Abstract
Mitochondrial dysfunction can arise from genetic defects or environmental exposures and impact a wide range of biological processes. Among these are metabolic pathways involved in glutamine catabolism, anabolism, and glutamine-glutamate cycling. In recent years, altered glutamine metabolism has been found to play important roles in the pathologic consequences of mitochondrial dysfunction. Glutamine is a pleiotropic molecule, not only providing an alternate carbon source to glucose in certain conditions, but also playing unique roles in cellular communication in neurons and astrocytes. Glutamine consumption and catabolic flux can be significantly altered in settings of genetic mitochondrial defects or exposure to mitochondrial toxins, and alterations to glutamine metabolism appears to play a particularly significant role in neurodegenerative diseases. These include primary mitochondrial diseases like Leigh syndrome (subacute necrotizing encephalopathy) and MELAS (mitochondrial myopathy with encephalopathy, lactic acidosis, and stroke-like episodes), as well as complex age-related neurodegenerative disorders such as Alzheimer’s and Parkinson’s diseases. Pharmacologic interventions targeting glutamine metabolizing and catabolizing pathways appear to provide some benefits in cell and animal models of these diseases, indicating glutamine metabolism may be a clinically relevant target. In this review, we discuss glutamine metabolism, mitochondrial disease, the impact of mitochondrial dysfunction on glutamine metabolic processes, glutamine in neurodegeneration, and candidate targets for therapeutic intervention.
Keywords: Mitochondrial disease, Glutamine toxicity, Neurodegenerative disease
1. Mitochondrial metabolism and glutamine
Mitochondria are the primary energy-generating organelles in most mammalian cells. Mitochondria orchestrate varied metabolic processes and provide adenine triphosphate (ATP), the primary chemical used for short-term cellular energy storage and transfer (McInnes, 2013). A greatly simplified summary of central mitochondrial metabolism is as follows (see Fig. 1): catabolic metabolism of carbohydrates, fatty acids (FAs), ketones, and amino acids (AAs) produces acetyl-CoA, which can enter the tricarboxylic acid (TCA) cycle. Metabolism of acetyl-CoA by the TCA cycle generates NADH and FADH2, which power pumping of protons across the mitochondrial inner membrane by the electron transport chain (ETC). This proton pumping generates a proton gradient across the inner membrane, which is harnessed by ATP synthase to drive ATP production (Kanter, 2018). Though some ATP is generated through glycolysis and substrate level phosphorylation in the TCA cycle, and more obscure processes, the ETC provides the bulk of ATP in most eukaryotic cells under normal conditions.
Fig. 1.
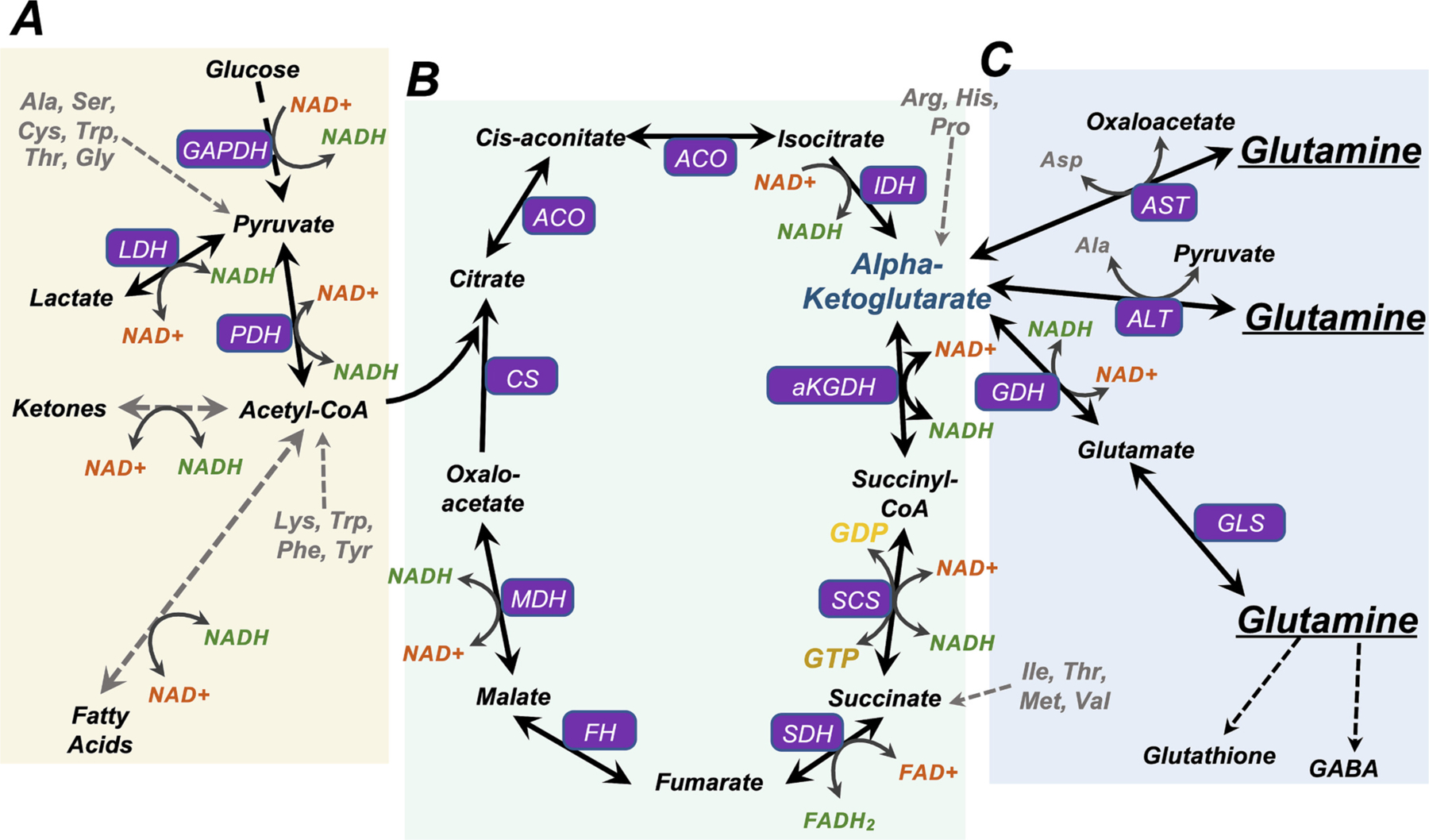
Glutamine and NADH in central metabolic pathways. (A) Catabolism of glucose, ketones, and fatty acids generates Acetyl-CoA. Glucose is broken down through glycolysis to form pyruvate. In settings of reduced flux through the TCA cycle, such as in anaerobic conditions or settings of increased NADH/NAD+, pyruvate generated through glycolysis can be reduced to lactate by LDH (lactate dehydrogenase) with concomitant oxidation of NADH to NAD+. Ketone catabolism converges at acetyl-CoA and NADH is produced from NAD+ in the process. Ketogenesis, the reverse process, primarily occurs in the liver, consuming acetyl-CoA and NADH. Beta-oxidation of fatty acids generates acetyl-CoA and NADH. Metabolism of the amino acids Lys, Trp, Phe, and Tyr also converges at acetyl-CoA, while Ala, Ser, Cys, Trp, Thr, and Gly can be metabolized to pyruvate. (B) An overview of the tri-carboxylic acid (TCA) cycle. Acetyl-CoA generated through glycolysis, ketone metabolism, fatty acid metabolism, lactate metabolism, and the metabolism of certain amino acids can feed the TCA cycle to sustain carbon flow and aerobic metabolism. NADH is generated from NAD+ at four steps in the cycle: isocitrate to alpha-ketoglutarate (aKG) catalyzed by isocitrate dehydrogenase (IDH), aKG to succinyl-CoA catalyzed by aKG dehydrogenase (aKGDH), succinyl-CoA to succinate catalyzed by succinyl-CoA synthetase (SCS), and malate to oxaloacetate catalyzed by malate dehydrogenase (MDH). (C) Central pathways of glutamine metabolism. Through the process of glutaminolysis, glutaminase (GLS) catalyzes the reaction of glutamine to glutamate, and glutamate is converted to aKG by glutamate dehydrogenase (GDH). Glutamine may also be converted directly to aKG by alanine or aspartate aminotransferase (ALT and AST), with concomitant production of alanine or aspartate, respectively. Glutamine is also a precursor to glutathione and GABA, which are critical for cellular antioxidant defenses and neurotransmission.
Glucose is a key molecule used for energy storage and transport in eukaryotes, and regulation of extracellular/circulating glucose is crucial in mammalian health. Extracellular glucose is transported into cells via GLUT/SLC2A family transporters. Most glucose imported into cells is either metabolized into pyruvate or incorporated into glycogen, a polysaccharide of glucose used for efficient osmo-normal storage. The major catabolic fates of pyruvate include conversion to acetyl-CoA; anaerobic metabolism to lactate by lactate dehydrogenase (LDH), producing ATP and oxidizing NADH to NAD+; or conversion to alanine by alanine aminotransferase (ALT) with a concomitant conversion of glutamine to alpha-ketoglutarate (Fig. 1A). Glycogen can be rapidly hydrolyzed to free glucose upon demand.
FAs provide an alternate form of energy storage. FAs are typically stored as triglycerides, three FA chains covalently attached to a glycerol molecule. Fat stored in lipid droplets can provide free FAs for metabolism, and lipolysis of triglycerides in adipose tissue provides free FAs and glycerol to the blood stream. These are transported bound to albumin and taken up by peripheral tissues for catabolism. Catabolism of fatty acids via beta-oxidation produces acetyl-CoA, available to enter the TCA cycle or be converted to ketones (Fig. 1A).
Acetyl-CoA can be used to generate ketone bodies through ketogenesis, providing an alternate circulating catabolic energy source. The majority of ketogenesis occurs in the liver, though neuroprotective ketogenesis has been reported to occur in astrocytes, a brain glial cell type important for neuronal metabolism, glutamine cycling (see below), and neuromodulation (Takahashi et al., 2014; Guzman and Blazquez, 2004; Guzman and Blazquez, 2001). Ketones are metabolized to acetyl-CoA by end organs and cells, such as neurons in the CNS. The brain is a major utilizer of ketones, which are a preferred energy source in the developing mammalian brain (Steiner, 2019; Yeh and Sheehan, 1985; Yeh et al., 1977). Evidence suggests ketone supplementation is beneficial in multiple neurologic diseases including epilepsy, traumatic brain injury, and Alzheimer’s disease (Jensen et al., 2020; Broom et al., 2019; Arisaka et al., 2020).
1.1. Glutamine metabolism
Amino acids (AAs) can also enter the TCA cycle, although most dietary AAs are first converted into glucose or ketones in the liver. Glutamine is the most abundant AA in both muscle and blood, and an important and versatile circulating and intracellular metabolite in mammals (Cruzat et al., 2018; Ling et al., 2019). In addition to involvement in protein synthesis, glutamine is a precursor for the antioxidant glutathione, which is critical for maintaining cellular redox status and protecting cells from oxidative damage, and glutamine acts as a key alternative substrate for supplying the TCA cycle and driving oxidative metabolism when glucose metabolism is disrupted (Newsholme et al., 2003; Chen et al., 2018). Glutamine also plays unique roles in neuronal activity, detailed below.
Glutamine is a key anaplerotic (TCA cycle-feeding) substrate (Brunengraber and Roe, 2006) (Fig. 1C). Glutamine is of particular importance to rapidly dividing cells, including neoplastic cells, feeding the TCA cycle, enabling protein synthesis, and providing a precursor for glutathione and for nucleotides necessary for cell replication (Liao et al., 2019; Bowtell and Bruce, 2002; Martins et al., 2020). Glutamine can enter the TCA cycle after conversion to glutamate and then alpha-ketoglutarate (aKG) through a process termed glutaminolysis, catalyzed by glutaminase (GLS) and glutamate dehydrogenase (GDH). Alternatively, alanine or aspartate aminotransferase (ALT and AST, respectively) can convert glutamine to aKG, with concomitant production of alanine or aspartate, respectively. A mitochondrial AST isozyme exists, which converts mitochondrial glutamine to aKG for TCA entrance. This AST reaction is a key part of the malate-aspartate shuttle, a key biochemical system for the net effect of moving transferring NADH equivalents from the cytosol to the mitochondria (increasing cytoplasmic NAD+ and mitochondrial NADH) (Borst, 2020). In addition, mitochondrial AST driven conversion of glutamate and oxaloacetate to aKG and aspartate has been found to be necessary for the survival of proliferating cells in the setting of ETC inhibition (Birsoy et al., 2015). ALT is expressed predominantly in the liver, while AST is expressed in multiple tissues, including brain (Kim et al., 2020).
