Abstract
Background
Alpha-1 antitrypsin deficiency (AATD) is the most common genetic risk factor for early-onset emphysema. However, AATD continues to be underrecognized and underdiagnosed. Provider awareness about AATD, concerns with testing costs, and limited understanding about therapeutic options contribute to its underdiagnosis. We hypothesized that provider education would improve awareness of AATD and improve screening.
Objective
To evaluate the impact of a targeted provider education module on AATD screening.
Methods
We developed a web-based education module to address barriers to screening for AATD, deployed the education module using the Medscape Education platform, assessed perceived healthcare provider confidence in AATD screening, and conducted a prospective pre and postintervention study of AATD testing practices at a high-volume academic outpatient subspecialty pulmonary clinic.
Results
A total of 11,385 healthcare providers, including eight pulmonologists at our institution, completed the web-based education module. Confidence in identifying patients at high risk for AATD improved after completing the module (“not confident” in AATD screening was 7.7% postintervention compared with 19.4% preintervention). The rate of screening patients at high risk for AATD improved more than twofold (AATD screening rate 9.7% preintervention vs. 20.4% postintervention; P = 0.004). Among patients screened for AATD in our cohort, 27.2% had a genotype/phenotype or low alpha-1 antitrypsin concentration consistent with AATD.
Conclusion
Targeted healthcare provider education can improve the confidence in testing for AATD. Improvements in provider confidence corresponded to improvements in AATD screening in a subspecialty pulmonary clinic. More than one-fourth of screening tests suggested AATD, underpinning the value of testing in high-risk individuals.
Keywords: alpha-1 antitrypsin deficiency, chronic obstructive pulmonary disease, education
Although the prevalence of alpha-1 antitrypsin deficiency (AATD) is estimated to be at least 1 in 5,000 Americans, and although AATD affects up to 5–10% of persons with chronic obstructive pulmonary disease (COPD), AATD remains underdiagnosed (1–5). Screening for AATD is inconsistent for many reasons, including limited knowledge of testing procedures, lack of healthcare provider awareness of screening modalities, and lack of familiarity with treatments (6). Undertesting and underdiagnosis of AATD lead to delays in the identification and care of patients with AATD (7). Although electronic medical record–level alerts have been trialed in the past (with some efficacy in increasing appropriate screening) (3), there remains a need to further increase appropriate AATD screening in applicable patients.
It is currently the recommendation of multiple professional societies to screen all patients with COPD or incompletely reversible asthma for AATD at least once (8, 9). This recommendation applies to a large portion of patients who present to a general pulmonary clinic. The current rate of screening for AATD in a real-world pulmonary practice is not known, but it has been shown that education interventions in screening for other disease types have been successful in increasing the number of appropriate screens (10). In the present study, we developed an education module targeting healthcare providers and measured perceptions about AATD testing; deployed the module in a single-center, high-volume pulmonary practice; and measured the impact of the intervention on AATD testing.
Methods
Study Setting, Design, and Data Sources
We performed a prospective pre- and postintervention study of AATD testing at an academic outpatient pulmonary clinic. We considered patients as eligible for AATD testing if they fulfilled the criteria of having both 1) a new-patient visit and 2) a diagnosis of COPD or asthma. The preintervention period was defined as the 6 months before deployment of the education module, and the postintervention period was the 6-month time frame after the education module was released. The education intervention was aimed at increasing awareness of AATD, detailing typical screening procedures, addressing barriers to testing, and providing a framework for test interpretation and next steps when encountering an eligible patient.
The outpatient pulmonary clinic (Kirklin Clinic at the University of Alabama at Birmingham) is a quaternary referral center for the state of Alabama and areas of Georgia, Mississippi, Louisiana, and Tennessee and sees more than 700,000 unique patient visits annually (11). The pulmonary clinic is staffed by attending pulmonologists, trainees in pulmonary medicine, and advanced practice providers. To identify patients eligible for AATD screening, we used a two-step approach to case identification with Informatics for Integrating Biology at the Bedside (i2b2) screening followed by second-level physician review (12). The i2b2 platform is designed primarily for cohort identification, allowing users to perform an enterprise-wide search of health information to determine the existence of a set of patients meeting certain inclusion or exclusion criteria for this study (12). We used the following groups of search terms within i2b2 to identify cohorts from the electronic data warehouse: Group 1 comprised International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, codes for COPD and asthma (J44, J44.9, J45.3, J45.30, J45.4, J45.40, J45.5, and J45.50); group 2 comprised medical service encounters to the pulmonary service within 6 months of the query date; group 3 comprised visit type of one-time or recurring outpatient visits; group 4 comprised those aged 18 years of age or older; and group 5 comprised those presumed to be living. This i2b2 query was repeated for the postintervention time frame.
