Abstract
Background
Interferon alpha is the only agent approved for the postoperative adjuvant treatment of high‐risk cutaneous melanoma. However, the survival advantage associated with this treatment is unclear, especially in terms of overall survival. Thus, adjuvant interferon is not universally considered a gold standard treatment by all oncologists.
Objectives
To assess the disease‐free survival and overall survival effects of interferon alpha as adjuvant treatment for people with high‐risk cutaneous melanoma.
Search methods
We searched the following databases up to August 2012: the Cochrane Skin Group Specialised Register, CENTRAL in The Cochrane Library (2012, issue 8), MEDLINE (from 2005), EMBASE (from 2010), AMED (from 1985), and LILACS (from 1982). We also searched trials databases in 2011, and proceedings of the ASCO annual meeting from 2000 to 2011. We checked the reference lists of selected articles for further references to relevant trials.
Selection criteria
We included only randomised controlled trials (RCTs) comparing interferon alpha to observation (or any other treatment) for the postoperative (adjuvant) treatment of patients with high‐risk skin melanoma, that is, people with regional lymph node metastasis (American Joint Committee on Cancer (AJCC) TNM (tumour, lymph node, metastasis) stage III) undergoing radical lymph node dissection, or people without nodal disease but with primary tumour thickness greater than 1 mm (AJCC TNM stage II).
Data collection and analysis
Two authors extracted data, and a third author independently verified the extracted data. The main outcome measure was the hazard ratio (HR), which is the ratio of the risk of the event occurring in the treatment arm (adjuvant interferon) compared to the control arm (no adjuvant interferon). The survival data were either entered directly into Review Manager (RevMan) or extrapolated from Kaplan‐Meier plots and then entered into RevMan. Based on the presence of between‐study heterogeneity, we applied a fixed‐effect or random‐effects model for calculating the pooled estimates of treatment efficacy.
Main results
Eighteen RCTs enrolling a total of 10,499 participants were eligible for the review. The results from 17 of 18 of these RCTs, published between 1995 and 2011, were suitable for meta‐analysis and allowed us to quantify the therapeutic efficacy of interferon in terms of disease‐free survival (17 trials) and overall survival (15 trials). Adjuvant interferon was associated with significantly improved disease‐free survival (HR (hazard ratio) = 0.83; 95% CI (confidence interval) 0.78 to 0.87, P value < 0.00001) and overall survival (HR = 0.91; 95% CI 0.85 to 0.97; P value = 0.003). We detected no significant between‐study heterogeneity (disease‐free survival: I² statistic = 16%, Q‐test P value = 0.27; overall survival: I² statistic = 6%; Q‐test P value = 0.38).
Considering that the 5‐year overall survival rate for TNM stage II–III cutaneous melanoma is 60%, the number needed to treat (NNT) is 35 participants (95% CI = 21 to 108 participants) in order to prevent 1 death. The results of subgroup analysis failed to answer the question of whether some treatment features (i.e. dosage, duration) might have an impact on interferon efficacy or whether some participant subgroups (i.e. with or without lymph node positivity) might benefit differently from interferon adjuvant treatment.
Grade 3 and 4 toxicity was observed in a minority of participants: In some trials, no‐one had fever or fatigue of Grade 3 severity, but in other trials, up to 8% had fever and up to 23% had fatigue of Grade 3 severity. Less than 1% of participants had fever and fatigue of Grade 4 severity. Although it impaired quality of life, toxicity disappeared after treatment discontinuation.
Authors' conclusions
The results of this meta‐analysis support the therapeutic efficacy of adjuvant interferon alpha for the treatment of people with high‐risk (AJCC TNM stage II‐III) cutaneous melanoma in terms of both disease‐free survival and, though to a lower extent, overall survival. Interferon is also valid as a reference treatment in RCTs investigating new therapeutic agents for the adjuvant treatment of this participant population. Further investigation is required to select people who are most likely to benefit from this treatment.
Keywords: Humans; Antineoplastic Agents; Antineoplastic Agents/therapeutic use; Chemotherapy, Adjuvant; Chemotherapy, Adjuvant/methods; Chemotherapy, Adjuvant/mortality; Disease‐Free Survival; Interferon‐alpha; Interferon‐alpha/therapeutic use; Melanoma; Melanoma/drug therapy; Melanoma/mortality; Melanoma/surgery; Randomized Controlled Trials as Topic; Skin Neoplasms; Skin Neoplasms/drug therapy; Skin Neoplasms/mortality; Skin Neoplasms/surgery
Plain language summary
Interferon for the treatment of melanoma patients after surgical removal of their tumour
Cutaneous melanoma is one of the deadliest types of skin cancer, and its incidence is rising in all Western countries. Furthermore, melanoma is one of the solid tumours most resistant to treatment with chemotherapy, which means that the outlook for people whose cancer has spread through their body (distant metastatic disease) is dismal, with only 10% of these patients surviving longer than 5 years.
After surgical removal of the primary tumour and in the absence of distant metastatic disease, people with melanoma have variable prognosis: In fact, between 40% to 90% of these patients are alive after 5 years. Therefore, adjuvant (i.e. postoperative) therapy has been proposed to reduce the risk of death in patients with high‐risk melanoma who have more aggressive tumours that are identified according to pathological features, such as the primary tumour thickness and regional lymph node status (disease stage).
The only compound that has shown some positive therapeutic effects in this patient group is interferon alpha, which is a protein produced by human macrophages (one type of white blood cell) and is known for its antiviral and antitumour activities.
In this review, we gathered evidence from 18 randomised controlled trials, enrolling more than 10,000 participants, testing the hypothesis that interferon treatment can improve the survival of people with melanoma at high risk of spreading after surgical removal of the tumour.
Whereas not all single studies demonstrated a survival benefit for patients treated with interferon, combining the available evidence, we found that the use of postoperative interferon improves the survival of those with high‐risk melanoma. On average, the toxicity associated with interferon administration (such as fever and fatigue) is limited; moreover, it is reversible when the treatment is stopped. Since interferon alpha is the only approved drug after surgery for those with high‐risk melanoma, efforts to identify those who might benefit most from this treatment are very important in order to avoid unnecessary toxicity for those who would not benefit from interferon alpha treatment. Combination of interferon with novel drugs is another field of ongoing research to improve the life expectancy of people with high‐risk melanoma.
Summary of findings
for the main comparison.
| Interferon alpha compared with treatment other than interferon (including observation) for the adjuvant treatment of melanoma | ||||||
|
Patient or population: high‐risk melanoma participants Settings: adjuvant Intervention: interferon alpha Comparison: observation, treatments other than interferon, or both | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Observation or treatment | Interferon alpha | |||||
| First recurrence | 50/100 | 44/100 | HR 0.83 (0.78 to 0.87) | 10,345 (17 studies) | High‐quality | Further research may provide information regarding patient selection and interferon schedule |
| Death | 40/100 | 37/100 | HR 0.91(0.85 to 0.97) | 9927 (15 studies) | High‐quality | Further research may provide information regarding patient selection and interferon schedule |
| *Assumed risk: For disease‐free survival outcome: 5‐year disease recurrence rate = 50%; for overall survival outcome: 5‐year death rate = 40% (in patients with TNM stage II‐III). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: Confidence interval; HR: Hazard ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
Background
The management of people with lymph node metastases from cutaneous melanoma is one of the most challenging issues for surgical and medical oncologists. In fact, the therapeutic role of sentinel node biopsy, radical lymph node dissection, and adjuvant therapies has never been clearly proven (Australian Cancer Network 2008; Thompson 2009).
After surgery, the only approved medical adjuvant therapy for people with lymph node metastases is interferon (IFN) alpha (Kirkwood 1996). After the first trial reporting an overall survival benefit (Kirkwood 1996), the FDA (the United States Food and Drug Administration) and the EMEA (European Medicines Agency) approved high‐dose interferon for the adjuvant treatment of high‐risk melanoma patients (i.e. TNM stage IIb‐IIIC) (Eggermont 2009). On the basis of this randomised controlled trial (RCT), the standard regimen is currently one month of high‐dose intravenous interferon, followed by one year of low‐dose IFN.
Following the RCT published by Kirkwood and Colleagues in 1996, many trials have been conducted to compare interferon versus observation, or different interferon schedules and dosages (Ascierto 2008). The results appear to confirm a benefit in terms of disease‐free survival, but the impact of adjuvant interferon on overall survival is quite unclear (Kirkwood 2004; Lens 2002; Pirard 2004; Wheatley 2003). The results of an individual patient data meta‐analysis on this subject have been published in the form of an abstract: They confirm longer disease‐free survival for those treated with IFN and, interestingly, pinpoint a small but significant advantage in terms of overall survival (Wheatley 2007).
Based on the disease‐free survival advantage, the small overall survival benefit, and the high toxicity, the role of interferon alpha in melanoma remains debatable (Ascierto 2008; Bajetta 2008; Eggermont 2008a; Janku 2010; Kirkwood 2009; Thirlwell 2008; Verma 2006). Accordingly, because the overall survival benefit may be small when weighed against possible toxicity, the international guidelines do not routinely recommend interferon. However, in those participants with metastatic regional lymph nodes who have had a lymphadenectomy, some guidelines suggest its use may be considered (Australian Cancer Network 2008; Dummer 2009; Fecher 2009; Saiag 2007).
We present a glossary of terms used in this review in Table 2.
