Abstract
Objective:
To assess disruption in healthcare services for HIV treatment by national emergency in response to the coronavirus disease 2019 (COVID-19) pandemic in the United States.
Design:
Time-series analysis
Methods:
We analyzed the IQVIA Real World Data–Longitudinal Prescriptions Database and calculated time trends in the weekly number of persons with active antiretroviral prescriptions for HIV treatment, and of persons who obtained antiretroviral prescriptions during January 2017–March 2021. We used interrupted time-series models to estimate the impact of the COVID-19 pandemic on antiretroviral therapy (ART) use between March 2020 and March 2021.
Results:
We found that the weekly number of persons with active antiretroviral prescriptions decreased by an average 2.5% (95% confidence interval [CI]: −3.8% to −1.1%), compared to predicted use, during March 2020 through March 2021. The weekly number of persons who obtained antiretroviral prescriptions decreased 4.5% (95% CI: −6.0% to −3.0%), compared to the predicted number. Men, persons aged ≤34 years, privately insured persons, and persons in medication assistance programs had greater decreases than other groups.
Conclusions:
We demonstrated a decrease in the number of persons with active antiretroviral prescriptions during the first year of the COVID-19 pandemic and the number did not return to levels expected in the absence of the pandemic. Disruptions in HIV care and decreased ART may lead to lower levels of viral suppression and immunologic control, and increased HIV transmission in the community.
Keywords: antiretroviral treatment, coronavirus disease 2019, United States
Background
The United States declared a national emergency in response to the coronavirus disease 2019 (COVID-19) pandemic on March 13, 2020. This response resulted in the closure of nonessential businesses and most non-emergency healthcare venues, and stay-at-home orders limited the movement of persons in their communities [1]. Many persons lost employer-sponsored health insurance as unemployment increased during the pandemic [2,3]. Since the emergency declaration, several waves of COVID-19 diagnoses have been observed in the United States resulting in more than 957 000 deaths [4]. With the availability and implementation of safe and effective vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), businesses and healthcare venues re-opened and persons resumed many of their usual activities outside of their homes.
HIV infection is usually life-threatening if untreated. HIV viral suppression protects the health of persons with HIV and prevents transmission of HIV [5]. Viral suppression can only be achieved by adherence to daily oral antiretroviral drugs, or monthly intramuscular injections available since early 2021. HIV care requires ongoing clinical monitoring to assess sustained viral suppression, assess the safety of antiretroviral treatment (ART), manage comorbid conditions, and to refill prescriptions for ART [6]. These healthcare visits also provide opportunities for providers to support adherence to ART and engagement in HIV care, and to provide any needed health services or other essential support services.
The COVID-19 pandemic disrupted HIV prevention, diagnosis, and treatment services, due to both closure of healthcare venues early in the pandemic and many persons’ sheltering at home. Disruptions were observed in the increasing trends of HIV testing and preexposure prophylaxis (PrEP) prescriptions [7,8], and HIV service disruptions were reported in a survey of HIV service-seeking behavior [9].
Objective
The impact of the COVID-19 pandemic on HIV treatment has not been evaluated at the national level. In this study, we assessed the effect of COVID-19 pandemic on antiretroviral treatment services for HIV in the United States. This information can help to estimate the impact of the pandemic on HIV care, and to guide interventions to prevent decreases in the use of ART in future pandemics and other public health emergencies.
Design
We designed an interrupted time-series analysis to estimate the impact of COVID-19 pandemic emergency on ART use in the United States.
Methods
We analyzed 2017–2021 data from the IQVIA Real-World Data–Longitudinal Prescription Database (IQVIA). The database included more than 92% of retail prescriptions and more than 60% of mail order prescriptions in the United States, except prescriptions from closed healthcare systems [10]. We also extracted data on demographic characteristics of persons with HIV in 2019 from the National Center for HIV, Viral Hepatitis, STD, and TB Prevention AtlasPlus Dashboard [11] to serve as an external benchmark for comparison with characteristics of persons in the IQVIA database.
We categorized antiretroviral prescriptions for HIV treatment using a series of exclusion steps. First, among all the antiretroviral prescription records of persons aged 13 years and older we excluded prescriptions for PrEP using a previously validated algorithm [12]. Among the remaining prescriptions, we excluded those for treatment of hepatitis B infection. These included lamivudine, tenofovir disoproxil fumarate, and tenofovir alafenamide fumarate [13] among persons without an HIV diagnosis record and without a prescription for any other concurrent antiretroviral drug. Finally, we excluded prescriptions with a cumulative supply of ≤28 days among persons without an HIV diagnosis as potential postexposure prophylaxis (PEP) prescriptions [14] (Figure, Supplemental Digital Content, http://links.lww.com/QAD/C568) We categorized the remaining prescriptions as ART for HIV treatment and these were included in our analytic sample.
We defined two outcome measures in this study: the weekly number of persons with active prescriptions to assess prevalent ART users and, the weekly number of persons who obtained antiretroviral prescriptions to estimate patient-provider encounters, including in-persons and virtual encounters. To estimate the weekly number of persons with active prescriptions, we used the prescription date and cumulative days of supply of all pharmacy transaction records to infer the dates of active antiretroviral prescriptions for each patient and calculated the number of persons with active antiretroviral prescription each week. To estimate the weekly number of persons who obtained antiretroviral prescriptions, we identified the first pharmacy transaction for every prescription and excluded transactions for refills, and calculated the number of persons who obtained antiretroviral prescriptions each week.
