ABSTRACT
With the widespread vaccination of COVID-19 vaccine, a few cases have been reported that COVID-19 vaccine may cause endocrine disorders. A 59-y-old man presented with a loss of appetite after the first COVID-19 vaccination, which resolved spontaneously after 3 d. After the second COVID-19 vaccination, the symptoms including the loss of appetite, nausea, and vomiting reappeared and worsened along with loss of vision. He was found to have severe hyponatremia, and further investigations revealed secondary adrenal insufficiency, secondary hypothyroidism and Rathke’s cleft cyst. The patient responded well to glucocorticoid and levothyroxine supplementation, and at 1-y follow-up the patient developed hypogonadism. We hypothesize that hypophysitis is probably induced by COVID-19 vaccine and report the rare but serious adverse reactions for early recognition and intervention.
KEYWORDS: COVID-19 vaccination, Rathke’s cleft cyst, hypophysitis, hyponatremia, adverse reaction
Introduction
A novel coronavirus disease (COVID-19) has become a major threat to global health. The pathogen is a novel enveloped RNAβ-coronavirus 2 that has been named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1 SARS-CoV-2 enters cells of human tissue through angiotensin-converting enzyme 2 (ACE2) receptor,2,3 and mainly infects the lungs, leading to viral pneumonia, often complicated by acute respiratory distress syndrome and sepsis. Timely and appropriate vaccination is the key to preventing COVID-19. Several types of COVID-19 vaccines, such as mRNA, adenoviral vectors and inactivated vaccines, have been marketed in various countries to reduce infection and mortality rates. It is not clear whether there is an increased risk of endocrine disease morbidity after vaccination, but some endocrine diseases were reported after vaccination, such as type 1 diabetes mellitus,4 thyroiditis,5 Graves’ disease,6 AVP deficiency (central urolithiasis),7 optic neuritis and pituitary inflammation comorbidities,8,9 and even adrenal crisis secondary to COVID-19 vaccination in patients with hypopituitarism.10 In this paper, we report a case of hypophysitis after COVID-19 vaccination in a patient with Rathke’s cleft cyst to raise attention to the rare but serious adverse reactions for early recognition and intervention.
Patient presentation
A 59-y-old man who was diagnosed with space-occupying lesion of unknown nature in sella turcica area 30 y ago suffered from loss of appetite after receiving the first COVID-19 vaccination, which resolved 3 d later. Twenty-six days later, he received the second COVID-19 vaccination. The symptoms of decreased appetite had worsened compared to the previous one, along with loss of vision, nausea, and vomiting, and he came to the clinic with a pulse rate of 58 beats per minute, a blood pressure of 114/68 mmHg, and a body weight of 83 Kg. He looked tired and spoke slowly. The skin was dry, there were no defects in the visual fields, the thyroid was not swollen, the hair distribution was normal, there was no mucous edema, and the physical examination was otherwise unremarkable.
Laboratory evaluation showed a serum sodium level of 116 mmol/L (reference range 137–147 mmol/L) suggestive of severe hyponatremia. Alanine aminotransferase (ALT) 53 U/L (reference range 5–40 U/L), aspartate aminotransferase (AST) 83 U/L (reference range 0–40 U/L), hemoglobin 127 g/L (reference range 120–160 g/L), serum potassium and the function of kidney was normal. Further laboratory tests of thyroid function suggested: triiodothyronine 0.77 nmol/L (reference range 0.92–2.79), thyroxine 58.00 nmol/L (reference range 58.10–165.20), free triiodothyronine 2.49 pmol/l (reference range 3.50–6.50), free thyroxine 9.50 pmol/l (reference range 10.00–23.00), thyrotropin 1.933 mlU/L (reference range 0.400–6.710), consistent with central hypothyroidism. Serum cortisol at 8am was 134.56 nmol/L (reference range 198.7–797.5), adrenocorticotropic hormone level at 8am was 5.51 pmol/L (reference range < 10.12), and 24-hour urinary UFC less than 13.79 nmol/24 h (reference range 98.0–500.1), suggesting secondary adrenal insufficiency. Testosterone level was 20.00 nmol/L (reference range 3.02–27.07), prolactin 98.74 uIU/mL (reference range 44.52–375.24), luteinizing hormone (LH) 1.07mIU/mL (reference range 1.50–9.30), and follicle-stimulating hormone (FSH) 1.76mIU/mL (reference range 1.40–18.10). The low level of LH and FSH indicated the gonadal axis may also had been impaired, although the patient did not complain of low libido or erectile dysfunction. IGF-1 level was 30.35 ng/mL (reference range 60–350), and growth hormone 0.030 ng/mL, indicating the growth hormone axis damaged. Enhanced MRI of the saddle region displayed a cystic lesion within the sella, indicating Rathke’s cleft cyst (RCC), no thickening of pituitary stalk (Figure 1).