After the i2b2 collection, a board-certified pulmonologist conducted a manual review of the identified patient charts in the electronic health record. This review encompassed all clinical encounter notes, laboratory test values, and outside facility records that were present in the record. We used the following rubric for classification as “high-suspicion patients”: a new patient encounter in the pulmonary service, the encounter occurred in the outpatient setting, the encounter occurred during the time window for the pre- or postintervention period, and there must be a physician diagnosis of either adult-onset asthma or COPD. Patients were excluded from the cohort if any of the following conditions were met: not seen as a new-patient visit by the pulmonary service during the window of time, encounters for follow-up visits, encounters for procedures (e.g., bronchoscopy, pulmonary function testing) without an outpatient clinic visit, or encounters for inpatient visits. The same method was used for the postintervention period.
Once a patient was deemed eligible for inclusion in the curated cohort, a structured review and abstraction by a pulmonologist reviewer (R.C.S.) ascertained the following information: confirmed clinical diagnosis of COPD or adult-onset asthma; collection of demographic data, including biological sex, age, and race; and presence of comorbid conditions. Comorbid conditions included emphysema, bronchiectasis, asthma, liver test abnormalities (defined either by a diagnosis code or by review of liver function laboratory test values that were above the upper limit of normal at our hospital laboratory), cirrhosis, panniculitis, vasculitis, and smoking status. Additional information related to screening included location where the AATD diagnosis took place (our institution or elsewhere), type of screening test used (genotype/phenotype vs. alpha-1 antitrypsin [AAT] concentration), and screening test results.
Education Intervention
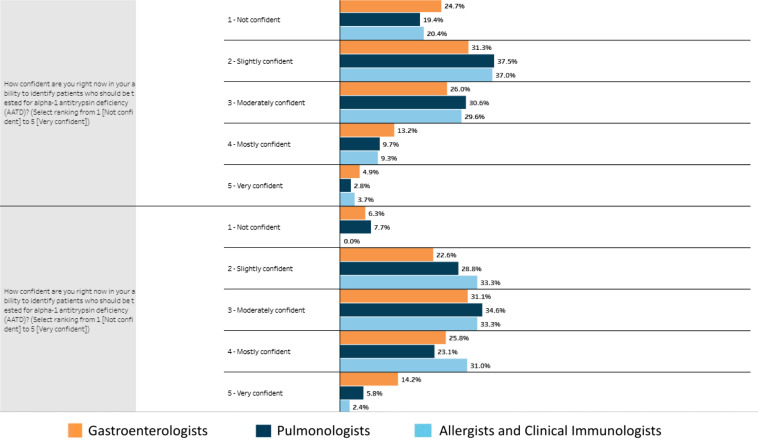
In conjunction with Medscape Education, an online education tool was created and used as our education intervention. Before developing the education tool, Medscape Education conducted a survey of its users from September 22, 2021, to September 29, 2021. This preintervention survey showed that 100% of pulmonologists surveyed considered themselves “very familiar” with the symptoms of AATD and 90% were “very familiar” with the screening guidelines for identifying patients with AATD (Figure 1). This tool, A Provider’s Guide to Detecting Alpha-1 Antitrypsin Deficiency, was eligible for completion starting January 6, 2022 (13). The tool focused on pathophysiology, prevalence, and therapeutics available for AATD, as well as helping providers identify patients who are eligible for AATD screening (Figure 2). This tool was an online video-based activity with concomitant and downloadable slides featuring multiple faculty voices and perspectives. It was shared with the pulmonary faculty at our institution and was also available for completion by Medscape Education members as a continuing medical education activity certified for 0.75 American Medical Association Physician’s Recognition Award Category 1 Credit and 0.75 American Nurses Credentialing Center Contact Hours. A pre– and post–continuing medical education question, with a simple 5-point Likert scale (not confident to very confident), was used to gauge physician confidence in identifying patients who should be tested for AATD.