We fitted quasi-Poisson models as interrupted time-series adjusted for seasonality to estimate the outcome number before and after the start of the COVID-19 pandemic [15]. In all models, the time before March 15 was used as the reference for the pre-pandemic period. Use of elective healthcare service typically decreases around major holidays. In the model for weekly number of persons who obtained an antiretroviral prescription, we also added dummy variables to indicate the weeks that included federal holidays to adjust for an expected holiday effect on health service utilization. We predicted the weekly number of persons who would have had active antiretroviral prescriptions assuming the pandemic did not occur and compared the predicted number to the actual weekly number observed. We reported the percentage change and 95% confidence intervals (CI) over the observation period between the predicted and observed values for both the weekly number persons with active an antiretroviral prescription, and the weekly number of persons who obtained an antiretroviral prescription. We also performed stratified analyses by sex, age group, race and ethnicity, U.S. Census region, and type of health insurance. All analyses were performed using SAS 9.4 (SAS Inc., Cary, North Carolina, USA) and R 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Table 1 compares the characteristics of persons prescribed ART for HIV treatment in the IQVIA database with those of persons living with diagnosed HIV by year-end 2019 reported to the CDC National HIV Surveillance System (NHSS). In 2019, 792 834 persons had active antiretroviral prescriptions for HIV treatment in the IQVIA database, representing about 76.0% of the 1 042 542 persons with diagnosed HIV reported to NHSS. The distributions of sex, age, and U.S. Census region were similar in both the IQVIA and NHSS databases. Limited race and ethnicity data in the IQVIA database did not allow a comparison with surveillance data (Table 1).
Table 1.
Characteristics of persons with diagnosed HIV infection and persons with active antiretroviral prescriptions for HIV treatment in the United States, 2019.
| Persons with diagnosed HIVa N (%) | Persons with HIV prescribed antiretroviral drugsb N (%) | |
|---|---|---|
| Total | 1 042 542 (100.0) | 792 834 (100.0) |
| Sex | ||
| Male | 799 500 (76.7) | 588 800 (74.3) |
| Female | 243 042 (23.3) | 203 434 (25.7) |
| Unknown | 0 (0.0) | 600 (0.1) |
| Age group (years) | ||
| 13–24 | 30 776 (3.0) | 20 698 (2.6) |
| 25–34 | 162 336 (15.6) | 127 198 (16.0) |
| 35–44 | 193 690 (18.6) | 155 106 (19.6) |
| 45–54 | 267 943 (25.7) | 178 536 (22.5) |
| ≥55 | 387 797 (37.2) | 311 296 (39.3) |
| Race and ethnicityc | ||
| White | 303 487 (29.1) | 131 291 (16.6) |
| Black | 421 778 (40.5) | 80 291 (10.1) |
| Hispanic | 248 889 (23.9) | 37 958 (4.8) |
| Asian/other | 68 388 (6.6) | 9 851 (1.2) |
| Unknown | Not available | 533 443 (67.3) |
| Census region | ||
| Northeast | 236 247 (22.7) | 172 035 (21.7) |
| Midwest | 124 805 (12.0) | 110 996 (14.0) |
| South | 474 786 (45.5) | 346 699 (43.7) |
| West | 207 446 (19.9) | 144 320 (18.2) |
| Other/unknown | 16 491 (1.6) | 18 784 (2.4) |
| Insurance typed | ||
| Public | Not available | 366 225 (46.2) |
| Private | Not available | 333 215 (42.0) |
| Cash | Not available | 37 739 (4.8) |
| Other/Assistance Program | Not available | 124 622 (15.7) |
| Unknown | Not available | 134 275 (16.7) |
Number of prevalent persons living with diagnosed HIV through December 2019 (https://www.cdc.gov/nchhstp/atlas/index.htm). Persons with diagnosed HIV include persons who were alive by the end of 2019, and PWH prescribed antiretroviral drugs might include persons who died during 2019.
Number of persons with active antiretroviral prescriptions for HIV treatment in 2019. (https://www.iqvia.com/-/media/iqvia/pdfs/uk/fact-sheets/iqvia-longitudinal-prescription-data.pdf).
Other race and ethnicity included American Indian or Alaska Native and Native Hawaiian or Other Pacific Islander.
Public insurance includes Medicare and Medicaid; Other/Assistance Program includes federal and state assistance programs, drug manufacturer medication assistance programs, and medication coupon programs. One person may have more than one type of insurance in the same year; each row represents persons who had any prescription paid by this source in 2019.
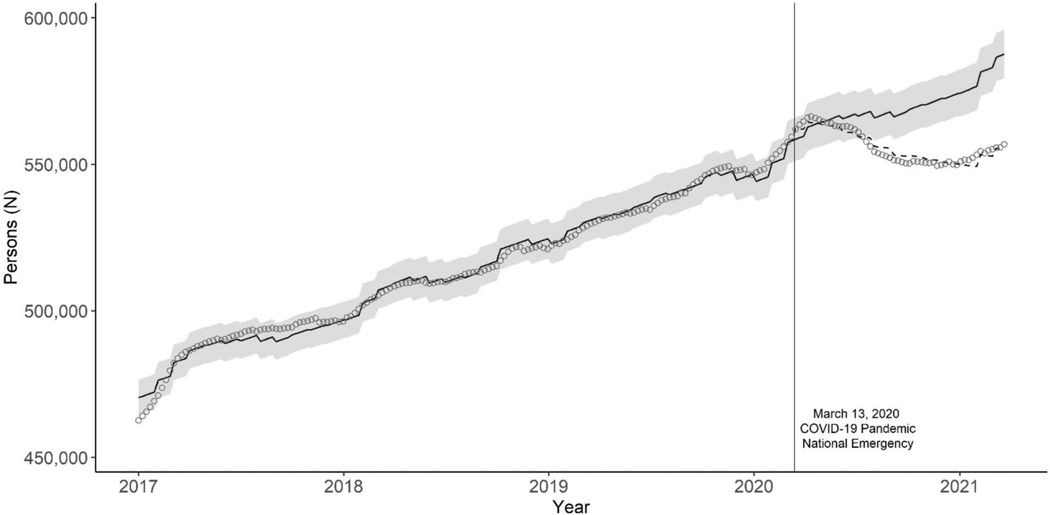
Prior to the national COVID-19 emergency declaration, during the week of March 8–14, 2020, there were 559 317 persons with active ART prescriptions. The number of persons continued to increase and peaked at 566 303 during the week of April 12–18, 2020, and then began to decrease. There was a slow rebound starting the week of November 22–28, 2020, but the number of persons with active antiretroviral prescriptions did not recover to the expected level by March 27, 2021, one year after the emergency declaration (Fig. 1). During March 15, 2020, to March 27, 2021, we estimated an average 2.5% decrease (95% CI:−3.8% to −1.1%) in the number of persons with active antiretroviral prescriptions compared to the predicted number. The relative difference between the observed and predicted numbers of persons with an antiretroviral prescription declined from 0.0% (95% CI: −1.4% to 1.5%) during the second quarter of 2020 to −4.6% (95% CI: −5.9% to −3.2%) during the first quarter of 2021. During the week of March 21–27, 2021, the last week of our observation period, we estimated a 5.3% decrease (95% CI: −6.6% to −3.9%) between the observed 556 791 and predicted 587 658 persons with active antiretroviral prescriptions (Table 2).