2. Metabolic sequelae of mitochondrial dysfunction
2.1. NADH and metabolism
The impact of mitochondrial dysfunction on cellular respiration depends on the precise mitochondrial defect, and in many cases is poorly understood. Rewiring of central carbon metabolism is common among genetic defects impacting ETC function. ETC defects can slow consumption of TCA cycle generated NADH, resulting in an increase in the NADH/NAD+ ratio. Increased NADH/NAD+ inhibits flux through NADH redox sensitive steps in the TCA cycle and glycolysis. This situation results in decreased glucose metabolism with a compensatory increase in the products of anaerobic glucose use, including lactate and alanine (Mukaneza et al., 2019; Aspuria et al., 2014).
NADH redox (the ratio of NADH/NAD+) is a major regulator of cellular metabolism, with multiple core metabolic reactions influenced by this ratio (Fig. 1). In glycolysis, G3P dehydrogenase (GAPDH) catalyzes the production of 1,3-bisphosphoglycerate from glyceraldehyde-3-phosphate (G3P), with the reaction concomitantly generating NADH from NAD+. Pyruvate dehydrogenase (PDH) catalyzes conversion of pyruvate into acetyl-CoA while consuming NAD+ to generate NADH. In fat metabolism, oxidation of L-β-hydroxyacyl CoA’s, catalyzed by 3-hydroxyacyl-CoA dehydrogenase, is also an NAD+ consuming reaction. Within the TCA cycle itself, NADH is produced (and NAD+ consumed) in three reactions: 1) the conversion of isocitrate to oxalosuccinate by isocitrate dehydrogenase (IDH), 2) the conversion of aKG to succinate by aKG dehydrogenase (a-KGDH), and 3) the conversion of malate to oxaloacetate by malate dehydrogenase (MDH). IDH isoform IDH3 functions in the context of the TCA cycle and is NAD+ reactive, while IDH1 and 2 utilize NADP+.
Each of these NAD+ dependent reactions and are slowed or inhibited by an elevated NADH/NAD+ ratio. The impact is most pronounced at PDH and IDH: PDH catalyzes the rate-limiting step for entry of glucose carbons into the TCA cycle, and NAD+ dependent IDH catalyzes a rate-limiting and irreversible step of the TCA cycle (Gabriel et al., 1986). When NADH/NAD+ is high, conversion of pyruvate to lactate by lactate-dehydrogenase (LDH) is favored over conversion to acetyl-CoA, while TCA cycle flux through IDH is inhibited. LDH consumes NADH, replenishing NAD+, contributing to the favorability of this lactate production in the setting of high NADH.
In conditions of increased NADH/NAD+, pyruvate can also be converted to alanine by alanine-aminotransaminase (ALT), concomitant with the consumption of glutamate and production of aKG (Figs. 1, 2). Increased NADH/NAD+ can impair carbon flux through the TCA cycle and leads to cataplerosis, the exit of intermediates from the cycle. Major cataplerotic products include pyruvate, aspartate, adenylosuccinate, and glutamate (Owen et al., 2002).
Fig. 2.
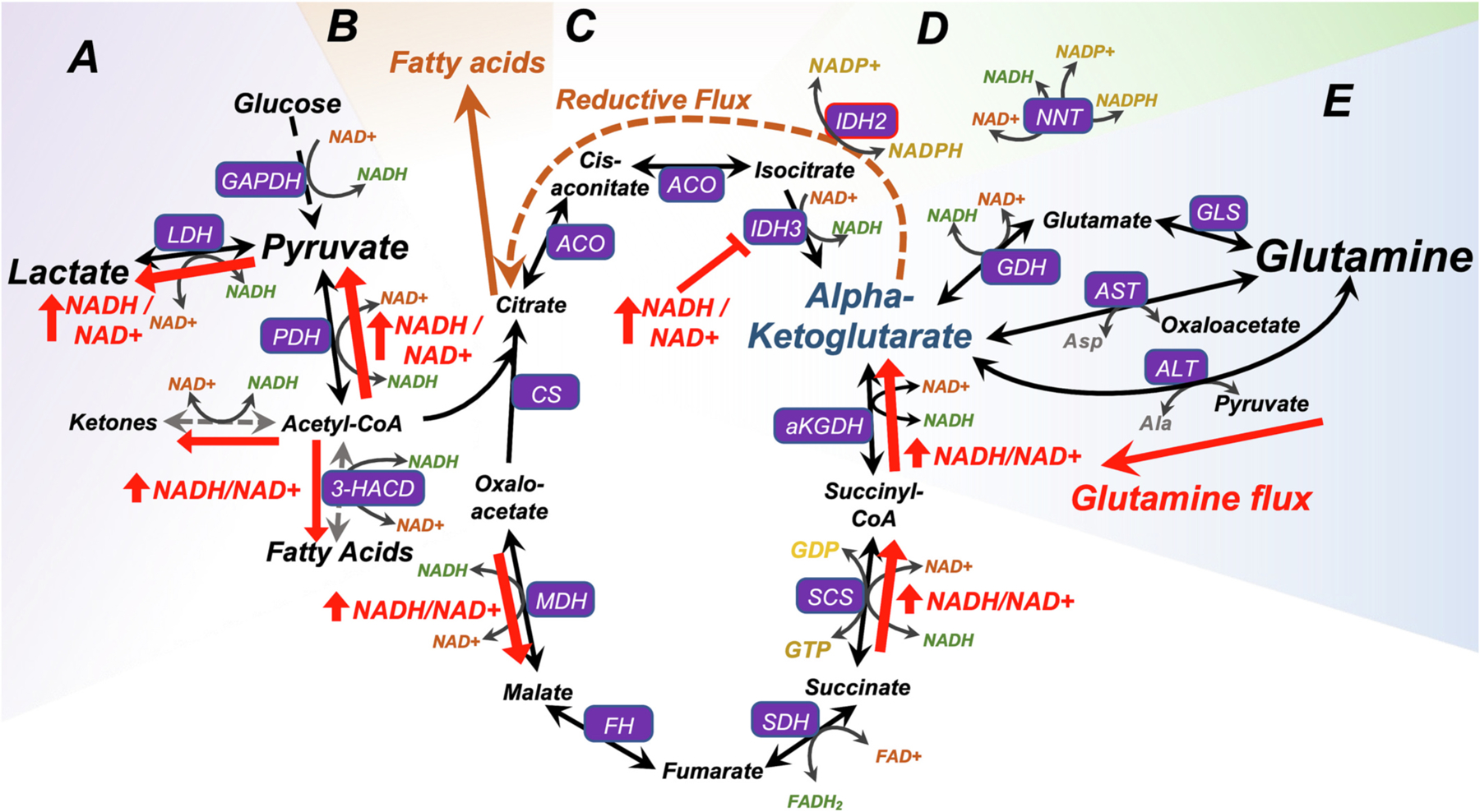
Tissue specific glutamine alterations in genetic mitochondrial disease. Reduced brain glutamine levels have been reported in the Ndufs4(KO) mouse model of Leigh syndrome, in MELAS patients, and in FA patients. Glutaminolysis enzymes have been found to be upregulated in the Purkinje cells of Mfn2 deficient mice. Glutamine/glutamate/aKG have been shown to support mitochondrial oxidative phosphorylation in brain mitochondria from Ndufs4 deficient mice where significant defects in ETC CI driven mitochondrial oxygen consumption are seen with other metabolic substrates, such as pyruvate and lactate. Glutamine levels are also reduced in OPA1 deficient patients. In contrast to these brain findings, glutamine levels are increased in both animal models of COX10 deficiency, and in both human patients and animal models of MM, and IOSCA resulting from deficiencies in the mitochondrial helicase Twinkle. These changes are thought to result from increased muscle catabolism.
The production of glutamate from aKG is favorable as a cataplerotic outlet in some high NADH contexts as the reaction, catalyzed by GDH, can consume NADH and generates NAD+. GDH is also linked to TCA cycle function through regulation by guanosine triphosphate (GTP) levels – GTP, generated by succinyl-CoA synthetase, is a potent inhibitor of GDH (Mastorodemos et al., 2005). Mitochondrial dysfunction can impact glutamine levels differentially depending on relative changes to NADH redox and TCA cycle flux: de-inhibition by low GTP would be expected to promote either anaplerosis or cataplerosis, depending on NADH redox. The NAD+ concentration also plays a role in GDH regulation via the post-translational modification ADP-ribosylation, catalyzed by the mitochondrial ADP-ribosyl transferase (mART). NAD+ is utilized by mART to covalently and reversibly inhibit GDH, and low NAD+ results in reduced inhibition (Herrero-Yraola et al., 2001). Accordingly, when TCA disruption with high NADH/NAD+ impairs flux to aKG, these modes of regulating GDH drive increased glutamine flux into the TCA cycle via de-inhibition of this enzyme (Fig. 2).
Citrate is critical for fatty acid synthesis. Under conditions where citrate is depleted but NADH is not high, glutamine-derived aKG can both replenish citrate through IDH2 dependent reductive metabolism which consumes NADPH (Wise et al., 2011; Mullen et al., 2014) (Fig. 2). NADH produced by oxidative flux of aKG can replenish NADPH via interconversion by nicotinamide nucleotide transferase (NNT) (Mullen et al., 2014). This allows glutamine to provide carbons for driving both cellular metabolism and biosynthesis.
ETC CI, an NADH dehydrogenase and coenzyme Q reductase, is a major NADH consuming enzyme. Defects in ETC CI can lead to increased NADH/NAD+, resulting in disruption of NADH redox regulated processes detailed above. Through this mechanism, ETC CI dysfunction can drive increases in lactate and alanine, reduce glucose carbon flux to the TCA cycle, increase cataplerosis, and reduce entry of pyruvate into the TCA cycle. Interestingly, genetic defects in both ETC CI and PDH can cause the genetic mitochondrial disease Leigh syndrome (LS, detailed above) (De Meirleir, 2013); this seems to indicate a special role for pyruvate flux to acetyl-CoA in the pathogenesis of LS, though a clear explanation for the link is lacking.
2.2. Impacts of altered glutamine metabolism in settings of mitochondrial dysfunction
As detailed above, glutamine can enter the TCA cycle as aKG, an anaplerotic process. This can be increased in settings where altered NADH/NAD+ redox drives reductive catabolism. In this context, aKG produced through glutaminolysis can alleviate bottlenecks at IDH, replenish citrate, and relieve NADH accumulation (see Fig. 2).
This glutamine anaplerosis has been observed in multiple models of mitochondrial dysfunction. Cells with mitochondrial defects have an increased dependence on glutamine for proliferation and survival, and increased glutaminolysis is a critical compensatory mechanism in certain forms of mitochondrial dysfunction (Chen et al., 2018; Motori et al., 2020). Mitochondrial respiratory capacity appears to dictate whether flux at the aKG junction will be oxidative or reductive, where severe defects in respiratory capacity drive more reductive flux (Chen et al., 2018). In rapidly proliferating cells with mitochondrial defects, reductive TCA cycle flux is crucial in part due to its role in aspartate synthesis; aspartate is a precursor for both proteins (as an amino acid) and nucleotides, providing a backbone for de novo pyrimidines synthesis (Birsoy et al., 2015; Sullivan et al., 2015). For example, anaplerosis of glutamine is necessary for proliferation of PDH deficient cells (Roma et al., 2022; Wang et al., 2019).
The importance of carbon entry via aKG in mitochondrial dysfunction is demonstrated by mitochondrial oxygen consumption assays in the setting of ETC CI defects: in ETC CI deficient mitochondria, pyruvate/malate driven oxygen consumption is significantly impaired, but oxygen consumption is partly rescued when glutamate/malate are provided as substrates and fully rescued with aKG/malate (Kayser et al., 2016; Johnson et al., 2020). These findings show that aKG (and glutamine, as a precursor to aKG) can compensate for certain disruptions of the TCA cycle and/or PDH.
Consistent with these data in isolated mitochondria, dimethyl-KG (DMKG), a cell permeable KG, has been found to attenuate (albeit modestly) disease in the Ndufs4(KO) (Lee et al., 2019). The NAD+ precursor nicotinamide riboside (NMN) was also found to modestly modify disease course, and the authors proposed a model whereby attenuation of NADH/NAD+ redox increases aKG and this attenuates disease progression (though redox changes were only detected in skeletal muscle). In support of this model, recent data has shown that expression of the yeast NADH dehydrogenase Ndi1 in the neurons of Ndufs4(KO) mice, using Nestin-Cre driven expression, can rescue NADH/NAD+ redox and many symptoms of disease (McElroy et al., 2020). Whether alterations to glutamine/glutamate/aKG metabolism play an important causal role in the link between altered redox and disease remains to be more directly demonstrated, but all evidence would appear to support such a connection.
