Abstract
FtsK is essential for Escherichia coli cell division. We report that cells lacking the C terminus of FtsK are defective in chromosome segregation as well as septation, often exhibiting asymmetrically positioned nucleoids and large anucleate regions. Combining the corresponding truncated ftsK gene with a mukB null mutation resulted in a synthetic lethal phenotype. When the truncated ftsK was combined with a minCDE deletion, chains of minicells were generated, many of which contained DNA. These results suggest that the C terminus of FtsK has an important role in chromosome partitioning.
FtsK was originally discovered to be an essential cell division protein in Escherichia coli. A mutation in the 5′ end of the gene ftsK44 resulted in a temperature-sensitive lethal phenotype, in which a blockage of a very late stage of septation occurred, resulting in cell chains (4). Recent FtsK depletion experiments have confirmed that FtsK is essential for septation and have suggested that it may act early (14). The C terminus of FtsK may be a separate functional domain, because an N-terminal fragment of FtsK was sufficient to complement the ftsK44 mutation (4, 18), to localize to the septum in a merodiploid (18), and to restore septation in a strain that lacks the native ftsK gene (7). Furthermore, the extreme C terminus of FtsK is separated from the N terminus by a large proline-glutamine-rich linker, and this C terminus is highly similar in sequence to the C termini of Bacillus subtilis SpoIIIE and other members of the SpoIIIE family (4). SpoIIIE of B. subtilis is involved in the rescue of chromosomes that are bisected by asymmetric septa, which normally arise only during sporulation (16). The C terminus of SpoIIIE is specifically involved in DNA recognition, and the N terminus is required for localization and anchoring of SpoIIIE to the septum (17). SpoIIIE is essential for sporulation because of its rescue function but is dispensable for vegetative growth, presumably because the normal preseptational partitioning process pulls the chromosomes sufficiently apart. However, a role for SpoIIIE in vegetative cells of B. subtilis was revealed artificially by introducing a min mutation; many of the resulting polar minicells in a spoIIIE min mutant contained chromosomal DNA, suggesting that the rescue function is needed whenever an asymmetric septum forms (12).
The similarity between FtsK and SpoIIIE suggested that FtsK had a chromosome partitioning function in addition to its unique septation function (4). However, several attempts to demonstrate such a function in E. coli were unsuccessful. For example, the cell chains of the ftsK44 mutant appeared to have normal chromosome segregation (4). A truncated FtsK that inactivated the C terminus but left the N terminus intact also was reported to exhibit normal chromosome segregation (6). Interestingly, this mutant, ftsK1::cat, forms cell chains under certain conditions, indicating that the C terminus also functions in late septation. In this paper, we show that the ftsK1::cat mutant indeed exhibits defects in chromosome segregation and provide supporting evidence by combining ftsK1::cat with other mutations that affect chromosome and septum placement.
Abnormal nucleoid segregation in the ftsK1::cat mutant.
Although previous studies of mutations in the N terminus and C terminus of FtsK suggested that both domains function in septation but not in chromosome segregation, the similarity between the C terminus of FtsK and members of the SpoIIIE family prompted us to examine chromosome segregation in more detail in the ftsK1::cat mutant. This mutation was transduced from AD10 (6) into the wild-type E. coli strain MG1655 to make WM974 and into strain MC1061 to make WM977. The tendency of ftsK1::cat cells to form chains facilitated the evaluation of nucleoid positioning patterns within aligned cells. We found that despite the presence of many normal nucleoid patterns, approximately 20% of individual cells and cells within chains of WM974 (of 1,591 cells counted) in logarithmic growth contained nucleoids at asymmetric positions, resulting in large cell segments that lacked chromosomal DNA (Fig. 1A). In addition to this positioning problem, about 1% of the cells were anucleate. Although this is a low proportion of cells, it is significantly higher than the proportion in wild-type cells in logarithmic growth (which exhibit <0.03% anucleate cells) (10) and provides additional evidence for a chromosome segregation defect. Interestingly, the ftsK1::cat-mediated segregation problems became more pronounced in cells within or entering stationary phase and was also more severe in WM977 (data not shown). For both the mutant and wild-type strains, DAPI (4′,6-diamidino-2-phenylindole) staining of live cells revealed nucleoid patterns similar to those of fixed cells (data not shown).
