SUMMARY
Objectives
Malignant minor salivary glands carcinomas (MiSGC) of the larynx and trachea are rare tumours and published evidence is sparse. We conducted a systematic review to describe shareable treatment strategies and oncological outcomes of these neoplastic entities.
Methods
Full text English manuscripts published from January 1st 2000 to December 14th 2022 were included. Data on demographics, treatments and outcomes were collected. A pooled analysis of 5-year overall survival (OS) was performed.
Results
Seventeen articles and 365 patients met the inclusion criteria. The most common subsites involved were subglottic and distal trachea. Adenoid cystic carcinoma was, by far, the most frequent histotype. The first-choice treatment strategy was surgery (86.8%), while adjuvant treatments were delivered in 57.4% of patients. Only 12.9% were treated with definitive radiotherapy with/without chemotherapy. The mean follow-up was 68.3 months. One hundred nine (34.9%) deaths were recorded and 62.4% were cancer-related. Five-year OS ranged from 20% to 100% and, at pooled analysis, it was 83% (range, 78-87%).
Conclusions
In case of MiSGC of the larynx and trachea, surgery remains the mainstay of treatment. Adjuvant treatments are frequently delivered. Survival estimates are good overall, but highly heterogeneous.
KEY WORDS: minor salivary gland tumors, larynx, trachea, treatment, survival
RIASSUNTO
Obiettivi
I tumori maligni delle ghiandole salivari minori laringo-tracheali sono rari e i dati pubblicati sono scarsi. Abbiamo condotto una revisione sistematica della letteratura per descrivere le strategie di trattamento e i risultati oncologici.
Metodi
Sono stati inclusi articoli in lingua inglese pubblicati dal 1° gennaio 2000 al 14 dicembre 2022. Abbiamo raccolto dati su trattamento e sopravvivenza ed è stata effettuata un’analisi aggregata della sopravvivenza complessiva (OS) a 5 anni.
Risultati
Sono stati inclusi 17 articoli e 365 pazienti. Le sottosedi più frequentemente coinvolte sono risultate la sottoglottide e la trachea distale, mentre il carcinoma adenoido-cistico si è confermato di gran lunga l’istotipo più frequente. La strategia di trattamento di prima scelta è stata la chirurgia (86,8%) e il 57,4% dei pazienti ha ricevuto un trattamento adiuvante. Solo il 12,9% è stato trattato con radioterapia o chemioradioterapia esclusive. Il follow-up medio è stato di 68,3 mesi. Centonove (34,9%) pazienti sono deceduti, di cui il 62,4% a causa della malattia. La sopravvivenza a 5 anni riportata varia dal 20% al 100% e nell’analisi aggregata è pari all’83% (range, 78-87%).
Conclusioni
Per i tumori delle ghiandole salivari minori laringo-tracheali la chirurgia è il trattamento di scelta, eventualmente associata a trattamenti adiuvanti. Le stime di sopravvivenza sono buone, ma altamente eterogenee.
PAROLE CHIAVE: tumori delle ghiandole salivari minori, laringe, trachea, trattamento, sopravvivenza
Introduction
The incidence of salivary glands carcinomas ranges from 4 to 135 cases per million per year: overall, 10-15% of cases arise in the minor salivary glands. Minor salivary glands cancers (MiSGC) are found mostly in the oral cavity and oropharynx, while the larynx and trachea are seldom involved 1. In particular, laryngeal MiSGC are extremely rare, accounting for less than 1% of all malignant tumors of this anatomical site 2. The most common histotypes are adenoid cystic (ACC, 32-69%) and mucoepidermoid carcinomas (MEC, 15-35%) 3. They often occur as submucosal masses found, in decreasing frequency, within the subglottis, supraglottis, and glottis. Likewise, the incidence of primary tracheal carcinoma is extremely low (0.1 to 0.26 per million), accounting for only 0.1-0.4% of all cancers 4,5. Primary tracheal ACC is the second most common malignant tracheal tumour after squamous cell carcinoma 6 and accounts for approximately 10-20% of all tracheal malignancies. Other salivary glands histotypes are exceedingly rare 6-8.
Oncologic treatment modalities have substantially improved in the last decades. However, the rarity of these cancers hinders the definition of a standard of care and survival analyses are flawed by the small size of published series, often represented by anecdotal experiences and case reports. Moreover, larger cohorts sometimes gather tumours from different sites without detailed data for the specific site(s) of origin, thus limiting the information available for laryngeal and tracheal MiSGC.
We carried out a systematic review on MiSGC of the larynx and trachea to describe treatment strategies and oncological outcomes.
Materials and methods
Objectives
The primary objective was definition of survival outcomes in patients affected by MiSGC of the larynx and trachea. Secondary objectives were description of applied treatment modalities.
Search strategy
This systematic review was performed and reported in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) checklist and statement recommendations 9. A comprehensive search on PubMed, Web of Science, and Scopus was conducted on December 14th 2022, using the following queries: “(Minor salivary gland* OR salivary) AND (tumor* OR neoplasm* OR malignancy OR malignancies OR cancer* OR neoplasia*) AND (Larynx OR laryngeal OR Trachea OR Tracheal OR laryngo-tracheal)”.
Selection criteria
Only original papers in English, published in a peer-reviewed journal from January 1st, 2000 to December 14th, 2022, were considered. Exclusion criteria were: a) impossibility to extrapolate specific data on treatments and survival of patients with MiSGC of the larynx or trachea from composite series; b) pediatric cohorts; c) series with less than or equal to 5 patients; d) non-human studies.
Two authors (CM and EM) independently screened all titles and abstracts, and full-texts were obtained for publications that fulfilled inclusion criteria. The references of all selected articles were reviewed to identify additional relevant articles. Disagreements were solved by discussion with a senior author (DM).