Figure 1.
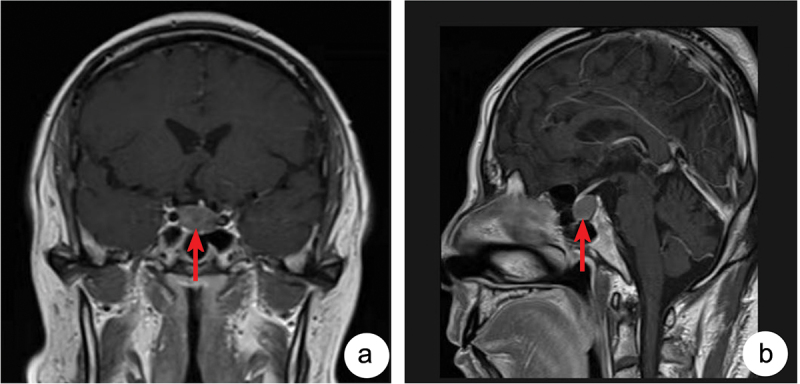
Enhanced MRI of the saddle region displayed a cystic lesion within the sella (red arrow indication) 12 × 12 × 18 mm. A:Coronal plane; B:Sagittal plane.
He was diagnosed with hypophysitis (secondary adrenal insufficiency, secondary hypothyroidism, growth hormone deficiency) and Rathke’s cleft cyst.
The patient was told that he might need to take prednisone and levothyroxine throughout his life, and he might need the surgical intervention of the Rathke’s cleft cyst. The patient was willing to receive medication but refused surgical treatment. He was given oral prednisone tablets of 5 mg and levothyroxine sodium tablets of 50ug per d. His blood sodium level gradually increased to normal, his appetite improved, and his symptoms of fatigue disappeared.
The patient was on regular follow-up. He appeared healthy without weight change, and his laboratory tests for serum sodium and glucose were in the normal range. At 1 y follow-up, his thyroid function was within normal range, his testosterone level decreased to 4.19 nmol/L with luteinizing hormone of 1.29 mIU/mL and follicle-stimulating hormone of 1.99 mIU/mL indicating secondary hypogonadism. The patient had no symptoms of polyuria or polydipsia. He had developed the whole anterior pituitary hypofunction without the involvement of the posterior pituitary.
Discussion
China’s COVID-19 vaccine is inactivated vaccine with high safety. Common adverse reactions are fever, fatigue, and loss of appetite. Most of the symptoms are mild and can be relieved on their own without medication. As Taieb A mentioned in five cases, the pituitary disorder developed after the first dose of the corresponding vaccine. Regarding the types of pituitary disorder, five were hypophysitis (variable clinical aspects ranging from pituitary lesion to pituitary stalk thickness) and three were pituitary apoplexy. The time period between vaccination and pituitary disorder ranged from 1 to 7 d. Depending on each case’s follow-up time, a complete remission was obtained in all the apoplexy cases but in only three patients with hypophysitis (persistence of the central diabetes insipidus).11 The patient‘s symptoms after the first vaccination were considered as an adverse reaction instead of hypopituitarism. He was not taken seriously until the occurrence of pituitary crisis was diagnosed. However, our patient did not obtain a complete remission and progressed to complete pituitary dysfunction without diabetes insipidus 1 y later.