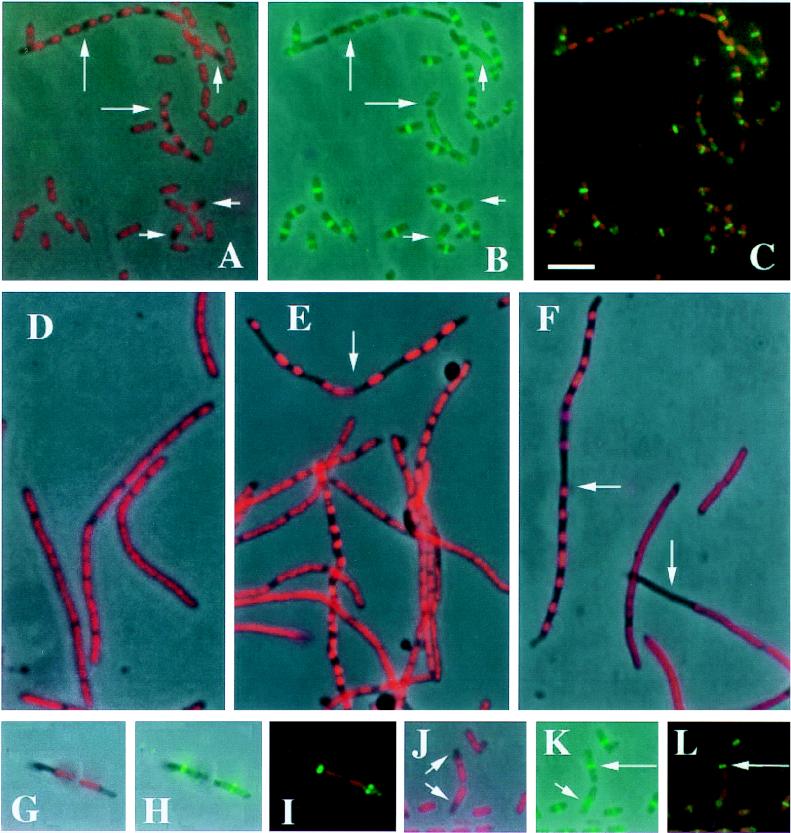
FIG. 1.
Localization of nucleoids and FtsZ in the ftsK1::cat mutant. Strain WM974 was grown in M9 medium supplemented with glucose and Casamino Acids (A to C and G to L) or Luria-Bertani medium (E and F) at 37°C. The wild-type parental control strain MG1655 was grown in Luria-Bertani medium at 37°C (D). Cells were grown to an optical density at 600 nm of approximately 0.2, fixed, stained with 0.5 μg of DAPI per ml, and visualized as described previously (13). Immunostaining with affinity-purified anti-FtsZ was also described previously (13). For panels D to F, cephalexin at 20 μg per ml was added to the culture, which was grown for an additional 2 h prior to fixation and DAPI staining. Panels A, D, E, F, G, and J show overlays of phase contrast plus DAPI staining (pseudocolored red); panels B, H, and K show overlays of phase contrast plus FtsZ immunostaining (green); and panels C, I, and L show overlays of DAPI (red) plus FtsZ immunostaining (green). Long arrows in panels A and B point to chains of cells with segregation defects, while short arrows in these panels highlight single cells with defects. Arrows in panels E and F point to filaments with severe segregation defects. Arrows in panel J highlight two cells, probably daughters, with misplaced nucleoids; the short arrow in panel K points to the FtsZ ring in the bottom cell that is off center relative to the cell and adjacent to the nucleoid; and the long arrows in panels K and L point to the FtsZ ring in the top cell that is asymmetric relative to the misplaced nucleoid but nevertheless at the cell midpoint. Bar, 5 μm.
