Abstract
Background
Previous studies have shown that intravenous normal saline (NS) may be associated with the incidence of acute kidney injury (AKI). This study aimed to evaluate the association between the volume of NS infusion and AKI in heat stroke (HS) patients.
Methods
This multicenter retrospective cohort study included 138 patients with HS. The primary outcome was the incidence of AKI. Secondary outcomes included the need for continuous renal replacement therapy (CRRT), admission to the intensive care unit (ICU), length of stay in the ICU and hospital, and in-hospital mortality. Multivariate regression models, random forest imputation, and genetic and propensity score matching were used to explore the relationship between NS infusion and outcomes.
Results
The mean volume of NS infusion in the emergency department (ED) was 3.02 ± 1.45 L. During hospitalization, 33 patients (23.91%) suffered from AKI. In the multivariate model, as a continuous variable (per 1 L), the volume of NS infusion was associated with the incidence of AKI (OR, 2.51; 95% CI, 1.43–4.40; p = .001), admission to the ICU (OR, 3.46; 95% CI 1.58–7.54; p = .002), and length of stay in the ICU (β, 1.00 days; 95% CI, 0.44–1.56; p < .001) and hospital (β, 1.41 days; 95% CI, 0.37–2.45; p = .008). These relationships also existed in the forest imputation cohort and matching cohort. There were no differences in the use of CRRT or in-hospital mortality.
Conclusions
The volume of NS infusion was associated with a significant increase in the incidence of AKI, admission to the ICU, and length of stay in the ICU and hospital among patients with HS.
Keywords: Normal saline, acute kidney injury, serum chloride, heat stroke, emergency department
Introduction
Heat stroke (HS) is a life-threatening illness caused by a rapid increase in one’s core temperature in excess of 40 °C from exposure to a hot and humid environment [1]. HS manifests as a systemic illness that includes encephalopathy, coagulopathy, respiratory failure, hypotension, liver, kidney and muscle injury, vomiting and diarrhea [2]. Acute kidney injury (AKI) is one of the most common complications of HS, with an incidence of up to 90.9% [3]. AKI following HS is a major factor associated with the need for renal replacement therapy, longer hospital stay, and hospital mortality [4–6].
Immediate cooling and support of organ system function are the two main therapeutic objectives for patients with HS [7]. Crystalloid solution administration is widely considered an essential part of the management of HS. Administering an intravenous cold infusion quickly is an effective cooling method in hospitals [8]. Fluid resuscitation with crystalloid solution is an important intervention to restore organ perfusion, improve tissue oxygenation, promote renal blood flow, and prevent myoglobin-induced renal injury [7]. Normal saline (NS), a common type of crystalloid solution, is widely used in patients with HS, especially in the emergency department (ED) where the patient is in the early and key stage of the disease.
However, the chloride concentration of NS (154 mmol/L) is higher than that of human plasma (94–111 mmol/L) [9]. Chloride-rich fluid administration may induce or exacerbate hyperchloremia and metabolic acidosis [10–12] and may produce renal vasoconstriction and a reduction in the glomerular filtration rate [13, 14]. Recent large-scale studies have brought forward compelling evidence on this matter. Some indicate that compared to balanced crystalloids, NS might be associated with a higher incidence of adverse kidney events in both critical and non-critical adult cases [9, 15]. There have been suggestions that moderating intravenous chloride intake might lead to a reduced AKI incidence and lowered necessity for continuous renal replacement therapy (CRRT) in critically ill patients [9, 16]. Yet, the debate continues. Other extensive trials have posited that balanced solutions might not significantly outperform NS in reducing mortality or AKI in critically ill patients [17]. Likewise, systematic reviews have also hinted at negligible differences in outcomes between NS and balanced solutions for septic adults [18].
Given this backdrop, the association between NS administration and AKI remains hotly debated. Complicating matters further is the fact that many large-scale studies have encompassed a diverse patient pool [9, 15–17], leaving gaps in our understanding regarding NS’s effects in specific conditions like HS. Recognizing the unique pathophysiological nuances of HS and the potential implications of crystalloid solution choice, our research endeavors to shed light on the relationship between NS infusion volume and HS outcomes. Our retrospective multicenter cohort study, spanning 2021 to 2022, delves into this relationship, aiming to offer valuable insights and guiding future HS management strategies.
Methods
This study was approved by the Ethics Committee of the Second Affiliated Hospital of Zhejiang University School of Medicine (approval number: 2022-0913), and it conforms to the provisions of the Declaration of Helsinki. The requirement for informed consent was waived due to the retrospective nature of the study. The STROBE guidelines for cohort studies were applied.