Figure 1.
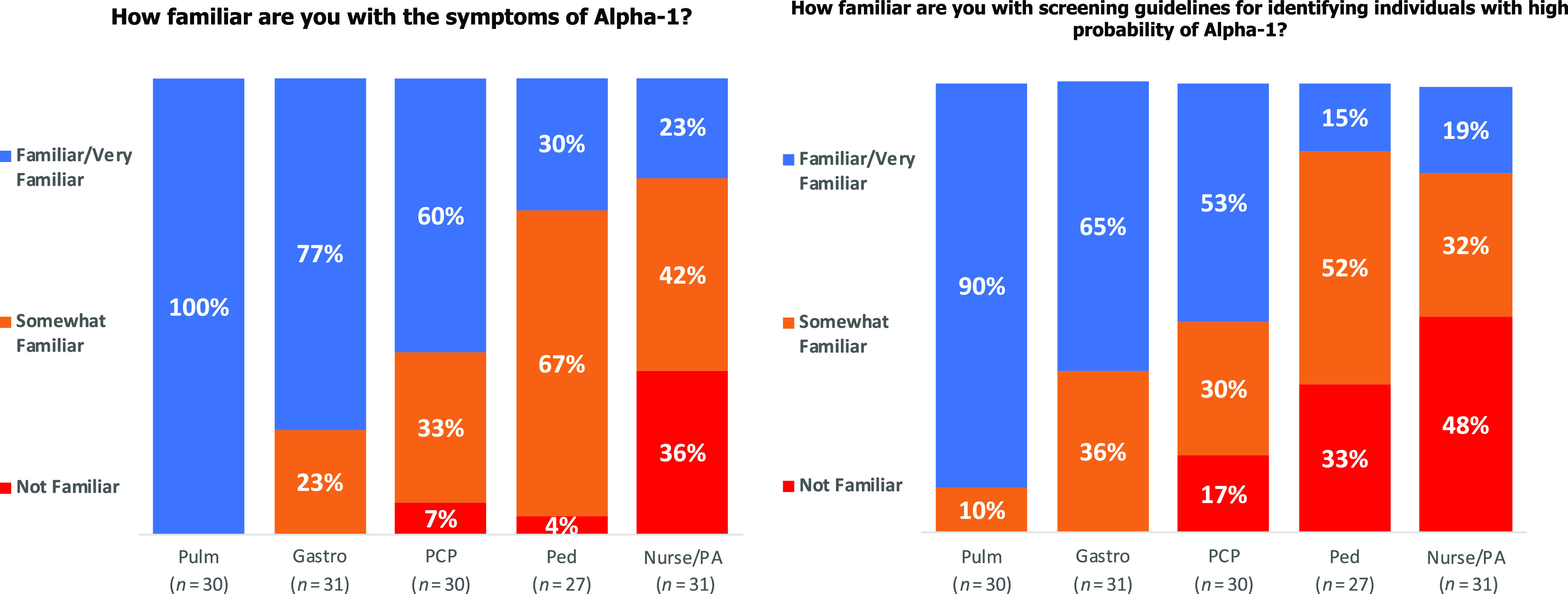
Medscape Education preintervention provider survey. Medscape Education conducted an online poll of existing users that posed questions about familiarity with alpha-1 antitrypsin deficiency. Answers were graded as “familiar/very familiar” (blue), “somewhat familiar” (orange), or “not familiar” (red). Gastro = Gastroenterologist; PA = physician assistant; Ped = Pediatrician; PCP = primary care provider; Pulm = pulmonologist.
Figure 2.
Module content overview.
Statistical Analyses
We used descriptive statistics to ascertain the rate of AATD screening among eligible patients, as well as to compare screening rates before and after the intervention. We used either Kruskal-Wallis or chi-square tests to evaluate the relationship between appropriately screened patients in the pre- and postintervention periods, to measure the differences in screening rates before and after intervention, and to measure associations between specific demographic factors and AATD screening.