Entry of glutamine into the TCA cycle as aKG has also been shown to enhance mTORC1 signaling, which is known to occur in the setting of mitochondrial disease (Yang et al., 2017; Johnson et al., 2019). The causal link between glutamine, aKG, mTOR, is complex; the role of mTOR in mitochondrial disease in whole organisms appears to be defined more by immune activity than metabolism, but cultured cell models support a cell-autonomous role for mTOR dysregulation, and this remains an active area of research (Johnson et al., 2019; Johnson et al., 2013; Stokes et al., 2022; Capristo et al., 2022).
3. Mitochondrial dysfunction and human disease
Mitochondrial dysfunction is an umbrella term used to refer to a variety of distinct defects in normal mitochondrial functions. Parameters of mitochondrial dysfunction include altered mitochondrial enzyme level or enzymatic activity, including in the electron transport chain (ETC) complexes; mitochondrial network alterations, including hyper-fragmentation or hyper-connectivity resulting from defective mitochondrial fission or fusion; mitochondrial structural or substructural abnormalities, such as abnormal cristae morphology or swelling; altered mitochondrial biogenesis or turnover, which can be related to changes in mitophagy; abnormal reactive oxygen species (ROS) production or neutralization; impaired function of the TCA cycle or related metabolic processes; and impaired ability to generate ATP, either overall capacity or from a specific substrate (Montgomery and Turner, 2015; Bornstein et al., 2020).
3.1. Genetic mitochondrial disease
Defects in mitochondrial DNA (mtDNA) or nuclear genes encoding mitochondrial factors can lead to genetic mitochondrial diseases. Many distinct forms of mitochondrial disease, grouped and defined based on clinical presentation, have been described. These include Leigh syndrome (LS), the most common pediatric presentation of genetic mitochondrial disease; Mitochondrial Encephalomyopathy Lactic Acidosis and Stroke-like Episodes (MELAS); Friedrich’s ataxia; Kearns-Sayre syndrome (KSS); and Leber’s Hereditary Optic Neuropathy (LHON) (Breuer et al., 2013; Orsucci et al., 2021; Frazier et al., 2019). While individually rare, mitochondrial diseases are the leading cause of inborn errors of metabolism, as well as a leading causes of genetic neurological dysfunction. Mitochondrial diseases are typically clinically defined with named syndromes representing constellations of symptoms which can arise from lesions in one of multiple genetically distinct loci. In LS, for example, more than 85 distinct genes have been causally associated with the disease, with many distinct disease causing variants in some of these genes (Kim et al., 2022).
Clinical presentation among genetic mitochondrial diseases is strikingly heterogenous; for example, LHON is primarily a single organ disorder, while LS is a complex multi-system disease impacting many organ systems. In general, there is no clear functional mechanism distinguishing genes causing one form of mitochondrial disease versus another. No current models exist to explain which genetic lesions will lead to which clinical manifestations.
3.2. Mitochondria in complex human diseases
Genetic lesions with weak functional consequences can contribute to complex multigenic diseases. Genetic loci associated with mitochondrial components have been linked to many such diseases through genome-wide association studies (GWAS) (Bornstein et al., 2020; Johnson et al., 2017; Gonzalez et al., 2023; Johnson et al., 2015). Mitochondrial dysfunction is thought to play a causal role in a wide range of human pathologies, from cancer to normative aging. Neurodegenerative diseases, in particular, have been widely associated with altered mitochondrial functions. Links between mitochondrial function and complex neurologic diseases are reviewed in detail elsewhere, but a discussion focused on the role of altered glutamine metabolism in some key diseases is provided in below.
3.3. Environmental toxins
Mitochondrial dysfunction can also result from environmental exposures to chemotherapeutics, pollutants, mitotoxic compounds found in foods or dietary supplements, and agricultural chemicals such as pesticides. Environmental toxins can impact mitochondrial phospholipids, damage mtDNA, inhibit mitochondrial enzymes including the ETC, or cause mitochondrial membrane depolarization (the latter best typified by the early 20th century diet drug 2,4-DNP) (Meyer et al., 2013; Gorini et al., 2018; Zolkipli-Cunningham and Falk, 2017; Grundlingh et al., 2011).
A small group of particularly well documented environmental mitotoxins have been directly linked to human neurodegenerative diseases. These include the ETC complex I (ETC CI) inhibiting pesticide/piscicide rotenone and mitochondrial ROS generating herbicides paraquat and diquat, both linked to Parkinson’s disease through both epidemiologic and pre-clinical studies (Spivey, 2011; Olubodun-Obadun et al., 2022; Miyazaki et al., 2020; Tanner et al., 2011; Tangamornsuksan et al., 2019; Drechsel and Patel, 2009; Yan et al., 2016). The ETC CI inhibiting plant toxin annonacin has similarly been linked to progressive supranuclear palsy (PSP), a disease with similarities to both PD and LS (Spivey, 2011; Olubodun-Obadun et al., 2022; Miyazaki et al., 2020). Ample experimental evidence links these and other mitochondrial toxins to various neurodegenerative disease forms in model systems.
Overall, these mitotoxins provide some of the most compelling evidence for mitochondrial origins of neurodegenerative disease: animal studies causally demonstrate mitochondrial toxin exposure leads to disease, while human epidemiologic data provides strong links between environmental exposure and disease risk. It is worth noting that although links between mitochondrial toxins and neurodegenerative disease are robustly supported by both animal and human studies, multiple well known mitotoxins continue to receive renewed approval for agricultural use. A good example is antimycin A, a compound used in the laboratory as a potent ETC CIII inhibitor and employed as a pesticide (specifically as a piscicide in fish management and aquaculture, see EPA 738-R-07-007).
4. Altered glutamine levels in mitochondrial disease
While roles for glutamine in metabolic responses to mitochondrial dysfunction in cultured cells are clear, the precise impact of mitochondrial defects is likely highly cell, tissue, and context specific. As with many cell-based findings, it is not entirely clear where or when reductive flux occurs in vivo, and what functional tipping points are important for NADH redox, aspartate synthesis, etc.
Some cell-specific data in LS and Friedrich’s Ataxia (FA, caused by defects in mitochondrial iron handling) implicate glutamine/glutamate metabolism in the pathogenesis of these diseases arising from mitochondrial dysfunction. In the Ndufs4 model of LS, cell-specific depletion of the ETC CI subunit Ndufs4 in glutamatergic neuron (using the VGlut2-Cre promoter) reproduces nearly the entire phenotype of whole-body Ndufs4(KO) model, including CNS lesions (Johnson et al., 2020; Bolea et al., 2019), while total neuron loss via Nestin-Cre mediated excision yields similar results (Quintana et al., 2010). In FA, VGLUT-positive terminals in the dentate nucleus are selectively depleted, while the majority of neurons are spared (Koeppen et al., 2011). In these two forms of mitochondrial dysfunction, disease appears to uniquely impact glutamatergic neurons.
Metabolomic studies have shown glutamine levels are depleted in specific tissues in certain mitochondrial diseases. Ndufs4(KO) brains are significantly deficient in glutamine, glutamate, and aKG in all brain regions (Johnson et al., 2013; Terburgh et al., 2021). Similarly, patients with MELAS and FA have reduced brain glutamate, and patients with hereditary optic atrophy type 1 caused by OPA1 mutations have reduced plasma glutamate (Moller et al., 2005; Huxtable et al., 1979; Chao de la Barca et al., 2019). In each of these diseases, the neuronal tissues are the most significant target of mitochondrial dysfunction.
In contrast to these findings, defects causing mainly muscle pathology generally appear to increase free glutamate in muscle and/or plasma. In the muscle specific COX10 (heme A:farnesyltransferase cytochrome c oxidase assembly factor) knockout mouse model of mitochondrial myopathy (MM), the amino acids (AAs) glutamine, glutamate, and alanine are significantly elevated in muscle, but not plasma, brain, or liver (Chen et al., 2018). Changes were attributed to muscle wasting, though it was unclear why glutamine, glutamate, and alanine were specifically elevated among AAs. In MM and infantile onset spinocerebellar ataxia (IOSCA) resulting from distinct mutations in TWINKLE (the replicative mitochondrial DNA helicase), patients show increased plasma glutamate (Nikkanen et al., 2016). Mouse models for these TWINKLE defects were found to have distinct skeletal muscles AA changes - all AAs were increased in the MM model, while only lysine, arginine, glutamine, and glutamate were elevated in the IOSCA model (Fig. 3).
Fig. 3.
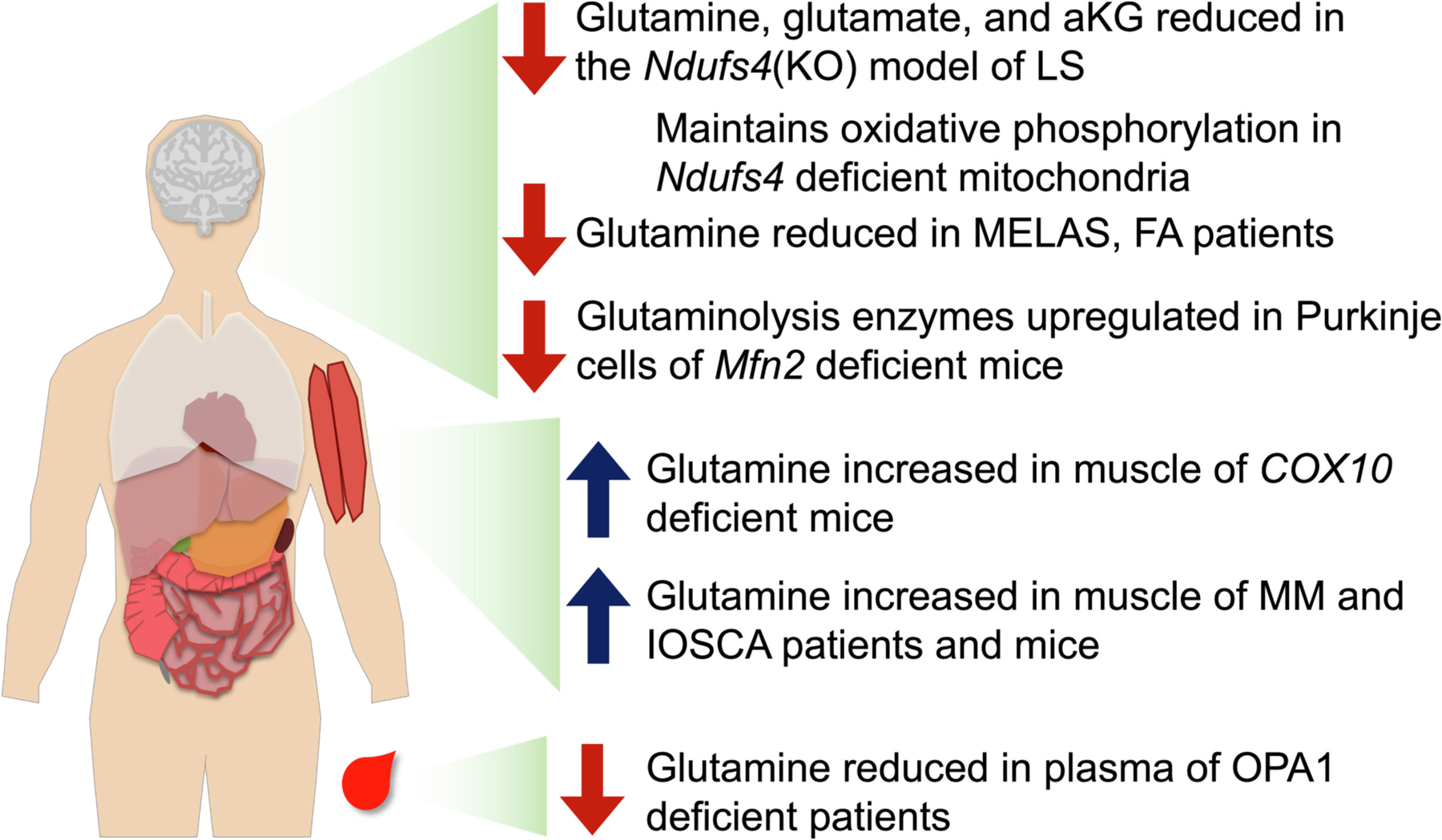
Glutamine metabolism in the setting of mitochondrial dysfunction. (A) Increased NADH/NAD+ resulting from ETC CI dysfunction can drive increased pyruvate and lactate levels by driving LDH and PDH reactions in an NAD+ regenerating direction. (B) Fatty acid synthesis is critical for cellular proliferation and, in some cases, function and survival. (C) Anaplerotic flux of glutamine into the TCA cycle both through oxidative and reductive flow can sustain fatty acid synthesis. (E) Mitochondrial NAD+ dependent IDH (IDH3) is the TCA cycle step most sensitive to inhibition by high NADH/NAD+, and the IDH3 reaction is generally irreversible. In conditions of high NADH, aKG can be metabolized to citrate via reductive flow that takes advantage of NADP+ dependent IDH. NNT can catalyze the interconversion of NADPH and NADH to regenerate NAD+. (E) Glutamine can provide anaplerotic input into the TCA cycle at aKG via multiple mechanisms, including conversion through ALT or AST, concomitant with the production of alanine or aspartate, or through conversion to glutamate then to aKG by way of glutaminase and glutamate dehydrogenase. Aspartate is a key nucleotide precursor, and production from glutamine is key to survival proliferating cells with mitochondrial defects. By both providing both NADH relief through reductive flux and sustaining flow through the TCA cycle downstream of IDH3 the glutamine/glutamine/aKG pathway can sustain mitochondrial oxidative metabolism in the face of altered NADH/NAD+ redox resulting from ETC dysfunction.