Most patients have an insidious onset and slow progression of hypopituitarism, and the clinical manifestations are related to the rapidity and extent of the pituitary lesions. Infections, trauma, vomiting, diarrhea, dehydration, starvation, cold and other stressful situations can induce pituitary crisis, which is life-threatening. This patient had a history of RCC, which is a congenital developmental anomaly. RCCs are non-growing or extremely slow-growing, with a mean diameter of usually <10 mm, most of which are clinically unremarkable, and often occupy the saddle space but may extend upwards in larger sizes. The clinical symptoms of RCC are mainly caused by localized occupations compressing adjacent important structures. Common symptoms include headache, visual field disorders, and pituitary endocrine dysfunction. The patient never complained of any discomfort before. Excluding other stress factors, we considered that the current occurrence of hypophysitis was directly or indirectly related to the COVID-19 vaccination. Hypophysitis can potentially manifest with one or more of the following four clinical features: mass effects such as headaches and visual symptoms; symptoms of deficiency of anterior pituitary hormones; central diabetes insipidus; and hyperprolactinemia. This patient only had pituitary dysfunction, no diabetes insipidus, no pituitary enlargement, and no hyperprolactinemia.
After the vaccine became available in December 2020, a number of adverse reactions to the vaccine had been reported, including endocrine impairment.12,13 Endocrine dysfunction after SARS-CoV-2 vaccination reported in the literature mainly involves the thyroid, pituitary and adrenal glands. In the thyroid gland, the most common disorder was subacute thyroiditis,5 followed by autoimmune thyroid disease, newly diagnosed or relapsed Graves’ disease,6 in addition to cases of painless thyroiditis,14 totaling more than 100 cases. Severe hyponatremia has been reported after immunization with COVID-19 vaccine,15,16 which was later confirmed to be syndrome of inappropriate antidiuresis.16 Murvelashvili N reported a case of pituitary inflammation following immunization with the mRNA-1273 SARS-CoV-2 vaccine, which is the first reported case of hypopituitarism possibly associated with immunization against COVID-19.9 Morita S reported the case of isolated adrenocorticotropic hormone deficiency and noted that rare but urgent adrenal crises might occur in patients with symptoms of common side effects after the new vaccine and pointed that endocrine adverse reactions might be induced even after injecting inactivated SARS-CoV-2 mRNA vaccine because of the disruption of the autoimmune system by adjuvants in the mRNA vaccine.17 Especially,the risk of post-vaccination pituitary damage increased in patients with previous pituitary adenomas or previous asymptomatic COVID-19 infection.18,19
Based on the fact that vaccine adjuvants have been widely used in human vaccines to enhance the immune response to vaccination, the critical role of adjuvants in augmenting adverse immune responses has been highlighted in recent reports,20,21 and in genetically susceptible individuals, adjuvants may disrupt the host’s immune homeostasis and trigger polyclonal activation of B lymphocytes through molecular mimicry or other similar pathogenic mechanisms.21
We hypothesized that the possible reasons of hypophysitis after vaccination as following. Firstly, induction by adjuvants added to the COVID-19 vaccine, which can enhance the body’s immune response to antigens or change the type of immune response to keep the body in an optimal state of immunity, but may likewise induce an immune response in susceptible individuals,22,23 and hypopituitarism occurs. Secondly, the virus itself had been shown in the literature to be responsible for triggering inflammatory and autoimmune diseases.24,25 The expression of ACE2 in the hypothalamus was confirmed, making it a probable target for SARS-CoV-2.26 A COVID-19-infected patient may present with panhypopituitarism or pituitary apoplexy. The concentration of viral protein may reach a certain peak a few days after vaccination, triggering an autoimmune response9 or systemic inflammatory response.27 Thirdly, pituitary hormone dysfunction can occur in patients with Rathke’s cleft cysts, which can be manifested clinically as hyperpituitary, hypopituitary, or normal pituitary function, which is closely related to the degree of physical compression and chemical stimulation of pituitary tissues by the RCC. This patient had a history of RCC. He usually lived and worked normally with no symptoms, but the diameter of the cyst was >10 mm, which had some compression on the pituitary tissue, and there might be an underlying impaired adeno-pituitary function, which was in the compensatory phase of daily life. The vaccine as a stress factor-induced hypophysitis after the vaccination. The three factors mentioned above might work together to cause pituitary crisis. Pharmacovigilance for post-vaccination must be patient-oriented and focus on the health of patients.28
In conclusion, COVID-19 vaccination may induce hypophysitis directly or indirectly. We should carefully observe the adverse reactions after vaccine injection and evaluate endocrine function if necessary, especially in patients with severe hyponatremia and hypotension to early recognize the rare but serious adverse reactions of COVID-19 vaccine.