Study design, setting, and participants
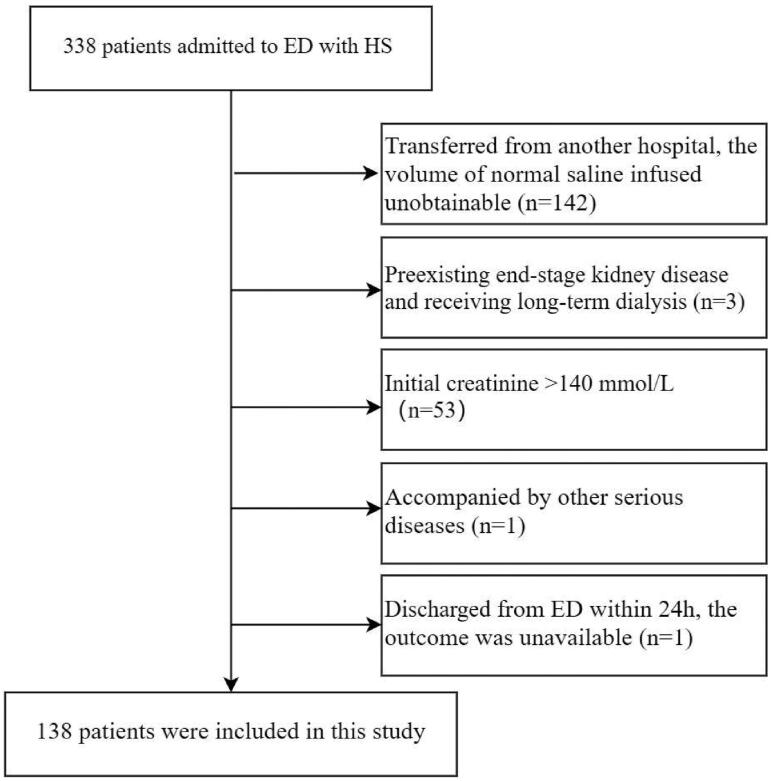
This retrospective cohort study was conducted in six tertiary care hospitals in China between 2021 and 2022. Consecutive adult patients with HS who were admitted to EDs were included in our study. HS was defined by the criteria according to the expert consensus on the diagnosis and treatment of HS in China [19]. Patients were excluded from the study for several reasons: (1) if they were transferred from another hospital or if the volume of NS infused prior to admission was undocumented; (2) if they had existing end-stage renal disease on chronic dialysis; and (3) if initial laboratory results at ED admission indicated an imminent risk for AKI. In line with the Clinical Practice Guidelines [20] and taking into account the typical hypovolemia in HS, the exclusion criteria were as follows: a serum creatinine level exceeding 140 mmol/L; (4) concurrent critical conditions such as severe trauma or substantial intracranial hemorrhage; and (5) self-discharge within the first 24 h of admission due to the severity of their condition, leaving outcome data incomplete.
Data collection and management
We collected information on patients’ baseline characteristics, underlying comorbidities, state of consciousness and vital signs obtained at ED admission, and length of stay in the ED. The National Early Warning Score (NEWS), a critical severity scoring system commonly used in EDs, was calculated based on vital signs and GCS obtained at ED admission, as well as supplemental oxygen [21]. Data from laboratory tests performed at ED admission were extracted. We retrieved the first serum chloride result after admission to the intensive care unit (ICU) or general ward. If the patient was discharged from the ED, then the last test results before discharge were retrieved. Specific treatments, such as the use of vasoactive drugs, and CRRT, regardless of whether it is due to AKI or non-AKI reasons, were also extracted from the electronic patient data. The volume of NS infusion in the ED was rechecked between the doctor’s orders and the drug administration list. That during prehospital care was calculated according to the nursing record sheet.
Outcomes
The primary outcome of this study was the incidence of AKI. AKI was defined as an increase in serum creatinine by 50% within seven days, an increase in serum creatinine by 0.3 mg/dL (26.5 μmol/L) within two days, or oliguria [22]. The secondary outcomes included admission to the ICU, the need for CRRT, length of stay in the ICU and hospital, and in-hospital mortality.
Statistical analysis
All statistical analyses were performed using Empower (R) (www.empowerstats.com, X&Y Solutions, Inc., Boston, MA). A two-tailed p Value <.05 was considered statistically significant.
The patients’ baseline characteristics were summarized using descriptive statistics. Categorical variables are reported as absolute numbers with percentages and were analyzed by Chi-square or Fisher’s exact test, as appropriate. Normally and nonnormally distributed continuous variables were compared using Student’s t-test and the Mann–Whitney’s U-test, respectively.
The incidence of AKI was analyzed using logistic regression, and the results are reported as odds ratios (ORs) with 95% confidence intervals (CIs). Multivariable sensitivity analysis was performed for all outcomes. After screening for collinearity between covariates according to the variance inflation factor, covariates were included as potential confounders in the adjusted models if the changes in the estimates of the NS infusion volume for the outcomes were more than 10% or were significantly associated with the outcomes. The associations of each confounder with the outcomes of interest and changes in the effect estimates are shown in Supplementary Tables S1–S3. The serum creatinine level obtained at ED admission was also included as a covariate for all outcomes since late renal function was greatly dependent on the starting level.
There was not a great deal of missing data in our study. The largest amount of missing data in potential confounders was lactic acid level (7, 5.07%). We used random forest imputation, based on five replications, to account for missing data on covariates. We repeated logistic regression analysis with the complete data cohort to assess the association between the NS infusion volume and AKI for sensitivity analyses. Supplementary Tables S4 and S5 give additional details of the statistical analyses.
Furthermore, we divided the participants into two groups: a group with a volume of NS >2.5 L and a group with a volume ≦2.5 L. As the baseline data between the groups with different volumes of NS were not balanced, the patients with a greater NS infusion volume showed more severe conditions, and genetic matching incorporating the estimated propensity score was performed to adjust for confounders and balance the observed covariates between these two groups. Specifically, we used institution and baseline characteristics, HS type, NEWS at admission, baseline laboratory results, length of stay in the ED, and use of vasoactive drugs as covariates, calculated the propensity score for each patient, and performed 1:1 matching using a genetic matching algorithm. Genetic matching, a method for multivariate matching, leverages an affinely invariant approach [23]. The method utilizes an evolutionary algorithm, meticulously assigning weights to a range of baseline covariates. This strategic weighting is designed to ensure the optimal balance of these covariates, thereby achieving a state of equilibrium between the matched treatment and control groups in observational studies. While the methodology is nonparametric and independent of the propensity score’s estimation or knowledge, its efficiency is significantly enhanced when the propensity score is either known or estimated [23]. Multivariate regression model was conducted to assess differences in outcomes between the two groups after matching. The propensity score was included as a covariate in the adjusted models.