1. Glossary of terms.
| Medical term | Explanation |
| Adjuvant treatment | Any medical oncology therapy used after surgery to kill microscopic tumour residues not removed by the surgeon |
| AJCC TNM stages I‐IV | Stage I and II = primary melanomas with negative regional lymph nodes Stage III = regional lymph node melanoma metastasis from known or unknown primary tumours Stage IV = melanoma metastasis at distant site (e.g. lung, liver, brain) |
| Apoptosis | Apoptosis is a genetically determined process of programmed cell death that may occur in cells. Apoptosis is a normal physiological process eliminating DNA‐damaged, superfluous, or unwanted cells and when halted may result in uncontrolled cell growth and tumour formation |
| Angiogenesis | Development of new blood vessels. It may occur in the healthy body for healing of wounds and restoring blood flow to tissues after injury, or in tumours, where it promotes the spread of cancer cells through new blood vessels |
| Lymphadenectomy | This surgical operation aims to remove the lymph nodes of 1 or more of the 3 main fields (neck, axilla, groin) where metastatic melanoma cells are present |
| Metastasis | The spread of a malignant tumour from its original site to any part of the body (such as lymph nodes, lungs, liver, brain, bones, and so on) |
| Neoadjuvant treatment | Any medical oncology therapy used before surgery to reduce the tumour bulk |
| Pegylated interferon | The addition of polyethylene glycol to the interferon molecule to improve the effectiveness of the drug |
| Randomised controlled trial | This is a particular design of study, in which participants are randomly assigned to different treatments. A randomised controlled trial provides the highest evidence for the use, or not, of a diagnostic or therapeutic intervention |
| Sentinel node biopsy | Surgical procedure to find the first lymph node that drains the skin area of the primary melanoma. The pathological evaluation of the sentinel node allows definition of the status of the lymphatic field when no clinically evident regional lymph node metastasis is present |
| TNM classification | This is the international classification of tumour spread issued by the American Joint Committee on Cancer (AJCC): "T" refers to the size of a tumour, "N" to the presence and extent of lymph node metastasis, and "M" indicates whether or not distant metastatic disease (that is, to lungs, liver, brain, bones) is present E.g. AJCC stage II (T2‐4N0M0): T2‐4 refers to the size and extent of the primary tumour; N0 refers to no regional lymph node involvement; M0 refers to no metastases AJCC stage III (TanyN+M0): Tany refers to any size and extent of the primary tumour, including thin (< 1.00 mm) and thick melanomas (> 4.00 mm); N+ refers to any number of positive regional lymph nodes |
| Grades of toxicity | Grade 0 = no adverse event or within normal limits Grade 1 = mild adverse event Grade 2 = moderate adverse event Grade 3 = severe and undesirable adverse event Grade 4 = life‐threatening or disabling adverse event |
Description of the condition
Although melanoma is not the most common form of skin cancer, it accounts for 80% of all skin cancer deaths (Jemal 2010; Lens 2004; Lui 2007; Tawbi 2007; Thompson 2005; Tsao 2004; Wang 2007). During the 20th century, the incidence of melanoma in white populations rose faster than the incidence of any other solid tumour, barring lung cancer (Ferlay 2010; Jemal 2010; Mocellin 2011). Data from the United States indicates that in 2001, 47,700 new cases were recorded compared to 59,940 new cases in 2007. Worldwide, 160,000 new cases and 41,000 deaths were reported in 2002. In 2007, the American Cancer Society estimated that the lifetime risk of melanoma is higher for men (1/49) than for women (1/73), the death rate from melanoma reaching 8110 cases in that same year (Wang 2007). These data clearly indicate that cutaneous melanoma represents a growing public health problem.
Early diagnosis and surgical removal of the primary tumour still represent the approach with the highest likelihood of cure for participants with early stage melanoma (Mocellin 2011a; Sladden 2009). In this setting, sentinel node biopsy provides patients with a significant benefit in terms of disease‐free survival, and when there is metastatic disease present in the regional lymph nodes, there is a possible overall survival advantage (Morton 2006; Pasquali 2010a).
In people with advanced melanoma, resection is recommended for selected cases with limited metastatic disease (Thompson 2005; Tsao 2004). Anti‐CTLA‐4 monoclonal antibodies and inhibitors of v‐Raf murine sarcoma viral oncogene homologue B1 (BRAF) recently gained the approval of drug agencies for AJCC stage IV disseminated melanoma (Chapman 2011; Hodi 2010), a condition considered as one of the most resistant to conventional chemotherapy (Crosby 2000; Gogas 2007; Lui 2007; Sasse 2007; Tawbi 2007).
In those with high‐risk melanoma, AJCC stage II (T2‐4N0M0) and AJCC stage III (TanyN+M0) disease, the 5‐year overall survival rate varies between 30% and 70% (Balch 2009), and the only agents currently approved for the treatment of these patients in an adjuvant setting after radical surgery are interferon alpha and pegylated interferon (Eggermont 2009; Garbe 2010; Kirkwood 2008).
Description of the intervention
Interferon alpha is a type I interferon mainly produced by macrophages (Theofilopoulos 2005). The interferon cluster region of chromosome 9p22 encodes 13 different interferon genes: Among them, the interferon‐2 gene presents 3 polymorphic variants, known as interferon alpha 2a, interferon alpha 2b, and interferon alpha 2c (Pestka 2007). Interferon alpha demonstrated anticancer effects in both experimental models and in the clinical setting, although its exact mechanism of action is still unclear (Moschos 2007; Pasquali 2010). With regard to the treatment of melanoma, only the proteins encoded by the interferon alpha 2a and interferon alpha 2b genes, which differ in a single amino acid at position 23 (lysine > arginine), have been tested as therapeutic agents in the clinical setting, and human recombinant interferon alpha 2b has been approved for the adjuvant treatment of this type of skin cancer.
After apparently radical surgery of primary melanoma without clinical evidence of distant metastases, the only approved medical adjuvant therapy for patients with high‐risk melanoma is interferon alpha (Kirkwood 2008). Based on the results from the first trial reporting an overall survival benefit (Kirkwood 1996), the US Food and Drug Administration (FDA) and the European Medicines Agency approved the high‐dose interferon (IFN) for the adjuvant treatment of high‐risk melanoma patients (i.e. AJCC TNM stage IIB‐IIIC) (Eggermont 2009). Recently, based on the EORTC (European Organisation for Research and Treatment of Cancer) trial 18991 (Eggermont 2008), the FDA approved pegylated interferon. Currently, the standard regimen is one month of high‐dose intravenous IFN, followed by one year of low‐dose interferon. However, different schedules of interferon therapy have also been tested (Lens 2006). There are four main approaches to interferon therapy:
high‐dose interferon alpha administered for one month (and followed by one year at a low dose),
interferon alpha at an intermediate dose (tested for one or two years),
interferon alpha at a low dose (tested for one or three years), or
pegylated interferon.
High‐dose interferon is burdened by the highest toxicity; however, it is believed to be the most active regimen (Kirkwood 2008).
How the intervention might work
The best known physiological activity of interferons is their antiviral function through their ability to inhibit the replication of different types of viruses, both by blocking the cell cycle machinery and by stimulating the immune response. Interferon has also long been recognised as an anticancer factor in both experimental and clinical models (e.g. myeloid leukaemia, renal cell carcinoma, and melanoma). However, the cellular and molecular mechanism underlying this activity is still incompletely elucidated (Borden 2007; Pasquali 2010). Interesting findings about the mechanism of action of interferon in melanoma came from an observational study in which interferon was administered as neoadjuvant treatment in participants with clinically detectable lymph node metastases (Moschos 2006). The subsequent radical lymph node dissection allowed the investigators to study the effects of interferon on tumour tissue and to examine immunologic and molecular biomarkers of tumour response. Interferon did not influence tumour cell phenotype, proliferation rate, apoptosis, or angiogenesis. On the other hand, a higher grade of tumour‐infiltrating‐mononuclear‐immune cell was observed in cases showing response to interferon treatment. Therefore, the main anticancer effect of interferon is currently believed to be linked to its immunostimulatory effects.
Why it is important to do this review
Several randomised studies have been designed and conducted on the use of interferon alpha as an adjuvant treatment for melanoma (Cascinelli 2001; Eggermont 2008; Garbe 2008; Kirkwood 2004). While the disease‐free survival benefit for interferon treatment was consistent across the trials, a meaningful overall survival benefit for interferon therapy has been reported in some studies only (Garbe 2008; Kirkwood 1996). According to 2 meta‐analyses published more than 6 years ago on the results of 12 (Wheatley 2003) and 9 (Pirard 2004) randomised controlled trials, respectively, interferon alpha appears to provide a statistically significant disease‐free survival advantage (mainly over observation) in participants with high‐risk cutaneous melanoma, whereas no impact on overall survival was demonstrated. Since then, 2 other large randomised controlled trials have been published that compared interferon and observation. These enrolled 1700 participants, but the findings have been conflicting in terms of overall survival benefit (1 trial is positive: Garbe 2008, and the other one showed no differences: Eggermont 2008).
With the aim of summarising the evidence on interferon, we carried out a meta‐analysis that compared interferon treatment versus any other comparator than interferon in participants with high‐risk cutaneous melanoma (Mocellin 2010). Our findings confirmed the disease‐free survival benefit reported by the previous meta‐analyses, and supported the significant 3% overall survival advantage reported by the individual patient data meta‐analysis of Wheatley (Wheatley 2007). These meta‐analyses did not include results for at least two more trials (the Nordic Trial and the Sunbelt Melanoma Trial), which are currently only available in abstract form (Hansson 2011; McMasters 2008).
The most recent meta‐analyses on interferon as adjuvant treatment for melanoma have not investigated adverse events and quality of life (Mocellin 2010; Wheatley 2007). Even if these topics are relevant in oncology, these end points are not suitable for a meta‐analysis. In fact, almost all trials compare interferon to observation. Interferon arms are intrinsically associated with lower quality of life and presence of adverse events when compared to control arms; thus, quality of life and adverse events data are hardly reported in terms of summary data suitable for meta‐analysis.
Therefore, we have performed this systematic review and meta‐analysed the available evidence in order to determine the true benefit of interferon in the adjuvant treatment of melanoma.
Objectives
To assess the disease‐free survival and overall survival effects of interferon alpha as adjuvant treatment for people with high‐risk cutaneous melanoma.
We have not addressed adverse events and quality of life, even if they represent a crucial issue in interferon alpha treatment, in this systematic review, as virtually all trials have compared interferon treatment with observation (i.e. no treatment).
Methods
Criteria for considering studies for this review
Types of studies
We considered as eligible all randomised controlled trials that compared interferon alpha with observation (or any regimen other than interferon alpha) for the adjuvant treatment of skin melanoma.
Types of participants
We included people with high‐risk skin melanoma, that is, those with regional lymph node metastasis undergoing radical lymph node dissection (AJCC stage III), or people without nodal disease but with primary tumour thickness greater than 1 mm (AJCC stage II).
Types of interventions
We included adjuvant (i.e. postoperative) interferon (experimental arm) versus observation or any treatment other than interferon (control arm).
Types of outcome measures
The main outcome measure was the hazard ratio (HR), defined as the ratio between the risk of event (disease recurrence or death) in the treatment arm (adjuvant interferon) and the same risk in the control arm (no adjuvant interferon).
Moreover, for overall survival data, we calculated the number needed to treat (NNT), defined as the number of participants to be treated with IFN to avoid one event (death).
Primary outcomes
The primary outcomes considered were disease‐free survival and overall survival. The survival time was calculated from the date of randomisation.
Search methods for identification of studies
We aimed to identify all relevant randomised controlled trials regardless of language, publication status (published, unpublished, in press, or in progress), or publication format (abstract, meeting presentation, full‐text article).