The relationship between changes in brain and muscle glutamine, glutamate, and related metabolites have not yet been directly explored, and even indirect evidence is lacking as MM models have tended to focus on muscle, and neurogenerative disease models on brain.
4.1. Metabolic dysfunction in mitochondrial disease
Systemic metabolic abnormalities are a common feature of genetic mitochondrial disease, with direct or indirect relations to glutamine. Glucose homeostasis, in particular, is impacted in multiple forms of mitochondrial dysfunction. Mitochondrial diabetes (mtDB) is a prominent clinic feature in some mitochondrial diseases including maternally inherited diabetes and deafness (MIDD), Kearns-Sayre syndrome (KSS), MELAS, and FA; an estimated ~22 % of all mitochondrial disease patients presenting with mtDB (Ashfaq et al., 2021). Unlike type 1 diabetes, caused by immune-mediated loss of pancreatic β-cells, or type 2, caused by insulin resistance in glucose consuming tissues, dysregulation of glucose homeostasis in mtDB appears to occur when mitochondrial dysfunction leads to an impairment of β-cell insulin secretion (Schaefer et al., 2013).
In addition to mtDB, in which glucose clearance from the bloodstream is impaired, hypoglycemia with normal hepatic function also often occurs in mitochondrial disease. The etiology of mitochondrial disease associated hypoglycemia is not well understood (Ashfaq et al., 2021; Mochel et al., 2005). In addition to pancreatic defects, central glucose sensing may be altered in both mitochondrial diabetes and mitochondrial hypoglycemia. Cell autonomous and systemic glucose regulation are impacted by mitochondrial dysfunction, but the precise relationship between cell-autonomous and systemic metabolic features of mitochondrial disease are often unclear.
Products of anaerobic respiration of glucose - pyruvate, lactate, and alanine - are frequently elevated in the blood of patients with mitochondrial disease (Smeitink et al., 2006; Smeitink, 2003). Increased lactate can also be detected in impacted brain regions in some mitochondrial diseases, such as Leigh syndrome, via proton magnetic resonance spectroscopy (MRS), contributing to clinical diagnosis (Lin et al., 2003; Chi et al., 2011; Delonlay et al., 2013). Hyperlactemia and lactic acidosis are severe acute metabolic sequelae in mitochondrial disease patients, but it is not clear whether increased levels of tissue or circulating lactate, pyruvate, or alanine play any causal role in the pathogenesis of progressive (non-acute) symptoms (Maassen et al., 2004). These metabolites are elevated in the Ndufs4(KO) mouse model of ETC CI Leigh syndrome, and levels are attenuated by disease-modulating treatments, suggesting some role in disease (Johnson et al., 2020; Johnson et al., 2013; Stokes et al., 2022; Bornstein et al., 2022).
The cellular origins of systemic metabolic derangements in mitochondrial disease are not easily established. For example, while muscle is the largest producer of lactate in normal conditions, recent data suggests that immune cells are the major source of blood lactate in at least certain settings (Stokes et al., 2022; Stokes et al., 2022). In other situations, such as altered glutamate and aKG in the brain of Ndufs4(KO) mice, the cellular source of metabolic dysfunction has not been established, and the diversity of, and complex relationships between, brain cell types complicates experimental approaches (Johnson et al., 2020).
5. Glutamine in the brain
5.1. Brain metabolism
The mammalian brain has unique metabolic requirements and energetic demands. In humans, the brain consumes more energy than any other organ in the body by mass, and is estimated to account for ~25 % of all glucose use and ~20 % of oxygen consumption (Steiner, 2019; Goyal and Raichle, 2018). Energetically costly neuronal activities include generation of transmembrane potentials through ion pumping, trafficking of neurotransmitter vesicles at the synapse, and synthesis of neurotransmitters (Mergenthaler et al., 2013; Tomasi et al., 2013; Du et al., 2008).
The brain has limited supplies of glycogen and lipid droplets (lipid-rich organelles) compared with other tissues (Fig. 4). FAs make up approximately half of the brain’s mass, but most are structural components rather than energy stores (Bruce et al., 2017; Barber and Raben, 2019). Some lipid droplets are present in astrocytes, microglia, and neurons (Farmer et al., 2020). Lipid droplets in astrocytes can support neurons by providing stores for oxidation to ketones for uptake by neurons (Barber and Raben, 2019). Lipid droplet accumulation in microglia is primarily associated with inflammation, rather than energetic stores (Farmer et al., 2020; Marschallinger et al., 2020).
Fig. 4.
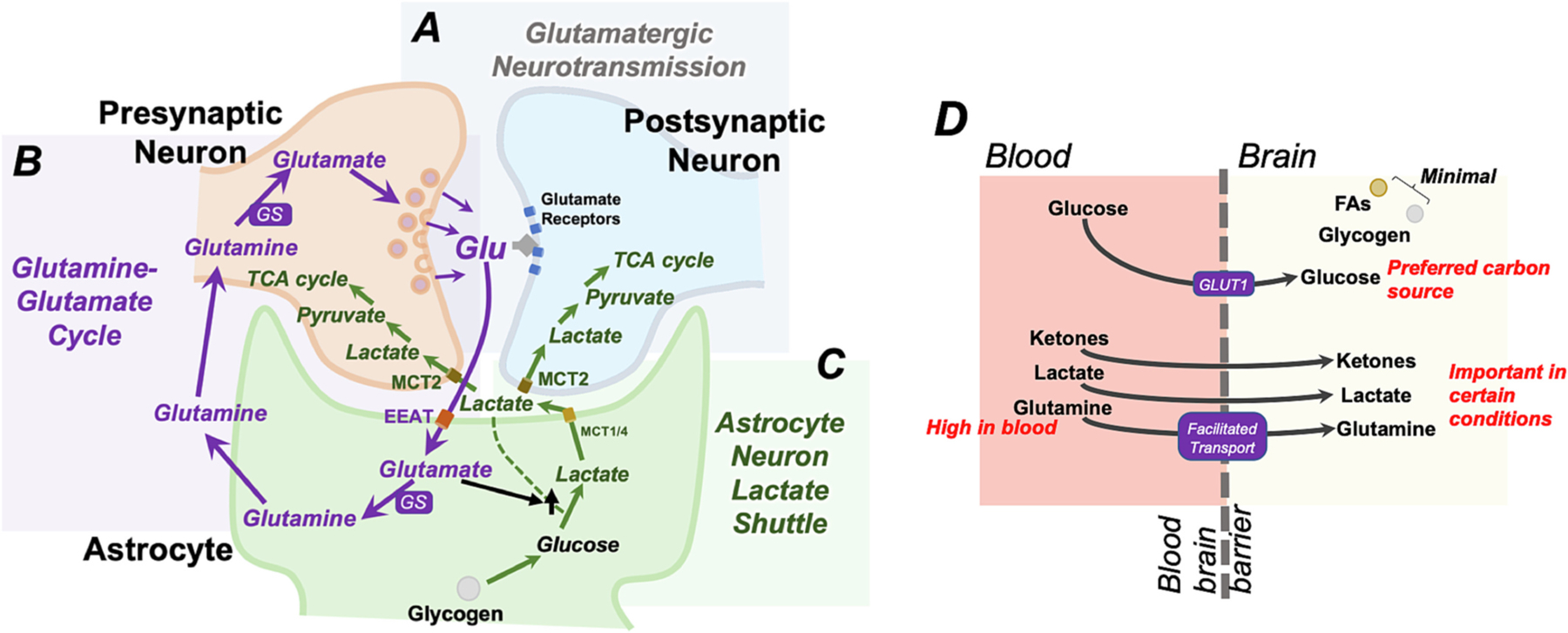
Glutamine in the brain. (A) Glutamate is the most abundant excitatory neurotransmitter. Glutamate in pre-synaptic glutamatergic neurons is packaged into vesicles at the synapse. Vesicular glutamate released into the synaptic cleft is acts on glutamate receptors at the postsynaptic neuron. (B) The astrocyte-neuron glutamine-glutamate cycle ensures that glutamate in the synapse is rapidly removed from the synapse to allow for subsequent synaptic events. In this cycle, glutamate imported into astrocytes through the EEAT glutamate transporters. Within astrocytes, glutamate is converted to glutamine, which is not a neurotransmitter, by glutaminase (GS). This is subsequently trafficked back to presynaptic neurons, where it is converted back into glutamate and re-packaged into synaptic vesicles to repeat the cycle. (C) Lactate produced in astrocytes can be exported via astrocyte-specific monocarboxylate transporters (MCT1 and MCT4) to subsequently be imported into neurons through MCT2 and supply the TCA cycle and oxidative metabolism. This process is termed the astrocyte neuron lactate shuttle (ANLS). Some evidence indicates that astrocyte lactate production is stimulated by intracellular glutamate levels and by glutamate receptors at the membrane, possibly linking ANLS flux to glutamatergic synaptic activity. (D) The brain has minimal local energy stores in forms of glycogen, which can be hydrolyzed to generate glucose, and lipid droplets, which can generate free fatty acids (FAs). The majority of brain metabolism is supported by circulating glucose, largely transported through the blood brain barrier by the glucose transporter GLUT-1. Ketones and lactate are important carbon sources for brain metabolism under certain conditions such as fasting/starvation and in the setting of strenuous exercise, respectively. Ketones are also an important metabolite during brain development. Glutamine is the most abundant amino acid in the blood and can feed central carbon metabolism. Glutamine concentrations in the brain are tightly regulated; glutamine is imported into the brain via facilitated and sodium dependent transport mechanisms.
Glycogen is present in astrocytes, and glucose released from glycogen can be converted to lactate to provide fuel to the TCA cycle in neurons via the astrocyte-neuron lactate shuttle (ANLS). Astrocyte lactate is transported out of astrocytes via monocarboxylate transporters 1 and 4 (MCT1 and MCT4) and into neurons via MCT2. Astrocyte lactate generated in this manner appears important in hypoglycemia or periods of intense neuronal activity (Falkowska et al., 2015; Sinadinos et al., 2014; Brown and Ransom, 2007). While various details of the ANLS are hotly debated (reviewed elsewhere, see (Magistretti and Allaman, 2018)), some evidence indicates astrocyte lactate production may be stimulated by astrocyte glutamate receptors and intracellular astrocyte glutamate concentrations, linking the ANLS to glutamatergic neuron activity. Supply of carbons to the TCA cycle in glutamatergic neurons via the ANLS is thought to preserve pentose-phosphate pathway mediated glucose metabolism, which generates NADPH and the nucleotide precursor ribose-5-phosphate, and provide cataplerotic flow into the TCA cycle without consumption of amino acids.