Results
Education Intervention
The education intervention, A Provider’s Guide to Detecting Alpha-1 Antitrypsin Deficiency, was eligible for completion starting January 6, 2022. More than 11,000 learners nationwide opened the education module, and more than one-third of participants completed the entire module. Our survey, within the education intervention, showed that nearly one-fifth (19.4%) of all pulmonologists surveyed did not feel confident in identifying who should be screened for AATD before completing the module, and this amount decreased to 7.7% of pulmonologists after the education intervention (Figure 3). Relevant to our pilot study, eight participants who completed the education intervention self-reported as being pulmonologists from our institution. Of the education module participants who completed the postmodule survey, 97% reported that the module modified or reinforced a portion of their screening practice, with the highest proportion (73%) reporting that the module changed their approach to patients with COPD whom they see as being at high risk for AATD. However, 21% of module-completing participants reported that continued lack of familiarity with guidelines could be a barrier to appropriate screening.
Figure 3.
Pre and posteducation intervention provider confidence in assessing alpha-1 antitrypsin deficiency (AATD). Medscape Education users who participated in the AATD provider education module completed surveys before and after completing the course. Questions about provider confidence and awareness of AATD were scored on the basis of a Likert scale (not confident through very confident). Results from pulmonologists (dark blue), allergists (light blue), and gastroenterologists (orange) are shown.
Cohort Characteristics
Between May 29, 2021, and November 29, 2021 (preintervention), and between January 28, 2022, and July 28, 2022 (postintervention), 5,295 and 4,560 patients, respectively, were identified using i2b2 software and system electronic medical records. After a second-level review using the criteria outlined above, these lists were filtered down to 229 and 113 new patients eligible for AATD testing during each respective cohort phase (see the figures in the data supplement).
Combining pre- and postintervention cohorts, the mean age was 62.9 ± 12.6 years, with 49.7% male and 50.3% female. In the sample, 84.5% of patients were White, 14.9% were African American, and 0.6% were of Hispanic ethnicity. The majority (76.6%) of patients had emphysema, whereas 29.5% had asthma, 2.6% had bronchiectasis, 2.6% had liver function abnormalities, and 0.9% had cirrhosis. No patients had comorbid panniculitis or vasculitis. The majority (54.4%) of our patients were former smokers, whereas 21.3% were current smokers and 24.3% were never smokers (Table 1).
Table 1.
Pre and postintervention cohort characteristics (of patients eligible for screening)
| Preintervention | Postintervention | Total | P Value | |
|---|---|---|---|---|
| No. of subjects | 229 | 113 | 342 | — |
| Age, yr | ||||
| Mean | 62.6 | 63.4 | 62.9 | 0.712 |
| SD | 11.69 | 12.58 | 12.55 | |
| Median (range) | 64 (25–92) | 64 (20–85) | 64 (20–92) | |
| Sex | ||||
| Male | 103 (45.0%) | 67 (59.3%) | 170 (49.7%) | 0.013 |
| Female | 126 (55.0%) | 46 (40.7%) | 172 (50.3%) | |
| Race/ethnicity | ||||
| White | 197 (86.0%) | 92 (81.4%) | 289 (84.5%) | 0.515 |
| Black | 31 (13.5%) | 20 (17.7%) | 51 (14.9%) | |
| Hispanic | 1 (0.4%) | 1 (0.9%) | 2 (0.6%) | |
| Comorbid conditions | ||||
| Emphysema | 160 (69.9%) | 102 (90.3%) | 262 (76.6%) | <0.001 |
| Asthma | 80 (34.9%) | 21 (18.6%) | 101 (29.5%) | 0.002 |
| Bronchiectasis | 6 (2.6%) | 3 (2.7%) | 9 (2.6%) | 0.985 |
| Liver test abnormalities* | 4 (1.7%) | 5 (4.4%) | 9 (2.6%) | 0.146 |
| Cirrhosis | 3 (1.3%) | 0 (0.0%) | 3 (0.9%) | 0.222 |
| Smoking status | ||||
| Current | 46 (20.1%) | 27 (23.9%) | 73 (21.3%) | 0.041 |
| Former | 118 (51.5%) | 68 (60.2%) | 186 (54.4%) | |
| Never | 65 (28.4%) | 18 (15.9%) | 83 (24.3%) |
Definition of abbreviations: SD = standard deviation.