Electronic searches
We searched the following databases up to 22 August 2012:
the Cochrane Skin Group Specialised Register using the following terms: melanoma* and (interferon* or interferon*);
the Cochrane Central Register of Controlled Trials (CENTRAL) 2012, issue 8, in The Cochrane Library using the search strategy in Appendix 1;
MEDLINE via OVID (from 2005) using the strategy in Appendix 2;
EMBASE via OVID (from 2010) using the strategy in Appendix 3;
AMED via OVID (Allied and Complementary Medicine, from 1985) using the strategy in Appendix 4;
LILACS (Latin American and Caribbean Health Science Information database, from 1982) using the strategy in Appendix 5; and
ASCO (American Society of Clinical Oncology) Annual Meeting Abstracts 2000 to 2011.
The UK and US Cochrane Centres have ongoing projects to systematically search MEDLINE and EMBASE for reports of trials that are then included in the Cochrane Central Register of Controlled Trials. Searching has currently been completed in MEDLINE to 2004 and in EMBASE to 2009. Further searches of these two databases were undertaken for this review by the Cochrane Skin Group to cover the years not searched by the UK and US Cochrane Centres for CENTRAL.
Trials registers
We searched the following trials registers using the terms "melanoma" and "interferon" during the second half of 2011:
The metaRegister of Controlled Trials (www.controlled‐trials.com).
The US National Institutes of Health Ongoing Trials Register (www.clinicaltrials.gov).
The Australian and New Zealand Clinical Trials Registry (www.anzctr.org.au).
The World Health Organization International Clinical Trials Registry platform (www.who.int/trialsearch).
Searching other resources
Reference lists
We checked the bibliographies of selected studies for further references to relevant trials.
Data collection and analysis
We performed meta‐analysis following the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) guidelines (Moher 2009). We applied standard meta‐analysis methods (Sutton 2000) to evaluate the summary effect of postoperative interferon on disease‐free and overall survival.
Selection of studies
We assessed eligibility of the retrieved articles by title and abstract (SP and SM). If this information was insufficient for eligibility assessment, we reviewed the full article.
Data extraction and management
Two authors extracted data (SP and ML), and a third author independently verified the extracted data (SM). After all eligible studies had been identified, two authors independently performed a quality evaluation of the heterogeneity between studies, randomisation, blinding, and follow‐up, as well as data extraction (SM and SP). The authors achieved consensus on all results considered for the final analysis.
The following data were retrieved (if reported): demographics, study design, eligibility criteria, randomisation method, allocation method, disease stage at enrolment, type of interferon alpha, interferon alpha dosage, treatment duration, number of participants enrolled, number of participants randomised, number evaluated for data analyses, cross‐over allowance and percentage, intention‐to‐treat versus per‐protocol findings, follow‐up period, disease‐free survival, overall survival, and loss to follow up.
Assessment of risk of bias in included studies
We assessed risk of bias according to The Cochrane Collaboration checklist (Higgins 2011):
(a) the method of generation of the randomisation sequence; (b) the method of allocation concealment; (c) the blinding of participants, clinicians, and outcome assessors; (d) the presence of incomplete outcome data; and (e) selective outcome reporting.
This information was recorded in a 'Risk of bias' table, which is part of the 'Characteristics of included studies' table for each study.
Measures of treatment effect
The main outcome measure was the hazard ratio (HR), defined as the ratio between the risk of event in the treatment arm (adjuvant interferon) and the same risk in the control arm (no adjuvant interferon). Hazard ratios were reported with their 95% confidence intervals (CIs). Survival data (HR) were either entered directly in RevMan or extrapolated from Kaplan‐Meier plots using dedicated methods (Parmar 1998; Tierney 2007). Moreover, for overall survival data, we calculated the number needed to treat (NNT), defined as the number of participants to be treated with IFN to avoid one event (death).
Unit of analysis issues
For multiple‐arm trials in which two (or more) interferon arms were compared with the same control arm, we took within‐study correlation into consideration. As suggested by Borenstein and colleagues (Borenstein 2009), we first computed a composite effect size for the comparison of each interferon arm versus the control arm. Next, we calculated the correlation factor (r) based on the number of cases in each arm, which allowed us to compute the variance (V) of the composite effect size according to the following formula: V = 1/4 [(V1 + V2) + (2r*√ V1*√ V2)], where V1 and V2 are the variances of the original comparisons between each treatment arm and the control arm. Using this variance, we finally computed the standard error and then the 95% CI of the composite effect.
Dealing with missing data
We contacted trial authors of included studies whenever data essential for the meta‐analysis were missing or unclear.
Assessment of heterogeneity
We assessed the consistency of results (effect sizes) among studies using the two standard heterogeneity tests: the Chi²‐based Cochran Q‐test and the I² statistic (Higgins 2002). To be more conservative, we considered that heterogeneity was statistically significant when the Cochran Q‐test P value was less than 0.1 (i.e. the alpha level of significance for this test was set at 10%). In addition, inconsistency across studies was considered low, moderate, and high for I² statistic values lower than 25%, between 25% and 50%, and greater than 50%, respectively. We considered heterogeneity significant either when the I² statistic was greater than 50%, the Q‐test P value was smaller than 0.1, or both: In this case, we applied the random‐effects model to calculate the overall effect (which assumes that studies do not share the same common effect, and assigns a weight to each study taking into account both within‐ and between‐study variance), using the inverse variance method of DerSimonian and Laird (DerSimonian 1986). In all other cases, we applied the fixed‐effect model.
Assessment of reporting biases
We used a funnel plot to detect the so‐called "small study effect". In fact, publication and selection biases might burden small studies, which may show a lower methodological quality, possibly leading to a small effect in a meta‐analysis, the smaller studies having larger effects. Furthermore, between‐trial heterogeneity, which is observed in studies with a relatively small number of participants, might lower the study effect (Sterne 2000). We formally investigated funnel plot asymmetry with the Egger linear regression approach and the Begg rank correlation test: To be more conservative, we considered these tests statistically significant when the P value was less than 0.1. We formally assessed the impact of a small study effect bias on the summary effects by means of the 'trim & fill' method described by Duval and Tweedie (Duval 2000).
In order to assess the impact of "small" studies, we also performed a sensitivity analysis (see Sensitivity analysis) by excluding these trials. To this aim, we defined "small" as any study with a sample size smaller than 400 based on the following parameters of an ideal trial: A) minimum hazard ratio (HR) = 1.5; alpha level of significance = 5%; statistical power = 80%; median survival in controls = 60 months; accrual time = 36 months; follow‐up time = 48 months; randomisation ratio = 1:1.
Data synthesis
We used hazard ratios (HR) and 95% confidence intervals (CI) to synthesise the summary effect of interferon in terms of both disease‐free survival and overall survival.
Moreover, based on the summary HR (calculated with the meta‐analysis) and the 5‐year OS rate in the control population of participants with AJCC stage II‐III melanoma (roughly 60%), we calculated the number needed to treat (NNT), which provides readers with the number of participants to be treated with interferon in order to avoid one event (i.e. death); to this aim, we followed the method proposed by Altman (Altman 1999).
Subgroup analysis and investigation of heterogeneity
In order to investigate potential sources of heterogeneity, we used subgroup analysis and metaregression (method of moments) for the following factors: interferon dosage, treatment duration, type of interferon alpha used, participants' AJCC TNM stage (studies have been conducted with the 5th and 6th edition of the TNM staging manual), type of comparator, and year of publication.
Sensitivity analysis
We used the 'leave‐one‐out' sensitivity analysis to systematically ascertain the impact of individual randomised studies on the overall effect. We employed the exclusion of randomised studies for specific reasons (e.g. differences in control arms or sample size) as a further type of sensitivity analysis.
Results
Description of studies
Since we systematically reported the details of the included and excluded studies in the dedicated 'Characteristics of included studies' and 'Characteristics of excluded studies' tables, here we discuss some general aspects of the trials included in the meta‐analysis, as well as some peculiarities of specific RCTs.
Results of the search
The literature searches yielded 640 papers plus 4 papers from other sources (we report the review's flow diagram in Figure 1). Of these 644 references, we excluded 609 and considered the full text of the remaining 35. We then excluded 17 and included 18.
1.
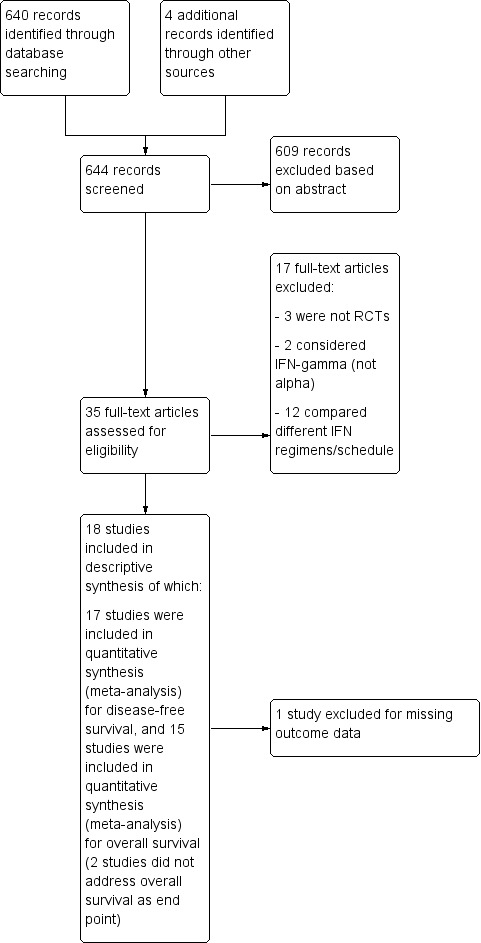
Study flow diagram
Included studies
We included 18 RCTs, with a total of 10,499 participants. These were published between 1995 and 2011. Table 3 summarises design, sample sizes, setting, participants, interventions, and outcomes of eligible studies. All but one study (a phase II RCT) were phase III RCTs.
