Abstract
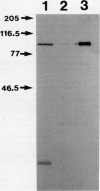
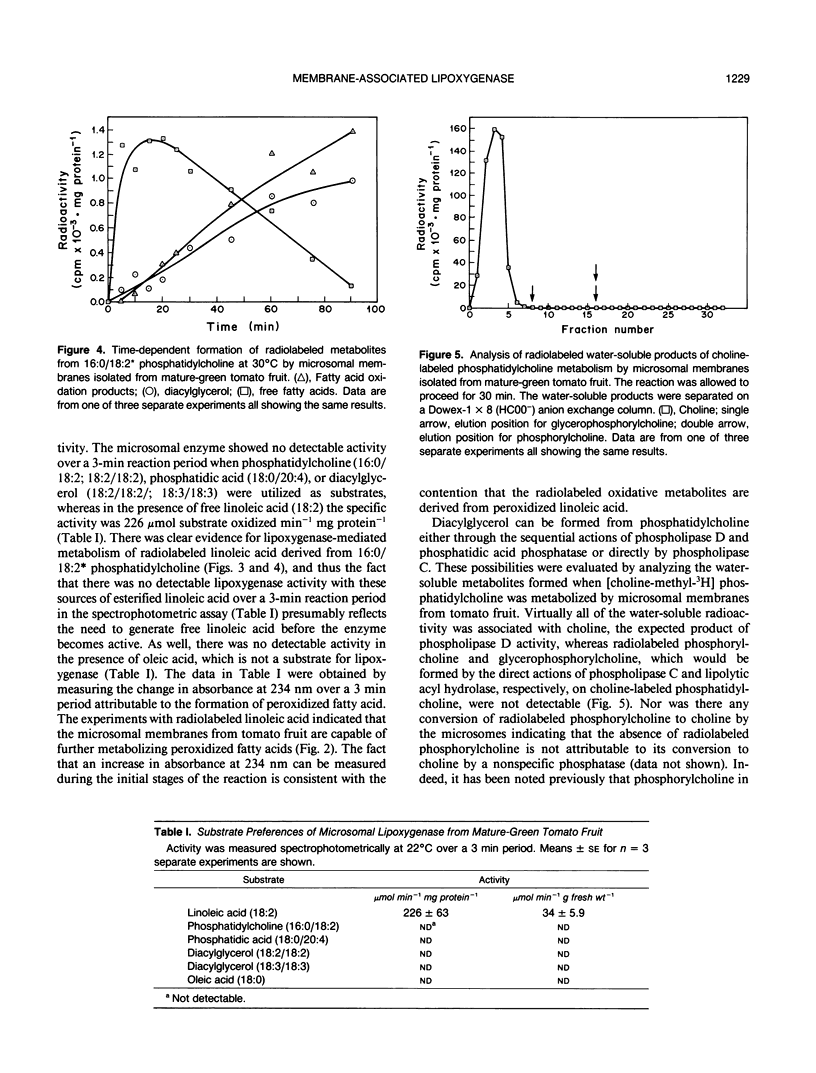
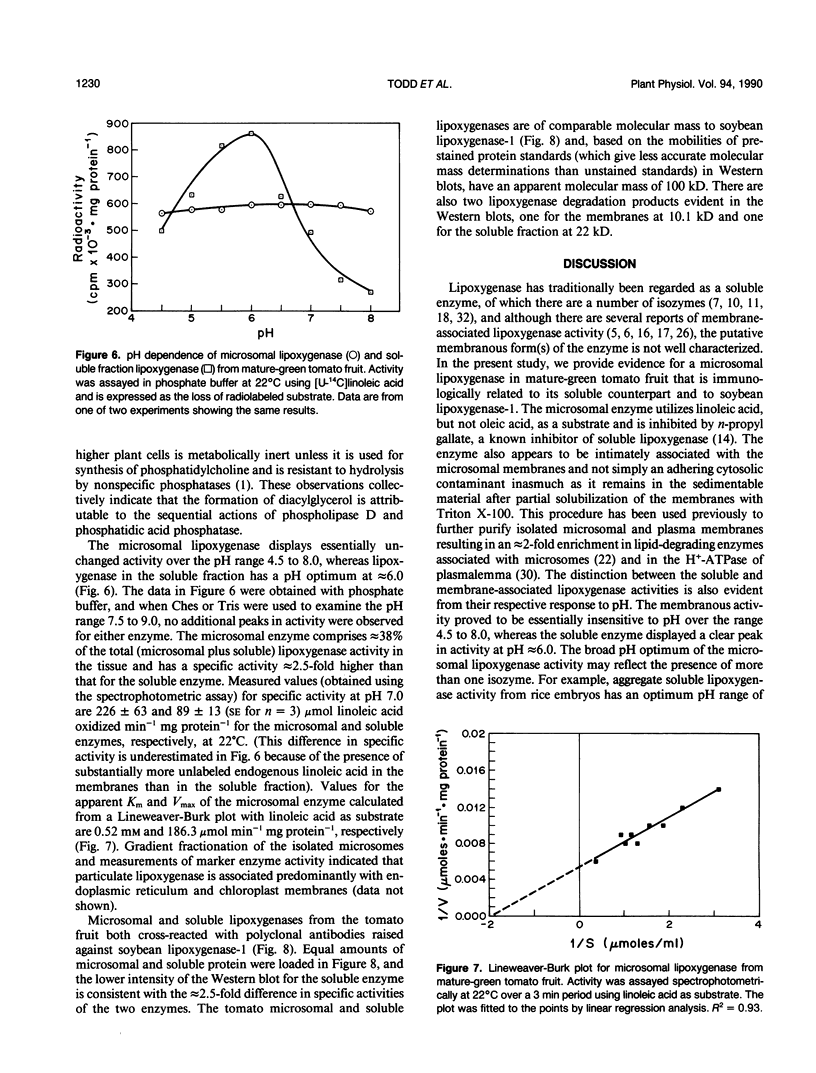
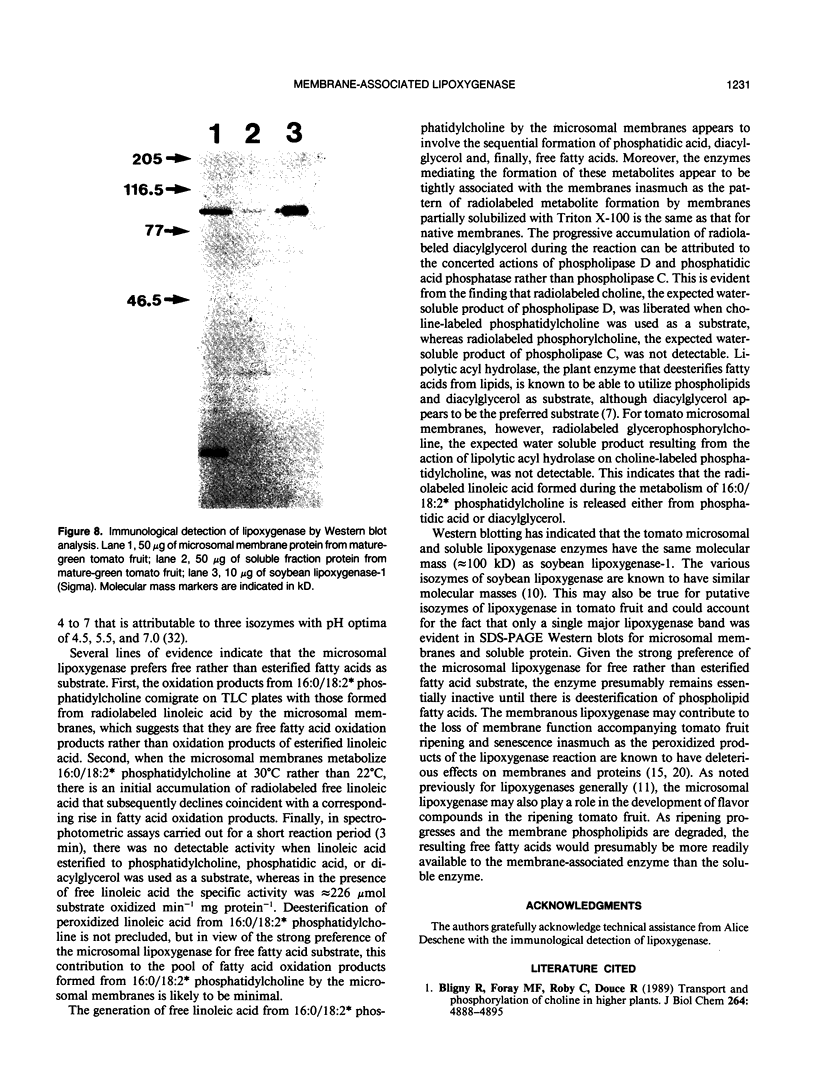
Microsomal membranes isolated from the pericarp of maturegreen tomato (Lycopersicon esculentum) fruit rapidly metabolize exogenous radiolabeled linoleic acid into fatty acid oxidation products at 22°C. The reaction is strongly inhibited by n-propyl gallate, an inhibitor of lipoxygenase. The membranes also rapidly metabolize 16:0/18:2* phosphatidylcholine into radiolabeled oxidation products that comigrate on TLC plates with those formed from free linoleic acid. At 30°C, the formation of fatty acid oxidation products from 16:0/18:2* phosphatidylcholine is slower, and there is an initial accumulation of radiolabeled linoleic acid that is not evident at 22°C, which can be attributed to the action of lipolytic acyl hydrolase. Radiolabeled phosphatidic acid and diacylglycerol are also formed during metabolism of 16:0/18:2* phosphatidylcholine by the microsomal membranes, and there is no breakdown of either linoleic acid or phosphatidylcholine by heat-denatured membranes. When Triton X-100 treated membranes were used, the same patterns of metabolite formation from radiolabeled linoleic acid and 16:0/18:2* phosphatidylcholine were observed. Thus, the enzymes mediating the breakdown of these radiolabeled compounds appear to be tightly associated with the membranes. Collectively, the data indicate that there is a lipoxygenase associated with microsomal membranes from tomato fruit that utilizes free fatty acid substrate released from