Given the minimal fuels stores, the brain requires constant supply via circulation (Fig. 4). The blood brain barrier (BBB) is impermeable to many metabolites, but allows glucose entry, mainly through the glucose transporter GLUT-1, and AA’s through a variety of transport systems (Keaney and Campbell, 2015; Zaragoza, 2020). Glucose is the primary carbon source for the mammalian brain under most conditions, but ketone bodies, lactate, and glutamine are also major substrates for metabolism. Metabolite preference is strongly influenced by nutritional status, age, health, and other physiologic factors. During strenuous physical activity, for example, lactate generated by skeletal muscle crosses the BBB and is catabolized in the brain. Ketone bodies, on the other hand, are present at low levels in the blood in adults, but high in neonates and in the settings of dietary ketosis and starvation (Proia et al., 2016; Riske et al., 2017). Circulating ketones are high during the neonatal growth period and a preferred substrate for the neonatal brain. In the developing brain, ketones serve as direct precursors for lipid synthesis during this period of active myelination, as well as a primary circulating energy source (Yeh and Sheehan, 1985; Cotter et al., 2011; Edmond et al., 1985; Stokes et al., 2021).
Glutamine is the most abundant amino acid in the blood, at 10–100 times the concentration of other amino acids (Cruzat et al., 2018; Ling et al., 2019). Glutamine can cross the BBB via facilitated transport and sodium-dependent transport, allowing dietary glutamine, and glutamine produced by muscle proteolysis, to supply the brain. Uptake is controlled at the abluminal membrane (brain-ward facing side of the BBB cells) to regulate CNS levels (Smith et al., 1987; Hawkins, 2009; Lee et al., 1998).
5.2. Glutamate neurotransmission and the glutamine/glutamate cycle
In addition to providing an energy source, glutamine is a precursor for glutathione, a key molecule involved in protection from oxidative damage, and is a precursor for both glutamate and GABA, the two most abundant neurotransmitters in the brain. Glutamate is a critical neurotransmitter: it is reported that more than 90 % of neurons have glutamate receptors and almost 40 % of neurons are classified as glutamatergic (Gasiorowska et al., 2021). While glutamine concentrations are about ten times higher than glutamate in the blood, glutamate is most abundant AA in the brain. Glutamate is highly concentrated in CNS tissue at 10,000–12,000 μmol/L, compared with 50–100 μmol/L in blood. However, for brain homeostasis and function, glutamate in extracellular spaces must be maintained at only 0.5–2 μM.
Synaptic glutamate transiently increases to millimolar concentrations during synaptic neurotransmission, and rapid removal of glutamate is critical to limit the length of signal at the synapse and allow for subsequent synaptic events (Conway, 2020; Wang and Zhang, 2005; Wang and Reddy, 2017). Astrocytes remove glutamate from the synaptic cleft and extracellular space and recycle it to neurons for re-use through a process called the glutamine-glutamate cycle (Fig. 4). Excitatory amino acid transporters (EAATs) transport synaptic glutamate into astrocytes, with EAAT2 responsible for ~90 % of transport (Kim et al., 2011). Within astrocytes, glutamate is then converted into the nonneurotransmitter glutamine by glutamine synthetase (GS), providing an intermediate for transport back to neurons. Glutamine export from astrocytes is facilitated by the glutamine transporter SN1 (Broer et al., 2002). Extracellular glutamine is imported into neurons via glutamine transporters such as SAT1 and SAT2 (Yao et al., 2000). Neuronal glutamine is subsequently used to synthesize glutamate via the enzyme glutaminase, whereafter it can be packaged into presynaptic vesicles for reuse (Conway, 2020).
The glutamine/glutamate cycle provides control over the length of stimulation and proper function is critical not only for neural activities, but also to prevent glutamate excitotoxicity, which is thought to result from chronic or over-stimulation of glutamate receptors (discussed below). Tight regulation of glutamine metabolism plays an important role in maintaining optimal intracellular and extracellular glutamate concentrations. Glutamate conversion to aKG is an anaplerotic reaction, as detailed, but can also contribute to regulation of overall glutamate levels.
6. Glutamine metabolism and neurodegenerative disease
6.1. Neurodegenerative mitochondrial diseases
Impaired glucose metabolism in the brain is a well-documented phenomena in genetic mitochondrial diseases. Patients with LS, MELAS, Friedrich’s ataxia (FA), and MM show reduced cerebral glucose metabolism and a shift from oxidative respiration to aerobic glycolysis by position emission tomography (PET) and carbon 13-labeled MRS (Lindroos et al., 2009; Haginoya et al., 2016; Otsuki et al., 2005; Molnar et al., 2000). Similarly, there is a significant increase (interpreted as an accumulation) of glycolytic intermediates in the brain of the Ndufs4 (KO) mouse model of ETC CI deficiency, neurodegeneration, and LS (Johnson et al., 2013).
Dysregulation of brain glutamine metabolism has also been reported in the setting of mitochondrial dysfunction. Glutamine, glutamate, and aKG are significantly reduced in all brain regions in the Ndufs4(KO) mouse model of CI deficient LS, and dimethyl-KG modestly attenuates disease in this model (Johnson et al., 2020; Lee et al., 2019; Johnson et al., 2013). Glutaminolysis is increased in neurons with oxidative phosphorylation deficiency, and glutaminolysis enzymes are upregulated in Purkinje cells isolated from the Mfn2 deficient mouse model of mitochondrial dysfunction show upregulation (Motori et al., 2020).
These data suggest that glutamine/glutamate/aKG support the TCA cycle in the brain when flux from glucose and through IDH is perturbed, a model directly supported by evidence mitochondrial oxygen consumption data. Pyruvate and glutamate driven complex I dependent (rotenone inhibited) mitochondrial oxygen consumption is 30–40 % reduced in isolated mitochondria from Ndufs4(KO) brain, but aKG fully supports normal maximal complex I dependent (rotenone inhibited) respiratory capacity (malate was included in each case) (Kayser et al., 2016; Johnson et al., 2013). Glutamate itself only partly rescued oxygen consumption in these experiments, likely due to the lack of cytoplasm (where glutamine synthetase is located) in the mitochondrial preparations. A comprehensive explanation for these findings remains to be determined, but they reveal that flux (at least in the ex vivo setting) through aKG can continue in a setting where ETC CI dysfunction has led to impairment of flux through PDH and IDH (Porpaczy et al., 1987).
6.2. Complex neurodegenerative diseases
Extensive research has been dedicated to studying the role of metabolism in complex neurodegenerative diseases with strong links to mitochondrial dysfunction. These include Alzheimer’s (AD), Parkinson’s (PD), amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS), and others (Bornstein et al., 2020; Johnson et al., 2017; Han et al., 2021; Barcelos et al., 2019). Patient and animal studies reporting decreases in cerebral glucose utilization by MRI and PET, reduced expression of glucose transporters, decreased levels of glycolytic proteins, and strong associations between alterations in glucose metabolism and associated neuropathology and dementia. Glutamine mishandling is also a well-documented in complex neurodegenerative diseases. The following is a brief overview of evidence linking glutamate metabolism to these diseases.
6.2.1. Alzheimer’ disease (AD)
AD is a progressive age-related neurodegenerative disorder involving severe cognitive impairment and memory loss coincident with amyloid plaque accumulation in hippocampal and neocortical tissue (Magalingam et al., 2018). AD is not generally thought to be a direct result of metabolic derangement, but aberrant glutamine metabolism has been reported in AD, and altered glutamate neurotransmission appears to play some role in pathology (Wang and Reddy, 2017; Liu et al., 2019).
Cortical tissue taken from AD patients showed decreased glutamate with increased glutamine (Griffin and Bradshaw, 2017). MRS studies of AD patients reported decreased glutamate in living cortex (Fayed et al., 2011; Rupsingh et al., 2011). In mice, the CRND8 mouse model of AD, which predisposes mice for amyloid accumulation, showed both glutamate and glutamine deficiencies in multiple brain regions (Salek et al., 2010).
Glutamate excitotoxicity is thought to play a role in AD pathobiology (Conway, 2020; Wang and Reddy, 2017; Liu et al., 2019; Lakhan et al., 2013). Signaling via the glutamate sensing NMDAR (N-methyl-d-aspartate receptor, a glutamate responsive receptor) is necessary for neuronal function, but the receptor also plays a key role in Ca2+ influx mediated excitotoxicity when overstimulated, and both insufficient and over-active NMDAR signaling can have catastrophic consequences to neurons (Wang and Reddy, 2017; Liu et al., 2019). NMDAR hypo- and hyper-functioning have both been described in AD, with amyloid deposits thought to directly impact NMDAR activity, and NMDAR activity contributing to cell death (Wang and Reddy, 2017; Lakhan et al., 2013). Amyloid accumulation appears to lead to a decrease in expression of EAAT2 on astrocytes, impairing glutamate reuptake via the glutamate-glutamine cycle and contributing to NMDAR-mediated excitotoxicity (Huang et al., 2018; Scott et al., 2011). Available data suggests that in early-stage AD, NMDAR hyper-functioning causes glutamate excitotoxicity, while loss of NMDA receptors and associated neurons subsequently contributes to overall hypo-functioning in late-stage disease (Lakhan et al., 2013).
Memantine is an FDA-approved mild affinity antagonist for the NMDAR specifically developed to interfere with glutamate excitotoxicity, one of a few pharmacological therapies for AD (Matsunaga et al., 2018). Unfortunately, though early trials suggest memantine provides some benefit, results are mixed, and even when benefits are reported they are extremely modest (Matsunaga et al., 2018; van Marum, 2009; Li et al., 2019). Whether targeting glutamine metabolism or signaling represents a viable strategy toward meaningful intervention in AD remains to be shown.
6.2.2. Parkinson’s disease (PD)
PD is one of the most common neurodegenerative disorders, involving loss of neurons in the substantia nigra, a brain region critical for production of dopamine (Magalingam et al., 2018; Zhang et al., 2019). In a subset of PD patients, impacted neurons accumulate intracellular inclusions called Lewy bodies (Spivey, 2011). Pharmacological treatment of PD symptoms involves dopamine receptor agonists and levodopa, a dopamine precursor, to compensate for dopaminergic signaling deficiencies (DeMaagd and Philip, 2015) (Spivey, 2011). While PD is most directly associated with dopaminergic neurons, glutamate may also contribute to neurodegeneration in PD. Evidence suggests that glutamate receptor expression and activity are dysregulated in PD, while administration of NMDAR antagonists in animal models reduces PD symptoms, such as akinesia and rigidy, and increases the effectiveness of levodopa (Zhang et al., 2019; Vanle et al., 2018; Ahmed et al., 2011; Greenamyre and O’Brien, 1991). However, clinical trials of an NMDAR antagonist in PD patients show limited efficacy, and glutamate likely only modifies disease (Zhang et al., 2019).
6.2.3. Amyotrophic lateral sclerosis (ALS)
ALS is an adult-onset neurodegenerative disease characterized by gliosis and neuron degeneration in the motor cortex, brainstem, and spinal cord (Kumar et al., 2013). Studies have found increased glutamate in ALS patient blood and cerebral spinal fluid. Post-mortem ALS patient brain samples were found to have increased glutamate but unchanged glutamine, but MRS studies of patients reported increases in both glutamate and glutamine (Kumar et al., 2013; Perry et al., 1987; Plaitakis et al., 1988; Fiszman et al., 2010; Spreux-Varoquaux et al., 2002). Altered glutamate receptor expression and activity have also been described in ALS patients and in models of disease, and genome-wide association studies have reported associations between glutamatergic neuron specific genes and ALS risk (Rosenblum and Trotti, 2017; van Rheenen et al., 2021). Despite this circumstantial evidence, pharmacologic and genetic studies manipulating glutamate receptor activity and glutamate signaling have led to only modest attenuation of outcomes in mouse models of ALS (Wang and Zhang, 2005; Blasco et al., 2014; Milanese et al., 2014; Bonifacino et al., 2017; Brownell et al., 2015; Guo et al., 2003; Joo et al., 2007; Hogg et al., 2018). Accordingly, as with AD and PD, altered glutamine metabolism may play a secondary or modifying role in disease, but available evidence does not a support a primary causal role in ALS disease pathogenesis.
6.2.4. Multiple sclerosis (MS)
MS is a neuroinflammatory disorder impacting the brain and spinal cord. While MS is most strongly associated with an autoimmune etiology that is not well understood, significant evidence supports a role for mitochondrial dysfunction in the underlying causes of this disease. Notably, MS occurs coincidentally with the primary genetic mitochondrial disease LHON (described above) significantly more frequently than would be expected by chance; the combined disease has been termed Harding’s syndrome or LHON-MS (Hanaford and Johnson, 2022; Cawley et al., 2010; Parry-Jones et al., 2008). Efforts to elucidate and target mitochondrial pathways involved in inflammation are an active focus of research in MS as well as primary mitochondrial disease and have been proposed to causally link them (Hanaford and Johnson, 2022; Bargiela and Chinnery, 2019).