Fig. 1. Observed and predicted weekly number of persons with active antiretroviral prescriptions for HIV treatment in the United States, January 2017–March 2021.
Circles: circles represent observed weekly number of persons. Dashed line: Predicted number of persons based on antiretroviral use from 2017 to March 2020. Blue solid line and band: Predicted number of persons and 95% confidence interval assuming absence of COVID-19 pandemic. During a given year, a person with an active antiretroviral prescription during the year might not have had an active prescription every week, resulting in a total number of persons with any antiretroviral prescription during the year (Table 1) being greater than the number of persons with an active antiretroviral prescription each week. ART, antiretroviral therapy; COVID-19, coronavirus disease 2019.
Table 2.
Estimated change in the number of persons with active antiretroviral prescriptions for HIV treatment in the United States, March 15, 2020–March 27, 2021.
| Average March 2020-March 2021 Relative change % (95% Cl) | 2020 Q2 March 15-Jun 27, 2020 Relative change % (95% Cl) | 2020 Q3 June 28-September 26, 2020 Relative change % (95% Cl) | 2020 Q4 September 27-December 26, 2020 Relative change % (95% Cl) | 2021 Q1 December 27, 2020-March 27, 2021 Relative change % (95% Cl) | End of 2021 Q1 March 21–27 2021 Relative Change % (95% Cl) | |
|---|---|---|---|---|---|---|
| Total | −2.5 (−3.8, −1.1) | 0.0 (−1.4, 1.5) | −2.1 (−3.5, −0.7) | −3.6 (−4.9, −2.3) | −4.6 (−5.9, −3.2) | −5.3 (−6.6, −3.9) |
| Sex | ||||||
| Male | −2.9 (−4.6, −1.2) | −0.1 (−1.9, 1.7) | −2.5 (−4.2, −0.8) | −4.1 (−5.8, −2.5) | −5.2 (−6.8, −3.5) | −5.8 (−7.4, −4.1) |
| Female | −1.2 (−2.7, 0.2) | 0.5 (−1.0, 2.0) | −0.9 (−2.3, 0.5) | −2.0 (−3.4, −0.5) | −2.8 (−4.2, −1.4) | −3.6 (−5.0, −2.2) |
| Age group (years) | ||||||
| 13–24 | −1.9 (−4.5, 0.8) | −1.7 (−4.4, 1.1) | −0.9 (−3.5, 1.8) | −1.1 (−3.6, 1.5) | −3.7 (−6.2, −1.1) | −4.2 (−6.7, −1.6) |
| 25–34 | −9.0 (−11.2, −6.8) | −4.1 (−6.4, −1.8) | −8.0 (−10.1, −5.8) | −10.7 (−12.8, −8.6) | −13.4 (−15.4, −11.3) | −14.7 (−16.7, −12.6) |
| 35–44 | −3.8 (−6.0, −1.5) | −0.3 (−2.7, 2.2) | −3.2 (−5.5, −0.9) | −5.2 (−7.4, −3.0) | −6.8 (−9.0, −4.5) | −7.9 (−10.0, −5.6) |
| 45–54 | −2.0 (−4.3, 0.4) | 0.6 (−1.8, 3.0) | −1.6 (−3.9, 0.8) | −3.1 (−5.4, −0.9) | −4.0 (−6.3, −1.7) | −4.7 (−6.9, −2.3) |
| 55–64 | −1.9 (−4.2, 0.5) | 0.3 (−2.1, 2.7) | −1.7 (−4.0, 0.6) | −3.0 (−5.3, −0.7) | −3.5 (−5.8, −1.1) | −4.0 (−6.3, −1.6) |
| ≥65 | −1.5 (−3.7, 0.7) | 0.0 (−2.2, 2.3) | −1.4 (−3.6, 0.8) | −2.2 (−4.3, −0.1) | −2.7 (−4.8, −0.5) | −3.3 (−5.4, −1.1) |
| Race and ethnicitya | ||||||
| White | −1.2 (−3.3, 0.9) | 0.9 (−1.3, 3.1) | −1.0 (−3.1, 1.1) | −2.3 (−4.3, −0.2) | −2.5 (−4.6, −0.4) | −2.6 (−4.6, −0.5) |
| Black | −1.2 (−3.4, 1.1) | 1.2 (−1.1, 3.6) | −0.7 (−2.9, 1.6) | −2.3 (−4.5, −0.1) | −3.3 (−5.4, −1.0) | −3.9 (−6.1, −1.7) |
| Hispanic | −0.6 (−3.6, 2.5) | 1.8 (−1.4, 5.0) | −0.3 (−3.3, 2.8) | −1.7 (−4.6, 1.3) | −2.5 (−5.5, 0.5) | −2.8 (−5.8, 0.2) |
| Asian/other | 9.7 (7.0, 12.5) | 7.7 (5.0, 10.5) | 8.8 (6.1, 11.6) | 9.6 (6.9, 12.3) | 13.3 (10.5, 16.2) | 15.6 (12.6, 18.6) |