Data extraction
The relevant features of each study included (publication year, country, study design, and period of observation) were collected along with demographics (age at presentation, gender), pre-operative imaging, tumor features (site and subsite, histotype, TNM classification, perineural invasion [PNI], lymphovascular invasion [LVI], grading, and margins status), treatment modality and therapeutic intent (curative vs palliative), post-operative complications, mean follow-up, deaths, recurrence rate (local, regional or distant), 5- and 10-year overall survival (OS), disease free survival (DFS), local (LRFS), and distant recurrence free survival (DRFS). Two authors (CM and EM) independently extracted data from eligible articles and created a dedicated database. Data analysis was performed with Microsoft Excel. A pooled analysis on 5-year OS retrieved from the selected articles was performed to obtain a general estimate of this outcome.
Results
General features
The initial search returned 3468 articles. Identification, screening, and inclusions/exclusions are detailed in Figure 1. A total of 17 articles, all based on retrospective series, with a total of 365 patients, were included in the present systematic review (Tab. I).
Figure 1.
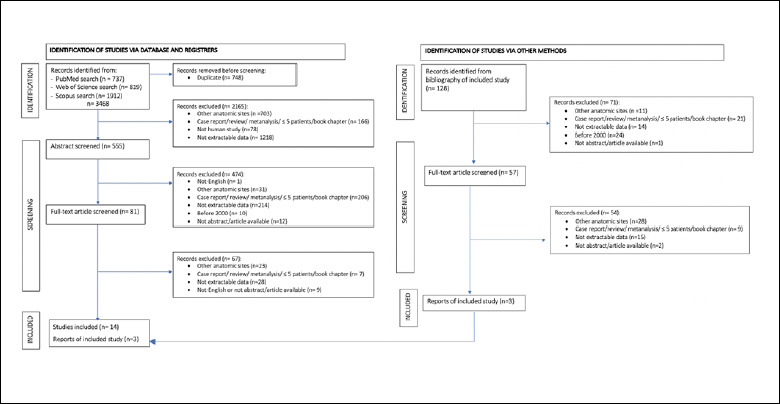
Identification, screening, and exclusion/inclusion of publications in the present systematic review.
Table I.
General features of the 17 articles included in the systematic review.
| First author | Publication year | Country | Study design | Observational period | No. of patients | Site |
|---|---|---|---|---|---|---|
| Mahlstedt 19 | 2002 | Germany | Retrospective | 1965-1998 | 15 | Larynx |
| Albers 42 | 2004 | Usa | Retrospective | 1992-2001 | 8 | Trachea |
| Wang 18 | 2006 | Taiwan | Retrospective | 1981-2000 | 11 | Larynx |
| Ganly 3 | 2006 | Usa | Retrospective | 1970-2003 | 12 | Larynx |
| Moukarbel 17 | 2008 | Canada | Retrospective | 1963-2005 | 15 | Larynx |
| Lucioni 14 | 2008 | Italy | Retrospective | 1990-2003 | 6 | Larynx |
| Wurtz 12 | 2010 | France | Retrospective | 2005-2007 | 6 | Trachea |
| Nielsen 11 | 2012 | Denmark | Retrospective | 1990-2007 | 6 | Larynx |
| Zhang 2 | 2014 | China | Retrospective | 2003-2010 | 15 | Larynx |
| Liu 15 | 2015 | China | Retrospective | 1998-2013 | 6 | Larynx |
| Chen 22 | 2015 | China | Retrospective | 1995-2012 | 52 | Trachea |
| Yang 20 | 2016 | China | Retrospective | 1995-2014 | 109 | Trachea |
| Je 21 | 2017 | South Korea | Retrospective | 1994-2008 | 22 | Trachea |
| Akbaba 16 | 2019 | Germany | Retrospective | 2002-2009 | 11 | Larynx |
| Högerle 13 | 2019 | Germany | Retrospective | 1991-2017 | 38 | Trachea |
| Chen 23 | 2020 | China-Usa | Retrospective | 2003-2014 | 16 | Larynx |
| Lionello 10 | 2021 | Italy | Retrospective | 1989-2020 | 17 | Larynx |
Demographics and tumour characteristics
Demographics and tumour features are outlined in Table II. Demographic characteristics were reported for all 365 patients. Mean age was 51.9 years (46.5 for tracheal and 53.6 for laryngeal localisation). The distribution was almost equal between genders. The most commonly involved site was the trachea with a trachea to larynx ratio of 1.9:1 (235 tracheal and 126 laryngeal MiSGC). Concerning the trachea, excluding 14% of patients with no specific subsite reported, the most common anatomical region of origin was distal/carinal trachea (23%), followed by middle (16.4%), and cervical trachea (10.9%). In contrast, the most common laryngeal subsite was the subglottis (14.9%), followed by supraglottis (13.4%), and glottis (4.1%). Only 12 cases (3.3%) had a transglottic localisation. Data on pre-operative imaging was reported for 204 cases and all underwent a CT scan.
Table II.
Demographics and tumour features.
