnosing and responding to violations in the positivity assumption. Stat Methods Med Res. 2012;21(1):31–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Young JG, Hernán MA, Robins JM. Identification, estimation and approximation of risk under interventions that depend on the natural value of treatment using observational data. Epidemiologic Methods. 2014;3(1):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Díaz I, Laan MJ. Assessing the causal effect of policies: an example using stochastic interventions. Int J Biostat. 2013;9(2):161–174. [DOI] [PubMed] [Google Scholar]
- 38. Haneuse S, Rotnitzky A. Estimation of the effect of interventions that modify the received treatment. Stat Med. 2013;32(30):5260–5277. [DOI] [PubMed] [Google Scholar]
- 39. Young JG, Logan RW, Robins JM, et al. Inverse probability weighted estimation of risk under representative interventions in observational studies. J Am Stat Assoc. 2019;114(526):938–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kennedy EH. Nonparametric causal effects based on incremental propensity score interventions. J Am Stat Assoc. 2019;114(526):645–656. [Google Scholar]
- 41. Díaz I, Williams N, Hoffman KL, et al. Nonparametric causal effects based on longitudinal modified treatment policies. J Am Stat Assoc. 2021;118(542):1–16. [Google Scholar]
- 42. Klompas M, Murphy M, Lankiewicz J, et al. Harnessing electronic health records for public health surveillance. Online J Public Health Inform. 2011;3(3):ojphi.v3i3.3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Klompas M, McVetta J, Lazarus R, et al. Integrating clinical practice and public health surveillance using electronic medical record systems. Am J Public Health. 2012;102(S3):S325–S332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Birkhead GS, Klompas M, Shah NR. Uses of electronic health records for public health surveillance to advance public health. Annu Rev Public Health. 2015;36(1):345–359. [DOI] [PubMed] [Google Scholar]
- 45. Mayer K, Appelbaum J, Rogers T, et al. The evolution of the Fenway community health model. Am J Public Health. 2001;91(6):892–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70(4):1–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Klompas M, Lazarus R, Daniel J, et al. Electronic Medical Record Support for Public Health (ESP): automated detection and reporting of statutory notifiable diseases to public health authorities. Advances in Disease Surveillance. 2007;3(3):1–5. [Google Scholar]
- 48. Krakower DS, Gruber S, Hsu K, et al. Development and validation of an automated HIV prediction algorithm to identify candidates for pre-exposure prophylaxis: a modelling study. Lancet HIV. 2019;6(10):e696–e704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. [Google Scholar]
- 50. Young JG, Cain LE, Robins JM, et al. Comparative effectiveness of dynamic treatment regimes: an application of the parametric g-formula. Stat Biosci. 2011;3(1):119–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Centers for Disease Control and Prevention (CDC) . US Public Health Service: Preexposure Prophylaxis for the Prevention of HIV Infection in the United States—2017 Update: a Clinical Practice Guideline. Technical report. Atlanta, GA: Centers for Disease Control and Prevention (CDC); 2018. [Google Scholar]
- 52. Centers for Disease Control and Prevention (CDC) . Monitoring Selected National HIV Prevention and Care Objectives by Using HIV Surveillance data—United States and 6 Dependent Areas, 2019. In: HIV Surveillance Supplemental Report 2021. Technical report. Atlanta, GA: Centers for Disease Control and Prevention (CDC); 2021. [Google Scholar]
- 53. Geskus RB, ed. Data Analysis With Competing Risks and Intermediate States. 1st ed. Boca Raton, FL: Chapman and Hall/CRC; 2019. [Google Scholar]
- 54. Young JG, Stensrud MJ, Tchetgen Tchetgen EJ, et al. A causal framework for classical statistical estimands in failure-time settings with competing events. Stat Med. 2020;39(8):1199–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Halloran ME, Struchiner CJ. Study designs for dependent happenings. Epidemiology. 1991;2(5):331–338. [DOI] [PubMed] [Google Scholar]
- 56. Fauci AS, Redfield RR, Sigounas G, et al. Ending the HIV epidemic: a plan for the United States. JAMA. 2019;321(9):844–845. [DOI] [PubMed] [Google Scholar]
- 57. Cahill S, Taylor SW, Elsesser SA, et al. Stigma, medical mistrust, and perceived racism may affect PrEP awareness and uptake in Black compared to White gay and bisexual men in Jackson, Mississippi and Boston, Massachusetts. AIDS Care. 2017;29(11):1351–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Marcus JL, Levine K, Sewell WC, et al. Switching from tenofovir disoproxil fumarate to tenofovir alafenamide for human immunodeficiency virus preexposure prophylaxis at a Boston community health center. Open Forum Infect Dis. 2021;8(8):ofab372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hernán MA, McAdams M, McGrath N, et al. Observation plans in longitudinal studies with time-varying treatments. Stat Methods Med Res. 2009;18(1):27–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Marcus JL, Hurley LB, Bradley Hare C, et al. Disparities in uptake of HIV preexposure prophylaxis in a large integrated healthcare system. Am J Public Health. 2016;106(10):e2–e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jenness SM, Maloney KM, Smith DK, et al. The PrEP care continuum and racial disparities in HIV incidence among men who have sex with men. Am J Epidemiol. 2019;188(4):743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rolle C-P, Rosenberg ES, Siegler AJ, et al. Challenges in translating PrEP interest into uptake in an observational study of young Black MSM. J Acquir Immune Defic Syndr. 2017;76(3):250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kelley CF, Kahle E, Siegler A, et al. Applying a PrEP continuum of care for men who have sex with men in Atlanta, Georgia. Clin Infect Dis. 2015;61(10):1590–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zivich PN, Breskin A. Machine learning for causal inference: on the use of cross-fit estimators. Epidemiology. 2021;32(3):393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Engebretsen S, Rø G, Freiesleben B, et al. A compelling demonstration of why traditional statistical regression models cannot be used to identify risk factors from case data on infectious diseases: a simulation study. BMC Med Res Methodol. 2022;22(1):146–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wen L, Marcus J, Young J. Intervention treatment distributions that depend on the observed treatment process and model double robustness in causal survival analysis [preprint]. arXiv. 2021. 10.48550/arXiv.2112.00807. Accessed January 4, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Naimi AI, Rudolph JE, Kennedy EH, et al. Incremental propensity score effects for time-fixed exposures. Epidemiology. 2021;32(2):202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Robins J, Li L, Tchetgen, Tchetgen E, et al. Quadratic semiparametric Von Mises calculus. Metrika. 2009;69(2–3):227–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Robins J, Li L, Tchetgen, Tchetgen ET, et al. Asymptotic normality of quadratic estimators. Stoch Process Their Appl. 2016;126(12):3733–3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chernozhukov V, Chetverikov D, Demirer M, et al. Double/debiased machine learning for treatment and structural parameters. Econom J. 2018;21(1):C1–C68. [Google Scholar]
- 71. Cai W, Laan MJ. One-step targeted maximum likelihood estimation for time-to-event outcomes. Biometrics. 2020;76(3):722–733. [DOI] [PubMed] [Google Scholar]
- 72. Rytgaard HCW, Laan MJ. One-step TMLE for targeting cause-specific absolute risks and survival curves [preprint]. arXiv. 2021. 10.48550/arXiv.2107.01537. Accessed January 4, 2023. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.