| Unknown | −3.6 (−5.6, −1.4) | −0.8 (−2.9, 1.5) | −3.1 (−5.2, −1.0) | −4.7 (−6.7, −2.6) | −6.0 (−8.1, −3.9) | −7.0 (−9.0, −4.9) |
| Census region | ||||||
| Northeast | −4.2 (−6.1, −2.2) | −1.4 (−3.4, 0.7) | −4.2 (−6.1, −2.2) | −5.3 (−7.2, −3.3) | −6.2 (−8.2, −4.2) | −6.8 (−8.7, −4.8) |
| Midwest | −3.3 (−5.3, −1.3) | −1.3 (−3.4, 0.8) | −3.2 (−5.2, −1.2) | −3.8 (−5.8, −1.9) | −5.0 (−7.0, −3.0) | −6.3 (−8.3, −4.3) |
| South | −2.1 (−4.4, 0.3) | 0.5 (−1.9, 3.0) | −1.3 (−3.7, 1.1) | −3.4 (−5.7, −1.1) | −4.3 (−6.6, −2.0) | −4.8 (−7.1, −2.5) |
| West | −1.2 (−3.2, 0.9) | 1.5 (−0.6, 3.7) | −0.9 (−3.0, 1.1) | −2.2 (−4.2, −0.2) | −3.4 (−5.4, −1.4) | −4.1 (−6.1, −2.1) |
| Insuranceb | ||||||
| Public | 1.9 (−0.1, 3.9) | 2.8 (0.7, 4.8) | 1.9 (0.0, 3.9) | 1.7 (−0.2, 3.7) | 1.2 (−0.8, 3.2) | 0.6 (−1.4, 2.6) |
| Private | −1.3 (−3.2, 0.7) | 2.1 (0.1, 4.2) | −0.7 (−2.6, 1.3) | −2.9 (−4.8, −1.0) | −4.3 (−6.2, −2.3) | −5.3 (−7.2, −3.4) |
| Cash | −13.2 (−15.6, −10.7) | −15.3 (−17.7, −12.8) | −14.1 (−16.6, −11.7) | −12.4 (−14.8, −10.0) | −11.0 (−13.5, −8.4) | −10.1 (−12.7, −7.5) |
| Other/Assistance Programs | −4.5 (−6.6, −2.4) | 2.8 (0.5, 5.1) | −3.4 (−5.5, −1.3) | −7.8 (−9.8, −5.8) | −10.1 (−12.0, −8.1) | −10.9 (−12.8, −8.9) |
| Unknown | −15.1 (−16.7, −13.5) | −12.2 (−13.8, −10.5) | −14.3 (−15.9, −12.7) | −16.2 (−17.8, −14.7) | −17.7 (−19.3, −16.2) | −18.0 (−19.5, −16.4) |
CI −confidence interval.
Other race and ethnicity included American Indian or Alaska Native and Native Hawaiian or Other Pacific Islander. Over 67% of persons had missing race and ethnicity information so these estimates might be unstable.
Public insurance includes Medicare and Medicaid; other includes federal and state assistance programs, drug manufacturer medication assistance programs, and medication coupon programs.
The average decrease in the weekly numberof persons with active antiretroviral prescriptions was larger among men (−2.9%, 95% CI: −4.6% to −1.2%) than women (−1.2%, 95% CI: −2.7% to −0.2%). The decrease was larger among those aged 25–34 years (−9.0%, 95% CI: −11.2% to −6.8%) compared with other age groups. We also found the average decrease was highest in the Northeast (−4.2%, 95% CI: −6.1% to −2.2%) compared with other regions. The number of persons with active antiretroviral prescription with public insurance (Medicare or Medicaid) increased over time with an average increase of 1.9% (95%CI: −0.1% to 3.9%). However, persons who paid with cash had an average 13.2% decrease over time (95% CI: −15.6% to −10.7%) (Table 2).
In an analysis stratified by age, sex, and health insurance type, the greatest decreases in persons with active antiretroviral prescriptions were observed among young men with private insurance ( − 4.6%, 95% CI: −7.1% to −2.1% in persons aged 13–24; −3.3%, 95% CI: −3.9% to −2.6% in persons aged 25–34) and young men insured by other assistance programs (−18.7%, 95% CI: −20.8% to −16.6% in persons aged 13–24, and −5.0%, 95% CI: −6.3% to −3.6% in persons aged 25–34) (Table, Supplemental Digital Content, http://links.lww.com/QAD/C568).