To amplify the chromosome segregation defect, and to determine if the defect depends on formation of the septum, we induced WM974 cells to produce filaments by treating them with cephalexin, which inactivates FtsI (11). Cephalexin-induced filaments of the wild-type strain, MG1655, exhibited uniform, well-segregated nucleoids throughout the length of the filaments (Fig. 1D). However, many filaments of WM974 containing ftsK1::cat displayed severe chromosome segregation defects, including nucleoid aggregates and large anucleate regions (Fig. 1E and F). Approximately 30% of the filaments contained at least one abnormally large anucleate region.
The FtsZ ring is essential for bacterial cell division (9). The positioning of the midcell FtsZ ring appears to be independent of the chromosome, although condensed, unsegregated chromosomes at the cell midpoint may prevent normal ring formation (13). To determine whether FtsZ rings are normally positioned in ftsK1::cat mutant cells with abnormally positioned chromosomes, we examined FtsZ localization in WM974 cells by immunofluorescence. As with chromosome segregation, most cells were normal, displaying a midcell FtsZ ring. However, cells with abnormally positioned chromosomes usually either lacked an FtsZ ring (Fig. 1B and C) or had a ring that was near the cell midpoint but to the side of a polarly localized nucleoid (Fig. 1G to L). Some anucleate cells (5 of 13 cells counted) also had central FtsZ rings (data not shown), consistent with previous studies of FtsZ rings in anucleate cells (13).
Lethality of an ftsK1::cat ΔmukB double mutant.
The results described above suggest that the C terminus of FtsK is involved in chromosome positioning. Nevertheless, this defect is not sufficiently severe to abolish viability, presumably because more cells appear to be unaffected by the mutation. Because a mukB null mutant has severe chromosome partitioning defects and yet is viable at temperatures below 28°C (10), we tested whether cells lacking both MukB and the FtsK C terminus would be viable. We attempted to combine the ΔmukB::kan (10) and ftsK1::cat mutations by phage P1 transduction. The ftsK and mukB genes are within cotransduction distance on the chromosome, at 20.1 and 21.0 min, respectively. Therefore, a small percentage of ftsK1::cat recipients (AD10 and WM974) receiving the ΔmukB::kan donor allele would be expected to become ftsK+ and chloramphenicol sensitive (Cms), and some of the ΔmukB::kan recipients (WM949, which is ΔmukB::kan in MG1655) receiving the ftsK1::cat allele would be expected to become mukB+ and kanamycin sensitive (Kms). However, when ftsK1::cat recipients AD10 and WM974 were transduced with ΔmukB::kan and grown at 22°C, 246 of 246 of the combined Kmr transductants were Cms. Similarly, when the ΔmukB::kan recipient WM949 was transduced with ftsK1::cat at 22°C, 200 of 200 of the Cmr transductants were Kms. These results suggest that the double mutants were not viable.
To confirm this suggestion and demonstrate that this synthetic lethality could be rescued, we transformed AD10 and WM974 with pAD12, an ampicillin-resistant (Apr) plasmid that encodes the C terminus of FtsK and which can partially rescue several defects of the ftsK1::cat mutation (6). These strains were then transduced with the ΔmukB::kan allele, selecting for Apr Kmr at 22°C. Distinct small- and large-colony transductants were obtained; we surmised that if pAD12 could only partially rescue the lethality, then the small colonies probably were enriched for the double mutants relative to the large colonies. In support of this idea, 92 of 356, or 26%, of the small-colony class were Apr Kmr Cmr, while only 9 of 272, or 3%, of the large-colony class were Apr Kmr Cmr. These results indicated that the ftsK gene on pAD12 could partially rescue the synthetic lethal phenotype conferred by the combined ftsK1::cat and ΔmukB::kan mutations.
Minicell chains in an ftsK1::cat ΔminB double mutant.