Acknowledgments
We thank the patient for allowing us to share his details.
Funding Statement
The work was supported by Air Force Logistics Department under Grant number BKJ20J004.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Authors’ contributions
Conceptualization, D.Z.; Investigation, Y.Y.Y., G.X.Z., and H.J.Z.; writing – original draft presentation, Y.Y.Y., and G.X.Z.; writing – review and editing, D.Z., and J.J.D.; visualization, H.J.G., and Y.M.B.; supervision, D.Z.
Ethical considerations
The patient signed written consent for publishing his information.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–4. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu N-H, Nitsche A, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ziegler CGK, Allon SJ, Nyquist SK, Mbano IM, Miao VN, Tzouanas CN, Cao Y, Yousif AS, Bals J, Hauser BM, et al. HCA lung biological network. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181(5):1016–1035.e19. doi: 10.1016/j.cell.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aydoğan Bİ, Ünlütürk U, Cesur M.. Type 1 diabetes mellitus following SARS-CoV-2 mRNA vaccination. Endocrine. 2022. Oct;78(1):42–46. doi: 10.1007/s12020-022-03130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Şendur SN, Oğuz SH, Ünlütürk U. COVID-19 vaccination and thyroiditis. Best Pract Res Clin Endocrinol Metab. 2023. Jul;37(4):101759. doi: 10.1016/j.beem.2023.101759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taieb A, Sawsen N, Asma BA, Ghada S, Hamza E, Yosra H, Amel M, Molka C, Maha K, Koussay A, et al. A rare case of grave’s disease after SARS-CoV-2 vaccine: is it an adjuvant effect? Eur Rev Med Pharmacol Sci. 2022. Apr;26(7):2627–2630. doi: 10.26355/eurrev_202204_28500. [DOI] [PubMed] [Google Scholar]
- 7.Partenope C, Pedranzini Q, Petri A, Rabbone I, Prodam F, Bellone S. AVP deficiency (central diabetes insipidus) following immunization with anti-COVID-19 BNT162b2 Comirnaty vaccine in adolescents: a case report. Front Endocrinol (Lausanne). 2023;14:1166953. doi: 10.3389/fendo.2023.1166953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsuo T, Okubo K, Mifune H, Imao T. Bilateral optic neuritis and hypophysitis with diabetes insipidus 1 month after COVID-19 mRNA vaccine: case report and literature review. J Invest Med High Impact Case Rep. 2023. Jan-Dec;11:23247096231186046. doi: 10.1177/23247096231186046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murvelashvili N, Tessnow A. A case of hypophysitis following immunization with the mRNA-1273 SARS-CoV-2 vaccine. J Invest Med High Impact Case Rep. 2021. Jan-Dec;9:23247096211043386. doi: 10.1177/23247096211043386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Markovic N, Faizan A, Boradia C, Nambi S. Adrenal crisis secondary to COVID-19 vaccination in a patient with hypopituitarism. AACE Clin Case Rep. 2022;8(4):171–3. doi: 10.1016/j.aace.2022.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taieb A, Mounira EE. Pilot findings on SARS-CoV-2 vaccine-induced pituitary diseases: a mini review from diagnosis to pathophysiology. Vaccines. 2022;10:2004. doi: 10.3390/vaccines10122004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menni C, Klaser K, May A, Polidori L, Capdevila J, Louca P, Sudre CH, Nguyen LH, Drew DA, Merino J, et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID symptom study app in the UK: a prospective observational study. Lancet Infect Dis. 2021;21(7):939–949. doi: 10.1016/S1473-3099(21)00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schimmel J, Alba EL, Chen A, Russell M, Srinath R. Letter to the editor: thyroiditis and thyrotoxicosis after the SARS-CoV-2 mRNA vaccine. Thyroid. 2021;31(9):1440. doi: 10.1089/thy.2021.0184. [DOI] [PubMed] [Google Scholar]