As in AD and ALS, circumstantial evidence supports some role for glutamine metabolism in MS, though the evidence is conflicting. Multiple studies have reported that glutamine and glutamate are increased in MS brains by MRS and that this increase is the best predictor of disease, while another study reported elevated plasma glutamine in MS patients (Tisell et al., 2013; Polacek et al., 2019; Yang et al., 2021a). However, others have reported decreased glutamine and glutamate in MS brains by MRS (MacMillan et al., 2016; Sastre-Garriga et al., 2005). Multivariate supervised analysis of brain MRS and a review of MRS literature both suggest glutamine and glutamate are among the most altered metabolites in MS brain, but change directionality depends on disease severity, as well as the region of white matter analyzed (i.e. unaffected brain vs lesion). Accuracy of determination is strongly dependent on the quality of the data (magnetic field strength, analysis methods, etc.) (Swanberg et al., 2019; Swanberg et al., 2022).
Glutamate excitotoxicity has been shown to contribute to disease progression, and glutamine antagonism attenuates disease, in mouse experimental autoimmune encephalitis models of MS, but the relevance of these models to MS is not clear (Hollinger et al., 2019; Pitt et al., 2000). Unfortunately, memantine (described above) trials in MS patients to date indicate that targeting glutamate excitotoxicity does not lessen cognitive decline or alter disease course in MS (Turalde et al., 2020).
7. Conclusions
Glutamine is central player in a wide range of biological processes. Glutamine is the most abundant amino acid in both muscle and blood, and a significant contributor to circulating metabolic homeostasis. Glutamine is a precursor for synthesis of the critical antioxidant glutathione and the most common neurotransmitter, glutamate, as well as an intermediate in glutamate recycling in the neuron-astrocyte glutamine-glutamate shuttle. Glutamine is also a major anaplerotic molecule, feeding the TCA cycle via input at aKG, enabling TCA cycle flux and feeding core TCA cycle driven biosynthetic pathways in settings of altered redox or low oxygen. Given these varied roles, it is perhaps of no surprise that experimental evidence has implicated glutamine metabolism in a wide range of pathologies, in particular those already linked to mitochondrial dysfunction.
Altered glutamine levels, or the levels or activity of glutamine metabolism related enzymes, are associated with a wide range of human pathologies. However, little evidence supports direct causality in links between altered glutamine metabolism and pathology. Moreover, while basic research links glutamine to a range of diseases, glutamine-targeting interventions have generally lacked efficacy in vivo. Memantine, designed to target NMDAR mediated glutamate excitotoxicity, is the best example of a glutamine/glutamate targeting therapy brought to the clinic and has proven to have little impact on disease course in AD (Matsunaga et al., 2018; van Marum, 2009; Li et al., 2019). This seems to suggest that if glutamine plays a significant role in AD, excitotoxicity is not a major mediating process. Efforts to promote TCA cycle entry through the glutamine/glutamate/aKG route using DMKG have shown some promise in both cell and mouse models of mitochondrial disease, but clinical translatability has not been tested. The modest benefits in a well-controlled mouse study may indicate that clinical efficacy will prove difficult to demonstrate, as with memantine.
The exception to the above statements is the setting of cancer, not discussed in this manuscript but the subject of multiple high-quality reviews (Souba, 1993; Li and Le, 2018; Li et al., 2021; Yang et al., 2021b). In cancer, reliance on glutamine catabolism for nucleotide precursors in rapidly dividing cells is a metabolic derangement amenable to pharmacologic targeting. Unfortunately, it is much easier to target a specific dysregulated metabolic process for inhibition with the aim of killing target cells than it is to promote intracellular utilization or to inhibit normal usage without significant off-target effects. The tissue and cell specificity of glutamine/glutamate metabolism in the brain, and extreme temporal and spatial specificity of glutamine/glutamate in glutamatergic synapse sub-structures, should give pause to any attempts to alter glutamine/glutamate metabolism through untargeted systemic approaches.
In summary, glutamine is pleiotropic molecule of key importance to a wide range of biological processes including antioxidant defense, neurotransmission, and cellular metabolism, and alterations in glutamine levels or metabolism have been reported in a wide range of diseases. However, causality is not often clear, and therapeutically beneficial targeting of glutamine metabolism has proven difficult. In the setting of neurodegenerative diseases, future efforts aimed at targeting therapies to neurons, or even synapses, may find efficacy where untargeted therapies have failed. In the setting of genetic mitochondrial disease, targeting glutamine-related metabolic pathways has shown some modest pre-clinical efficacy, but clinical transl. Ongoing work aimed at identifying additional therapeutic targets in glutamine metabolism and improving current intervention approaches may yield clinically relevant advances. Further study is needed to better understand the role and therapeutic potential of glutamine in human disease, including the cell and tissue specific roles of this metabolite.
Funding
SCJ was funded by NIH R01GM133865, NIH R01NS119426, and NIH R01GM144368. MS was funded by NIH R01GM133865. RB was funded by NIH T32GM095421 and NIH 1F31NS120442. PM was supported by NIH R35 GM139566.
Footnotes
Declaration of competing interest
None.
Data availability
No data was used for the research described in the article.
References
- Ahmed I, et al. , 2011. Glutamate NMDA receptor dysregulation in Parkinson’s disease with dyskinesias. Brain 134, 979–986. [DOI] [PubMed] [Google Scholar]
- Arisaka O, Ichikawa G, Imataka G, Koyama S, Sairenchi T, 2020. Iron, ketone bodies, and brain development. J. Pediatr 222, 262–263. [DOI] [PubMed] [Google Scholar]
- Ashfaq M, et al. , 2021. Hypoglycemia in mitochondrial disorders. Mitochondrion 58, 179–183. [DOI] [PubMed] [Google Scholar]
- Aspuria PP, et al. , 2014. Succinate dehydrogenase inhibition leads to epithelial-mesenchymal transition and reprogrammed carbon metabolism. Cancer Metab. 2, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber CN, Raben DM, 2019. Lipid metabolism crosstalk in the brain: glia and neurons. Front. Cell. Neurosci 13, 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcelos IP, Troxell RM, Graves JS, 2019. Mitochondrial dysfunction and multiple sclerosis. Biology (Basel) 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargiela D, Chinnery PF, 2019. Mitochondria in neuroinflammation - multiple sclerosis (MS), leber hereditary optic neuropathy (LHON) and LHON-MS. Neurosci. Lett 710, 132932. [DOI] [PubMed] [Google Scholar]
- Birsoy K, et al. , 2015. An essential role of the mitochondrial electron transport chain in cell proliferation is to enable aspartate synthesis. Cell 162, 540–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasco H, Mavel S, Corcia P, Gordon PH, 2014. The glutamate hypothesis in ALS: pathophysiology and drug development. Curr. Med. Chem 21, 3551–3575. [DOI] [PubMed] [Google Scholar]
- Bolea I, et al. , 2019. Defined neuronal populations drive fatal phenotype in a mouse model of Leigh syndrome. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino T, et al. , 2017. In-vivo effects of knocking-down metabotropic glutamate receptor 5 in the SOD1(G93A) mouse model of amyotrophic lateral sclerosis. Neuropharmacology 123, 433–445. [DOI] [PubMed] [Google Scholar]
- Bornstein R, Gonzalez B, Johnson SC, 2020. Mitochondrial pathways in human health and aging. Mitochondrion 54, 72–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein R, et al. , 2022. Differential effects of mTOR inhibition and dietary ketosis in a mouse model of subacute necrotizing encephalomyelopathy. Neurobiol. Dis 163, 105594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst P, 2020. The malate-aspartate shuttle (Borst cycle): how it started and developed into a major metabolic pathway. IUBMB Life 72, 2241–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowtell JL, Bruce M, 2002. Glutamine: an anaplerotic precursor. Nutrition 18, 222–224. [DOI] [PubMed] [Google Scholar]
- Breuer ME, et al. , 2013. The role of mitochondrial OXPHOS dysfunction in the development of neurologic diseases. Neurobiol. Dis 51, 27–34. [DOI] [PubMed] [Google Scholar]
- Broer A, et al. , 2002. Regulation of the glutamine transporter SN1 by extracellular pH and intracellular sodium ions. J. Physiol 539, 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broom GM, Shaw IC, Rucklidge JJ, 2019. The ketogenic diet as a potential treatment and prevention strategy for Alzheimer’s disease. Nutrition 60, 118–121. [DOI] [PubMed] [Google Scholar]
- Brown AM, Ransom BR, 2007. Astrocyte glycogen and brain energy metabolism. Glia 55, 1263–1271. [DOI] [PubMed] [Google Scholar]
- Brownell AL, et al. , 2015. PET imaging studies show enhanced expression of mGluR5 and inflammatory response during progressive degeneration in ALS mouse model expressing SOD1-G93A gene. J. Neuroinflammation 12, 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce KD, Zsombok A, Eckel RH, 2017. Lipid processing in the brain: a key regulator of systemic metabolism. Front. Endocrinol. (Lausanne) 8, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunengraber H, Roe CR, 2006. Anaplerotic molecules: current and future. J. Inherit. Metab. Dis 29, 327–331. [DOI] [PubMed] [Google Scholar]
- Capristo M, et al. , 2022. Rapamycin rescues mitochondrial dysfunction in cells carrying the m.8344A > G mutation in the mitochondrial tRNA(Lys). Mol. Med 28, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawley N, Molloy A, Cassidy L, Tubridy N, 2010. Late-onset progressive visual loss in a man with unusual MRI findings: MS, Harding’s, Leber’s or Leber’s Plus? Ir. J. Med. Sci 179, 599–601. [DOI] [PubMed] [Google Scholar]
- Chao de la Barca JM, et al. , 2019. The metabolomic signature of Opa1 deficiency in rat primary cortical neurons shows aspartate/glutamate depletion and phospholipids remodeling. Sci. Rep 9, 6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, et al. , 2018. Rewiring of glutamine metabolism is a bioenergetic adaptation of human cells with mitochondrial DNA mutations. Cell Metab. 27, 1007–1025 e1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi CS, et al. , 2011. Lactate peak on brain MRS in children with syndromic mitochondrial diseases. J. Chin. Med. Assoc 74, 305–309. [DOI] [PubMed] [Google Scholar]
- Conway ME, 2020. Alzheimer’s disease: targeting the glutamatergic system. Biogerontology 21, 257–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter DG, d’Avignon DA, Wentz AE, Weber ML, Crawford PA, 2011. Obligate role for ketone body oxidation in neonatal metabolic homeostasis. J. Biol. Chem 286, 6902–6910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruzat V, Macedo Rogero M, Noel Keane K, Curi R, Newsholme P, 2018. Glutamine: metabolism and immune function, supplementation and clinical translation. Nutrients 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Meirleir L, 2013. Disorders of pyruvate metabolism. Handb. Clin. Neurol 113, 1667–1673. [DOI] [PubMed] [Google Scholar]
- Delonlay P, Rotig A, Sarnat HB, 2013. Respiratory chain deficiencies. Handb. Clin. Neurol 113, 1651–1666. [DOI] [PubMed] [Google Scholar]
- DeMaagd G, Philip A, 2015. Parkinson’s disease and its management: part 1: disease entity, risk factors, pathophysiology, clinical presentation, and diagnosis. P T 40, 504–532. [PMC free article] [PubMed] [Google Scholar]
- Drechsel DA, Patel M, 2009. Differential contribution of the mitochondrial respiratory chain complexes to reactive oxygen species production by redox cycling agents implicated in parkinsonism. Toxicol. Sci 112, 427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du F, et al. , 2008. Tightly coupled brain activity and cerebral ATP metabolic rate. Proc. Natl. Acad. Sci. U. S. A 105, 6409–6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmond J, Auestad N, Robbins RA, Bergstrom JD, 1985. Ketone body metabolism in the neonate: development and the effect of diet. Fed. Proc 44, 2359–2364. [PubMed] [Google Scholar]