To further explore the possible mechanism between the NS infusion volume and outcomes, we analyzed the correlation between the changes in chloride and the volume of saline infusion to assess the possible immediate effect of saline infusion. Then, we performed analyses of the association between serum chloride, including the baseline level and the level tested after NS infusion, and the incidence of AKI. Moreover, we used a smoothing spline curve to characterize the risk of AKI associated with chloride after saline infusion.
Results
In the final analysis, 138 patients were included (Figure 1). Baseline demographics, vital signs obtained at ED admission, laboratory tests, use of CRRT, use of vasoactive drugs, and complications are outlined in Table 1.
Figure 1.
Flowchart.
Table 1.
Characteristics of the patients.
| Variables | All (n = 138) | Acute kidney injury |
p | |
|---|---|---|---|---|
| No (n = 105) | Yes (n = 33) | |||
| Baseline characteristics | ||||
| Age (years) | 65.26 ± 14.84 | 66.46 ± 14.57 | 61.45 ± 15.26 | .068 |
| Female, n (%) | 55 (39.86) | 42 (40.00) | 13 (39.39) | .951 |
| Comorbidities, n (%) | ||||
| Diabetes | 9 (6.52) | 5 (4.76) | 4 (12.12) | .135 |
| Chronic cardiovascular disease | 40 (28.99) | 31 (29.52) | 9 (27.27) | .977 |
| Mental disorder | 14 (10.14) | 10 (9.52) | 4 (12.12) | .666 |
| Dementia | 5 (3.62) | 4 (3.81) | 1 (3.03) | .834 |
| Institution, n (%) | .030 | |||
| 1 | 47 (34.0) | 40 (38.10) | 7 (21.21) | |
| 2 | 19 (13.77) | 13 (12.38) | 6 (18.18) | |
| 3 | 22 (15.94) | 17 (16.19) | 5 (15.15) | |
| 4 | 15 (10.87) | 14 (13.33) | 1 (3.03) | |
| 5 | 22 (15.94) | 15 (14.29) | 7 (21.21) | |
| 6 | 13 (9.42) | 6 (5.71) | 7 (21.21) | |
| Type of heat stroke, n (%) | .555 | |||
| Classic | 52 (37.68) | 41 (39.05) | 11 (33.33) | |
| Exertional | 86 (62.32) | 64 (60.95) | 22 (66.67) | |
| Chief complaints, n (%) | ||||
| Coma | 113 (81.88) | 82 (78.10) | 31 (93.94) | .039 |
| Epileptic seizure | 19 (13.77) | 12 (11.43) | 7 (21.21) | .155 |
| Onset time (h) | 1.00 (1.00–3.00) | 1.50 (1.00–3.00) | 1.00 (1.00–3.00) | .470 |
| Monitoring parameters at admission | ||||
| Temperature (°C) | 40.80 ± 0.81 | 40.77 ± 0.77 | 40.89 ± 0.95 | .452 |
| Heart rate (beat/min) | 128.57 ± 24.08 | 124.41 ± 23.64 | 141.82 ± 20.68 | <.001 |
| Systolic pressure (mmHg) | 126.62 ± 31.54 | 130.30 ± 31.42 | 114.94 ± 29.44 | .014 |
| Glasgow Coma Scale | 8.80 ± 4.19 | 9.50 ± 4.29 | 6.58 ± 2.94 | <.001 |
| NEWS | 12.51 ± 3.59 | 11.93 ± 3.69 | 14.36 ± 2.55 | <.001 |
| Body temperature after cooling (°C) | ||||
| Temperature (0.5 h) | 39.50 ± 0.99 | 39.32 ± 0.93 | 40.01 ± 0.90 | <.001 |
| Temperature (2 h) | 37.92 ± 1.00 | 37.73 ± 0.91 | 38.49 ± 1.05 | <.001 |
| Blood test at admission | ||||
| C-reactive protein (mg/L) | 0.62 (0.30–4.40) | 0.70 (0.30–5.80) | 0.30 (0.30–2.0) | .458 |
| Potassium (mmol/L) | 3.64 ± 0.63 | 3.58 ± 0.60 | 3.83 ± 0.70 | .053 |
| Sodium (mmol/L) | 130.46 ± 13.07 | 130.86 ± 7.67 | 129.21 ± 23.19 | .529 |
| Chloride (mmol/L) | 97.54 ± 9.06 | 97.11 ± 8.93 | 98.89 ± 9.49 | .329 |
| Blood glucose (mmol/L) | 11.35 ± 4.87 | 10.90 ± 4.80 | 12.70 ± 4.90 | .065 |
| Alanine aminotransferase (IU/L) | 23 (16.25–35.00) | 22.50 (16.25–35) | 23 (17.00–32.58) | .620 |
| Creatinine (mmol/L) | 98.11 ± 23.35 | 93.45 ± 23.26 | 112.92 ± 16.72 | <.001 |
| Blood urea nitrogen (mmol/L) | 6.86 ± 2.73 | 6.88 ± 2.82 | 6.80 ± 2.45 | .882 |
| Creatine kinase (U/L) | 265.50 (141.80–703.75) | 256.00 (133.20–546.55) | 395.00(159.50–930.00) | .656 |
| Troponin I (ng/mL) | 0.03 (0.01–0.07) | 0.02 (0.01–0.06) | 0.04 (0.02–0.15) | .061 |
| Prothrombin time (s) | 13.25 ± 2.01 | 13.29 ± 1.90 | 13.12 ± 2.37 | .681 |
| Lactic acid (mmol/L) | 3.42 ± 2.00 | 3.18 ± 2.08 | 4.15 ± 1.53 | .016 |
| White blood cell count (1 × 109/L) | 10.24 ± 4.71 | 10.25 ± 4.70 | 10.23 ± 4.82 | .983 |
| Platelet (1 × 109/L) | 197.01 ± 71.15 | 194.94 ± 69.07 | 203.55 ± 78.12 | .547 |
| Serum chlorine after normal saline infusion (mmol/L) | 107.67 ± 6.96 | 106.30 ± 6.53 | 110.83 ± 6.99 | .008 |
| Volume of normal saline infusion in ED (L) | 3.02 ± 1.45 | 2.82 ± 1.34 | 3.66 ± 1.61 | .006 |
| Total infusion volume (L) | 3.69 ± 1.99 | 3.48 ± 1.82 | 4.36 ± 2.19 | .043 |
| Use of vasoactive drugs, n (%) | 28 (20.29) | 13 (12.38) | 15 (45.45) | <.001 |
| Length of stay in ED (h) | 10.00 (4.50–19.88) | 10.00 (4.50–22.00) | 8.00 (4.00–16.50 | .160 |
| Outcomes | ||||
| RRT, n (%) | 7 (5.07) | 2 (1.90) | 5 (15.15) | .002 |
| Admitted to ICU, n (%) | 52 (37.68) | 25 (23.81) | 27 (81.82) | <.001 |
| Length of stay in hospital (d) | 5.00 (2.00–8.00) | 5.00 (0.00–7.00) | 7.00 (5.00–12.00) | .005 |