| Characteristics | Entire series | Larynx | Tracheal |
|---|---|---|---|
| Patients, no. | 365 | 130 | 235 |
| Age, years (mean) | 51.9 | 53.6 | 46.5 |
| Gender, no. | |||
| Male | 183 | 75 | 108 |
| Female | 176 | 49 | 127 |
| Anatomic site, no. | |||
| Trachea | 235 | - | 235 |
| Larynx | 126 | 126 | - |
| Subsites, no. | |||
| Supraglottis | 49 | 49 | - |
| Glottis | 15 | 15 | - |
| Subglottis | 54 | 54 | - |
| Transglottis | 12 | 12 | - |
| Trachea NOS | 51 | - | 51 |
| Cervical trachea | 40 | - | 40 |
| Middle trachea | 60 | - | 60 |
| Distal/carinal trachea | 84 | - | 84 |
| Histotypes, no. | |||
| ACC | 324 | 90 | 234 |
| MEC | 24 | 23 | 1 |
| Adenocarcinoma | 12 | 12 | - |
| Adenosquamous carcinoma | 3 | 3 | - |
| Myoepithelial | 2 | 2 | - |
| pTNM, (no. of pts. with available data) | |||
| T3-T4 | 51(86) | 28 | 23 |
| N+ | 16(85) | 16 | 0 |
| M0 | 131(142) | 92 | 39 |
| M1 | 11(142) | 4 | 7 |
| PNI-LVI, (no. of pts. surgically treated with available data) | |||
| PNI+ | 47(82) | 35 | 11 |
| LVI+ | 13(33) | 13 | - |
| Margins, (no. of pts. surgically treated with available data) | |||
| R0 | 76(273) | 34 | 42 |
| R0 close | 9(273) | 7 | 2 |
| R1 | 187(273) | 36 | 151 |
| R2 | 1(273) | 0 | 1 |
| NOS: not otherwise specified; ACC: adenoid cystic carcinoma; MEC: mucoepidermoid carcinoma; PNI: perineural invasion; LVI: lymphovascular invasion. | |||
The most common histotype was ACC (88.9%), followed by MEC (6.6%), with few cases of adenocarcinoma (AC, 3.2%), adenosquamous carcinoma (ASCC, 0.8%), and myoepithelial carcinoma (MC, 0.5%). The larynx showed a higher degree of histologic variability: 90 ACC (69.2% of all laryngeal cases), 23 MEC (18.2%), 12 AC (9.3%), 3 ASCC (2.3%), and 2 MC (1%) were reported. Conversely, tracheal localisation was dominated by ACC (99.5% of all tracheal cases) with only 1 MEC (0.5%). The TNM classification was reported in a few articles 2,3,10-19; in most cases tumours were advanced, while nodal involvement was rare. PNI was observed in 57.3% of patients and LVI was present in 39.4% (all had laryngeal MiSGC). Tumour grading was rarely reported. Resection margin status was detailed for 273 patients surgically treated and microscopically positive (R1) in the majority (68.5%). Only 0.4% had macroscopic positive margins (R2).
Treatment
Treatment modalities were described in all studies and are detailed in Table III. Surgery was the most common choice. Three-hundred seventeen patients (64.4% tracheal and 35.6% laryngeal MiSGC) were treated by different surgical procedures according to the tumour site and histotype. For laryngeal malignancies, 59.2% underwent total laryngectomy, 31.9% open partial horizontal laryngectomies or other forms of partial laryngectomies, and 8% laryngo-tracheal resections and anastomoses, while only 0.9% underwent endoscopic resections. When the distal trachea was involved, tracheal resection was associated with pulmonary resection or lobectomy in 22 and 1 patient, respectively. Only one study 12 reported on 6 patients treated by tracheal and carinal replacement with aortic allografts.
Table III.
Treatment strategies in the reported series of patients.
| First author | No. of patients | Site | Surgery | PL | TL | LTRA | ELR | TS | TS + lobectomy | TS + pulmonary resection | Adjuvant therapy |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Liu 15 | 6 | Larynx | 6 | 2 | 4 | 0 | 0 | 0 | 0 | 0 | 6 RT |
| Chen 23 | 16 | Larynx | 15 | 4 | 11 | 0 | 0 | 0 | 0 | 0 | 4 RT, 1 CRT |
| Lionello 10 | 17 | Larynx | 17 | 9 | 3 | 5 | 0 | 0 | 0 | 0 | 3 CRT |
| Zhang 2 | 15 | Larynx | 13 | 4 | 9 | 0 | 0 | 0 | 0 | 0 | 6 RT |
| Nielsen 11 | 6 | Larynx | 4 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 2 RT |
| Akbaba 16 | 11 | Larynx | 6 | 3 | 3 | 0 | 0 | 0 | 0 | 0 | 6 RT |
| Lucioni 14 | 6 | Larynx | 6 | 3 | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| Moukarbel 17 | 15 | Larynx | 12 | 1 | 8 | 2 | 1 | 0 | 0 | 0 | 8 RT |
| Wang 18 | 11 | Larynx | 9 | 2 | 7 | 0 | 0 | 0 | 0 | 0 | 3 RT, 1 CRT |
| Mahlstedt 19 | 15 | Larynx | 15 | 2 | 13 | 0 | 0 | 0 | 0 | 0 | 5 RT |
| Ganly 3 | 12 | Larynx | 10 | 2 | 6 | 2 | 0 | 0 | 0 | 0 | 6 RT |
| Yang 20 | 109 | Trachea | 109 | 0 | 0 | 5 | 0 | 100 | 0 | 4 | 73 RT |
| Chen 22 | 52 | Trachea | 48 | 0 | 0 | 5 | 0 | 30 | 0 | 13 | 12 RT, 12 CRT |
| Wurtz 12 | 6 | Trachea | 6 | 0 | 0 | 1 | 0 | 6 | 1 | 1 | 0 |
| Je 21 | 22 | Trachea | 13 | 0 | 0 | 0 | 0 | 8 | 0 | 4 | 13 RT |
| Högerle 13 | 38 | Trachea | 20 | 0 | 0 | - | 0 | - | - | - | 1 CIRT, 12 RT |
| Albers 42 | 8 | Trachea | 8 | 0 | 0 | 0 | 0 | 8 | 0 | 0 | 8 RT |
| PL: partial laryngectomy; TL: total laryngectomy; LTRA: laryngo-tracheal resection and anastomosis; ELR: endoscopic laryngeal resection; TS: tracheal surgery; RT: radiotherapy; CRT: chemoradiotherapy; CIRT: carbon-ion radiotherapy. | |||||||||||
Neck dissection was rarely performed (9.1% of patients), and all had laryngeal malignancies. Out of 29 patients, only for 10 and 2 patients the therapeutic or elective role of neck dissection was respectively specified. Analysing patients who underwent surgery, 52% (119 tracheal and 46 laryngeal tumours) were managed by adjuvant radiotherapy (RT), and only 5.4% (12 tracheal and 5 laryngeal) by adjuvant chemo RT (CRT): in almost all cases photons were delivered, while only one patient was treated with carbon-ion RT (CIRT). Neoadjuvant therapy was never performed. Only 12.3% of patients were treated by exclusive RT and 2 lesions (0.5%), one tracheal and one laryngeal, with concurrent CRT. Among these, only 6 tracheal MiSGC were treated with CIRT, and the others with conventional RT.