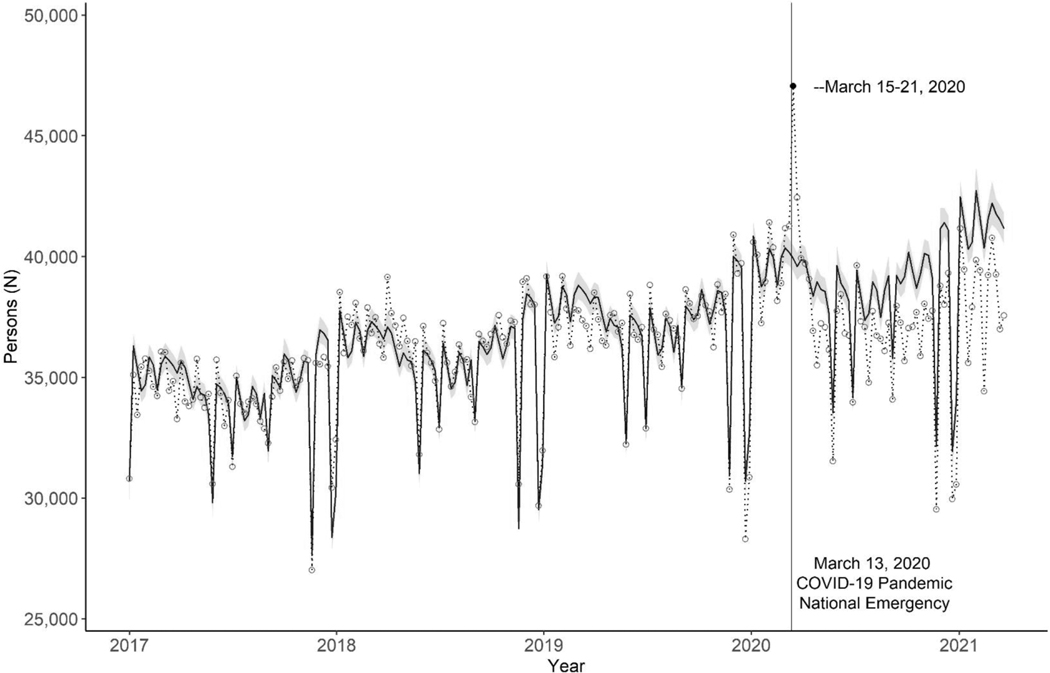
The weekly numbers of persons obtaining antiretroviral prescriptions decreased an average of 4.5% (95% CI: −6.0% to −3.0%) during March 15, 2020 through March 27, 2021. During the week of March 15–21, 2020, the first week after the emergency declaration, we observed a 17.8% increase (95% CI: 16.3% to 19.4%) in the number of persons obtaining antiretroviral prescriptions compared to the predicted 39 929 persons (Fig. 2, Table 3). The relative difference between the observed and predicted weekly numbers of persons with a new prescription declined progressively from a 1.2% decrease (95% CI: −2.7% to 0.3%) during the second quarter of 2020, to a 7.4% decrease (95% CI: −8.9% to −5.9%) during the first quarter of 2021 (Table 3).
Fig. 2. Observed and predicted weekly number of persons who obtained a new antiretroviral prescription for HIV treatment in the United States, January 2017–March 2021.
Circles and dotted line: Circles represent observed weekly number of persons, and the dotted line connects consecutive weeks. Blue solid line and band: Predicted number of persons and 95% confidence interval assuming the absence of the COVID-19 pandemic. Federal holidays with large decreases in antiretroviral prescriptions include New Year’s day, Memorial Day, Independence Day, Labor Day, Thanksgiving Day, and Christmas Day. These holidays were included as dummy variables in the model. We did not include other federal holidays as dummy variables in the model because large decreases in antiretroviral prescriptions were not observed. ARV, active antiretroviral; COVID-19, coronavirus disease 2019.
Table 3.
Estimated change in the number of persons who obtained antiretroviral prescriptions for HIV treatment in the United States, March 15, 2020–March 27, 2021.
| Average March 2020-March 2021 Relative change % (95% CI) | Week 1 March 15–21, 2020 Relative change % (95% CI) | 2020 Q2 March 15-June 27, 2020 Relative change % (95% CI) | 2020 Q3 June 28-September 26, 2020 Relative change % (95% CI) | 2020 Q4 September 27-December 26, 2020 Relative change % (95% CI) | 2021 Q1 December 27, 2020-March 27, 2021 Relative change % (95% CI) | |
|---|---|---|---|---|---|---|
| Total | −4.5 (−6.0, −3.0) | 17.8 (16.3, 19.4) | −1.2 (−2.7, 0.3) | −3.5 (−5.0, −1.9) | −6.3 (−7.8, −4.8) | −7.4 (−8.9, −5.9) |
| Sex | ||||||
| Male | −4.5 (−6.4, −2.6) | 17.6 (15.6, 19.6) | −1.1 (−2.9, 0.8) | −3.5 (−5.4, −1.6) | −6.4 (−8.2, −4.5) | −7.4 (−9.3, −5.5) |
| Female | −4.6 (−6.3, −2.8) | 18.6 (16.8, 20.5) | −1.5 (−3.2, 0.2) | −3.5 (−5.3, −1.7) | −6.1 (−7.8, −4.3) | −7.5 (−9.2, −5.7) |
| Age group (years) | ||||||
| 13–24 | −7.9 (−11.9, −3.9) | 16.4 (12.3, 20.7) | −6.0 (−9.8, −1.9) | −6.5 (−10.5, −2.3) | −7.4 (−11.4, −3.2) | −11.5 (−15.4, −7.5) |
| 25–34 | −11.5 (−14.0, −8.9) | 15.1 (12.4, 17.9) | −6.2 (−8.8, −3.6) | −10.5 (−13.1, −7.9) | −13.2 (−15.7, −10.7) | −16.0 (−18.4, −13.4) |
| 35–44 | −6.1 (−8.7, −3.4) | 20.3 (17.5, 23.1) | −1.5 (−4.1, 1.2) | −5.1 (−7.8, −2.4) | −7.8 (−10.4, −5.2) | −10.1 (−12.7, −7.5) |
| 45–54 | −3.7 (−6.4, −1.0) | 17.1 (14.4, 19.9) | −0.1 (−2.7, 2.6) | −2.7 (−5.4, 0.1) | −5.8 (−8.4, −3.1) | −6.8 (−9.4, −4.0) |
| 55–64 | −3.4 (−6.0, −0.6) | 17.9 (15.2, 20.6) | −0.7 (−3.3, 2.0) | −2.4 (−5.0, 0.4) | −5.3 (−7.9, −2.6) | −5.4 (−8.1, −2.7) |