- Falkowska A, et al. , 2015. Energy metabolism of the brain, including the cooperation between astrocytes and neurons, especially in the context of glycogen metabolism. Int. J. Mol. Sci 16, 25959–25981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer BC, Walsh AE, Kluemper JC, Johnson LA, 2020. Lipid droplets in neurodegenerative disorders. Front. Neurosci 14, 742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayed N, Modrego PJ, Rojas-Salinas G, Aguilar K, 2011. Brain glutamate levels are decreased in Alzheimer’s disease: a magnetic resonance spectroscopy study. Am. J. Alzheimers Dis. Other Dement 26, 450–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiszman ML, Ricart KC, Latini A, Rodriguez G, Sica RE, 2010. In vitro neurotoxic properties and excitatory aminoacids concentration in the cerebrospinal fluid of amyotrophic lateral sclerosis patients. Relationship with the degree of certainty of disease diagnoses. Acta Neurol. Scand 121, 120–126. [DOI] [PubMed] [Google Scholar]
- Frazier AE, Thorburn DR, Compton AG, 2019. Mitochondrial energy generation disorders: genes, mechanisms, and clues to pathology. J. Biol. Chem 294, 5386–5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel JL, Zervos PR, Plaut GW, 1986. Activity of purified NAD-specific isocitrate dehydrogenase at modulator and substrate concentrations approximating conditions in mitochondria. Metabolism 35, 661–667. [DOI] [PubMed] [Google Scholar]
- Gasiorowska A, et al. , 2021. The biology and pathobiology of glutamatergic, cholinergic, and dopaminergic signaling in the aging brain. Front. Aging Neurosci 13, 654931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez B, et al. , 2023. High-throughput sequencing analysis of nuclear-encoded mitochondrial genes reveals a genetic signature of human longevity. Geroscience 45, 311–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorini S, et al. , 2018. Chemotherapeutic drugs and mitochondrial dysfunction: focus on doxorubicin, Trastuzumab, and Sunitinib. Oxidative Med. Cell. Longev 2018, 7582730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal MS, Raichle ME, 2018. Glucose requirements of the developing human brain. J. Pediatr. Gastroenterol. Nutr 66 (Suppl. 3), S46–S49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenamyre JT, O’Brien CF, 1991. N-methyl-D-aspartate antagonists in the treatment of Parkinson’s disease. Arch. Neurol 48, 977–981. [DOI] [PubMed] [Google Scholar]
- Griffin JW, Bradshaw PC, 2017. Amino acid catabolism in Alzheimer’s disease brain: friend or foe? Oxidative Med. Cell. Longev 2017, 5472792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundlingh J, Dargan PI, El-Zanfaly M, Wood DM, 2011. 2,4-dinitrophenol (DNP): a weight loss agent with significant acute toxicity and risk of death. J. Med. Toxicol 7, 205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, et al. , 2003. Increased expression of the glial glutamate transporter EAAT2 modulates excitotoxicity and delays the onset but not the outcome of ALS in mice. Hum. Mol. Genet 12, 2519–2532. [DOI] [PubMed] [Google Scholar]
- Guzman M, Blazquez C, 2001. Is there an astrocyte-neuron ketone body shuttle? Trends Endocrinol. Metab 12, 169–173. [DOI] [PubMed] [Google Scholar]
- Guzman M, Blazquez C, 2004. Ketone body synthesis in the brain: possible neuroprotective effects. Prostaglandins Leukot. Essent. Fat. Acids 70, 287–292. [DOI] [PubMed] [Google Scholar]
- Haginoya K, et al. , 2016. FDG-PET study of patients with Leigh syndrome. J. Neurol. Sci 362, 309–313. [DOI] [PubMed] [Google Scholar]
- Han R, Liang J, Zhou B, 2021. Glucose metabolic dysfunction in neurodegenerative diseases-new mechanistic insights and the potential of hypoxia as a prospective therapy targeting metabolic reprogramming. Int. J. Mol. Sci 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanaford A, Johnson SC, 2022. The immune system as a driver of mitochondrial disease pathogenesis: a review of evidence. Orphanet. J. Rare Dis 17, 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins RA, 2009. The blood-brain barrier and glutamate. Am. J. Clin. Nutr 90, 867S–874S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero-Yraola A, et al. , 2001. Regulation of glutamate dehydrogenase by reversible ADP-ribosylation in mitochondria. EMBO J. 20, 2404–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg MC, Halang L, Woods I, Coughlan KS, Prehn JHM, 2018. Riluzole does not improve lifespan or motor function in three ALS mouse models. Amyotroph. Lateral Scler. Frontotemporal Degener 19, 438–445. [DOI] [PubMed] [Google Scholar]
- Hollinger KR, et al. , 2019. Glutamine antagonism attenuates physical and cognitive deficits in a model of MS. Neurol. Neuroimmunol. Neuroinflamm 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, et al. , 2018. Astrocytic glutamatergic transporters are involved in Abeta-induced synaptic dysfunction. Brain Res. 1678, 129–137. [DOI] [PubMed] [Google Scholar]
- Huxtable R, et al. , 1979. Regional distribution of amino acids in Friedreich’s ataxia brains. Can. J. Neurol. Sci 6, 255–258. [DOI] [PubMed] [Google Scholar]
- Jensen NJ, Wodschow HZ, Nilsson M, Rungby J, 2020. Effects of ketone bodies on brain metabolism and function in neurodegenerative diseases. Int. J. Mol. Sci 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, et al. , 2013. mTOR inhibition alleviates mitochondrial disease in a mouse model of Leigh syndrome. Science 342, 1524–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Dong X, Vijg J, Suh Y, 2015. Genetic evidence for common pathways in human age-related diseases. Aging Cell 14, 809–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, et al. , 2017. Network analysis of mitonuclear GWAS reveals functional networks and tissue expression profiles of disease-associated genes. Hum. Genet 136, 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, et al. , 2019. mTOR inhibitors may benefit kidney transplant recipients with mitochondrial diseases. Kidney Int. 95, 455–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, et al. , 2020. Regional metabolic signatures in the Ndufs4(KO) mouse brain implicate defective glutamate/alpha-ketoglutarate metabolism in mitochondrial disease. Mol. Genet. Metab 130, 118–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo IS, Hwang DH, Seok JI, Shin SK, Kim SU, 2007. Oral administration of memantine prolongs survival in a transgenic mouse model of amyotrophic lateral sclerosis. J. Clin. Neurol 3, 181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanter M, 2018. High-quality carbohydrates and physical performance: expert panel report. Nutr. Today 53, 35–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser EB, Sedensky MM, Morgan PG, 2016. Region-specific defects of respiratory capacities in the Ndufs4(KO) mouse brain. PLoS One 11, e0148219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keaney J, Campbell M, 2015. The dynamic blood-brain barrier. FEBS J. 282, 4067–4079. [DOI] [PubMed] [Google Scholar]
- Kim K, et al. , 2011. Role of excitatory amino acid transporter-2 (EAAT2) and glutamate in neurodegeneration: opportunities for developing novel therapeutics. J. Cell. Physiol 226, 2484–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, et al. , 2020. Immunological measurement of aspartate/alanine aminotransferase in predicting liver fibrosis and inflammation. Korean J. Intern. Med 35, 320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Lee J, Jang DH, 2022. NDUFAF6-related Leigh syndrome caused by rare pathogenic variants: a case report and the focused review of literature. Front. Pediatr 10, 812408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeppen AH, Davis AN, Morral JA, 2011. The cerebellar component of Friedreich’s ataxia. Acta Neuropathol. 122, 323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Ghosh D, Singh RL, 2013. Amyotrophic lateral sclerosis and metabolomics: clinical implication and therapeutic approach. J. Biomark 2013, 538765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhan SE, Caro M, Hadzimichalis N, 2013. NMDA receptor activity in neuropsychiatric disorders. Front. Psychiatry 4, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WJ, Hawkins RA, Vina JR, Peterson DR, 1998. Glutamine transport by the blood-brain barrier: a possible mechanism for nitrogen removal. Am. J. Phys 274, C1101–C1107. [DOI] [PubMed] [Google Scholar]
- Lee CF, Caudal A, Abell L, Nagana Gowda GA, Tian R, 2019. Targeting NAD(+) metabolism as interventions for mitochondrial disease. Sci. Rep 9, 3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Le A, 2018. Glutamine metabolism in cancer. Adv. Exp. Med. Biol 1063, 13–32. [DOI] [PubMed] [Google Scholar]
- Li DD, Zhang YH, Zhang W, Zhao P, 2019. Meta-analysis of randomized controlled trials on the efficacy and safety of donepezil, Galantamine, Rivastigmine, and Memantine for the treatment of Alzheimer’s disease. Front. Neurosci 13, 472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Copeland C, Le A, 2021. Glutamine metabolism in cancer. Adv. Exp. Med. Biol 1311, 17–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao GY, et al. , 2019. Blockage of glutamine-dependent anaplerosis affects mTORC1/2 activity and ultimately leads to cellular senescence-like response. Biol. Open 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin DD, Crawford TO, Barker PB, 2003. Proton MR spectroscopy in the diagnostic evaluation of suspected mitochondrial disease. AJNR Am. J. Neuroradiol 24, 33–41. [PMC free article] [PubMed] [Google Scholar]
- Lindroos MM, et al. , 2009. Cerebral oxygen and glucose metabolism in patients with mitochondrial m.3243A>G mutation. Brain 132, 3274–3284. [DOI] [PubMed] [Google Scholar]
- Ling HH, et al. , 2019. Clinical significance of serum glutamine level in patients with colorectal Cancer. Nutrients 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Chang L, Song Y, Li H, Wu Y, 2019. The role of NMDA receptors in Alzheimer’s disease. Front. Neurosci 13, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maassen JA, et al. , 2004. Mitochondrial diabetes: molecular mechanisms and clinical presentation. Diabetes 53 (Suppl. 1), S103–S109. [DOI] [PubMed] [Google Scholar]
- MacMillan EL, et al. , 2016. Progressive multiple sclerosis exhibits decreasing glutamate and glutamine over two years. Mult. Scler 22, 112–116. [DOI] [PubMed] [Google Scholar]
- Magalingam KB, Radhakrishnan A, Ping NS, Haleagrahara N, 2018. Current concepts of neurodegenerative mechanisms in Alzheimer’s disease. Biomed. Res. Int 2018, 3740461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistretti PJ, Allaman I, 2018. Lactate in the brain: from metabolic end-product to signalling molecule. Nat. Rev. Neurosci 19, 235–249. [DOI] [PubMed] [Google Scholar]
- Marschallinger J, et al. , 2020. Lipid-droplet-accumulating microglia represent a dysfunctional and proinflammatory state in the aging brain. Nat. Neurosci 23, 194–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins F, Goncalves LG, Pojo M, Serpa J, 2020. Take advantage of glutamine anaplerosis, the kernel of the metabolic rewiring in malignant gliomas. Biomolecules 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastorodemos V, Zaganas I, Spanaki C, Bessa M, Plaitakis A, 2005. Molecular basis of human glutamate dehydrogenase regulation under changing energy demands. J. Neurosci. Res 79, 65–73. [DOI] [PubMed] [Google Scholar]
- Matsunaga S, et al. , 2018. The efficacy and safety of memantine for the treatment of Alzheimer’s disease. Expert Opin. Drug Saf 17, 1053–1061. [DOI] [PubMed] [Google Scholar]
- McElroy GS, et al. , 2020. NAD+ regeneration rescues lifespan, but not ataxia, in a mouse model of brain mitochondrial complex I dysfunction. Cell Metab. 32, 301–308 e306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInnes J, 2013. Mitochondrial-associated metabolic disorders: foundations, pathologies and recent progress. Nutr. Metab. (Lond.) 10, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergenthaler P, Lindauer U, Dienel GA, Meisel A, 2013. Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends Neurosci. 36, 587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JN, et al. , 2013. Mitochondria as a target of environmental toxicants. Toxicol. Sci 134, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milanese M, et al. , 2014. Knocking down metabotropic glutamate receptor 1 improves survival and disease progression in the SOD1(G93A) mouse model of amyotrophic lateral sclerosis. Neurobiol. Dis 64, 48–59. [DOI] [PubMed] [Google Scholar]
- Miyazaki I, et al. , 2020. Chronic systemic exposure to low-dose rotenone induced central and peripheral neuropathology and motor deficits in mice: reproducible animal model of Parkinson’s disease. Int. J. Mol. Sci 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochel F, et al. , 2005. Respiratory chain defects may present only with hypoglycemia. J. Clin. Endocrinol. Metab 90, 3780–3785. [DOI] [PubMed] [Google Scholar]