2. Included studies.
| Study | Design | Sample size | Setting | Participants (AJCC TNM stage) | Intervention (interferon schedule) | Outcome (P value) |
| Creagan 1995 | Phase III RCT | 264 | Adjuvant | II‐III (T2‐4N0M0/TanyN+M0) |
IFNa(2a): 20 MU/sqm x 3/week for 4 months (route: i.m.) | DFS: not significant OS: not significant |
| Kirkwood 1996 | Phase III RCT | 287 | Adjuvant | II‐III (T4N0M0/TanyN+M0) |
IFNa(2b): 20 MU/sqm x 5/week (1 month, route: i.v.) + 10 MU/sqm x 3/week (48 weeks, route: s.c.) | DFS: 0.0013 OS: 0.015 |
| Rusciani 1997 | Phase III RCT | 154 | Adjuvant | I‐II (TanyN0M0) |
IFNa(2a): 3 MU x 3/week (3 weeks, route: i.m.) x 6 months, followed by a month with no treatment x 3 years | DFS: not reported OS: not reported |
| Pehamberger 1998 | Phase III RCT | 311 | Adjuvant | II (T2‐4N0M0) |
IFNa(2a): 3 MU x 7/week (3 weeks, route: s.c.) + 3 MU sc x 3/week (12 months, route: s.c.) | DFS: 0.02 OS: not evaluated |
| Grob 1998 | Phase III RCT | 499 | Adjuvant | II (T2‐4N0M0) |
IFNa(2a): 3 MU x 3/week (18 months, route: s.c.) | DFS: 0.033 OS: 0.046 |
| Kirkwood 2000 | Phase III RCT | 642 | Adjuvant | II‐III (T4N0M0/TanyN+M0) |
IFNa(2b) (high): 20 MU/sqm x 5/week (1 month, route: i.v.) + 10 MU/sqm x 2/week (48 weeks, route: s.c.). IFNa(2b) (low): 3 MU x 2/week (2 years, route: s.c.) | DFS (high): 0.03 DFS (low): not significant OS: not significant |
| Cameron 2001 | Phase III RCT | 96 | Adjuvant | II‐III (T3‐4N0M0/TanyN+M0) |
IFNa(2b): 3 MU x 3/week (6 months, route: s.c.) | DFS: not significant OS: not significant |
| Kirkwood 2001 | Phase III RCT | 880 | Adjuvant | II‐III (T4N0M0/TanyN+M0) |
IFNa(2b): 20 MU/sqm x 5/week (1 month, route: i.v.) + 10 MU/sqm x 2/week (48 weeks, route: s.c.) | DFS: 0.0027 OS: 0.0147 |
| Cascinelli 2001 | Phase III RCT | 444 | III (TanyN+M0) |
IFNa(2a): 3 MU x 3/week (36 months, route: s.c.) | DFS: not significant OS: not significant |
|
| Kirkwood 2001 | Phase II RCT | 107 | Adjuvant | II‐III‐IV (stage IV: resectable metastatic disease) |
IFNa(2b) (d1): IFNa (from day 1) 20 MU/sqm x 5/week (1 month, route: i.v.) + 10 MU/sqm x 3/week (48 weeks, route: s.c.). IFNa(2b) (d28): IFNa as above (from day 28) | DFS (d1): 0.016 DFS (d28): 0.03 OS: not significant |
| Hancock 2004 | Phase III RCT | 674 | Adjuvant | II‐III (T4N0M0/TanyN+M0) |
IFNa(2a): 3 MU x 3/week (2 years, route: s.c.) | DFS: not significant OS: not significant |
| Kleeberg 2004 | Phase III RCT | 830 | Adjuvant | II‐III (T3‐4N0M0/TanyN+M0) |
IFNa(2b): 1 MU every other day (12 months, route: s.c.) | DFS: not significant OS: not significant |
| Eggermont 2005 | Phase III RCT | 1388 | Adjuvant | II‐III (T4N0M0/TanyN+M0) |
IFNa(2b) (1 year): 10 MU x 5/week (4 weeks, route: s.c.) + 10 MU x 3/week (12 months, route: s.c.) IFNa(2b) (2 years): 10 MU x 5/week (4 weeks, route: s.c.) + 5 MU x 3/week (24 months, route: s.c.) |
DFS: not significant OS: not significant |
| Garbe 2008 | Phase III RCT | 444 | Adjuvant | III (TanyN+M0) |
IFNa(2a): 3 MU x 3/week (2 years, route: s.c.) | DFS: 0.018 OS: 0.005 |
| Eggermont 2008 | Phase III RCT | 1256 | Adjuvant | III (TanyN+M0) |
Pegylated IFNa(2b): 6 ug/Kg/week (8 weeks, route: s.c.) + 3 ug/Kg/w (5 years, route: s.c.) | DFS: 0.02 OS: not significant |
| McMasters 2008 | Phase III RCT | 774 | Adjuvant | II‐III (T2‐4N0M0/TanyM0)* |
IFNa(2b): 20 MU/sqm x 5/week (1 month, route: i.v.) + 10 MU/sqm x 3/week (48 weeks, route: s.c.) | DFS: not significant OS: not significant |
| Hansson 2011 | Phase III RCT | 855 | Adjuvant | II–III (T4N0M0/TanyN+M0) |
IFNa (2b) (1 year): 10 MU x 5/week (4 weeks, route: s.c.) + 10 MU x 3/week (12 months, route: s.c.) IFNa (2b) (2 years): 10 MU x 5/week (4 weeks, route: s.c.) + 10 MU x 3/week (24 months, route: s.c.) |
DFS: 0.034 OS: not significant |
| Agarwala 2011 | Phase III RCT | 1150 | Adjuvant | II‐III (T3‐4N0M0/TanyN1a‐N2aM0) |
IFNa(2b): 20MU/sqm x 5/week (1 month, route: i.v.) | DFS: not significant OS: not significant |
*Negative sentinel lymph node after standard pathological evaluation (H&E and immunohistochemistry) were tested with polymerase chain reaction (PCR) analysis
Sample sizes ranged between 96 and 1388 participants.
Studies were conducted across Europe (mainly within centres belonging to the European Organisation for Research and Treatment of Cancer (EORTC)), the United States (mainly within centres belonging to the Eastern Cooperative Oncology Group (ECOG)), and Australia.
Included studies investigated the impact of interferon alpha on disease‐free survival and overall survival of people with cutaneous melanoma after surgery. All participants underwent radical surgery for AJCC stage II (n = 810; 7.8%), AJCC stage III (n = 3512; 33.9%), or AJCC stages II–III (n = 6023; 58.3%) cutaneous melanoma. Participants were both men and women, generally aged 18 to 70 years.
Out of 18 trials, 16 compared interferon to observation, while 2 trials (Kirkwood 2001; Kirkwood 2001a) compared interferon to the GMK antimelanoma vaccine.
Interferon regimens varied in terms of dosage (high‐dose (20 mega‐units (MU)/m²), intermediate‐dose (10 MU/m²), and low‐dose (1 to 3 MU/m²)), administration route (subcutaneously (s.c.), intramuscularly (i.m.), and intravenously (i.v.)), and duration of treatment (4 months to 5 years), as detailed in the dedicated section: 'Characteristics of included studies'.
The two main causes of interruption to interferon treatment were disease progression or toxic effects: In the latter case, rates of treatment discontinuation (or dose reduction/delay) ranged from 0% to 58% (median 15%).
In terms of surgery, all participants received primary tumour excision in both interferon and control arms. Radical lymph node dissection was performed upon clinical and pathological evidence of lymph node metastasis in all RCTs, except for the E1684 trial (Kirkwood 1996), in which the enrolled participants underwent elective lymphadenectomy. Moreover, in trial EORTC18871 (Kleeberg 2004), the physician in charge performed elective lymph node dissection at their own discretion. The spread to regional lymph nodes was assessed mainly by means of physical examination (clinically evident metastatic disease) until the year 2000. Thereafter, sentinel node biopsy for the detection of subclinical metastatic disease was allowed by trial protocols. Many study designs did not consider sentinel node biopsy, thus, precluding the performance of subgroup meta‐analysis based on the type of lymph node positivity (i.e. macrometastasis (clinically palpable lymph nodes) versus micrometastasis (i.e. revealed by pathological assessment of the sentinel node biopsy)).
In one RCT (Kirkwood 2001a), the treatment arm regimen consisted of interferon combined with GMK (a ganglioside‐based anticancer vaccine). Because GMK alone was used in the comparison treatment arm, this study allowed the evaluation of any therapeutic benefit of IFN treatment; thus, we included it in the meta‐analysis. In addition, we used the 'leave‐one‐out' procedure as a sensitivity analysis to determine whether this trial had any impact on the overall effect estimated by the meta‐analysis (see results in Effects of interventions).
Since the findings from 2 trials, EORTC18871 and DKG80‐1, were reported in a single article as pooled results (and consequently as pooled HRs) (Kleeberg 2004), we included these 2 trials as a single study in the present meta‐analysis.
Excluded studies
We reported the reasons for the exclusion of 17 studies in the 'Characteristics of excluded studies' tables.
In one trial (Kokoschka 1990), although the authors mentioned that participants were randomly selected to receive interferon therapy, there was no mention of a corresponding control arm, and it seemed that surgically treated melanoma participants who were diagnosed at the time of the study were considered as a non‐randomised control group. In addition, participants with both AJCC TNM stage II and stage III melanoma were enrolled without mentioning any randomisation, and the results were represented separately as Kaplan–Meier curves for disease‐free survival (DFS), with no log‐rank P values, for the two stages. Also, the authors of this trial claimed that both stage I and stage II participants benefited from IFN therapy, although no difference in DFS or OS was detected between treatment and observation groups. All attempts to contact principal investigators of this trial were unsuccessful.
For two RCTs, the results were partially excluded. In a three‐arm RCT (Garbe 2008), the observation group was compared with both interferon alone and a combination of interferon and dacarbazine. In this case, we included only the comparison of IFN alone versus observation. The IFN plus dacarbazine arm was designed specifically to answer whether dacarbazine adds any therapeutic advantage to IFN; thus, we did not include results from this arm in the present meta‐analysis. One RCT (McMasters 2008) included two types of participants with AJCC TNM stage III disease: those with positive sentinel lymph node based on standard pathological evaluation and those classified as positive based on the results of polymerase chain reaction (PCR) for melanoma‐associated antigens. Although sentinel lymph node ultra staging by means of PCR methods is claimed to be associated with participant prognosis (Mocellin 2007), we decided to exclude the PCR arm since this was the only RCT including participants with this type of classification (that is, all the other RCTs enrolled TNM stage III participants exclusively on the basis of pathological evaluation).
Studies awaiting classification
There are no studies awaiting classification.
Ongoing studies
Two ongoing studies are comparing interferon treatment with new drugs: ipilimumab (ECOG E1609), an anti‐CTLA‐4 monoclonal antibody, and imatinib (NCT01782508), a c‐KIT inhibitor. Another trial is investigating the effect of interferon in participants with ulcerated primary melanomas (EORTC 18081).
Risk of bias in included studies
Figure 2 summarises the risk of bias for each included study. Although detailed descriptions of random sequence generation and allocation concealment were often missing, the quality of the available evidence was generally good except for Rusciani 1997.
2.