| ≥65 | −3.0 (−5.6, −0.4) | 14.9 (12.4, 17.6) | −0.9 (−3.4, 1.7) | −1.7 (−4.4, 1.0) | −4.9 (−7.5, −2.3) | −5.0 (−7.6, −2.3) |
| Race and ethnicitya | ||||||
| White | −2.7 (−5.2, 0.0) | 19.5 (16.8, 22.2) | −0.8 (−3.3, 1.8) | −2.3 (−4.9, 0.4) | −4.2 −6.8, −1.6) | −3.5 −6.1, −0.8) |
| Black | −2.9 (−5.6, 0.0) | 16.8 (14.0, 19.6) | 0.6 (−2.2, 3.4) | −2.0 (−4.8, 0.8) | −4.7 (−7.4, −1.9) | −5.6 (−8.4, −2.8) |
| Hispanic | −2.2 (−6.0, 1.8) | 22.4 (18.3, 26.6) | 0.0 (−3.7, 3.9) | −1.9 (−5.8, 2.1) | −3.0 (−6.8, 1.0) | −4.0 (−7.9, 0.0) |
| Asian/other | 5.6 (0.0, 11.5) | 30.6 (24.8, 36.8) | 8.2 (2.7, 14.0) | 5.1 (−0.6, 11.0) | 4.4 (−1.3, 10.3) | 4.4 (−1.4, 10.4) |
| Unknown | −5.7 (−8.0, −3.3) | 17.0 (14.5, 19.5) | −1.9 (−4.2, 0.5) | −4.4 (−6.8, −1.9) | −7.6 (−9.9, −5.2) | −9.3 (−11.6, −6.9) |
| Census region | ||||||
| Northeast | −6.9 (−9.2, −4.6) | 20.3 (17.8, 22.8) | −3.0 (−5.2, −0.7) | −6.3 (−8.6, −3.9) | −8.8 (−11.0, −6.5) | −10.2 (−12.5, −7.9) |
| Midwest | −5.7 (−8.1, −3.2) | 13.7 (11.2, 16.2) | −2.9 (−5.3, −0.4) | −4.5 (−7.0, −1.9) | −6.2 (−8.7, −3.7) | −9.3 (−11.7, −6.8) |
| South | −3.7 (−6.3, −1.0) | 16.8 (14.1, 19.6) | −0.8 (−3.4, 1.8) | −2.2 (−4.9, 0.6) | −5.8 (−8.4, −3.1) | −6.1 (−8.7, −3.4) |
| West | −2.8 (−5.2, −0.3) | 20.2 (17.7, 22.7) | 1.3 (−1.1, 3.8) | −2.5 (−4.9, 0.0) | −4.4 (−6.8, −2.0) | −6.0 (−8.4, −3.5) |
| Insurance | ||||||
| Public | −1.0 (−3.2, 1.3) | 16.4 (14.2, 18.6) | 0.0 (−2.2, 2.1) | −0.2 (−2.5, 2.1) | −1.7 (−3.9, 0.6) | −2.2 (−4.4, 0.1) |
| Private | −4.1 (−6.3, −1.9) | 19.7 (17.4, 22.0) | −0.3 (−2.4, 1.9) | −2.5 (−4.7, −0.2) | −5.8 (−8.0, −3.7) | −8.2 (−10.3, −6.0) |
| Cash | −2.1 (−7.7, 3.8) | 32.9 (26.4, 39.7) | −0.8 (−6.3, 4.9) | −3.8 (−9.4, 2.0) | −1.8 (−7.4, 4.1) | −2.1 (−7.8, 4.0) |
| Other/Assistance Programs | −9.1 (−11.7, −6.4) | 21.9 (19.0, 24.9) | −1.6 (−4.3, 1.1) | −8.5 (−11.1, −5.7) | −13.2 (−15.7, −10.6) | −13.6 (−16.1, −11.0) |
| Unknown | −19.8 (−21.9, −17.7) | 9.5 (7.1, 11.9) | −11.2 (−13.4, −8.9) | −18.5 (−20.7, −16.4) | −23.8 (−25.8, −21.8) | −25.6 (−27.6, −23.6) |
CI, confidence interval.
Other race/ethnicity included American Indian or Alaska Native and Native Hawaiian or Other Pacific Islander. Over 67% of persons had missing race and ethnicity information so these estimates might be unstable.
Public insurance includes Medicare and Medicaid; other includes federal and state assistance programs, drug manufacturer medication assistance programs, and medication coupon programs.
The number of persons who obtained antiretroviral prescriptions decreased more among persons aged 25–34 years ( −11.5%, 95% CI: −14.0% to −8.9%) compared with those aged 13–24 years ( − 7.9%, 95% CI: −11.9% to −3.9%). The greatest decrease was observed in the Northeast (− 6.9%, 95% CI: −9.2% to −4.6%), and the smallest decrease was observed in the West ( −2.8%, 95% CI: −5.2% to −0.3%). The number of persons with Medicare or Medicaid insurance were relatively stable during the year ( −1.0%, 95% CI: −3.2% to −1.3%), while a large decrease was observed in persons with private insurance ( − 4.1%, 95% CI: −6.3% to −1.9%) and persons supported by other federal, state, or coupon assistance programs (−9.1%, 95% CI: −11.7% to −6.4%) (Table 3).