- Moller HE, et al. , 2005. Magnetic resonance spectroscopy in patients with MELAS. J. Neurol. Sci 229-230, 131–139. [DOI] [PubMed] [Google Scholar]
- Molnar MJ, et al. , 2000. Cerebral blood flow and glucose metabolism in mitochondrial disorders. Neurology 55, 544–548. [DOI] [PubMed] [Google Scholar]
- Montgomery MK, Turner N, 2015. Mitochondrial dysfunction and insulin resistance: an update. Endocr. Connect 4, R1–R15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motori E, et al. , 2020. Neuronal metabolic rewiring promotes resilience to neurodegeneration caused by mitochondrial dysfunction. Sci. Adv 6, eaba8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukaneza Y, et al. , 2019. mTORC1 is required for expression of LRPPRC and cytochrome-c oxidase but not HIF-1alpha in Leigh syndrome French Canadian type patient fibroblasts. Am. J. Physiol. Cell Physiol 317, C58–C67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen AR, et al. , 2014. Oxidation of alpha-ketoglutarate is required for reductive carboxylation in cancer cells with mitochondrial defects. Cell Rep. 7, 1679–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsholme P, et al. , 2003. Glutamine and glutamate as vital metabolites. Braz. J. Med. Biol. Res 36, 153–163. [DOI] [PubMed] [Google Scholar]
- Nikkanen J, et al. , 2016. Mitochondrial DNA replication defects disturb cellular dNTP pools and remodel one-carbon metabolism. Cell Metab. 23, 635–648. [DOI] [PubMed] [Google Scholar]
- Olubodun-Obadun TG, Ishola IO, Adeyemi OO, 2022. Impact of environmental toxicants exposure on gut-brain axis in Parkinson disease. Drug Metab. Pers. Ther [DOI] [PubMed] [Google Scholar]
- Orsucci D, Caldarazzo Ienco E, Rossi A, Siciliano G, Mancuso M, 2021. mitochondrial syndromes revisited. J. Clin. Med 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuki T, et al. , 2005. Carbon 13-labeled magnetic resonance spectroscopy observation of cerebral glucose metabolism: metabolism in MELAS: case report. Arch. Neurol 62, 485–487. [DOI] [PubMed] [Google Scholar]
- Owen OE, Kalhan SC, Hanson RW, 2002. The key role of anaplerosis and cataplerosis for citric acid cycle function. J. Biol. Chem 277, 30409–30412. [DOI] [PubMed] [Google Scholar]
- Parry-Jones AR, Mitchell JD, Gunarwardena WJ, Shaunak S, 2008. Leber’s hereditary optic neuropathy associated with multiple sclerosis: Harding’s syndrome. Pract. Neurol 8, 118–121. [DOI] [PubMed] [Google Scholar]
- Perry TL, Hansen S, Jones K, 1987. Brain glutamate deficiency in amyotrophic lateral sclerosis. Neurology 37, 1845–1848. [DOI] [PubMed] [Google Scholar]
- Pitt D, Werner P, Raine CS, 2000. Glutamate excitotoxicity in a model of multiple sclerosis. Nat. Med 6, 67–70. [DOI] [PubMed] [Google Scholar]
- Plaitakis A, Constantakakis E, Smith J, 1988. The neuroexcitotoxic amino acids glutamate and aspartate are altered in the spinal cord and brain in amyotrophic lateral sclerosis. Ann. Neurol 24, 446–449. [DOI] [PubMed] [Google Scholar]
- Polacek H, et al. , 2019. Increased glutamate and deep brain atrophy can predict the severity of multiple sclerosis. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub 163, 45–53. [DOI] [PubMed] [Google Scholar]
- Porpaczy Z, Sumegi B, Alkonyi I, 1987. Interaction between NAD-dependent isocitrate dehydrogenase, alpha-ketoglutarate dehydrogenase complex, and NADH: ubiquinone oxidoreductase. J. Biol. Chem 262, 9509–9514. [PubMed] [Google Scholar]
- Proia P, Di Liegro CM, Schiera G, Fricano A, Di Liegro I, 2016. Lactate as a metabolite and a regulator in the central nervous system. Int. J. Mol. Sci 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana A, Kruse SE, Kapur RP, Sanz E, Palmiter RD, 2010. Complex I deficiency due to loss of Ndufs4 in the brain results in progressive encephalopathy resembling Leigh syndrome. Proc. Natl. Acad. Sci. U. S. A 107, 10996–11001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riske L, Thomas RK, Baker GB, Dursun SM, 2017. Lactate in the brain: an update on its relevance to brain energy, neurons, glia and panic disorder. Ther. Adv. Psychopharmacol 7, 85–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roma A, et al. , 2022. Glutamine metabolism mediates sensitivity to respiratory complex II inhibition in acute myeloid leukemia. Mol. Cancer Res 20, 1659–1673. [DOI] [PubMed] [Google Scholar]
- Rosenblum LT, Trotti D, 2017. EAAT2 and the molecular signature of amyotrophic lateral sclerosis. Adv. Neurobiol 16, 117–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupsingh R, Borrie M, Smith M, Wells JL, Bartha R, 2011. Reduced hippocampal glutamate in Alzheimer disease. Neurobiol. Aging 32, 802–810. [DOI] [PubMed] [Google Scholar]
- Salek RM, et al. , 2010. A metabolomic study of the CRND8 transgenic mouse model of Alzheimer’s disease. Neurochem. Int 56, 937–947. [DOI] [PubMed] [Google Scholar]
- Sastre-Garriga J, et al. , 2005. Metabolite changes in normal-appearing gray and white matter are linked with disability in early primary progressive multiple sclerosis. Arch. Neurol 62, 569–573. [DOI] [PubMed] [Google Scholar]
- Schaefer AM, Walker M, Turnbull DM, Taylor RW, 2013. Endocrine disorders in mitochondrial disease. Mol. Cell. Endocrinol 379, 2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott HA, Gebhardt FM, Mitrovic AD, Vandenberg RJ, Dodd PR, 2011. Glutamate transporter variants reduce glutamate uptake in Alzheimer’s disease. Neurobiol. Aging 32, 553 e551–511. [DOI] [PubMed] [Google Scholar]
- Sinadinos C, et al. , 2014. Neuronal glycogen synthesis contributes to physiological aging. Aging Cell 13, 935–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeitink JA, 2003. Mitochondrial disorders: clinical presentation and diagnostic dilemmas. J. Inherit. Metab. Dis 26, 199–207. [DOI] [PubMed] [Google Scholar]
- Smeitink JA, Zeviani M, Turnbull DM, Jacobs HT, 2006. Mitochondrial medicine: a metabolic perspective on the pathology of oxidative phosphorylation disorders. Cell Metab. 3, 9–13. [DOI] [PubMed] [Google Scholar]
- Smith QR, Momma S, Aoyagi M, Rapoport SI, 1987. Kinetics of neutral amino acid transport across the blood-brain barrier. J. Neurochem 49, 1651–1658. [DOI] [PubMed] [Google Scholar]
- Souba WW, 1993. Glutamine and cancer. Ann. Surg 218, 715–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivey A, 2011. Rotenone and paraquat linked to Parkinson’s disease: human exposure study supports years of animal studies. Environ. Health Perspect 119, A259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreux-Varoquaux O, et al. , 2002. Glutamate levels in cerebrospinal fluid in amyotrophic lateral sclerosis: a reappraisal using a new HPLC method with coulometric detection in a large cohort of patients. J. Neurol. Sci 193, 73–78. [DOI] [PubMed] [Google Scholar]
- Steiner P, 2019. Brain fuel utilization in the developing brain. Ann. Nutr. Metab 75 (Suppl. 1), 8–18. [DOI] [PubMed] [Google Scholar]
- Stokes J, et al. , 2021. Mechanisms underlying neonate-specific metabolic effects of volatile anesthetics. Elife 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes JC, et al. , 2022. Leukocytes mediate disease pathogenesis in the Ndufs4(KO) mouse model of Leigh syndrome. JCI Insight 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan LB, et al. , 2015. Supporting aspartate biosynthesis is an essential function of respiration in proliferating cells. Cell 162, 552–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanberg KM, Landheer K, Pitt D, Juchem C, 2019. Quantifying the metabolic signature of multiple sclerosis by in vivo proton magnetic resonance spectroscopy: current challenges and future outlook in the translation from proton signal to diagnostic biomarker. Front. Neurol 10, 1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanberg KM, Kurada AV, Prinsen H, Juchem C, 2022. Multiple sclerosis diagnosis and phenotype identification by multivariate classification of in vivo frontal cortex metabolite profiles. Sci. Rep 12, 13888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Iizumi T, Mashima K, Abe T, Suzuki N, 2014. Roles and regulation of ketogenesis in cultured astroglia and neurons under hypoxia and hypoglycemia. ASN Neuro 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangamornsuksan W, et al. , 2019. Paraquat exposure and Parkinson’s disease: a systematic review and meta-analysis. Arch. Environ. Occup. Health 74, 225–238. [DOI] [PubMed] [Google Scholar]
- Tanner CM, et al. , 2011. Rotenone, paraquat, and Parkinson’s disease. Environ. Health Perspect 119, 866–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terburgh K, Coetzer J, Lindeque JZ, van der Westhuizen FH, Louw R, 2021. Aberrant BCAA and glutamate metabolism linked to regional neurodegeneration in a mouse model of Leigh syndrome. Biochim. Biophys. Acta Mol. basis Dis 1867, 166082. [DOI] [PubMed] [Google Scholar]
- Tisell A, et al. , 2013. Increased concentrations of glutamate and glutamine in normal-appearing white matter of patients with multiple sclerosis and normal MR imaging brain scans. PLoS One 8, e61817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Wang GJ, Volkow ND, 2013. Energetic cost of brain functional connectivity. Proc. Natl. Acad. Sci. U. S. A 110, 13642–13647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turalde CWR, Espiritu AI, Anlacan VMM, 2020. Memantine for multiple sclerosis: a systematic review and meta-analysis of randomized trials. Front. Neurol 11, 574748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Marum RJ, 2009. Update on the use of memantine in Alzheimer’s disease. Neuropsychiatr. Dis. Treat 5, 237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rheenen W, et al. , 2021. Common and rare variant association analyses in amyotrophic lateral sclerosis identify 15 risk loci with distinct genetic architectures and neuron-specific biology. Nat. Genet 53, 1636–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanle B, et al. , 2018. NMDA antagonists for treating the non-motor symptoms in Parkinson’s disease. Transl. Psychiatry 8, 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Reddy PH, 2017. Role of glutamate and NMDA receptors in Alzheimer’s disease. J. Alzheimers Dis 57, 1041–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Zhang D, 2005. Memantine prolongs survival in an amyotrophic lateral sclerosis mouse model. Eur. J. Neurosci 22, 2376–2380. [DOI] [PubMed] [Google Scholar]
- Wang H, et al. , 2019. Metabolic and oncogenic adaptations to pyruvate dehydrogenase inactivation in fibroblasts. J. Biol. Chem 294, 5466–5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise DR, et al. , 2011. Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of alpha-ketoglutarate to citrate to support cell growth and viability. Proc. Natl. Acad. Sci. U. S. A 108, 19611–19616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan D, Zhang Y, Liu L, Yan H, 2016. Pesticide exposure and risk of Alzheimer’s disease: a systematic review and meta-analysis. Sci. Rep 6, 32222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Venneti S, Nagrath D, 2017. Glutaminolysis: a hallmark of cancer metabolism. Annu. Rev. Biomed. Eng 19, 163–194. [DOI] [PubMed] [Google Scholar]
- Yang F, et al. , 2021a. Altered plasma metabolic profiles in Chinese patients with multiple sclerosis. Front. Immunol 12, 792711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WH, Qiu Y, Stamatatos O, Janowitz T, Lukey MJ, 2021b. Enhancing the efficacy of glutamine metabolism inhibitors in cancer therapy. Trends Cancer 7, 790–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao D, et al. , 2000. A novel system a isoform mediating Na+/neutral amino acid cotransport. J. Biol. Chem 275, 22790–22797. [DOI] [PubMed] [Google Scholar]
- Yeh YY, Sheehan PM, 1985. Preferential utilization of ketone bodies in the brain and lung of newborn rats. Fed. Proc 44, 2352–2358. [PubMed] [Google Scholar]
- Yeh YY, Streuli VL, Zee P, 1977. Ketone bodies serve as important precursors of brain lipids in the developing rat. Lipids 12, 957–964. [DOI] [PubMed] [Google Scholar]
- Zaragoza R, 2020. Transport of amino acids across the blood-brain barrier. Front. Physiol 11, 973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, et al. , 2019. Roles of glutamate receptors in Parkinson’s disease. Int. J. Mol. Sci 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolkipli-Cunningham Z, Falk MJ, 2017. Clinical effects of chemical exposures on mitochondrial function. Toxicology 391, 90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.