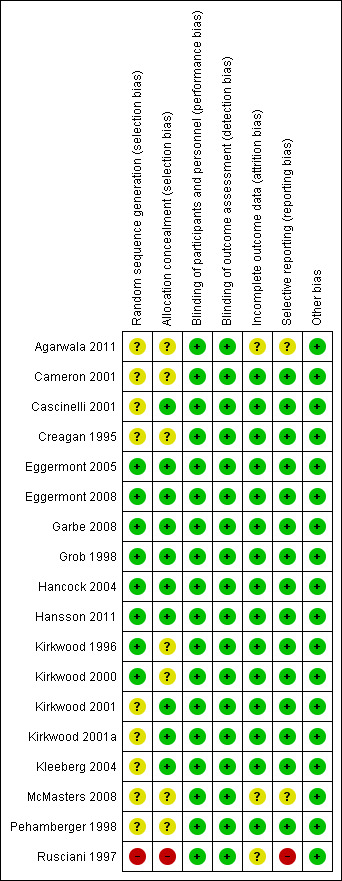
'Risk of bias' summary: review authors' judgments about each 'Risk of bias' item for each included study
Random sequence generation (selection bias)
Information regarding random sequence generation was unclear in nine studies. In the other eight studies, we judged this item at 'low risk of bias'. In Rusciani 1997, the risk of selection bias was high as it was unclear whether participants were randomly assigned to control or treatment. Because of this lack of clarity, the authors were contacted by phone and confirmed the randomised design, but omitted information regarding the possible risk of selection bias.
Allocation
Seven of the eligible studies under‐reported allocation concealment, so we judged the risk of this type of bias as 'unclear'. In the other 10 studies, we judged this item at 'low risk of bias'. In Rusciani 1997, the risk of selection bias was high, as we have reported above.
Blinding
Trials were not conducted in a blinded fashion, neither for the participants nor for the investigators. However, this is not expected to affect the primary aims of the study, i.e. disease‐free and overall survival, although some concerns may arise for other end points, such as toxicity and quality of life.
Incomplete outcome data
The risk of attrition bias was low for the majority of the studies. Missing outcome data were balanced in numbers across the intervention and observation groups, with similar reasons for missing data across groups.
Selective reporting
The main outcomes of the studies were disease‐free survival, overall survival, or both: One or both of these outcomes have been investigated according to the study design of each trial. In particular, two trials did not report data about the effect of interferon alpha on participant overall survival as they had been designed by the investigators to address only the issue of disease‐free survival (Kirkwood 2001a; Pehamberger 1998). Finally, the above‐mentioned Rusciani 1997 did not report any survival data (i.e. time‐to‐event data).
Other potential sources of bias
Since we did not identify any other potential sources of bias, we judged all the trials as at low risk of bias for this domain.
Effects of interventions
See: Table 1
Disease‐free survival (DFS)
All 17 eligible trials (Agarwala 2011; Cameron 2001; Cascinelli 2001; Creagan 1995; Eggermont 2005; Eggermont 2008; Garbe 2008; Grob 1998; Hancock 2004; Hansson 2011; Kirkwood 1996; Kirkwood 2000; Kirkwood 2001; Kirkwood 2001a; Kleeberg 2004; McMasters 2008; Pehamberger 1998), enrolling 10,345 participants, reported data on DFS.
The meta‐analysis of these 17 RCTs (Analysis 1.1) showed a significant risk reduction (17%) in participants undergoing postoperative interferon compared to those undergoing observation (15 trials) or other adjuvant therapy (HR = 0.83; CI 0.78 to 0.87; Z‐test P value < 0.0001). Between‐study heterogeneity was not statistically significant (I² statistic = 16%; Q‐test P value = 0.27).
1.1. Analysis.
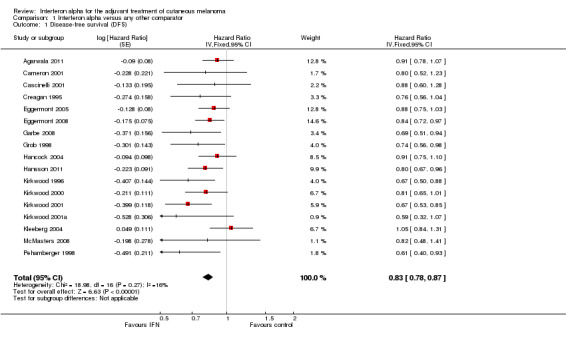
Comparison 1 Interferon alpha versus any other comparator, Outcome 1 Disease‐free survival (DFS).
The 'leave‐one‐out' sensitivity analysis showed no dominant RCT, which demonstrates that the overall effect estimate is not affected by the findings of a single RCT.
Upon exclusion of the 2 RCTs with an active control arm (Kirkwood 2001; Kirkwood 2001a), the results were very similar to those obtained including all 17 RCTs (HR = 0.84; CI 0.79 to 0.89; Z‐test P value < 0.0001; I² statistic = 2%, Q‐test P value = 0.43).
As shown in Figure 3, a small study effect was likely to be present (Begg test P = 0.17; Egger test P = 0.03), and the 'trim & fill' procedure revealed that 6 studies might be missing: Their addition to the meta‐analysis would reduce, but not nullify, the overall effect of interferon on DFS (adjusted HR = 0.87; CI 0.83 to 0.92). On the other hand, upon exclusion of RCTs enrolling fewer than 400 participants (Cameron 2001; Creagan 1995; Kirkwood 1996; Kirkwood 2001a; McMasters 2008; Pehamberger 1998), the meta‐analysis yielded results very similar to those obtained including all RCTs (HR = 0.85; CI 0.80 to 0.90; Z‐test P value < 0.0001; I² statistic = 20%; Q‐test P value = 0.25).
3.
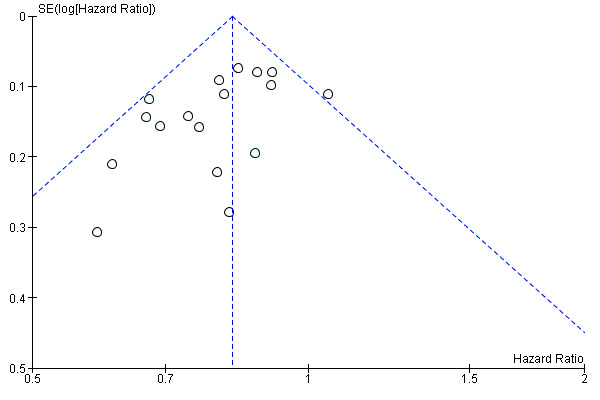
Funnel plot of comparison: 1 Interferon alpha versus any other comparator; outcome: 1.1 Disease‐free survival (DFS)
Finally, although we found no significant heterogeneity, we used subgroup analysis and metaregression to investigate whether some study design features might have affected trial outcomes. We report the results of all these analyses in Table 4 (for metaregression, see also Figure 4 and Figure 5). Overall, we found that none of the assessable factors we considered (interferon dose, TNM stage, year of publication, and treatment duration) significantly affected the impact of interferon on participants' DFS.
3. Additional analyses: disease‐free survival (DFS).
| Trial feature | RCT | HR (95% CI) # | Z‐test P value | I² statistic | Q‐test P value | Type of analysis | References |
| Interferon dose: high | 8* | 0.80 (0.74 to 0.87) | < 0.0001 | 14% | 0.32 | Subgroup meta‐analysis | 1 to 8 |
| Interferon dose: low | 8* | 0.85 (0.77 to 0.94) | 0.001 | 25% | 0.23 | Subgroup meta‐analysis | 8 to 15 |
| Interferon dose: intermediate | 2 | 0.84 (0.75 to 0.95) | 0.005 | 0% | 0.43 | Subgroup meta‐analysis | 16, 17 |
| Interferon dose | 17 | Q‐value = 0.99 | ‐ | 0% | 0.61 | Heterogeneity | 1 to 17 |
| TNM stage: II | 2 | 0.70 (0.55 to 0.88) | 0.002 | 0% | 0.46 | Subgroup meta‐analysis | 12, 15 |
| TNM stage: III | 5 | 0.85 (0.77 to 0.94) | 0.001 | 0% | 0.61 | Subgroup meta‐analysis | 1, 3, 6, 10, 11 |
| TNM stage: II‐III | 10 | 0.83 (0.77 to 0.89) | < 0.0001 | 32% | 0.15 | Subgroup meta‐analysis | 2, 4, 5, 7 to 9, 13, 14, 16, 17 |
| TNM stage | 17 | Q‐value = 2.23 | ‐ | 10% | 0.33 | Heterogeneity | 1 to 17 |
| Publication year | 17 | Slope = 0.01 (‐0.0004 to 0.02) | 0.06 | ‐ | ‐ | Metaregression | 1 to 17 |
| Treatment duration | 17 | Slope = 0.0001 (‐0.003 to 0.003) | 0.09 | ‐ | ‐ | Metaregression | 1 to 17 |
Statistics: Z‐test: tests the significance of meta‐analysis summary effect;I² statistic: measures between‐study heterogeneity in meta‐analysis; Q‐test: tests the significance of heterogeneity; Q‐value: Q‐test statistic; slope: coefficient of metaregression model
Abbreviations : interferon: interferon alpha; RCT: randomised controlled trial; HR: hazard ratio (from meta‐analysis); CI: hazard ratio confidence interval
References:1: Agarwala 2011; 2: Creagan 1995; 3: Eggermont 2008; 4: Kirkwood 1996; 5: Kirkwood 2001; 6: McMasters 2008; 7: Kirkwood 2001a; 8: Kirkwood 2000; 9: Cameron 2001; 10: Cascinelli 2001; 11: Garbe 2008; 12: Grob 2010; 13: Hancock 2004; 14: Kleeberg 2004; 15: Pehamberger 1998; 16: Eggermont 2005; 17: Hansson 2011
Notes : *: The starred values indicate that one study (Kirkwood 2000) is represented twice (in both high‐ and low‐dose interferon groups) because this trial had three arms: observation, high‐dose interferon, and low‐dose interferon. #: The third column displays hazard ratios if not otherwise specified
4.
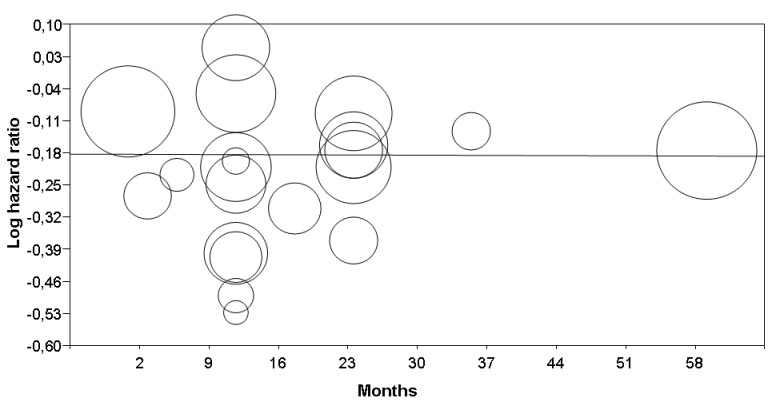
Metaregression: interferon effect (log hazard ratio, Y‐axis) on disease‐free survival versus treatment duration (months, X‐axis). See Table 4 for statistical details. Each circle represents a randomised controlled trial (the circle size is proportional to the trial weight)
5.