Defined either by a diagnosis code or by review of liver function laboratory test values that were above the upper limit of normal at our hospital laboratory.
Comparing the pre- and postintervention cohorts, there was a higher percentage of males in the postintervention cohort (45.0% vs. 59.3%; P = 0.013), but the mean age and racial demographics were similar. The most common comorbidity for both groups was emphysema (69.9% vs. 90.3%; P < 0.001), with asthma (34.9% vs. 18.6%; P = 0.002) and bronchiectasis (2.6% vs. 2.7%; P = 0.98) being less common. The majority of patients in both groups were former smokers (51.5% vs. 60.2%; P = 0.041) (Table 1).
Screening Practices before and after Intervention
AATD testing was performed in 44 (12.9%) patients in the combined pre- and postintervention cohort. We observed a greater than 120% increase in AATD testing in the cohort after the intervention (9.17% preintervention vs. 20.4% postintervention; P = 0.004). This increase in testing was driven by both an increase in AATD testing within our institution (7.9% preintervention vs. 15.0% postintervention; P = 0.039) and through an increase in reporting of AATD testing performed at outside institutions (1.4% preintervention vs. 6.3% postintervention; P = 0.020) (Table 2).
Table 2.
Pre and postintervention screening practices
| Preintervention | Postintervention | Combined | P Value | |
|---|---|---|---|---|
| No. of subjects | 229 | 113 | 342 | — |
| Patients screened for AATD (any screen) | 21 (9.2%) | 23 (20.4%) | 44 (12.8%) | 0.004 |
| Screen type* | ||||
| AAT concentration | 5 (2.2%) | 14 (12.4%) | 19 (5.6%) | <0.001 |
| Average concentration, mg/dl (range) | 130.2 (75.0–115.0) | 140.4(52.0–184.0) | 137.7 (52.0–184.0) | 0.354 |
| >80 mg/dl | 4 | 13 | 17 | |
| <80 mg/dl | 1 | 1 | 2 | |
| Phenotype/genotype | 17 (7.4%) | 11 (9.7%) | 28 (8.2%) | 0.046 |
| M/M | 11 | 5 | 16 | |
| M/S | 3 | 1 | 4 | |
| M/Z | 1 | 2 | 3 | |
| S/Z | 0 | 1 | 1 | |
| Z/Z | 2 | 2 | 4 | |
| Location of screen* | ||||
| At UAB | 18 (7.9%) | 17 (15.0%) | 35 (10.2%) | 0.039 |
| Outside UAB† | 3 (1.3%) | 6 (5.3%) | 9 (2.6%) | 0.020 |
Definition of abbreviations: AAT = alpha-1 antitrypsin; AATD = alpha-1 antitrypsin deficiency; UAB = University of Alabama at Birmingham.
Patients could have multiple types of screens performed.
Reported by our documentation/outside record gathering.
Factors associated with providers sending AATD testing included younger age (58.8 ± 11.69 vs. 63.4 ± 12.58; P = 0.011), comorbid liver function abnormalities (9.1% vs. 1.7%; P = 0.004), or cirrhosis (4.5% vs. 0.3%; P = 0.005). Variables including COPD versus asthma status, tobacco history, sex, or race did not influence the odds of screening for AATD in our cohorts.
Results of AATD Screening
Of the 44 patients screened for AATD, phenotype/genotype testing was performed in 28 and AAT concentrations were measured in 19 (some patients had both screens performed). Of screened patients, 12 (27.2%) of the 44 had abnormal phenotyping, including 4 with Pi*ZZ, 4 with Pi*MS, 3 with Pi*MZ, and 1 with Pi*SZ (Table 2). Two patients had low AAT concentrations, and both of these patients had abnormal genotypes (MZ and SZ, respectively).