phospholipids. The microsomal lipoxygenase is strongly active over a pH range of 4.5 to 8.0, comprises approximately 38% of the total (microsomal plus soluble) lipoxygenase activity in the tissue, has an apparent Km of 0.52 millimolar and an apparent Vmax of 0.186 millimoles per minute per milligram of protein. The membranous enzyme also cross-reacts with polyclonal antibodies raised against soybean lipoxygenase-1 and has an apparent molecular mass of 100 kilodaltons.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bligny R., Douce R. Calcium-dependent lipolytic acyl-hydrolase activity in purified plant mitochondria. Biochim Biophys Acta. 1978 Jun 23;529(3):419–428. doi: 10.1016/0005-2760(78)90086-3. [DOI] [PubMed] [Google Scholar]
- Bligny R., Foray M. F., Roby C., Douce R. Transport and phosphorylation of choline in higher plant cells. Phosphorus-31 nuclear magnetic resonance studies. J Biol Chem. 1989 Mar 25;264(9):4888–4895. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Fobel M., Lynch D. V., Thompson J. E. Membrane deterioration in senescing carnation flowers : coordinated effects of phospholipid degradation and the action of membranous lipoxygenase. Plant Physiol. 1987 Sep;85(1):204–211. doi: 10.1104/pp.85.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk M. O., Whitney M. A., Hausknecht E. C., O'Brien E. M. Resolution of the isoenzymes of soybean lipoxygenase using isoelectric focusing and chromatofocusing. Anal Biochem. 1985 Apr;146(1):246–251. doi: 10.1016/0003-2697(85)90422-1. [DOI] [PubMed] [Google Scholar]