Post-operative complications were described for 176 patients treated with surgery (55.5% of all patients treated with surgery) with tracheal MiSGC 12,20-22 and included 25.9% of non-fatal complications and one death for anastomotic problems (Tab. IV). No perioperative death was described for laryngeal MiSGC.
Table IV.
Post-operative complication.
| Author | No. surgical patients | Site | Post-operative complication | ||
|---|---|---|---|---|---|
| Death | Yes | Non-fatal complications | |||
| Yang 20 | 109 | Trachea | 1 | 24 | Unilateral recurrent laryngeal nerve palsy (9), anastomotic stenosis (4), dyspnoea (2), granulation stenosis (2), permanent tracheostomy (2), pneumonia (2), emphysema (1), tracheoesophageal fistula (1), incision infection (1) |
| Chen 22 | 48 | Trachea | 0 | 5 | Drinking cough (2), laryngeal nerve paralysis (1), tracheal stenosis (2) |
| Wurtz 12 | 6 | Trachea | 0 | 5 | Dehiscence anastomosis + sternoplasty for incomplete separation of the sternum (1), pneumonia + tracheotomy and ventilatory support (1), necrosis of the graft (treated with transplantation) + tracheotomy and ventilatory support + oesophageal fistula (1), anterior spinal cord ischaemia + purulent stent retention (required additional bronchial stenting) (1), sternal dehiscence (treated with sternoplasty) + ventilatory support (1) |
| Je 21 | 13 | Trachea | 0 | 14 | Tracheal stenosis (2), oesophagitis (3), pulmonary fibrosis (9) |
Follow-up and outcomes
The follow-up time was available for 320 patients (mean, 68.3 months; range, 34-123). Patient status was obtainable in 312 cases. One-hundred nine (34.9%) patients died, and the most frequent cause of death was disease-related (62.4%) due to loco-regional recurrence or distant metastasis. Laryngeal localisation was associated with a slightly higher rate of cancer-related death (52.9% vs 47%). During follow-up, the recurrence rate was 49.5%: local recurrence occurred in 25.7% of patients (40 with laryngeal and 16 with tracheal localisation), and distant metastasis in 23.8% (33 with laryngeal and 19 with tracheal tumours).
Survival estimates in terms of 5- and 10-year OS, DFS, LRFS, RRFS, and DRFS were frequently unavailable. The available information is reported in Table IV. Based on the data collected 2,13,16-21,23, 5-year OS ranged from 20% to 100%. Good survival rates were described by Akbaba 16 for laryngeal MiSGC (5-year OS 100%) and by Högerle 13 for the trachea (5-year OS 95%). The worse 5-year OS (20%) was described by Zhang 2 reporting on laryngeal MiSGC.
Due to the scarcity of available data in the published literature, we aimed to obtain an estimate of 5-year OS by performing a pooled statistical analysis. The average 5-year OS for laryngeal and tracheal MiSGC was 83%. The 95% confidence interval for the 5-year OS was calculated using a weighted mean based on the inverse of the standard errors derived from individual study proportions, resulting in an estimated range of 78-87%.
Five-year DFS was reported in three laryngeal 16-18 and two tracheal series 20,21, and ranged from 69% to 100% for the larynx, and from 47.8% to 62% for the trachea. The 5-year LRFS was 100% for the larynx 15 and 96% for the trachea 13. Five-year DRFS was reported only for tracheal MiSGC 13,21, and ranged from 52.6% to 69%.
Ten-year oncological outcomes were available in fewer studies 13,17,18,20,21. Ten-year OS ranged from 43.2% 20 to 81% 13 for the trachea, and from 46% 17 to 83% 18 for laryngeal MiSGC, while 10-year DFS 20,21 was 20-28.7%. A 10-year LRFS of 83% was reported by Högerle 13.
The same authors 13,20,21 reported specific oncologic outcomes for patients with tracheal MiSGC treated with surgery and adjuvant RT. Outcomes are detailed in Table V.
Table V.
Oncologic outcomes.