Discussion
We found that the rate of AATD screening in high-risk patients more than doubled at a high-volume quaternary subspecialty referral center after deploying a provider-focused education intervention. This change in AATD screening was statistically significant and correlated with an increase in provider confidence in identifying patients at high risk for AATD. Even though AATD can cause excess morbidity, a population-based study in Denmark suggested that only ∼25% of individuals with AATD are aware that they have the disease (14). The rise in direct-to-consumer genetic testing among the public has led to a new wave of AATD identification, with a prevalence higher than previously hypothesized (15). Although screening high-risk individuals can happen in a variety of ways, we believe that medical providers should take ownership for screening and diagnosing AATD.
Medscape Education survey data confirm that there are ongoing education gaps among pulmonologists regarding AATD screening. Although a majority of pulmonologists report knowing which patients to screen, providers in real-world practice are not screening the majority of high-risk patients. Some of the reluctance by providers is due in part to lack of awareness of existing therapies for AATD (7). Although interventions such as augmentation have efficacy, multiple pipeline products that are currently in phase 2/3 clinical trials show promise for additional therapeutic approaches. Even in the absence of a pharmacologic intervention for AATD, early diagnosis of AATD positively affects lung function and quality of life and influences patient-initiated behavior. For example, previous studies have shown that for each year of diagnostic delay, forced expiratory volume in 1 second percentage predicted decreases by 0.3%, St. George’s Respiratory Questionnaire score increases by 1.6 points, and the COPD Assessment Test score increases by 0.7 points per year (15–17). Putting these findings into context and knowing that the average diagnostic delay for AATD is approximately 5 years, each of these metrics will surpass the minimal clinically important difference for a decline in status during this time. In addition, smokers with AATD are more likely than their non-AATD counterparts to stop smoking after a diagnosis (17). Similarly, in a study of direct-to-consumer AATD testing, there was a 70% increased odds of reporting smoking reduction efforts and a 400% increased odds for reducing alcohol consumption (15).
We found that emphysema was the most common pulmonary condition in our pre- and postintervention cohorts. Although this finding is not surprising on the basis of our study design, it supports findings from population-based studies suggesting that emphysema is the most common respiratory diagnosis among individuals with AATD (18). To our knowledge, there is no algorithm to identify patients with a high likelihood of having AATD, although investigators have developed electronic health record–based classifiers for other rare conditions (19). If similar algorithms are developed to identify patients at risk for AATD, we anticipate that emphysema will be heavily weighted in modeling. It is also worth pointing out that our study demographics closely mirrored our local population, with a significant proportion of individuals of Black race and few with Hispanic ethnicity. Because of the increasing diversity of the U.S. population, it is important to consider AATD testing in patients other than those with northern European genetic ancestry.
We observed that a patient presenting for care in the postintervention cohort had a twofold higher chance than a patient presenting for care in the preintervention period of having an AATD screening test sent. The overall rate of testing for AATD remained abysmally low at approximately 20% of high-risk patients in the postintervention period. As seen in our intervention survey, although this module reinforced many participants’ screening practices, there still was some lingering concern that complexity of guidelines could be a barrier to appropriate screening.
There were a variety of lessons learned throughout the conduct of this study and implementation of the education intervention. We had a large discrepancy between the i2b2-generated list of patients and the curated list of patents after clinician review. There are several possibilities for this discrepancy, including an ineffective algorithm, the inability of the i2b2 program to discern inpatient and outpatient encounters with the pulmonary service line, or something else entirely. However, we erred on the side of having an overly sensitive screening tool to ensure that all potentially eligible patients were captured in our data abstraction, despite an increased workload for the pulmonologist reviewer. Future studies should explore refining the i2b2 sensitivity to decrease the amount of time spent in manual second-line review.
The findings from this study have uncovered additional unmet education needs among healthcare providers. Importantly, we observed that much of the improvement in screening was driven by increased testing using only alpha-1 antitrypsin concentrations, not by genotype or phenotype testing, and this was in contrast to the recommendations by the American Thoracic Society and the European Respiratory Society. Our education module focused primarily on increasing awareness of AATD and providing a rationale for testing to an audience with some awareness of the condition. We attempted to use the message that “every patient with COPD needs an AATD screening at least once in their life,” but it is possible that some of the nuance in our education module was overwhelming to the providers taking the module or that the portions which discussed testing algorithms lacked clarity. Our education module did warn against using solely alpha-1 concentrations as a screening tool for AATD. The variability in selecting the appropriate test, not just “any” test, needs to be explored in future studies.