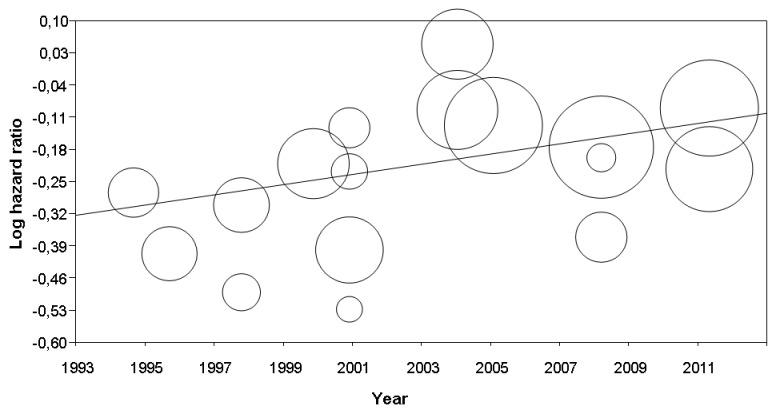
Metaregression: interferon effect (log hazard ratio, Y‐axis) on disease‐free survival versus publication year (year, X‐axis). See Table 4 for statistical details. Each circle represents a randomised controlled trial (the circle size is proportional to the trial weight)
Overall survival (OS)
Fifteen out of 17 eligible trials (Agarwala 2011; Cameron 2001; Cascinelli 2001; Creagan 1995; Eggermont 2005; Eggermont 2008; Garbe 2008; Grob 1998; Hancock 2004; Hansson 2011; Kirkwood 1996; Kirkwood 2000; Kirkwood 2001; Kleeberg 2004; McMasters 2008), enrolling 9927 participants, reported data on OS.
The meta‐analysis of these 15 RCTs (Analysis 1.2) showed a significant risk reduction (9%) in participants undergoing postoperative interferon compared to those undergoing observation (14 trials) or other adjuvant therapy (HR = 0.91; CI 0.85 to 0.97; Z‐test P value = 0.003). Between‐study heterogeneity was not statistically significant (I² statistic = 6%; Q‐test P value = 0.38).
1.2. Analysis.
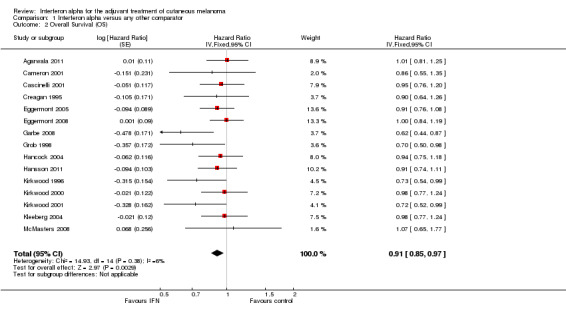
Comparison 1 Interferon alpha versus any other comparator, Outcome 2 Overall Survival (OS).
Considering a 5‐year OS rate of 60% for participants with TNM stage II–III cutaneous melanoma (Balch 2009), the number needed to treat (NNT) is approximately 35 participants (95% CI = 21 to 108 participants). This means that, given a group of 35 participants with high‐risk melanoma treated with interferon, after 5 years of follow‐up, 22 participants would be alive; in contrast, in a group of 35 participants not treated with interferon, 21 participants would be alive.
The 'leave‐one‐out' sensitivity analysis showed no dominant RCT, which demonstrates that the overall effect estimate is not affected by the findings of a single RCT.
Upon exclusion of the RCT with an active control arm (Kirkwood 2001), the results were very similar to those obtained including all 15 RCTs (HR = 0.92; CI 0.86 to 0.98; Z‐test P value = 0.01; I² statistic = 0%; Q‐test P value = 0.46).
As shown in Figure 6, a small study effect was likely to be present (Begg test P = 0.07; Egger test P = 0.06); however, the 'trim & fill' procedure suggested that no study is missing. On the other hand, upon exclusion of RCTs enrolling fewer than 400 participants (Cameron 2001; Creagan 1995; Kirkwood 1996; McMasters 2008), the meta‐analysis yielded results very similar to those obtained including all RCTs (HR = 0.92; CI 0.86 to 0.98; Z‐test P value = 0.012; I² statistic = 19%; Q‐test P value = 0.26).
6.
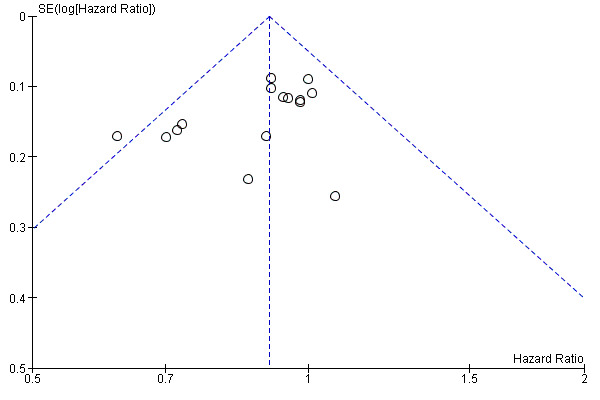
Funnel plot of comparison: 1 Interferon alpha versus any other comparator; outcome: 1.2 Overall survival (OS)
Finally, although we found no significant heterogeneity, we used subgroup analysis and metaregression to investigate whether some study design features might have affected trial outcomes. We report the results of all of these analyses in Table 5. According to subgroup analysis, interferon is not associated with an OS benefit in participants with AJCC TNM stage III melanoma (that is, in participants with regional lymph node metastasis), whereas the OS advantage is maintained in trials including both participants with stage III and those with stage II disease (that is, without lymph node metastasis). Only one trial enrolled exclusively AJCC TNM stage II participants and reported OS data, which did not enable us to perform subgroup analysis in this subset of participants. It should also be noted that the heterogeneity analysis did not show any significant difference between the summary hazard ratios (HR) of the three groups of trials (i.e. those enrolling participants with AJCC TNM stage II, stage III, and stage II‐III, respectively). With regard to interferon dose, subgroup analysis suggested that interferon is not associated with an OS benefit in participants treated with high‐ or intermediate‐dose, whereas the OS advantage is maintained in trials enrolling participants treated with low‐dose interferon. Also in this case, the heterogeneity analysis did not show any significant difference between the summary HR of the three groups of trials (i.e. those treating participants with high‐, intermediate‐, or low‐dose interferon, respectively). A metaregression showed neither year of publication nor treatment duration appeared to have any significant impact on the magnitude of treatment effect (Table 5).
4. Additional analyses: overall survival (OS).
| Feature | RCT | HR (95% CI) # | Z‐test P value | I² statistic | Q‐test P value | Type of analysis | References |
| Interferon dose: high | 7* | 0.93 (0.84 to 1.03) | 0.16 | 11% | 0.34 | Subgroup meta‐analysis | 1 to 7 |
| Interferon dose: low | 7* | 0.88 (0.79 to 0.98) | 0.02 | 23% | 0.25 | Subgroup meta‐analysis | 7 to 13 |
| Interferon dose: intermediate | 2 | 0.91 (0.80 to 1.04) | 0.16 | 0% | 1.00 | Subgroup meta‐analysis | 14, 15 |
| Interferon dose | 15 | Q‐value = 0.53 | ‐ | 0% | 0.76 | Heterogeneity | 1 to 15 |
| TNM stage: II | 1 | 0.70 (0.50 to 0.98) | ‐ | ‐ | ‐ | N/A | 11 |
| TNM stage: III | 5 | 0.95 (0.85 to 1.05) | 0.32 | 43% | 0.13 | Subgroup meta‐analysis | 1, 3, 6, 9, 10 |
| TNM stage: II‐III | 9 | 0.90 (0.83 to 0.98) | 0.01 | 0% | 0.77 | Subgroup meta‐analysis | 2, 4, 5, 7, 8, 12 to 15 |
| TNM stage | 15 | Q‐value = 3.11 | ‐ | 36% | 0.21 | Heterogeneity | 1 to 15 |
| Publication year | 15 | Slope = 0.01 (‐0.004 to 0.023) | 0.16 | ‐ | ‐ | Metaregression | 1 to 15 |
| Treatment duration | 15 | Slope = 0.001 (‐0.002 to 0.005) | 0.52 | ‐ | ‐ | Metaregression | 1 to 15 |
Statistics: Z‐test: tests the significance of meta‐analysis summary effect;I² statistic: measures between‐study heterogeneity in meta‐analysis; Q‐test: tests the significance of heterogeneity; Q‐value: Q‐test statistic; slope: coefficient of metaregression model
Abbreviations : interferon: interferon alpha; RCT: randomised controlled trial; HR: hazard ratio (from meta‐analysis); CI: hazard ratio confidence interval; N/A: not applicable
References:1: Agarwala 2011; 2: Creagan 1995; 3: Eggermont 2008; 4: Kirkwood 1996; 5: Kirkwood 2001; 6: McMasters 2008; 7: Kirkwood 2000; 8: Cameron 2001; 9: Cascinelli 2001; 10: Garbe 2008; 11: Grob 2010; 12: Hancock 2004; 13: Kleeberg 2004; 14: Eggermont 2005; 15: Hansson 2011
Notes : *: The starred values indicate that one study (Kirkwood 2000) is represented twice (in both high‐ and low‐dose interferon groups) because this trial had three arms: observation, high‐dose interferon, and low‐dose interferon. #: The third column displays hazard ratios if not otherwise specified
Discussion
Summary of main results
The results of this meta‐analysis support the efficacy of adjuvant interferon alpha (interferon) for the treatment of people with high‐risk (AJCC TNM stage II‐III) cutaneous melanoma in terms of both disease‐free survival (DFS) and ‐ to a lesser extent ‐ overall survival (OS). In fact, the risk reduction associated with interferon administration was statistically significant for both DFS (17%, 95% CI 13 to 22%) and OS (9%, 95% CI 3 to 15%) (Table 1).
The results of subgroup analysis failed to answer the questions of whether some treatment features (i.e. dosage, duration) might have an impact on interferon efficacy or whether some participant subgroups (i.e. with or without lymph node positivity) might benefit more from this postoperative adjuvant therapy.
High‐grade toxicity (i.e. Grades 3 and 4) was observed in a minority of participants: In some trials, no‐one had fever or fatigue of Grade 3 severity, but in other trials, up to 8% had fever and up to 23% had fatigue of Grade 3 severity. Less than 1% of participants had fever and fatigue of Grade 4 severity. Although it impaired quality of life, toxicity disappeared after treatment discontinuation.