| First author | No. of patients | Site | Histotype | All treated patients | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-year | 10-year | |||||||||||||
| ACC | MEC | AC | ASCC | OS | DFS | LRFS | DRFS | OS | DFS | LRFS | DRFS | |||
| Yang 20 | 109 | Trachea | 109 | 0 | 0 | 0 | 88.7 | 62 | - | - | 43.2 | 20 | - | - |
| Je 21 | 22 | Trachea | 22 | 0 | 0 | 0 | 81.8 | 47.8 | 100 | 52.6 | 54.2 | 28.7 | 70.2 | 33.5 |
| Högerle 13 | 38 | Trachea | 38 | 0 | 0 | 0 | 95 | - | 96 | 69 | 81 | - | 83 | 53 |
| Liu 15 | 6 | Larynx | 6 | 0 | 0 | 0 | - | - | 100 | - | - | - | - | - |
| Chen 23 | 16 | Larynx | 8 | 4 | 2 | 2 | 60 | - | - | - | - | - | - | - |
| Zhang 2 | 15 | Larynx | 6 | 6 | 3 | 0 | 20 | - | - | - | - | - | - | - |
| Akbaba 16 | 11 | Larynx | 11 | 0 | 0 | 0 | 100 | 100 | 100 | - | - | - | - | - |
| Moukarbel 17 | 15 | Larynx | 15 | 0 | 0 | 0 | 64 | 69 | - | - | 49 | - | - | - |
| Wang 18 | 11 | Larynx | 4 | 3 | 3 | 1 | 71 | 83 | - | - | 63 | - | - | - |
| Mahlstedt 19 | 15 | Larynx | 6 | 5 | 4 | 0 | 47 | - | - | - | - | - | - | - |
| Surgery + RT | ||||||||||||||
| 5-year | 10-year | |||||||||||||
| OS | DFS | LRFS | DRFS | OS | DFS | LRFS | DRFS | |||||||
| Yang 20 | 109 | Trachea | 109 | 0 | 0 | 0 | 88.4 | 67.8 | - | - | 37.6 | 14 | - | 35 |
| Je 21 | 22 | Trachea | 22 | 0 | 0 | 0 | 92.3 | 67.7 | 100 | 67.7 | 76.9 | 42.3 | 70.2 | 42.3 |
| Högerle 13 | 38 | Trachea | 38 | 0 | 0 | 0 | 84 | - | 100 | 65 | 84 | - | 100 | 65 |
| Liu 15 | 6 | Larynx | 6 | 0 | 0 | 0 | - | - | 100 | - | - | - | - | - |
| ACC: adenoid cystic carcinoma; MEC: mucoepidermoid carcinoma; AC: adenocarcinoma; ASCC: adenosquamous cell carcinoma; OS: overall survival; DFS: disease free survival; LRFS: local recurrence free survival; DRFS, distant recurrence free survival; RT: radiotherapy. | ||||||||||||||
Discussion
Due to the rarity of laryngeal and tracheal MiSGC, published evidence is sparse and of low quality, being mostly represented by retrospective relatively small cohort studies or even case reports. Accordingly, data on treatment and oncological outcomes are highly biased by the characteristics of the series analysed, and therefore poorly generalisable. In this systematic review we aimed to provide a general picture of laryngeal and tracheal MiSGC, with particular focus on treatment strategies and oncological outcomes.
Patients and tumour features
Our review included 365 patients with an equal distribution between genders and a peak at diagnosis in the adult age. A tracheal site was by far more frequent than a laryngeal one, and distal/carinal trachea and subglottis were the most involved subsites, respectively. The diagnostic delay of these lesions is mirrored by the relevant prevalence of advanced tumours (T3-T4) and possibly explained by the indolent and slow growth that often characterises MiSGC. Accordingly, symptoms such as dyspnoea are highly non-specific and arise so gradually that the patient tends to overlook them until they are extremely severe, while even specialists may initially misdiagnose such a clinical scenario as asthma.
Radiological work-up mostly included CT scan due to its ability to assess the primary tumour location, extra-luminal extension, and regional/distant metastasis 24. Moreover, image acquisition is quicker, a pro that is extremely relevant in case of pending airway problems, especially in critical conditions or in presence of a tracheostomy which greatly hampers MRI performance. Therefore, different from other locations such as the sinonasal region, MRI can be considered a second-choice examination, required only in case of specific tumour extensions and clinically-relevant issues (demonstration of cartilage involvement, perineural spread or extra-airway soft tissues invasion, e.g. into digestive tracts).
From a histological standpoint, apart from very few cases of MEC, rare laryngeal AC, ASCC and MC, ACC almost represents the totality of the series (88.9%): of note, this trend is more pronounced than in other head and neck sites (e.g., within the sinonasal region ACC represents about only two thirds of all MiSGC) 25. This predominance highly impacted the delayed presentation of laryngo-tracheal MiSGC (due to the indolent and slow growth typical of ACC), surgical outcomes (high frequency of R1 margins), pattern of tumour spread (high rate of PNI), and modes of recurrence (both local and distant).
Treatment
In our systematic review, the most common treatment applied was surgery, with or without post-operative RT. In fact, 86.4% of patients were treated with surgery, and different procedures based on the site and level of invasiveness were described: from total or partial laryngectomies to laryngo-tracheal resection and anastomosis. Sometimes tracheal surgery was associated with pulmonary resections or lobectomy. Only one study 12 reported 6 cases of tracheal and carinal replacement with aortic allografts. Conservative laryngeal surgical techniques were less often performed as first-choice treatment (36 cases compared to 67 total laryngectomies chosen especially for high-grade MiSGC).
Cumulative rate of positive margins (almost all microscopic, R1) is high (68%), and in line with the vast prevalence of ACC and its well-known tendency to develop PNI travelling at distance from macroscopic site of origin of the tumour itself. However, it is noteworthy that its distribution according to the site of origin is highly asymmetric, with a relevant prevalence of R1 in the tracheal cohort (55% vs 13%).
The relatively low rate of positive margins after laryngeal surgery is intriguing in view of the non-marginal rate of conservative surgery (about one of three cases). Moreover, this value is far lower than those reported for sinonasal ACCs, ranging from 60.6% to 65% 26. This discrepancy can be explained by the anatomical complexity of the sinonasal district, which limits wide surgical excisions and offers various nervous and vascular bundles to tumour microscopic escape. In contrast, the laryngeal box is a fairly well-confined anatomical compartment, with relatively few (and involved late) possible avenues for extra-laryngeal diffusion.
The choice of RT alone or CRT without surgery is rare and often reserved to patients with unresectable tumours or distant metastasis at diagnosis. The role of adjuvant RT has been debated in the literature over the past few years: adverse prognostic factors, such as positive or close surgical margins 27-30, high-grade malignant histology 27,30,31, PNI 30,32-34, and high T-N categories 27,35 are all indications for such a complementary treatment.
According to our data, about half of patients who underwent surgery received adjuvant RT (rarely CRT), due to the high rate of R1 and pT3-T4 lesions. Conventional photon therapy was mostly used, with few patients treated with CIRT, probably due to the relatively recent introduction of this treatment modality and its reduced overall availability in many countries, compared to conventional photon RT. Moreover, sound data about the real effectiveness of CIRT as adjuvant therapy for laryngeal and tracheal MiSGC are still not available in the literature, and the risks of subsequent stenosis or chondronecrosis at these sites are definitely not negligible. Instead, several studies reported promising results with proton beam therapy as adjuvant treatment for nasopharyngeal ACC compared to photon RT 36 and in association to conventional RT for oral cavity ACC, with good local control and relatively low toxicity 37.