Given that tests across the entire cohort identified an abnormal allele in nearly one-third of the cases, one could theorize that up to 93 patients in the combined preintervention and postintervention cohort could have abnormal AATD screening results. Put another way, the overwhelming majority of patients with undiagnosed AATD who presented for care during the study window remained undiagnosed. Of note, our screening positivity rate correlates with rates in previous international studies of similar high-risk patients that were conducted using a similar methodology (4, 5).
Limitations
Our study has certain limitations. Because of the anonymous nature of the education module, we were able to confirm provider participation in the module only by self-report. Furthermore, some of the providers could have participated in the module outside the time windows we selected for the pre- and postintervention periods. Cohort studies by nature have unaccounted-for confounding, and we cannot say with certainty that our increased screening rates were not influenced by some factor outside of our education intervention. Our strict inclusion and exclusion criteria were used in an attempt to ensure that the same types of patients, presenting in the same stage of their diagnostic evaluation, were being evaluated in both phases of this study.
Conclusion
In conclusion, we found that the rate of AATD screening for high-risk patients more than doubled after an education intervention. A rollout of education interventions to other centers could increase the number of AATD screenings and thereby directly increase the identification of patients with AATD and improve the generalizability of our findings. The overall rates of screening high-risk patients for AATD remain low at our center. Given the rate of detecting abnormal alleles in our small study, it is likely that a substantial number of patients with AATD have yet to be identified. However, improving provider awareness about screening for AATD is an important first step in addressing the unmet need for diagnosing AATD in high-volume quaternary medical centers in both future studies and clinical practice.
Footnotes
Supported by Medscape, LLC, and by National Heart, Lung, and Blood Institute grant 1R01HL148215. The funders of the study did not have a role in the study design, data analysis, data interpretation, or writing of the report.
Author Contributions: The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors. The authors had full access to the data in this study, take complete responsibility for the integrity of the data and accuracy of the data analysis, and take responsibility for the integrity of the work as a whole. All authors discussed and interpreted the results, contributed to the data analyses, contributed to the writing and reviewing of the manuscript, and gave final approval for the version to be published.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Lieberman J, Winter B, Sastre A. Alpha 1-antitrypsin Pi-types in 965 COPD patients. Chest . 1986;89:370–373. doi: 10.1378/chest.89.3.370. [DOI] [PubMed] [Google Scholar]
- 2. Silverman EK, Miletich JP, Pierce JA, Sherman LA, Endicott SK, Broze GJ, Jr, et al. Alpha-1-antitrypsin deficiency. High prevalence in the St. Louis area determined by direct population screening. Am Rev Respir Dis . 1989;140:961–966. doi: 10.1164/ajrccm/140.4.961. [DOI] [PubMed] [Google Scholar]
- 3. Rahaghi F, Ortega I, Rahaghi N, Oliveira E, Ramirez J, Smolley L, et al. Physician alert suggesting alpha-1 antitrypsin deficiency testing in pulmonary function test (PFT) results. COPD . 2009;6:26–30. doi: 10.1080/15412550802587927. [DOI] [PubMed] [Google Scholar]