Overall completeness and applicability of evidence
Although this meta‐analysis showed that interferon is associated with a better survival, it failed to identify the optimal interferon dosage and schedules. The available evidence is unsuitable to fully address the issue of the impact of interferon on the quality of life of the treated participants.
Interferon dosage and schedules
While the studies included in this meta‐analysis are sufficient to prove the effect of interferon in reducing risk of disease progression and death among high‐risk people, it is still necessary to identify optimal treatment dosages and schedules and to improve patient selection. With regard to interferon dosage, findings from our subgroup analyses (see Table 4 and Table 5) indicate that interferon is equally effective in terms of DFS when different doses (i.e. high versus low versus intermediate) are administered.
In contrast, when it comes to OS, the therapeutic benefit remains statistically significant only when considering trials that used low‐dose interferon. This finding is in contrast with the common belief that high‐dose interferon is the most effective, which has led the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) to approve high‐dose interferon and pegylated interferon. This is supported by the results of a RCT addressing this issue, which compared high‐dose versus low‐dose interferon, and showed that the low‐dose interferon regimen was associated with advantage in DFS, but not in OS (Kirkwood 2000). Our findings appear to challenge this but leave the debate open, since the low statistical power might be the reason why the subgroup analysis of our meta‐analysis failed to detect a significant risk reduction: In other words, the number of RCTs needed to detect a relatively small OS advantage (such as that observed in this meta‐analysis) might be higher than the number of RCTs currently available.
It is important to remember that some RCTs (Hansson 2011; Kirkwood 1996) directly addressed this issue by randomly assigning participants to different interferon doses, but we had to exclude the following from this review as they did not fulfil the inclusion criteria: Chiarion‐Sileni 2011; Grob 2010; Hauschild 2009; Pectasides 2009. However, none of them demonstrated that the classical E1684 regimen (i.e. high‐dose induction + low‐dose maintenance) (Kirkwood 1996) yields results different from the E1684 maintenance phase alone (Hauschild 2009) or that an intensified high‐dose interferon regimen (4 courses of induction phase every other month) is any better than the classical E1684 full regimen (1‐month induction + 11‐month maintenance) (Chiarion‐Sileni 2011), or that the E1684 full regimen is superior to the E1684 induction phase alone (Pectasides 2009). The results of these trials cannot be pooled in a meta‐analysis since the study designs are too heterogeneous.
Analogously, we could not define whether the duration of interferon treatment impacts on its efficacy: In fact, metaregression analysis did not show any significant relationship between interferon treatment duration and DFS or OS benefit (see Table 4 and Table 5). The results of the EORTC trial published in 2005 (Eggermont 2005) showed that intermediate‐dose interferon prolonged DFS (as compared to observation) only when administered for 2 years (and not for 1 year), although no effect on OS was observed. Nevertheless, four other RCTs that addressed this issue did not detect any statistically significant difference between the following:
the original E1684 full regimen (high‐dose for 1 month + low‐dose for 11 months) versus an intermittent schedule (3 courses of high‐dose for 1 month repeated every 4 months) (Mohr 2008);
intermediate‐dose interferon for 1 year compared to 2 years (Hansson 2011);
a low‐dose interferon regimen for 18 months versus the same for 60 months (Hauschild 2010); or
low‐dose interferon for 18 months versus a similar regimen (pegylated interferon 100 mcg subcutaneously once weekly) for 36 months (Grob 2010).
Also, the results of these trials cannot not be pooled in a meta‐analysis since the study designs are too heterogeneous.
Finally, we could not exhaustively answer the question of whether interferon treatment is more effective in a specific disease stage (i.e. AJCC TNM stage II (negative lymph nodes) versus stage III (positive nodes)). In fact, considering disease‐free survival as the end point, interferon appeared to be equally effective. However, when overall survival was taken into consideration, RCTs enrolling exclusively stage III participants showed no survival advantage (Table 5), whereas RCTs indiscriminately enrolling participants with stage II or stage III disease did demonstrate an overall survival benefit (Table 5), and the only RCT exclusively enrolling stage II participants was positive in terms of overall survival (Grob 2010). Overall, our findings might suggest that interferon adjuvant therapy is more effective in stage II melanoma patients, but the evidence supporting this hypothesis is too scarce to draw any definitive conclusion.
Toxity and quality of life
Severe adverse events (Grades 3 and 4 according to the scale adopted by the World Health Organization) after treatment with interferon occurred in the minority of the included studies (Table 6), with Grade 3 fever (0% to 8%) and fatigue (0% to 23%) being the most frequently observed adverse events.
5. Grade 3 to 4 adverse events after treatment with adjuvant interferon alpha.
| Study | Arm | Fever | Fatigue | Myalgia | Arthralgia | Anorexia | Dizziness | Headache | Mood | ||||||||
| Grade 3 (%) | Grade 4 (%) | Grade 3 (%) | Grade 4 (%) | Grade 3 (%) | Grade 4 (%) | Grade 3 (%) | Grade 4 (%) | Grade 3 (%) | Grade 4 (%) | Grade 3 (%) | Grade 4 (%) | Grade 3 (%) | Grade 4 (%) | Grade 3 (%) | Grade 4 (%) | ||
| Grob 1998 | IFN | < 1 | 0 | NR | NR | NR | NR | NR | NR | NR | NR | 0 | NR | 1 | NR | < 1 | < 1 |
| Observation | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | |
| Kirkwood 2000 | High‐dose IFN | NR | NR | 23 | 1 | 16 | 1 | NR | NR | NR | NR | NR | NR | NR | NR | 8 | 1 |
| Low‐dose IFN | NR | NR | 3 | 0 | 8 | 1 | NR | NR | NR | NR | NR | NR | NR | NR | 2 | 0 | |
| Observation | NR | NR | 0 | 0 | 0 | 0 | NR | NR | NR | NR | NR | NR | NR | NR | 0 | 0 | |
| Kirkwood 2001 | IFN | NR | NR | 20.6 | 0.3 | 3.8 | 0 | NR | NR | NR | NR | NR | NR | NR | NR | 8.6 | 1.3 |
| GSK | NR | NR | 1.2 | 0 | 0.5 | 0 | NR | NR | NR | NR | NR | NR | NR | NR | 0.5 | 0.2 | |
| Hancock 2004 | IFN | 1 | 0 | 6.7 | 0 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 3.1 | 0 |
| Observation | 0 | 0 | 1.3 | 0 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 1.6 | 0 | |
| Eggermont 2005 | 13‐month IFN | 6 | 1 | 14 | 1 | 7 | 1 | 6 | 1 | 6 | 1 | 4 | 1 | 5 | 1 | 10 | 2 |
| 25‐month IFN | 8 | 1 | 12 | 1 | 2 | 1 | 2 | 1 | 6 | 1 | 4 | 1 | 5 | 1 | 9 | 1 | |
| Observation | 0 | 0 | 2 | 0 | 2 | 0 | 2 | 1 | 1 | 0 | 2 | 1 | 2 | 0 | 2 | 1 | |
| Garbe 2008 | IFN | 0 | 0 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 1.6 | 0.8 |
| IFN + DTIC | 0 | 0 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 1.6 | 0 | |
| Observation | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | |
| Eggermont 2008 | Peg‐IFN | 4 | 1 | 15 | 1 | 4 | 1 | NR | NR | NR | NR | NR | NR | 4 | 1 | 6 | 1 |
| Observation | 0 | 0 | 1 | 0 | 1 | 0 | NR | NR | NR | NR | NR | NR | 0 | 0 | 1 | 1 | |
| Hansson 2011 | 13‐month IFN | 1.1 | 0.4 | 9.8 | NR | 5.3 | NR | 2.8 | NR | 3.5 | NA | NR | NR | 3.5 | NR | 4.9 | 1.1 |
| 25‐month IFN | 1 | 0 | 11.2 | NR | 4.9 | NR | 4.6 | NR | 3.5 | NA | NR | NR | 3.1 | NR | 2.4 | 0 | |
| Observation | 0.4 | 0 | 1.8 | NR | 1.1 | NR | 1.4 | NR | 0 | NA | NR | NR | 0.4 | NR | 0.4 | 0 | |
NR: not reported
Not available for Creagan 1995 (grades 3 and 4 not reported), Kirkwood 1996 (adverse events grouped as "constitutional symptoms"), Pehamberger 1998 (grades 3 and 4 not reported), Cameron 2001, Cascinelli 2001, Kirkwood 2001 (E2696), Kleeberg 2004, McMaster 2008, Agarwala 2011
Side‐effects such as these impair quality of life (QoL) as reported by some other studies that investigated quality of life after treatment with interferon (Bottomley 2009 based on Eggermont 2008; Ziefle 2011a based on Hauschild 2010; and Brandberg 2012 based on Hansson 2011). Results favoured the treatment with interferon over observation in terms of quality of life‐adjusted survival (Cole 1996 based on Kirkwood 1996; Kilbridge 2001 and Kilbridge 2002 based on both Kirkwood 1996 and Kirkwood 2000; and Caraceni 1998 based on Cascinelli 2001). In the pivotal trial of the Eastern Cooperative Oncology Group, the E1684 (Kirkwood 1996), Cole et al studied the effect of quality of life on patient survival and showed that although interferon improved disease‐free and overall survival by 9 and 7 months, respectively, it caused 6 months of significant toxicity (Cole 1996). However, participants treated with interferon had a better quality of life‐adjusted survival than participants who were observed only, regardless of their relative valuations for toxicity and disease‐free survival. This difference was statistically significant only for participants who considered toxicity to have a high relative value and disease relapse to have a low relative value, but not for participants who valued toxicity the same as relapse. Another study conducted on participants of the E1684 trial compared the quality of life of participants who experienced severe toxicity during treatment with interferon (Grade 4) with that of participants who had a recurrence in the observation arm, showing that participants rated quality of life with melanoma recurrence lower than even severe toxicity (Kilbridge 2001).
A quality of life‐adjusted survival analysis conducted in a subgroup of participants enrolled in 2 of the included studies (Kirkwood 1996; Kirkwood 2000) suggested that interferon may improve quality of life‐adjusted survival for some, but only a minority (16%) showed a statistically significant benefit. The change in quality of life‐adjusted survival depends more on the measure for interferon toxicity than on the measure for melanoma recurrence (Kilbridge 2002). Taken together, these data showed that although interferon alpha negatively affects quality of life, there is an improvement in quality of life‐adjusted survival for those who undergo treatment with interferon. The occurrence of toxicity and its negative effect on quality of life can be managed as suggested by expert authors (Bottomley 2009; Hauschild 2008), and it is reversible when the treatment is stopped (Brandberg 2012).