Post-operative complication rates reported in these studies were generally low, but it should be noted that they mainly refer to tracheal procedures. Unfortunately, no detailed data about laryngeal surgery were provided. However, treatment-related mortality was anecdotal.
Chemotherapy is mainly restricted to metastatic or relapsing disease with limited success rate 38. In our systematic review, few patients were treated with chemotherapy, but today new therapeutic strategies are taken in consideration, due to the evolving understanding of biology of salivary glands tumours. A precise diagnosis and knowledge of tumour mutations may lead to development of new treatments and personalised medicine approaches. Indeed, on the top of traditional diagnostic tools, such as immunohistochemistry and fluorescence in situ hybridisation, the next-generation sequencing approach has been successfully applied in recent years to extend and refine our insights on salivary glands tumours 39, carrying to the identification of previously unknown fusion genes, potentially usable as diagnostic tools as well as novel targets for future therapeutic developments. For example, MEC is associated to high level of EGFR expression with poor prognosis and cetuximab has been used as a complementary therapy in low-grade tumours or as single therapy in metastatic or recurrent MEC 40. ACC frequently presents MYB altered pathway and molecular inhibitors are currently under development; in particular, multiple kinase inhibitors showed a promising response in advanced ACC 40,41.
Oncological outcomes
Mean follow-up was 68.3 months. In view of the notorious tendency of ACC to recur at 10, 15 or even 20 years after primary treatment, follow-up should be regarded as limited and prompt the reader to cautiously interpret the survival outcomes reported herein.
In this regard, 62.4% of all deaths were caused by the disease, and laryngeal localisation was associated with a higher rate of cancer-related death. The pattern of recurrence was typical of ACC, with a prevalence of local and distant sites. Five-year OS was worse for laryngeal MiSGC (Tab. IV), while 5-year OS collected for tracheal cases ranged between 81.8% to 95%. On the other hand, 5-year DFS, described in only 5 studies (3 laryngeal 16-18 and 2 tracheal 20,21 series), was worse for tracheal malignancies (62% and 47.8% for the trachea compared to 100%, 83%, and 69% for the larynx). This finding is in line with the different rates of positive margins at these different sites.
We also collected data on specific oncologic outcomes for patients with tracheal MiSGC treated with multimodal therapy (surgery and adjuvant RT). While 5-year oncological outcomes are quite similar when considering all patients versus those treated with surgery and RT, 10-year oncological outcomes improved with the use of multimodal therapy.
Based on the data collected, surgery associated with RT had a better impact on outcomes, mainly in terms of 5- and 10-year LRFS and DRFS, and adjuvant RT may have also helped in delaying recurrence. Due to the limited data available, in order to obtain an estimate of 5-year OS, we performed a pooled analysis yielding a value of 83% (ranging from 78% to 87%). The same statistical analysis was not applicable to the other outcomes due to the lack of precise data.
Limitations
The present systematic review has some limitations. Considering all articles included, due to the rarity of these malignancies and the considerable span of time in which these series were frequently collected, we were not able to find complete oncologic outcomes and, because ACC was the most common represented histotype, most of the results were referred to this tumour. The pooled analysis was appliable only to 5-year OS for which we collected more data while, for the other outcomes, for which very few details were available, it could not be applied due to poor reliability.
Conclusions
MiSGC of the larynx and trachea are very rare tumours, often presenting as locally advanced and invasive diseases. To date there are no guidelines about their treatment. Surgery followed by adjuvant (C)RT is the most common first-choice treatment. However, few studies are available in the literature and with a small number of patients or with insufficiently long follow-up. The literature shows that distant metastases may occur up to more than 5 years after treatment. Multicentric prospective studies with long-term follow-up are required to better define treatment strategies and possibly improve oncological outcomes.
Conflict of interest statement
The authors declare no conflict of interest.
Author contributions
CM, DM, CP: study design, article selection, review drafting and critical revision; CM, EM: article search and selection, data extraction; AP: pooled analysis and critical revision; DL, MT, VR: critical revision of the article.
Ethical consideration
No formal ethics committee approval was required for this article as it is based on already published clinical data from other studies available in the literature.