To examine the effects of loss of ftsK function on formation of minicells, we combined a deletion of the entire minB locus (genes minCDE) with the ftsK1::cat mutation. The minB deletion was derived from PB114 (minB::kan) (5). The double ΔminB::kan ftsK1::cat mutant was constructed by sequentially transducing MG1655 to kanamycin and chloramphenicol resistance producing strain WM975. The viability of this strain indicated that the loss of minB had no synthetic phenotype. As expected, WM975 exhibited a combination of minicells and filaments typical of minB mutants. However, because the ftsK1::cat mutation delayed cell separation, many minicells were found still attached to cell poles or, more strikingly, to each other (Fig. 2A and D). The minicell chains were therefore a convenient record of several sequential rounds of minicell septation events, consistent with previous observations of sequential minicell formation in microculture (2).
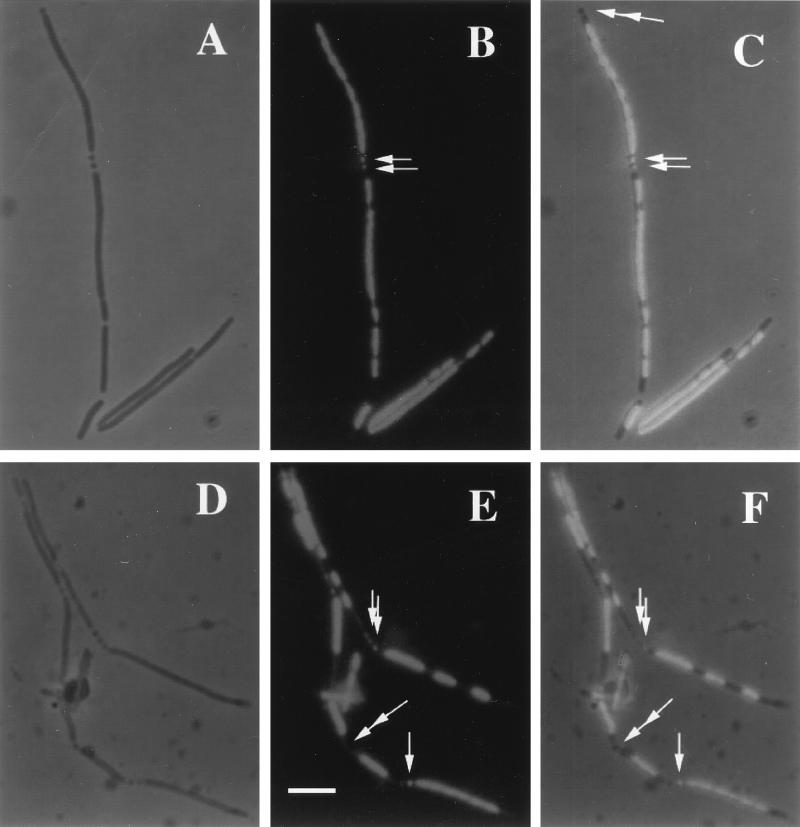
FIG. 2.
Chromosomal DNA in minicells of the ftsK1::cat strain. Strain WM975 (minB::kan ftsK1::cat) was grown in Luria-Bertani medium to an optical density at 600 nm of approximately 0.2. The cells were then fixed and stained with DAPI as described in the legend to Fig. 1. Panels A to C and D to F represent two different fields of cells, shown by phase contrast (A and D), DAPI staining (B and E), which appears bright on a dark background, and combined phase contrast and DAPI staining (C and F). Single arrows point to minicells that contain chromosomal DNA, while double arrows point to minicells lacking DNA. Bar, 5 μm.
DNA in minicells of the ftsK1::cat minB double mutant.
Minicells generated by a min mutant (1) or by overproduction of FtsZ or FtsZ and FtsA (3, 15) normally do not contain chromosomal DNA. This is presumably because septation events leading to minicell formation normally occur away from nucleoid regions. However, the chromosome positioning defect of the ftsK1::cat mutant prompted us to test whether chromosomal DNA might be abnormally partitioned into minicells in WM975. Examination of minicells revealed that 28% of all attached minicells (of 177) were strongly stained for DNA. This is probably an underestimate, because minicells with weak DAPI staining were not scored. Examples of minicells with and without DNA are shown in Fig. 2B, C, E, and F. In general, minicells with DNA seemed to occur most frequently when they were adjacent to a nucleate region of the mother cells and rarely when adjacent to an anucleate region. This phenomenon of minicells containing DNA is precisely what was observed in vegetatively growing spoIIIE min mutants of B. subtilis (12), further supporting the idea that FtsK and SpoIIIE may have similar roles in chromosome dynamics.