- 4. Calle Rubio M, Soriano JB, López-Campos JL, Soler-Cataluña JJ, Alcázar Navarrete B, Rodríguez González-Moro JM, et al. EPOCONSUL Study Testing for alpha-1 antitrypsin in COPD in outpatient respiratory clinics in Spain: a multilevel, cross-sectional analysis of the EPOCONSUL study. PLoS One . 2018;13:e0198777. doi: 10.1371/journal.pone.0198777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Soriano JB, Lucas SJ, Jones R, Miravitlles M, Carter V, Small I, et al. Respiratory Effectiveness Group Trends of testing for and diagnosis of α1-antitrypsin deficiency in the UK: more testing is needed. Eur Respir J . 2018;52:1800360. doi: 10.1183/13993003.00360-2018. [DOI] [PubMed] [Google Scholar]
- 6. Taliercio RM, Chatburn RL, Stoller JK. Knowledge of alpha-1 antitrypsin deficiency among internal medicine house officers and respiratory therapists: results of a survey. Respir Care . 2010;55:322–327. [PubMed] [Google Scholar]
- 7. Greulich T, Ottaviani S, Bals R, Lepper PM, Vogelmeier C, Luisetti M, et al. Alpha1-antitrypsin deficiency – diagnostic testing and disease awareness in Germany and Italy. Respir Med . 2013;107:1400–1408. doi: 10.1016/j.rmed.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 8. American Thoracic Society; European Respiratory Society. American Thoracic Society/European Respiratory Society statement: standards for the diagnosis and management of individuals with alpha-1 antitrypsin deficiency. Am J Respir Crit Care Med . 2003;168:818–900. doi: 10.1164/rccm.168.7.818. [DOI] [PubMed] [Google Scholar]
- 9. Sandhaus RA, Turino G, Brantly ML, Campos M, Cross CE, Goodman K, et al. The diagnosis and management of alpha-1 antitrypsin deficiency in the adult. Chronic Obstr Pulm Dis . 2016;3:668–682. doi: 10.15326/jcopdf.3.3.2015.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Noman S, Shahar HK, Abdul Rahman H, Ismail S, Abdulwahid Al-Jaberi M, Azzani M. The effectiveness of educational interventions on breast cancer screening uptake, knowledge, and beliefs among women: a systematic review. Int J Environ Res Public Health . 2020;18:263. doi: 10.3390/ijerph18010263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.University of Alabama at Birmingham. https://www.uabmedicine.org/locations/the-kirklin-clinic-of-uab-hospital/
- 12.National Center for Biomedical Computing. https://www.i2b2.org
- 13.Wells JM, Pirozzi CS, Schumacher RC, Kirkpatrick DP, Goldklang M.https://www.medscape.org/viewarticle/965614
- 14. Seersholm N, Kok-Jensen A, Dirksen A. Survival of patients with severe alpha 1-antitrypsin deficiency with special reference to non-index cases. Thorax . 1994;49:695–698. doi: 10.1136/thx.49.7.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ashenhurst JR, Nhan H, Shelton JF, Wu S, Tung JY, Elson SL, et al. 23andMe Research Team Prevalence of alpha-1 antitrypsin deficiency, self-reported behavior change, and health care engagement among direct-to-consumer recipients of a personalized genetic risk report. Chest . 2022;161:373–381. doi: 10.1016/j.chest.2021.09.041. [DOI] [PubMed] [Google Scholar]
- 16. Tejwani V, Nowacki AS, Fye E, Sanders C, Stoller JK. The impact of delayed diagnosis of alpha-1 antitrypsin deficiency: the association between diagnostic delay and worsened clinical status. Respir Care . 2019;64:915–922. doi: 10.4187/respcare.06555. [DOI] [PubMed] [Google Scholar]
- 17. Carpenter MJ, Strange C, Jones Y, Dickson MR, Carter C, Moseley MA, et al. Does genetic testing result in behavioral health change? Changes in smoking behavior following testing for alpha-1 antitrypsin deficiency. Ann Behav Med . 2007;33:22–28. doi: 10.1207/s15324796abm3301_3. [DOI] [PubMed] [Google Scholar]
- 18. Acquavella J, Vágó E, Sorensen HT, Horváth-Puhó E, Hess GP. Registry-based cohort study of alpha-1 antitrypsin deficiency prevalence, incidence and mortality in Denmark 2000-2018. BMJ Open Respir Res . 2022;9:e001281. doi: 10.1136/bmjresp-2022-001281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ehsani-Moghaddam B, Queenan JA, MacKenzie J, Birtwhistle RV. Mucopolysaccharidosis type II detection by naïve Bayes classifier: an example of patient classification for a rare disease using electronic medical records from the Canadian Primary Care Sentinel Surveillance Network. PLoS One . 2018;13:e0209018. doi: 10.1371/journal.pone.0209018. [DOI] [PMC free article] [PubMed] [Google Scholar]