Figures and tables
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Vander Poorten V, Hunt J, Bradley PJ, et al. Recent trends in the management of minor salivary gland carcinoma. Head Neck 2014;36:444-455. https://doi.org/10.1002/hed.23249 10.1002/hed.23249 [DOI] [PubMed] [Google Scholar]
- 2.Zhang M, Li KN, Li C, et al. Malignant minor salivary gland carcinomas of the larynx. ORL J Otorhinolaryngol Relat Spec 2014;76:222-226. https://doi.org/10.1159/000368322 10.1159/000368322 [DOI] [PubMed] [Google Scholar]
- 3.Ganly I, Patel SG, Coleman M, et al. Malignant minor salivary gland tumors of the larynx. Arch Otolaryngol Head Neck Surg 2006;132:767-770. https://doi.org/10.1001/archotol.132.7.767 10.1001/archotol.132.7.767 [DOI] [PubMed] [Google Scholar]
- 4.Kumar NS, Iype EM, Thomas S, et al. Adenoid cystic carcinoma of the trachea. Indian J Surg Oncol 2016;7:62-66. https://doi.org/10.1007/s13193-015-0453-5 10.1007/s13193-015-0453-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Honings J, van Dijck JAAM, Verhagen AFTM, et al. Incidence and treatment of tracheal cancer: a nationwide study in the Netherlands. Ann Surg Oncol 2007;14:968-976. https://doi.org/10.1245/s10434-006-9229-z 10.1245/s10434-006-9229-z [DOI] [PubMed] [Google Scholar]
- 6.Ning Y, He W, Bian D, et al. Tracheo-bronchial adenoid cystic carcinoma: a retrospective study. Asia Pac J Clin Oncol 2019;15:244-249. https://doi.org/10.1111/ajco.13162 10.1111/ajco.13162 [DOI] [PubMed] [Google Scholar]
- 7.Huo Z, Wu H, Li S, et al. Molecular genetic studies on EGFR, KRAS, BRAF, ALK, PIK3CA, PDGFRA, and DDR2 in primary pulmonary adenoid cystic carcinoma. Diagn Pathol 2015;10:161. https://doi.org/10.1186/s13000-015-0409-7 10.1186/s13000-015-0409-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan WL, Lee VHF, Siu SWK, et al. Inoperable adenoid cystic carcinoma of trachea: complete remission after multi-modality treatment. Hong Kong J Radiol 2014;17:203-207. https://doi.org/10.12809/hkjr1413202 10.12809/hkjr1413202 [DOI] [Google Scholar]
- 9.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. https://doi.org/10.1136/bmj.b2700 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lionello M, Canal F, Presotto F, et al. Laryngeal adenoid cystic carcinoma: radical or conservative surgery? Am J Otolaryngol 2021;42:102974. https://doi.org/10.1016/j.amjoto.2021.102974 10.1016/j.amjoto.2021.102974 [DOI] [PubMed] [Google Scholar]
- 11.Nielsen TK, Bjørndal K, Krogdahl A, et al. Salivary gland carcinomas of the larynx: a national study in Denmark. Auris Nasus Larynx 2012;39:611-614. https://doi.org/10.1016/j.anl.2012.02.003 10.1016/j.anl.2012.02.003 [DOI] [PubMed] [Google Scholar]
- 12.Wurtz A, Porte H, Conti M, et al. Surgical technique and results of tracheal and carinal replacement with aortic allografts for salivary gland-type carcinoma. J Thorac Cardiovasc Surg 2010;140:387-393.e2. https://doi.org/10.1016/j.jtcvs.2010.01.043 10.1016/j.jtcvs.2010.01.043 [DOI] [PubMed] [Google Scholar]
- 13.Högerle BA, Lasitschka F, Muley T, et al. Primary adenoid cystic carcinoma of the trachea: clinical outcome of 38 patients after interdisciplinary treatment in a single institution. Radiat Oncol 2019;14:117. https://doi.org/10.1186/s13014-019-1323-z 10.1186/s13014-019-1323-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lucioni M, Marioni G, Libera DD, et al. Treatment of unusual or rare laryngeal nonsquamous primary malignancies: radical (total/extended total laryngectomy) or conservative surgery? Am J Otolaryngol 2008;29:106-112. https://doi.org/10.1016/j.amjoto.2007.02.007 10.1016/j.amjoto.2007.02.007 [DOI] [PubMed] [Google Scholar]
- 15.Liu W, Chen X. Adenoid cystic carcinoma of the larynx: a report of six cases with review of the literature. Acta Otolaryngol 2015;135:489-493. https://doi.org/10.3109/00016489.2014.990583 10.3109/00016489.2014.990583 [DOI] [PubMed] [Google Scholar]
- 16.Akbaba S, Lang K, Bulut OC, et al. The role of organ- and function-preserving radiotherapy in the treatment of adenoid cystic carcinoma of the larynx. Head Neck 2019;41:2208-2214. https://doi.org/10.1002/hed.25678 10.1002/hed.25678 [DOI] [PubMed] [Google Scholar]
- 17.Moukarbel RV, Goldstein DP, O’Sullivan B, et al. Adenoid cystic carcinoma of the larynx: a 40-year experience. Head Neck 2008;30:919-924. https://doi.org/10.1002/hed.20802 10.1002/hed.20802 [DOI] [PubMed] [Google Scholar]
- 18.Wang MC, Liu CY, Li WY, et al. Salivary gland carcinoma of the larynx. J Chin Med Assoc 2006;69:322-325. https://doi.org/10.1016/S1726-4901(09)70266-7 10.1016/S1726-4901(09)70266-7 [DOI] [PubMed] [Google Scholar]
- 19.Mahlstedt K, Ussmüller J, Donath K. Malignant sialogenic tumours of the larynx. J Laryngol Otol 2002;116:119-122. https://doi.org/10.1258/0022215021910078 10.1258/0022215021910078 [DOI] [PubMed] [Google Scholar]
- 20.Yang H, Yao F, Tantai J, et al. Resected tracheal adenoid cystic carcinoma: improvements in outcome at a single institution. Ann Thorac Surg 2016;101:294-300. https://doi.org/10.1016/j.athoracsur.2015.06.073 10.1016/j.athoracsur.2015.06.073 [DOI] [PubMed] [Google Scholar]
- 21.Je HU, Song SY, Kim DK, et al. A 10-year clinical outcome of radiotherapy as an adjuvant or definitive treatment for primary tracheal adenoid cystic carcinoma. Radiat Oncol 2017;12:196. https://doi.org/10.1186/s13014-017-0933-6 10.1186/s13014-017-0933-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen F, Huang M, Xu Y, et al. Primary tracheal adenoid cystic carcinoma: adjuvant treatment outcome. Int J Clin Oncol 2015;20:686-692. https://doi.org/10.1007/s10147-014-0771-6 10.1007/s10147-014-0771-6 [DOI] [PubMed] [Google Scholar]