Conclusions.
Using several approaches, we have discovered a role for FtsK in chromosome segregation in E. coli. We have shown that about one-fifth of cells containing a truncated FtsK have obvious chromosome positioning defects. These defects were most easily observed in cell chains or in filaments, where the positioning problems were compounded over a longer distance. The abnormal state of the chromosomes in these cells correlated with the failure to form proper FtsZ ring structures, and when FtsZ rings did form, they often were located at one side of the misplaced nucleoid. The lack of FtsZ rings might be caused in part by induction of the SOS response or by blockage of the division site by the abnormal nucleoid.
The hypothesis that FtsK and MukB may function together to partition chromosomes is supported by the synthetic lethality of a ΔmukB ftsK1::cat double mutant. The phenotype suggests that MukB and the C terminus of FtsK have some redundant functions, allowing the single mutants to survive. We speculate that in the presence of functional MukB, chromosomes are properly condensed and are often in positions sufficient for segregation of intact chromosomes to daughter cells after cell division, even without the postulated positioning function of FtsK. Likewise, in the absence of MukB, chromosomes become disorganized, but FtsK may be able to position them in enough cells to achieve viability at lower temperatures. In cells lacking both the FtsK C terminus and MukB, on the other hand, the positioning and condensation defects may be compounded sufficiently to preclude viability of a colony.
In the absence of the SpoIIIE-like portion of FtsK, many minicells contained fragments of the chromosome, just like in B. subtilis spoIIIE min double mutants. This and the aberrant localization of nucleoids in cell chains are presumably due to trapping of nucleoids by invaginating septa, which normally is prevented by the C terminus of FtsK. The abnormal nucleoid localization in some of the cephalexin-induced ftsK1::cat filaments might also be explained if a subset of cells had their nucleoids trapped by septa prior to addition of the drug. However, the role of FtsK in E. coli must be somewhat different from that of B. subtilis SpoIIIE because FtsK possesses a septation function that has not been demonstrated for SpoIIIE. Further studies of the role of FtsK in chromosome segregation should allow a clearer picture of the mechanistic aspects of the process to emerge.
While this paper was under review, Liu et al. published a report describing chromosome segregation defects associated with a C-terminal deletion of FtsK that are very similar to the defects observed here (8). They also reported that the SOS response is induced by an FtsK deficiency, presumably because of DNA damage incurred during nucleoid trapping by the septum in some cells. SOS induction of SulA might explain why FtsZ rings are often lacking in cells with aberrantly positioned DNA.
Acknowledgments
We are grateful to W. Cook and L. Rothfield for the PB114 strain and to A. Diez and T. Nyström for strain AD10 and the pAD12 plasmid.
This work was supported by National Science Foundation grant MCB-9513521. E.K.W. was also supported by the University of Texas–Houston Summer Research Program.