| Length of stay in ICU (d) | 0.00 (0.00–4.00) | 0.00 (0.00–0.00) | 5.00 (3.00–8.00) | <.001 |
| Coagulation impairment, n (%) | 20 (14.49) | 6 (5.71) | 14 (42.42) | <.001 |
| Rhabdomyolysis, n (%) | 8 (5.80) | 6 (5.71) | 2 (6.06) | .941 |
| Mortality, n (%) | 10 (7.25) | 5 (4.76) | 5 (15.15) | .045 |
| Central nervous system damage at discharge, n (%) | 16 (11.59) | 9 (8.57) | 7 (21.21) | .048 |
NEWS: National Early Warning Score; ED: emergency department; ICU: intensive care unit; RRT: renal replacement therapy.
The mean volume of NS infusion in the ED was 3.02 ± 1.45 L. During hospitalization, 33 patients (23.9%) suffered from AKI, 7 patients (5.07%) needed CRRT, 52 patients (37.68%) were admitted to the ICU, and 10 patients (7.25%) died from all causes. Compared with non-AKI patients, AKI patients had a faster heart rate, lower systolic pressure, lower Glasgow Coma Scale (GCS) score, and higher NEWS. After cooling, AKI patients had higher body temperature at 0.5 h and 2 h. They also had higher levels of creatinine and lactic acid. Patients with AKI were more likely to use vasoactive drugs, receive RRT, be admitted to the ICU, suffer from central nervous system damage at discharge, and have a longer length of stay in the hospital and ICU.
The association between the NS infusion volume and the incidence of AKI was revealed by univariate and multivariate logistic regression (Table 2). As a continuous variable, each liter increase in NS administered was associated with a 46% increase in the odds of AKI in univariate analysis (OR, 1.46; 95% CI, 1.12–1.92; p = .005). In the multivariate model, this relationship between the NS infusion volume and the incidence of AKI appeared more significant (OR, 2.51; 95% CI, 1.43–4.40; p = .001). The finding was consistent in the random forest imputation cohort (Supplementary Table S5). In addition, all participants were stratified into two groups according to the volume of NS infusion (>2.5 L and ≦2.5 L). The OR of the incidence of AKI was significantly higher in the high volume group than in the low volume group in univariate and adjusted model logistic regression analyses (p < .05).
Table 2.
Logistic regression of normal saline infusion volume in emergency department for acute kidney injury in patients with heat stroke.
| Volume of normal saline infusion | Acute kidney injury |
|||||
|---|---|---|---|---|---|---|
| Univariate |
Adjust model I |
Adjust model II |
||||
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Per 1 L increase | 1.46 (1.12, 1.92) | .005 | 1.39 (1.03, 1.89) | .033 | 2.51 (1.43, 4.40) | .001 |
| ≦2.5 L | Reference | Reference | Reference | |||
| >2.5 L | 4.25 (1.62, 11.14) | .003 | 5.29 (1.66, 16.85) | .004 | 59.90 (7.55, 475.08) | <.001 |
NEWS: National Early Warning Score; OR: odds ratio; CI: confidence interval.
Adjust model I adjusted for sex, age, type of heat stroke, and creatinine. Adjust model II adjusted for institution, NEWS, creatinine, troponin I, lactic acid, chlorine, coronary heart disease, use of vasoactive drugs, and length of stay in emergency department.
The relationships between the NS volume and use of CRRT, admission to the ICU, length of stay in the ICU and hospital, and hospital mortality were revealed by univariate and multivariate regression (Table 3). With increasing volume of NS, the incidence of ICU admission increased, with an OR of 3.46 (95% CI, 1.58–7.54; p = .02), while the length of stay in the ICU and hospital also increased, with β values of 1.00 days (95% CI, 0.44–1.56; p < .001) and 1.41 days (95% CI, 0.37–2.45; p = .008), respectively. There were no differences in the use of CRRT or hospital mortality.
Table 3.
Multivariate regression of normal saline infusion in emergency department for the second outcomes in patients with heat stroke.
| Volume of normal saline infusion (L) | OR/β (95% CI) p |
||
|---|---|---|---|
| Univariate | Adjust model I | Adjust model II | |
| RRT | 0.93 (0.54, 1.61) .793 | 0.70 (0.37, 1.34) .280 | 0.40 (0.13, 1.22) .107a |
| Admitted to ICU | 1.50 (1.16, 1.94) .002 | 1.43 (1.08, 1.91) .013 | 3.46 (1.58, 7.54) .002b |
| Length of stay in ICU | 1.18 (0.70, 1.67) <.001 | 1.22 (0.70, 1.74) <.001 | 1.00 (0.44, 1.56) <.001c |
| Length of stay in hospital | 2.52 (1.74, 3.29) <.001 | 2.64 (1.85, 3.43) <.001 | 1.41 (0.37, 2.45) .008d |
| Mortality | 1.54 (1.04, 2.27) .030 | 2.08 (1.23, 3.53) .006 | 1.96 (0.72, 5.35) .189e |
NEWS: National Early Warning Score; RRT: renal replacement therapy; ICU: intensive care unit; OR: odds ratio; CI: confidence interval.