Discussion
We found that during the COVID-19 pandemic, the number of persons with active antiretroviral prescriptions for ART decreased in the United States from March 2020 through March 2021. This decrease suggests that fewer persons with HIV were using ART than expected, and that a large number of persons with HIV had an interruption in their continuous use of ART. This decrease might be attributed to missed HIV diagnoses because of decreased HIV testing during the pandemic [7], as well as interrupted ART among persons with diagnosed HIV before the pandemic. It is encouraging that the decrease in antiretroviral prescriptions was smaller compared with large service disruptions observed for HIV testing and PrEP prescriptions [7,8].
The decreased number of persons with active antiretroviral prescriptions might have a negative impact on both person- and population-level health. Our population level observations suggest a potential impact at person-level that should be evaluated in future studies. ART disruptions might result in a person not achieving and/or maintaining viral suppression and maintaining immune system function. Also, with potentially more persons with unsuppressed HIV viral loads, it is possible that increased HIV transmission occurred during the pandemic.
Immediately after the declaration of the COVID-19 emergency, we observed that healthcare providers proactively prescribed large quantities of antiretroviral drugs to their patients, resulting in a spike in the number of persons who obtained antiretroviral prescriptions in mid-March 2020. The quantities of these stockpiled prescriptions were insufficient to offset decreased antiretroviral prescriptions during our study period. The observed number of persons with active antiretroviral prescriptions started to decrease in April 2020 and did not recover to the predicted number by March 2021.
The decreased numbers of persons who obtained a new antiretroviral prescription suggests that healthcare encounters by persons with diagnosed HIV were negatively affected during the COVID-19 pandemic emergency declaration. Healthcare encounters are opportunities not only for antiretroviral prescription refills but also for monitoring the safety and efficacy of HIV treatment and to provide other healthcare needs, for treatment of comorbidities, and to provide access to essential support services. Telehealth and virtual healthcare increased in the United States since the beginning of the pandemic [16], and many health systems and providers piloted various telehealth models to support ongoing HIV care [17–19]. The gap in service encounters found in our study indicates a potential opportunity for expansion of telehealth services. However, because HIV care also requires regularly scheduled laboratory tests to monitor HIV viral load, CD4+ cell count, and other tests [6], self-collected specimens and novel laboratory collection models are needed to ensure high quality uninterrupted HIV care during public health and other emergencies.
We found Medicare and Medicaid served as an effective healthcare safety net during the pandemic, preventing tens of thousands of persons with HIV from losing access to antiretroviral treatment. The number of persons with active antiretroviral prescriptions covered by Medicare or Medicaid was stable over the months of our study, while the number of those with private insurance or medication assistance programs declined substantially. This is probably because some persons with private insurance had employer-sponsored insurance that was lost with the pandemic shutdowns and unemployment [2,3]. Many persons enrolled in Medicaid during the pandemic with a 17.3% increase in enrollment from February 2020 through March 2021 [3,20], ensuring access to healthcare services during the pandemic. However, the decline in the overall number of persons prescribed ART indicates that increased Medicaid enrollment did not entirely close the access gap.
Particularly alarming is that the largest decrease was among younger men, the group with the highest HIV diagnosis rate [11]. Young men with private insurance or medication assistance programs had the largest antiretroviral prescription decreases during the COVID-19 pandemic. Several factors might have been associated with their decreased ART use. By May 2020, 25.3% of young persons were unemployed [21], leading to a loss of employer-sponsored health insurance and decreased access to HIV treatment services. In addition, many HIV clinics were closed during the early months of the pandemic [22]. Finally, venues that support newly diagnosed persons to initiate ART using medication assistance programs, such as community-based organizations and nonemergent health clinics, were also closed [23].
Our study included a national estimate of antiretroviral drugs prescribed in the United States but has some limitations. First, we might have underestimated the number of persons prescribed antiretroviral drugs because the IQVIA database included 92% of prescriptions in retail pharmacies and about 60% of mail order pharmacies, and it did not include some closed healthcare systems such as health maintenance organizations. Second, race and ethnicity data were missing for more than half of persons prescribed antiretroviral drugs, limiting our ability to study trends in antiretroviral prescriptions by race and ethnicity. Third, we likely underestimated the number of persons prescribed antiretroviral drugs who were covered by Medicare or Medicaid and private insurance, as third-party payer information was completely missing for 8% of persons with antiretroviral prescriptions. Fourth, some excess deaths among people with HIV (PWH) might have occurred during the COVID-19 pandemic resulting in an overestimation of decreased ARV use. However, 16 230 deaths occurred among PWH in 2019 and 18 160 in 2020 [11]. Potential excess death may be about 2000 persons, which is a small proportion of the estimated 31 000 persons who did not have antiretroviral prescriptions during the pandemic suggesting that bias due to excess deaths is likely minimal.
We observed a decrease in the number of persons with active antiretroviral prescriptions in the first year of the COVID-19 pandemic and the observed trend did not return to levels that would have been predicted in the absence of the pandemic. Disruptions in HIV care services might lead to morbidity and missed opportunities for engagement and retention in care for other services. Furthermore, a delay in HIV diagnosis and lower rates of viral suppression caused by interrupted ART may have increased HIV transmission in the United States. Increased efforts are needed to prevent disruptions in HIV services during the ongoing COVID-19 pandemic and future public health and other crises. Interventions are needed to support continuous access to HIV healthcare services during times of shutdowns and unemployment. Studies that estimate the impact of COVID-19 on CD4+ cell count and viral load among PWH would provide more direct evidence, and innovative models of HIV care are also needed that use telehealth and other nontraditional access to HIV testing, and to clinical and laboratory monitoring of persons with HIV using ART. Without a strong safety net to preserve access to HIV services, setbacks can occur in achieving the goals of the Ending the HIV Epidemic in the US initiative [24].