REFERENCES
- 1.Adler H I, Fisher W, Cohen A, Hardigree A. Miniature E. coli cells deficient in DNA. Proc Natl Acad Sci USA. 1967;57:321–326. doi: 10.1073/pnas.57.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akerlund T, Bernander R, Nordström K. Cell division in Escherichia coli minB mutants. Mol Microbiol. 1992;6:2073–2083. doi: 10.1111/j.1365-2958.1992.tb01380.x. [DOI] [PubMed] [Google Scholar]
- 3.Begg K, Nikolaichik Y, Crossland N, Donachie W D. Roles of FtsA and FtsZ in activation of division sites. J Bacteriol. 1998;180:881–884. doi: 10.1128/jb.180.4.881-884.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Begg K J, Dewar S J, Donachie W D. A new Escherichia coli cell division gene, ftsK. J Bacteriol. 1995;177:6211–6222. doi: 10.1128/jb.177.21.6211-6222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Boer P A J, Crossley R E, Rothfield L I. A division inhibitor and a topological specificity factor coded for by the minicell locus determine the proper placement of the division site in Escherichia coli. Cell. 1989;56:641–649. doi: 10.1016/0092-8674(89)90586-2. [DOI] [PubMed] [Google Scholar]
- 6.Diez A A, Farewell A, Nannmark U, Nyström T. A mutation in the ftsK gene of Escherichia coli affects cell-cell separation, stationary-phase survival, stress adaptation, and expression of the gene encoding the stress protein UspA. J Bacteriol. 1997;179:5878–5883. doi: 10.1128/jb.179.18.5878-5883.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Draper G C, McLennan N, Begg K, Masters M, Donachie W D. Only the N-terminal domain of FtsK functions in cell division. J Bacteriol. 1998;180:4621–4627. doi: 10.1128/jb.180.17.4621-4627.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu G, Draper G C, Donachie W D. FtsK is a bifunctional protein involved in cell division and chromosome localization in Escherichia coli. Mol Microbiol. 1998;29:893–903. doi: 10.1046/j.1365-2958.1998.00986.x. [DOI] [PubMed] [Google Scholar]
- 9.Lutkenhaus J, Addinall S G. Bacterial cell division and the Z ring. Annu Rev Biochem. 1997;66:93–116. doi: 10.1146/annurev.biochem.66.1.93. [DOI] [PubMed] [Google Scholar]
- 10.Niki H, Jaffe A, Imamura R, Ogura T, Hiraga S. The new gene mukB codes for a 177-kD protein with coiled-coil domains involved in chromosome partitioning of Escherichia coli. EMBO J. 1991;10:183–194. doi: 10.1002/j.1460-2075.1991.tb07935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pogliano J, Pogliano K, Weiss D S, Losick R, Beckwith J. Inactivation of FtsI inhibits constriction of the FtsZ cytokinetic ring and delays the assembly of FtsZ rings at potential division sites. Proc Natl Acad Sci USA. 1997;94:559–564. doi: 10.1073/pnas.94.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharpe M E, Errington J. Postseptational chromosome partitioning in bacteria. Proc Natl Acad Sci USA. 1995;92:8630–8634. doi: 10.1073/pnas.92.19.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun Q, Yu X-C, Margolin W. Assembly of the FtsZ ring at the central division site in the absence of the chromosome. Mol Microbiol. 1998;29:491–504. doi: 10.1046/j.1365-2958.1998.00942.x. [DOI] [PubMed] [Google Scholar]
- 14.Wang L, Lutkenhaus J. FtsK is an essential cell division protein that is localized to the septum and induced as part of the SOS response. Mol Microbiol. 1998;29:731–740. doi: 10.1046/j.1365-2958.1998.00958.x. [DOI] [PubMed] [Google Scholar]
- 15.Ward J E, Lutkenhaus J. Overproduction of FtsZ induces minicells in E. coli. Cell. 1985;42:941–949. doi: 10.1016/0092-8674(85)90290-9. [DOI] [PubMed] [Google Scholar]
- 16.Wu L J, Errington J. A conjugation-like mechanism for prespore chromosome partitioning during sporulation in Bacillus subtilis. Genes Dev. 1995;9:1316–1326. doi: 10.1101/gad.9.11.1316. [DOI] [PubMed] [Google Scholar]
- 17.Wu L J, Errington J. Septal localization of the SpoIIIE chromosome partitioning protein in Bacillus subtilis. EMBO J. 1997;16:2161–2169. doi: 10.1093/emboj/16.8.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu X-C, Tran A H, Sun Q, Margolin W. Localization of cell division protein FtsK to the Escherichia coli septum and identification of a potential N-terminal targeting domain. J Bacteriol. 1998;180:1296–1304. doi: 10.1128/jb.180.5.1296-1304.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]