Adjust model I adjusted for sex, age, type of heat stroke, creatinine.
Adjust model II adjusted for:
Institution, NEWS, creatinine, lactic acid, and use of vasoactive drugs.
Institution, sex, age, NEWS, chlorine, creatinine, lactic acid, use of vasoactive drugs, and length of stay in emergency department.
Institution, type of heat stroke, age, NEWS, creatinine, lactic acid, use of vasoactive drugs, and length of stay in emergency department.
Institution, type of heat stroke, age, NEWS, creatinine, lactic acid, use of vasoactive drugs, and length of stay in emergency department.
Institution, type of heat stroke, sex, age, NEWS, chlorine, creatinine, troponin I, use of vasoactive drugs, and length of stay in emergency department.
Genetic and propensity score matching were used to determine the optimal balance between the NS >2.5 L and ≦2.5 L groups. In total, 108 pairs were matched. Table S6 shows the characteristics of the initial and matched cohorts, and Figure S1 is the histogram of PS for two groups. Apart from institution, all other confounders were similar in the matched cohort. Multivariate regression analysis was performed to assess the association between the volume of NS and outcomes. After adjusting for propensity scores, patients who received more than 2.5 L of NS exhibited a higher incidence of AKI (OR, 7.39; 95% CI, 3.01–18.11; p < .001), ICU admission (OR, 3.37; 95% CI, 1.91–5.97; p < .001), along with longer ICU (β, 3.06 days; 95% CI, 2.06–4.05; p < .001) and hospital stays (β, 3.70 days; 95% CI, 2.34–5.07; p < .001), compared to those who received less than 2.5 L of NS (Table 4).
Table 4.
Multivariate regression of volume of normal saline infusion with outcomes in the genetic matching cohorta.
| Volume of normal saline infusion | Univariate |
Adjust model |
||
|---|---|---|---|---|
| OR/β (95% CI) | p Value | OR/β (95% CI) | p Value | |
| ≦2.5 L | Reference | Reference | ||
| >2.5 L | ||||
| AKI | 6.35 (2.66, 15.13) | <.001 | 7.39 (3.01, 18.11) | <.001 |
| Admitted to ICU | 3.17 (1.81, 5.54) | <.001 | 3.37 (1.91, 5.97) | <.001 |
| Length of stay in ICU | 2.81 (1.78, 3.85) | <.001 | 3.06 (2.06, 4.05) | <.001 |
| Length of stay in hospital | 3.57 (2.21, 4.94) | <.001 | 3.70 (2.34, 5.07) | <.001 |
OR: odds ratio; CI: confidence interval: ICU: intensive care unit.
Adjust model adjusted for: propensity-score.
The genetic matching cohort included 108 patients with volume of normal saline infusion ≦2.5 L, and 108 volume of normal saline infusion >2.5 L.
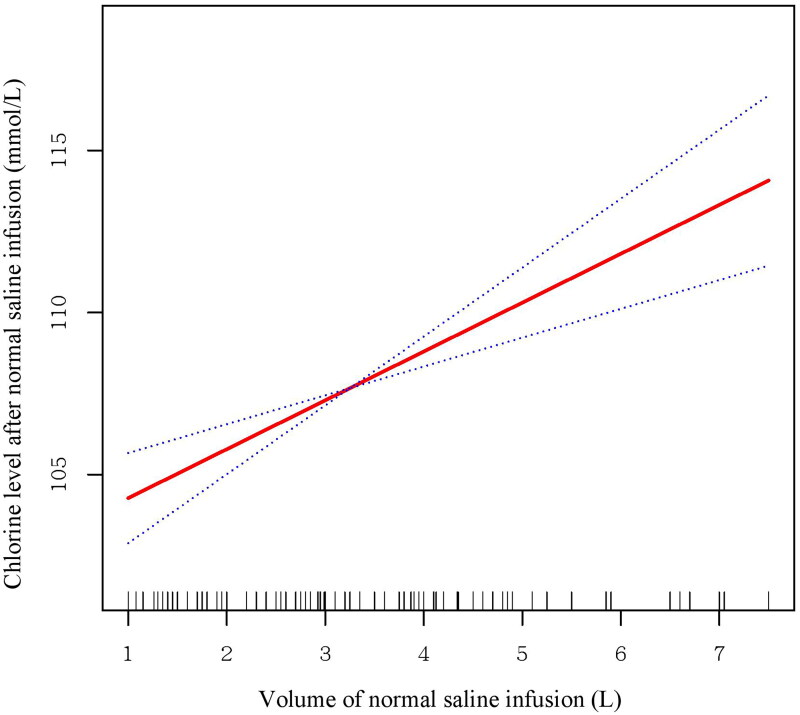
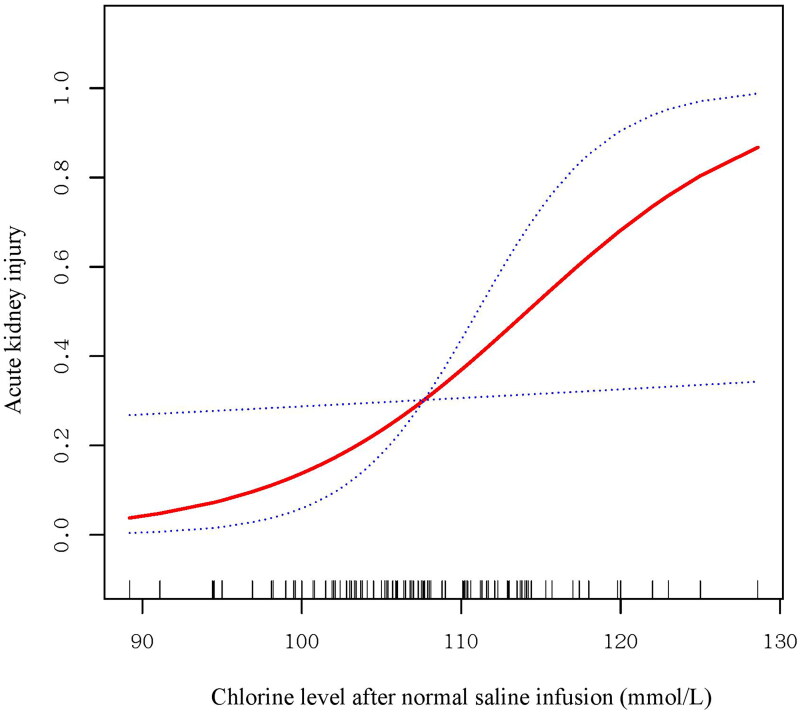
Moreover, we found that the chloride level significantly increased with increasing NS volume (Figure 2). The chloride level after NS infusion was significantly associated with the incidence of AKI (OR 1.14; 95% CI, 1.01–1.28; p = .040) (Table S7). As shown in Figure 3, the relationship between chloride and AKI showed a nearly S-shaped curve. The incidence of AKI increased sharply with increasing chloride level when the chloride level was over 100 mmol/L. However, a relationship could not be found between baseline chloride level and the incidence of AKI in either the univariate or adjusted model.
Figure 2.
Smoothing spline curve of the relationship between the NS infusion volume and serum chloride levels after saline infusion. Increasing NS infusion volume was significantly associated with increasing serum chloride levels after NS infusion.
Figure 3.
Smoothing spline curve of the association between serum chloride levels after saline infusion and AKI. The relationship between chloride and AKI showed a nearly S-shaped curve. The incidence of AKI increased sharply with increasing chloride when the chloride level was over 100 mmol/L.
Discussion