- Jensenius J. C., Andersen I., Hau J., Crone M., Koch C. Eggs: conveniently packaged antibodies. Methods for purification of yolk IgG. J Immunol Methods. 1981;46(1):63–68. doi: 10.1016/0022-1759(81)90333-1. [DOI] [PubMed] [Google Scholar]
- Kanofsky J. R., Axelrod B. Singlet oxygen production by soybean lipoxygenase isozymes. J Biol Chem. 1986 Jan 25;261(3):1099–1104. [PubMed] [Google Scholar]
- Leibowitz M. E., Johnson M. C. Relation of lipid peroxidation to loss of cations trapped in liposomes. J Lipid Res. 1971 Nov;12(6):662–670. [PubMed] [Google Scholar]
- Mack A. J., Peterman T. K., Siedow J. N. Lipoxygenase isozymes in higher plants: biochemical properties and physiological role. Isozymes Curr Top Biol Med Res. 1987;13:127–154. [PubMed] [Google Scholar]
- Martin T. W. Formation of diacylglycerol by a phospholipase D-phosphatidate phosphatase pathway specific for phosphatidylcholine in endothelial cells. Biochim Biophys Acta. 1988 Oct 14;962(3):282–296. doi: 10.1016/0005-2760(88)90258-5. [DOI] [PubMed] [Google Scholar]
- Matsushita S. Specific interactions of linoleic acid hydroperoxides and their secondary degraded products with enzyme proteins. J Agric Food Chem. 1975 Mar-Apr;23(2):150–154. doi: 10.1021/jf60198a049. [DOI] [PubMed] [Google Scholar]
- Paliyath G., Thompson J. E. Calcium- and calmodulin-regulated breakdown of phospholipid by microsomal membranes from bean cotyledons. Plant Physiol. 1987 Jan;83(1):63–68. doi: 10.1104/pp.83.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paliyath G., Thompson J. E. Senescence-Related Changes in ATP-Dependent Uptake of Calcium into Microsomal Vesicles from Carnation Petals. Plant Physiol. 