Supplementary Material
Acknowledgements
Disclaimer: The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.Moreland A, Herlihy C, Tynan M, Sunshine G, McCord R, Hilton C, Poovey J, et al. Timing of state and territorial COVID–19 stay-at-home orders and changes in population movement–−United States, March 1–May 31, 2020. Morb Mortal Wkly Report 2020; 69:1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith S, Edwards R, Duong H. Unemployment rises in 2020, as the country battles the COVID-19 pandemic. Monthly Lab Rev 2021; 144:1. [Google Scholar]
- 3.Bundorf M, Gupta S, Kim C. Trends in US health insurance coverage during the COVID–19 pandemic. JAMA Health Forum 2021; 2:e212487American Medical Association. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. COVID Data Tracker. Available at: https://covid.cdc.gov/covid-data-tracker/#datatracker-home.
- 5.Cohen M, Chen Y, McCauley M, Gamble T, Hosseinipour M, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panel on Antiretroviral Guidelines for Adults and Adolescents, Department of Health and Human Services. Laboratory testing schedule for monitoring patients with HIV before and after initiation of antiretroviral therapy, guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Available at: https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/ [updated on August 16, 2021].
- 7.Delaney K, Jayanthi P, Emerson B, Zhu W, Pitasi M, Huang Y, et al. Impact of COVID–19 on commercial laboratory testing for HIV in the United States. Top Antivir Med 2021; 29:288–289. [Google Scholar]
- 8.Huang Y, Zhu W, Wiener J, Kourtis A, Hall H, Hoover K. Impact of coronavirus disease 2019 (COVID-19) on human immune-deficiency virus (HIV) pre-exposure prophylaxis prescriptions in the United States–−a time-series analysis. Clin Infect Dis 2022:ciac038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanchez T, Zlotorzynska M, Rai M, Baral S. Characterizing the impact of COVID–19 on men who have sex with men across the United States in April, 2020. AIDS Behav 2020; 24:2024–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.IQVIA. IQVIA longitudinal prescription data. Available at: https://www.iqvia.com/-/media/iqvia/pdfs/uk/fact-sheets/iqvia-longitudinal-prescription-data.pdf [Accessed April 21, 2021].
- 11.Centers for Disease Control and Prevention, National Center for HIV, Viral Hepatitis, STD, and TB Prevention AtlasPlus Dashboard. Available at: https://www.cdc.gov/nchhstp/atlas/index.htm [Accessed on 1 June 2022].
- 12.Furukawa N, Smith D, Gonzalez C, Huang Y, Hanna D, Felsen U, et al. Evaluation of algorithms used for PrEP surveillance using a reference population from New York City, July 2016–June 2018. Public Health Rep 2020; 135:202–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Terrault N, Lok A, McMahon B, Chang K, Hwang J, Jonas M, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018; 67:1560–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dominguez K, Smith D, Thomas V, Crepaz N, Lang K, Heneine W, et al. Updated guidelines for antiretroviral postexposure prophylaxis after sexual, injection drug use, or other nonoccupational exposure to HIV--United States, 2016. Available at: https://stacks.cdc.gov/view/cdc/38856. [DOI] [PubMed]
- 15.Bernal J, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol 2017; 46:348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koonin L, Hoots B, Tsang C, Leroy Z, Farris K, Jolly B, et al. Trends in the use of telehealth during the emergence of the COVID–19 pandemic–−United States, January–March 2020. Morb Mortal Wkly Rep 2020; 69:1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yelverton V, Qiao S, Weissman S, Olatosi B, Li X. Telehealth for HIV care services in South Carolina: utilization, barriers, and promotion strategies during the COVID-19 pandemic. AIDS Behav 2021; 25:3909–3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Auchus I, Jaradeh K, Tang A, Marzan J, Boslett B. Transitioning to telehealth during the COVID-19 pandemic: patient perspectives and attendance at an HIV clinic in San Francisco. AIDS Patient Care STDs 2021; 35:249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brody J, Rajabiun S, Strupp Allen H, Baggett T. Enhanced telehealth case management plus emergency financial assistance for homeless-experienced people living with HIV during the COVID-19 pandemic. American Journal of Public Health 2021; 111:835–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corallo B, Rudowitz R. Analysis of recent national trends in Medicaid and CHIP enrollment. Washington, DC: Kaiser Family Foundation. 2021. Aug 16. Available at: https://www.kff.org/coronavirus-covid-19/issue-brief/analysis-of-recent-national-trends-in-medicaid-and-chip-enrollment/. [Google Scholar]
- 21.Kochhar R. Unemployment rose higher in three months of COVID-19 than it did in two years of the great recession (Pew Research Center, 2020). Available at: https://www.pewresearch.org/fact-tank/2020/06/11/unemployment-rose-higher-in-three-months-of-covid-19-than-it-did-in-two-years-of-the-great-recession/. [Google Scholar]
- 22.Qiao S, Li Z, Weissman S, Li X, Olatosi B, Davis C, et al. Disparity in HIV service interruption in the outbreak of COVID-19 in South Carolina. AIDS Behav 2021; 25:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stephenson R, Walsh A, Chavanduka T, Sallabank G, Horvath K, Castel A, et al. Widespread closure of HIV prevention and care services places youth at higher risk during the COVID-19 pandemic. PLoS One 2021; 16:e0249740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Department of Health and Human Services. Ending the HIV epidemic in the U.S. Available at: https://www.hiv.gov/federal-response/ending-the-hiv-epidemic/overview.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.