We performed a retrospective multicenter cohort study to explore the association between the volume of NS infusion in the ED and the incidence of AKI among patients with HS. We found that NS volume was associated with a significant increase in the incidence of AKI. In addition, we found that the NS infusion volume was associated with admission to the ICU and the length of stay in the ICU and hospital. Moreover, the chloride level after NS infusion was significantly associated with the incidence of AKI. These findings remained significant after adjusting for baseline variables.
HS is often accompanied by serious clinical syndromes and multiorgan dysfunction and has a high fatality rate [19, 1]. Renal dysfunction is common in both exertional HS (EHS) and classic HS (CHS) [3, 24–26]. In our study cohort, after excluding patients with significant abnormalities in the initial creatinine level, 33 (23.91%) patients suffered from AKI, 11 (21.15%) from CHS, and 22 (25.58%) from EHS. In HS management, blood purification plays a crucial role. It serves not just for AKI management but also as an efficacious intravascular cooling method. Additionally, it aids in the elimination of proinflammatory cytokines associated with HS and can benefit patients with severe HS-induced liver damage [1, ]. Consequently, two (1.9%) non-AKI patients underwent RRT in our cohort.
Renal damage in HS patients is multifactorial, involving direct thermal injury, hypovolemia, renal hypoperfusion, rhabdomyolysis, and DIC [8]. In our study’s cohort, the body temperatures at 0.5 h and 2 h for the AKI group were significantly elevated compared to the non-AKI group, highlighting the impact of hyperthermia on organ damage. Patients with AKI displayed notably more rapid heart rate, reduced systolic blood pressure, and a greater propensity for the administration of vasoactive agents, suggesting inadequate blood volume and renal hypoperfusion. Additionally, the likelihood of coagulopathy was markedly increased in AKI patients. Interestingly, rhabdomyolysis rates did not differ significantly between patients with and without AKI, possibly due to our study’s exclusion criteria. Among the 53 excluded patients, eight (15.09%) developed rhabdomyolysis, which was a higher incidence rate than in the cohort of this study.
Rapid administration of cold NS is a highly effective in vivo cooling technique for HS patients [2, ], notably decreasing body temperature and hospital stay durations [27]. This method, particularly recommended for dehydrated EHS patients, is endorsed by experts in China for its safety and efficiency [3]. Our study found that 97.1% of HS patients received this treatment. Moreover, HS often leads to hypovolemic shock due to factors like excessive sweating and diarrhea [2, 28]. Thus, intravenous isotonic crystalloids, especially sodium-containing solutions like NS, are key for effective volume resuscitation [28]. NS is also crucial for preventing myoglobin-induced renal injury in EHS patients, a group prone to rhabdomyolysis [7]. This practice is widely recommended and used in clinical settings. In our study, NS was the most common intravenous fluid used across different treatment facilities. Patients in our study received an average of 3.02 ± 1.45 L of NS in the ED, a significantly larger volume than the 1.60 ± 1.10 L reported in Self et al.’s study [9].
However, the high chloride content in NS raises concerns. NS infusion appears to increase the need for intensive care, renal failure, prolonged hospital stays, and higher mortality rates [30–34]. Compared to balanced crystalloid, NS is associated with more frequent hyperchloremic metabolic acidosis and more frequent receipt and higher overall doses of vasopressors [30]. Critically ill patients receiving saline before ICU admission show a higher incidence of death and the need for new renal replacement therapies [31]. Limiting chloride-rich infusions in the ICU can reduce AKI and CRRT usage [16]. In our study, we found that the volume of NS infusion in the ED was associated with the incidence of AKI, ICU admission, and the length of ICU stay and hospitalization, even after adjusting for baseline serum creatinine level.