- 23.Chen L, Deng W, Li C, et al. Clinical outcome and comparison between squamous and non-squamous cell carcinoma of the larynx. Acta Otolaryngol 2020;140:195-201. https://doi.org/10.1080/00016489.2019.1700305 10.1080/00016489.2019.1700305 [DOI] [PubMed] [Google Scholar]
- 24.Zvrko E, Golubović M. Laryngeal adenoid cystic carcinoma. Acta Otorhinolaryngol Ital 2009;29:279-282. [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrari M, Mattavelli D, Tomasoni M, et al. The MUSES*: a prognostic study on 1360 patients with sinonasal cancer undergoing endoscopic surgery-based treatment: *MUlti-institutional collaborative study on endoscopically treated sinonasal cancers. Eur J Cancer 2022;171:161-182. https://doi.org/10.1016/j.ejca.2022.05.010 10.1016/j.ejca.2022.05.010 [DOI] [PubMed] [Google Scholar]
- 26.Michel G, Joubert M, Delemazure AS, et al. Adenoid cystic carcinoma of the paranasal sinuses: retrospective series and review of the literature. Eur Ann Otorhinolaryngol Head Neck Dis 2013;130:257-262. https://doi.org/10.1016/j.anorl.2012.09.010 10.1016/j.anorl.2012.09.010 [DOI] [PubMed] [Google Scholar]
- 27.Garden AS, Weber RS, Ang KK, et al. Post-operative radiation therapy for malignant tumors of minor salivary glands. Outcome and patterns of failure. Cancer 1994;73:2563-2569. https://doi.org/10.1002/1097-0142(19940515)73:10<2563::aid-cncr2820731018>3.0.co;2-x [DOI] [PubMed] [Google Scholar]
- 28.Sadeghi A, Tran LM, Mark R, et al. Minor salivary gland tumors of the head and neck: treatment strategies and prognosis. Am J Clin Oncol 1993;16:3-8. https://doi.org/10.1097/00000421-199302000-00002 10.1097/00000421-199302000-00002 [DOI] [PubMed] [Google Scholar]
- 29.van der Wal JE, Snow GB, van der Waal I. Histological reclassification of 101 intraoral salivary gland tumours (new WHO classification). J Clin Pathol 1992;45:834-835. https://doi.org/10.1136/jcp.45.9.834 10.1136/jcp.45.9.834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jenkins DW, Spaulding CA, Constable WC, et al. Minor salivary gland tumors: the role of radiotherapy. Am J Otolaryngol 1989;10:250-256. https://doi.org/10.1016/0196-0709(89)90004-5 10.1016/0196-0709(89)90004-5 [DOI] [PubMed] [Google Scholar]
- 31.Waldron CA, el-Mofty SK, Gnepp DR. Tumors of the intraoral minor salivary glands: a demographic and histologic study of 426 cases. Oral Surg Oral Med Oral Pathol 1988;66:323-333. https://doi.org/10.1016/0030-4220(88)90240-x 10.1016/0030-4220(88)90240-x [DOI] [PubMed] [Google Scholar]
- 32.Beckhardt RN, Weber RS, Zane R, et al. Minor salivary gland tumors of the palate: clinical and pathologic correlates of outcome. Laryngoscope 1995;105:1155-1160. https://doi.org/10.1288/00005537-199511000-00003 10.1288/00005537-199511000-00003 [DOI] [PubMed] [Google Scholar]
- 33.Chen AM, Garcia J, Granchi P, et al. Base of skull recurrences after treatment of salivary gland cancer with perineural invasion reduced by post-operative radiotherapy. Clin Otolaryngol 2009;34:539-545. https://doi.org/10.1111/j.1749-4486.2009.02036.x 10.1111/j.1749-4486.2009.02036.x [DOI] [PubMed] [Google Scholar]
- 34.Gil Z, Carlson DL, Gupta A, et al. Patterns and incidence of neural invasion in patients with cancers of the paranasal sinuses. Arch Otolaryngol Head Neck Surg 2009;135:173-179. https://doi.org/10.1001/archoto.2008.525 10.1001/archoto.2008.525 [DOI] [PubMed] [Google Scholar]
- 35.Vander Poorten VL, Balm AJ, Hilgers FJ, et al. Stage as major long term outcome predictor in minor salivary gland carcinoma. Cancer 2000;89:1195-1204. https://doi.org/10.1002/1097-0142(20000915)89:6<1195::aid-cncr2>3.3.co;2-a [DOI] [PubMed] [Google Scholar]
- 36.Akbaba S, Ahmed D, Lang K, et al. Results of a combination treatment with intensity modulated radiotherapy and active raster-scanning carbon ion boost for adenoid cystic carcinoma of the minor salivary glands of the nasopharynx. Oral Oncol 2019;91:39-46. https://doi.org/10.1016/j.oraloncology.2019.02.019 10.1016/j.oraloncology.2019.02.019 [DOI] [PubMed] [Google Scholar]
- 37.Lang K, Baur M, Akbaba S, et al. Intensity Modulated Radiotherapy (IMRT) + Carbon Ion Boost for adenoid cystic carcinoma of the minor salivary glands in the oral cavity. Cancers 2018;10:488. https://doi.org/10.3390/cancers10120488 10.3390/cancers10120488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shekar K, Singh M, Godden D, et al. Recent advances in the management of salivary gland disease. Br J Oral Maxillofac Surg 2009;47:594-597. https://doi.org/10.1016/j.bjoms.2009.07.006 10.1016/j.bjoms.2009.07.006 [DOI] [PubMed] [Google Scholar]
- 39.Todorovic E, Dickson BC, Weinreb I. Salivary gland cancer in the era of routine next-generation sequencing. Head Neck Pathol 2020;14:311-320. https://doi.org/10.1007/s12105-020-01140-4 10.1007/s12105-020-01140-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Porcheri C, Meisel CT, Mitsiadis TA. Molecular and cellular modelling of salivary gland tumors open new landscapes in diagnosis and treatment. Cancers 2020;12:3107. https://doi.org/10.3390/cancers12113107 10.3390/cancers12113107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Corrêa TS, Matos GDR, Segura M, et al. Second-line treatment of HER2-positive salivary gland tumor: Ado-Trastuzumab Emtansine (T-DM1) after progression on trastuzumab. Case Rep Oncol 2018;11:252-257. https://doi.org/10.1159/000488669 10.1159/000488669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Albers E, Lawrie T, Harrell JH, et al. Tracheobronchial adenoid cystic carcinoma: a clinicopathologic study of 14 cases. Chest 2004;125:1160-1165. https://doi.org/10.1378/chest.125.3.1160 10.1378/chest.125.3.1160 [DOI] [PubMed] [Google Scholar]