1988 Oct;88(2):295–302. doi: 10.1104/pp.88.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterman T. K., Siedow J. N. Behavior of Lipoxygenase during Establishment, Senescence, and Rejuvenation of Soybean Cotyledons. Plant Physiol. 1985 Aug;78(4):690–695. doi: 10.1104/pp.78.4.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterman T. K., Siedow J. N. Immunological comparison of lipoxygenase isozymes-1 and -2 with soybean seedling lipoxygenases. Arch Biochem Biophys. 1985 May 1;238(2):476–483. doi: 10.1016/0003-9861(85)90190-0. [DOI] [PubMed] [Google Scholar]
- Reddanna P., Whelan J., Reddy P. S., Reddy C. C. Isolation and characterization of 5-lipoxygenase from tulip bulbs. Biochem Biophys Res Commun. 1988 Dec 30;157(3):1348–1351. doi: 10.1016/s0006-291x(88)81023-4. [DOI] [PubMed] [Google Scholar]
- Schewe T., Rapoport S. M., Kühn H. Enzymology and physiology of reticulocyte lipoxygenase: comparison with other lipoxygenases. Adv Enzymol Relat Areas Mol Biol. 1986;58:191–272. doi: 10.1002/9780470123041.ch6. [DOI] [PubMed] [Google Scholar]
- Siedow J. N., Girvin M. E. Alternative Respiratory Pathway: ITS ROLE IN SEED RESPIRATION AND ITS INHIBITION BY PROPYL GALLATE. Plant Physiol. 1980 Apr;65(4):669–674. doi: 10.1104/pp.65.4.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernooy-Gerritsen M., Leunissen J. L., Veldink G. A., Vliegenthart J. F. Intracellular localization of lipoxygenases-1 and -2 in germinating soybean seeds by indirect labeling with protein a-colloidal gold complexes. Plant Physiol. 1984 Dec;76(4):1070–1078. doi: 10.1104/pp.76.4.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]