NS resuscitation may lead to hyperchloremic metabolic acidosis, contributing to renal vasoconstriction and reduced renal function [33–35]. Moreover, resuscitation with NS fails to repair endothelial glycocalyx thickness, inhibits syndecan-1 shedding, decreases tissue perfusion, and increases leukocyte rolling and adhesion [34], which aggravate tissue and organ damage. We observed that chloride levels rose with increased NS volumes, which is consistent with the previous studies [9, 15]. Furthermore, the chloride level after NS infusion, not the baseline chloride level, was significantly associated with the incidence of AKI. This suggests managing chloride levels is crucial in HS treatment because hyperchloremia is closely associated with metabolic acidosis and may lead to renal vasoconstriction and decreased glomerular filtration rate. Regarding AKI, in patients with HS, it is more reasonable to control the serum chloride level under 100 mmol/L. Numerous studies suggest that balanced crystalloids outperform NS, but their effectiveness specifically for HS patients remains uncertain. This area merits further investigation to determine the most effective treatment for HS.
This study is the first to investigate the relationship between NS infusion volume in the ED and AKI incidence in HS patients. We also examined the correlation between post-infusion blood chloride levels and AKI, revealing a near S-shaped curve. Given that completely avoiding NS infusion is challenging, managing blood chloride levels may be a more practical approach. Considering NS’s prevalence in EDs, healthcare providers should be aware of its safety implications. Effective HS treatment should balance immediate improvements with potential long-term effects. However, our study has limitations. Its retrospective design allows only for association analysis, not causality. We did not account for NS volumes administered post-ICU or general ward admission, nor did we consider chloride from other solutions, which could influence outcomes. Despite several sensitivity analyses, including genetic matching and random forest imputation, unobserved confounders might exist. We also lacked data on long-term outcomes post-discharge, important for understanding AKI’s progression and implications for quality of life and survival. Lastly, without classification by Acute Kidney Disease Network criteria [35], the study could not precisely delineate the relationship between NS volume and kidney damage.
Conclusions
We conducted a multicenter retrospective cohort study to evaluate the association between the volume of NS infusion and AKI in adults with HS. We found that the volume of NS infusion was associated with a significant increase in the incidence of AKI, ICU admission, and length of stay in the ICU and hospital. Whether it would be beneficial to change chloride-rich intravenous fluids to chloride-poor fluids or to adopt a chloride-restrictive intravenous fluid strategy for HS patients needs further study.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
Lan Chen: conceptualization, visualization, formal analysis, writing – original draft, writing – review and editing; Junlu Zhao: data curation; Liyun Lu: data curation; Zhumei Gong: data curation; Shuying Xu: data curation; Xiaoling Yang: data curation; Yuping Zhang: conceptualization and methodology; Xiuqin Feng: conceptualization, methodology, validation, writing – review and editing, supervision, and project administration. The authors read and approved the final manuscript.
Ethical approval
This study was approved by the Ethics Committee of the Second Affiliated Hospital of Zhejiang University School of Medicine (approval number: 2022-0913), and the requirement for informed consent was waived.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.People’s Liberation Army Professional Committee of Critical Care Medicine . Expert consensus on standardized diagnosis and treatment for heat stroke. Mil Med Res. 2016;3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouchama A, Abuyassin B, Lehe C, et al. . Classic and exertional heatstroke. Nat Rev Dis Primers. 2022;8(1):8. doi: 10.1038/s41572-021-00334-6. [DOI] [PubMed] [Google Scholar]
- 3.Satirapoj B, Kongthaworn S, Choovichian P, et al. . Electrolyte disturbances and risk factors of acute kidney injury patients receiving dialysis in exertional heat stroke. BMC Nephrol. 2016;17(1):55. doi: 10.1186/s12882-016-0268-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Misset B, De Jonghe B, Bastuji-Garin S, et al. . Mortality of patients with heatstroke admitted to intensive care units during the 2003 heat wave in France: a National Multiple-Center Risk-Factor Study. Crit Care Med. 2006;34(4):1087–9. doi: 10.1097/01.CCM.0000206469.33615.02. [DOI] [PubMed] [Google Scholar]
- 5.Bagshaw SM, George C, Bellomo R, et al. . Changes in the incidence and outcome for early acute kidney injury in a cohort of Australian intensive care units. Crit Care. 2007;11(3):R68. doi: 10.1186/cc5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu M, Wang C, Liu Z, et al. . Clinical characteristics and risk factors associated with acute kidney injury inpatient with exertional heatstroke: an over 10-year intensive care survey. Front Med. 2021;8:678434. doi: 10.3389/fmed.2021.678434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouchama A, Knochel JP.. Heat stroke. N Engl J Med. 2002;346(25):1978–1988. doi: 10.1056/NEJMra011089. [DOI] [PubMed] [Google Scholar]
- 8.Liu SY, Song JC, Mao HD, et al. . Expert consensus on the diagnosis and treatment of heat stroke in China. Mil Med Res. 2020;7(1):1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Self WH, Semler MW, Wanderer JP, et al. . Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med. 2018;378(9):819–828. doi: 10.1056/NEJMoa1711586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith RJ, Reid DA, Delaney EF, et al. . Fluid therapy using a balanced crystalloid solution and acid–base stability after cardiac surgery. Crit Care Resus. 2010;12(4):235–241. doi: 10.1016/S1441-2772(23)01342-X. [DOI] [PubMed] [Google Scholar]