- 14.Siolos A, Gartzonika K, Tigas S. Thyroiditis following vaccination against COVID-19: report of two cases and review of the literature. Metabol Open. 2021. Dec;12:100136. doi: 10.1016/j.metop.2021.100136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chienwichai K, Sriinkua P, Chang A. Symptomatic hyponatremia after ChAdOx1 nCoV-19 coronavirus disease-19 vaccination. Clin Nephrol. 2022;98(3):162–166. doi: 10.5414/CN110906. [DOI] [PubMed] [Google Scholar]
- 16.Lindner G, Ryser B. The syndrome of inappropriate antidiuresis after vaccination against COVID-19: case report. BMC Infect Dis. 2021;21(1):1000. doi: 10.1186/s12879-021-06690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morita S, Tsuji T, Kishimoto S, Uraki S, Takeshima K, Iwakura H, Furuta H, Nishi M, Inaba H, Matsuoka T-A, et al. Isolated ACTH deficiency following immunization with the BNT162b2 SARS-CoV-2 vaccine: a case report. BMC Endocr Disord. 2022;22(1):185. doi: 10.1186/s12902-022-01095-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aliberti L, Gagliardi I, Rizzo R, Bortolotti D, Schiuma G, Franceschetti P, Gafà R, Borgatti L, Cavallo MA, Zatelli MC, et al. Pituitary apoplexy and COVID-19 vaccination: a case report and literature review. Front Endocrinol (Lausanne). 2022. Nov 17;13:1035482. doi: 10.3389/fendo.2022.1035482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taieb A, Asma BA, Mounira EE. Evidences that SARS-CoV-2 vaccine-induced apoplexy may not be solely due to ASIA or VITT syndrome’, commentary on pituitary apoplexy and COVID-19 vaccination: a case report and literature review. Front Endocrinol (Lausanne). 2023. Jan 25;14:1111581. doi: 10.3389/fendo.2023.1111581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.İ̇remli BG, Şendur SN, Ünlütürk U. Three cases of subacute thyroiditis following SARS-CoV-2 vaccine: postvaccination ASIA syndrome. J Clin Endocrinol Metab. 2021;106(9):2600–2605. doi: 10.1210/clinem/dgab373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bragazzi NL, Hejly A, Watad A, Adawi M, Amital H, Shoenfeld Y. ASIA syndrome and endocrine autoimmune disorders. Best Pract Res Clin Endocrinol Metab. 2020;34(1):101412. doi: 10.1016/j.beem.2020.101412. [DOI] [PubMed] [Google Scholar]
- 22.Liang Z, Zhu H, Wang X, Jing B, Li Z, Xia X, Sun H, Yang Y, Zhang W, Shi L, et al. Adjuvants for coronavirus vaccines. Front Immunol. 2020 Nov 6;11:589833. doi: 10.3389/fimmu.2020.589833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garvey LH, Nasser S. Anaphylaxis to the first COVID-19 vaccine: is polyethylene glycol (PEG) the culprit? Br J Anaesth. 2021;126(3):e106–e108. doi: 10.1016/j.bja.2020.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mirza SA, Sheikh AAE, Barbera M, Ijaz Z, Javaid MA, Shekhar R, Pal S, Sheikh AB. COVID-19 and the endocrine system: a review of the current information and misinformation. Infect Dis Rep. 2022. Mar 11;14(2):184–97. doi: 10.3390/idr14020023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gorchane A, Ach T, Sahli J, Abdelkrim AB, Mallouli M, Bellazreg F, Hachfi W, Chaieb MC, Ach K. Uncovering the alarming rise of diabetic ketoacidosis during COVID-19 pandemic: a pioneer African study and review of literature. Front Endocrinol (Lausanne). 2023. Jul 26;14:1234256. doi: 10.3389/fendo.2023.1234256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chigr F, Merzouki M, Najimi M. Autonomic brain centers and pathophysiology of COVID-19. ACS Chem Neurosci. 2020 Jun 3;11(11):1520–1522. doi: 10.1021/acschemneuro.0c00265. [DOI] [PubMed] [Google Scholar]
- 27.Liu B, Li M, Zhou Z, Guan X, Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J Autoimmun. 2020. Jul;111:102452. doi: 10.1016/j.jaut.2020.102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montastruc JL. Pharmacovigilance and drug safety: fair prescribing and clinical research. Therapie. 2022. May-Jun;77(3):261–3. doi: 10.1016/j.therap.2022.03.001. [DOI] [PubMed] [Google Scholar]