- 11.Yunos NM, Kim IB, Bellomo R, et al. . The biochemical effects of restricting chloride-rich fluids in intensive care. Crit Care Med. 2011;39(11):2419–2424. [DOI] [PubMed] [Google Scholar]
- 12.Jahangir A, Sahra S, Niazi M, et al. . Comparison of normal saline solution with low-chloride solutions in renal transplants: a meta-analysis. Kidney Res Clin Pract. 2021;40(3):484–495. doi: 10.23876/j.krcp.21.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yunos NM, Bellomo R, Story D, et al. . Bench-to-bedside review: chloride in critical illness. Crit Care. 2010;14(4):226. doi: 10.1186/cc9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quilley CP, Lin YS, McGiff JC.. Chloride anion concentration as a determinant of renal vascular responsiveness to vasoconstrictor agents. Br J Pharmacol. 1993;108(1):106–110. doi: 10.1111/j.1476-5381.1993.tb13447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Semler MW, Self WH, Wanderer JP, et al. . Balanced crystalloids versus saline in critically ill adults. N Engl J Med. 2018;378(9):829–839. doi: 10.1056/NEJMoa1711584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yunos NM, Bellomo R, Hegarty C, et al. . Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA. 2012;308(15):1566–1572. doi: 10.1001/jama.2012.13356. [DOI] [PubMed] [Google Scholar]
- 17.Zampieri FG, Machado FR, Biondi RS, et al. . Effect of intravenous fluid treatment with a balanced solution vs 0.9% saline solution on mortality in critically ill patients: the BaSICS randomized clinical trial. JAMA. 2021;326(9):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beran A, Altorok N, Srour O, et al. . Balanced crystalloids versus normal saline in adults with sepsis: a comprehensive systematic review and meta-analysis. J Clin Med. 2022;11(7):1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shu-Yuan L, Qian W, Yun-Peng L, et al. . Interpretations and comments for expert consensus on the diagnosis and treatment of heat stroke in China. Mil Med Res. 2020;7(3):371–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palevsky PM, Liu KD, Brophy PD, et al. . KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis. 2013;61(5):649–672. doi: 10.1053/j.ajkd.2013.02.349. [DOI] [PubMed] [Google Scholar]
- 21.Chen L, Zheng H, Chen L, et al. . National Early Warning Score in predicting severe adverse outcomes of emergency medicine patients: a retrospective cohort study. J Multidiscip Healthc. 2021;14:2067–2078. doi: 10.2147/JMDH.S324068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barry R, James MT.. Guidelines for classification of acute kidney diseases and disorders. Nephron Clin Pract. 2015;131(4):221–226. doi: 10.1159/000441425. [DOI] [PubMed] [Google Scholar]
- 23.Alexis D, Jasjeet SS.. Genetic matching for estimating causal effects: a general multivariate matching method for achieving balance in observational studies. Rev Econ Stat. 2013;95(3):932–945. [Google Scholar]
- 24.Fan H, Zhao Y, Zhu JH, et al. . Thrombocytopenia as a predictor of severe acute kidney injury in patients with heat stroke. Ren Fail. 2015;37(5):877–881. doi: 10.3109/0886022X.2015.1022851. [DOI] [PubMed] [Google Scholar]
- 25.Donham BP, Frankfurt SB, Cartier RA, et al. . Low incidence of death and renal failure in United States military service members hospitalized with exertional heat stroke: a retrospective cohort study. Mil Med. 2020;185(Suppl. 1):362–367. doi: 10.1093/milmed/usz214. [DOI] [PubMed] [Google Scholar]
- 26.Wang C, Yu B, Chen R, et al. . Association of D-dimer and acute kidney injury associated with rhabdomyolysis in patients with exertional heatstroke: an over 10-year intensive care survey. Ren Fail. 2021;43(1):1561–1568. doi: 10.1080/0886022X.2021.2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mok G, Degroot D, Hathaway NE, et al. . Exertional heat injury: effects of adding cold (4 °C) intravenous saline to prehospital protocol. Curr Sports Med Rep. 2017;16(2):103–108. doi: 10.1249/JSR.0000000000000345. [DOI] [PubMed] [Google Scholar]
- 28.Leon LR, Bouchama A.. Heat stroke. Compr Physiol. 2015;5(2):611–647. doi: 10.1002/cphy.c140017. [DOI] [PubMed] [Google Scholar]
- 29.Lee A, Ko C, Buitrago C, et al. . Lactated ringers vs normal saline resuscitation for mild acute pancreatitis: a randomized trial. Gastroenterology. 2021;160(3):955–957.e4. doi: 10.1053/j.gastro.2020.10.044. [DOI] [PubMed] [Google Scholar]
- 30.Pfortmueller CA, Funk GC, Reiterer C, et al. . Normal saline versus a balanced crystalloid for goal-directed perioperative fluid therapy in major abdominal surgery: a double-blind randomised controlled study. Br J Anaesth. 2018;120(2):274–283. doi: 10.1016/j.bja.2017.11.088. [DOI] [PubMed] [Google Scholar]
- 31.Semler MW, Wanderer JP, Ehrenfeld JM, et al. . Balanced crystalloids versus saline in the intensive care unit. The SALT randomized trial. Am J Respir Crit Care Med. 2017;195(10):1362–1372. doi: 10.1164/rccm.201607-1345OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Semler MW, Kellum JA.. Balanced crystalloid solutions. Am J Respir Crit Care Med. 2019;199(8):952–960. doi: 10.1164/rccm.201809-1677CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilcox CS. Regulation of renal blood flow by plasma chloride. J Clin Invest. 1983;71(3):726–735. doi: 10.1172/jci110820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Torres LN, Chung KK, Salgado CL, et al. . Low-volume resuscitation with normal saline is associated with microvascular endothelial dysfunction after hemorrhage in rats, compared to colloids and balanced crystalloids. Crit Care. 2017;21(1):160. doi: 10.1186/s13054-017-1745-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehta RL, Kellum JA, Shah SV, et al. . Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.