Of note, differences in assessment of quality of life prevented meta‐analysis of quality of life measures. Currently, there is still a lack of a melanoma‐oriented questionnaire that could account for changes in quality of life in subsets of patients (Cormier 2012). Beside the cancer questionnaire QLQ‐C30 of the European Organisation for Research and Treatment of Cancer (EORTC), only two QoL questionnaires have been designed specifically for people who have melanoma: the FACT‐Melanoma, which does not include evaluation of psychological domains, and the Malignant Melanoma Module, which has not been validated yet (Cormier 2011; Winstanley 2013). A new instrument for assessing quality of life of people with melanoma, but which is not addressing interferon and adjuvant treatment specifically, is under evaluation within the Quality of Life Group of the EORTC.
Quality of the evidence
This body of evidence allows us to draw robust conclusions regarding the impact of interferon alpha on disease‐free and overall survival of high‐risk melanoma patients. We included 18 studies, with 10,499 participants: Of these, we were able to perform meta‐analyses for disease‐free survival on 10,345 participants from 17 studies and overall survival on 9927 participants from 15 studies; 2 studies had not been designed to investigate overall survival, and 1 included study of 154 participants did not assess either outcome. The evidence we collected comes from well‐designed randomised controlled trials (RCTs) most often conducted by international organisations, e.g. the European Organization for Research and Treatment of Cancer (EORTC), and the Eastern Cooperative Oncology Group (ECOG). Therefore, the quality of the evidence meta‐analysed in the present work can be considered high (Table 1).
Potential biases in the review process
There was a generally low risk of bias in all the eligible studies, but one (Rusciani 1997) that was not suitable for inclusion in the meta‐analysis. Selection bias was difficult to ascertain in roughly half of the studies as no information was reported in the manuscripts. Finally, no trial was conducted in a blinded fashion. Although this is not supposed to affect survival, which is the primary end point of all studies, issues may arise regarding toxicity and quality of life.
Agreements and disagreements with other studies or reviews
Our findings support the efficacy of adjuvant interferon alpha (interferon) for the treatment of people with high‐risk (AJCC TNM stage II‐III) cutaneous melanoma in terms of both disease‐free survival (DFS) and, to a lesser extent, overall survival (OS). These results represent a significant update of the existing literature on this subject: In fact, compared to previous meta‐analyses, the present work includes 3 new RCTs (Agarwala 2011; Hansson 2011; McMasters 2008) and can count on the survival data of 10,345 participants.
Out of three previous meta‐analyses of adjuvant interferon for melanoma patients (Pirard 2004; Mocellin 2010; Wheatley 2003), the first two reported a disease‐free survival advantage but not an overall survival benefit (Pirard 2004; Wheatley 2003). In particular, Pirard and colleagues (Pirard 2004) considered nine trials including the two studies with severe drawbacks in their design, which we excluded from the present meta‐analysis (see Excluded studies). These investigators measured the risk of both disease recurrence and death by calculating odds ratios (OR: the ratio between odds, where "odds" indicates the ratio between events and non‐events in a given participant group): This is an inappropriate method to study survival data (also called time‐to‐event data), which are better described by hazard ratios (HR: the ratio between the risks of an event occurring over a given time span, where "hazard" indicates the risk in a given participant population). This is especially true when considering the OS data because in the long‐term, all participants of both comparison arms will die of disease or any other cause; thus, no therapy‐induced benefit could ever be detected using statistics based exclusively on the number of events.
In the second full‐text meta‐analysis, Wheatley and colleagues (Wheatley 2003) used time‐to‐event data and found a DFS (but not OS) advantage including 12 RCTs, but not the 2 trials we judged ineligible (Kokoschka 1990; Rusciani 1997). For three included RCTs (Eggermont 2005; Hancock 2004; Kleeberg 2004), these authors could not use the definitive data because the articles reporting them were published after their meta‐analysis was published. Later on, the same authors published an abstract describing an individual patient data meta‐analysis on the same subject (Wheatley 2007), considering 13 RCTs and 6067 participants; these investigators showed a benefit in terms of both DFS (OR = 0.87; 95% CI 0.81 to 0.93) and OS (OR = 0.9; 95% CI 0.84 to 0.97).
Finally, in a meta‐analysis published in 2010, Mocellin and colleagues considered 14 RCTs (including 2 trials published after the last work of Wheatley and colleagues (Eggermont 2008; Garbe 2008) and including 8122 participants: They also found that interferon significantly improves both DFS (risk reduction = 18%) and OS (risk reduction = 11%) of participants with high‐risk cutaneous melanoma (Mocellin 2010).
Authors' conclusions
Implications for practice.
Our findings support the efficacy of adjuvant interferon alpha (interferon) for the treatment of participants with high‐risk (AJCC TNM stage II‐III) cutaneous melanoma in terms of both disease‐free survival and, to a lesser extent, overall survival. High‐grade toxicity was observed in a minority of participants, who reported impairment in their quality of life. Nevertheless, participants reported relief from symptoms and improvement in quality of life after treatment discontinuation.
Until better selection methods or more effective therapies are available, the findings of the present meta‐analysis lend support to the use of interferon in the routine clinical setting to provide patients with the best chance of survival.
Implications for research.
The results reported above cannot be fully satisfying in terms of antimelanoma efficacy. In fact, considering a 5‐year OS rate of 60% for participants with TNM stage II–III cutaneous melanoma (Balch 2009), the number needed to treat (NNT) is approximately 35 participants (95% CI 21 to 108 participants). Therefore, much more effort is clearly warranted to improve these results. This might be achieved in three main ways.
A) By identifying participants who actually need adjuvant treatment:
About 40% of AJCC TNM stage II‐III participants are likely to be cured with surgery alone; thus, it is unnecessary to expose them to the toxicity of interferon therapy. Moreover, their inclusion in interferon trials could represent a bias in evaluating interferon efficacy since they would never benefit from this treatment and thus would only dilute the survival advantage associated with the administration of this cytokine to those who do harbour minimal residual disease after surgery. This issue can be addressed by developing effective methods to detect minimal residual disease after apparently radical surgery of high‐risk melanoma: In this regard, detection of circulating tumour cells in the peripheral blood is being advocated as a promising tool to select melanoma patients most likely to need adjuvant treatments (Mocellin 2006). As a further example, some investigators have proposed ulceration as a histopathological marker of tumour aggressiveness (and thus as a predictor of minimal residual disease) (Eggermont 2011). A planned trial will test the hypothesis that interferon might be more effective in this subset of participants (the EORTC 18081, which will compare adjuvant pegylated interferon in sentinel node‐negative AJCC stage II and IIIA participants with ulcerated primary tumours).
B) By elucidating the molecular mechanisms underlying melanoma responsiveness to interferon:
This would allow physicians to administer interferon selectively to people most likely to be responsive to interferon (Brown 2006; Foser 2006; Kumar 2007; Lesinski 2008; Lesinski 2010; Rao 2010; Wang 2008). For instance, in a recent report it has been suggested that multiplexed analysis of serum biomarkers (interleukin (IL)‐1alpha, IL‐1beta, IL‐6, IL‐8, IL‐12p40, IL‐13, granulocyte‐macrophage colony‐stimulating factor (GM‐CSF), monocyte chemoattractant protein‐1 (MCP‐1), macrophage inflammatory protein (MIP)‐1alpha, MIP‐1beta, interferon, tumour necrosis factor (TNF)‐alpha, epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), and TNF receptor II) might become a useful tool for predicting response to interferon of patients with high‐risk operable melanoma (Yurkovetsky 2007a).
C) By developing new treatments that are more effective than interferon against minimal residual disease.
In this regard, great expectations are obviously set on anti‐melanoma molecularly targeted therapies (Friedlander 2010; Mocellin 2010a); recent successes with small‐molecule BRAF inhibitors (Hodi 2010) and anti‐CTLA‐4 monoclonal antibodies (Chapman 2011) in people with metastatic melanoma are fuelling new hopes, although no data are yet available in the adjuvant setting.
Until better selection methods or more effective therapies are available, the findings of the present meta‐analysis lend support to the use of interferon in the routine clinical setting to provide patients with the best chance of survival. Moreover, we must remember that other well‐established adjuvant treatments, such as those routinely administered to people with breast, colorectal, and ovarian carcinomas, are associated with risk reductions very similar to those found in this meta‐analysis for those with high‐risk melanoma treated with interferon (Ascierto 2008). Therefore, the need for better therapeutic strategies is an urgent issue for virtually all tumour types.
Finally, the findings of this meta‐analysis support interferon alpha as the reference treatment in RCTs investigating new therapeutic agents for the adjuvant treatment of high‐risk melanoma participants. One such trial, comparing interferon and ipilimumab in AJCC TNM stage III‐IV melanoma participants after surgery (an immunostimulating agent acting by inhibiting CTLA‐4), has been designed by ECOG and is currently ongoing (ECOG E1609, clinicaltrial.gov identifier: NCT01274338).
What's new
| Date | Event | Description |
|---|---|---|
| 12 October 2015 | Review declared as stable | A search in September 2015 found no relevant new results. Our Co‐ordinating Editor confirmed that there is currently no new data of relevance. Thus, this review has been marked stable because an update has not been considered necessary for two successive years. Our Trials Search Co‐ordinator will run a new search in September 2016 to re‐assess whether an update is needed. |
History
Protocol first published: Issue 1, 2011 Review first published: Issue 6, 2013
| Date | Event | Description |
|---|---|---|
| 7 October 2015 | Amended | Author information (affiliation) updated. |
| 2 October 2014 | Amended | Although there were 3 ongoing studies listed in the last published review, a search of MEDLINE and Embase in 2014 found some relevant results, and this is a rapidly moving field, this review has currently been deemed not in need of an update because the review team assessed the aforementioned search results and found that only 1 trial (Eggermont AM, Suciu S, Testori A, Santinami M, Kruit WH, Marsden J, et al. Long‐term results of the randomized phase III trial EORTC 18991 of adjuvant therapy with pegylated interferon alfa‐2b versus observation in resected stage III melanoma. Journal of Clinical Oncology 2012;30(31):3810‐8. (DOI: 10.1200/JCO.2011.41.3799)) would be eligible for the update. However, the review authors deemed that the data from this article were just an update of a trial that they had already included in the review (Eggermont 2008), with the findings being virtually identical to the original findings. Thus, an update has not been considered necessary at this time. Our Trials Search Co‐ordinator will run a new search towards the end of 2015 to re‐assess whether an update is needed. |
Notes
A search in September 2015 found no relevant new results. Our Co‐ordinating Editor confirmed that there is currently no new data of relevance. Thus, this review has been marked stable because an update has not been considered necessary for two successive years. Our Trials Search Co‐ordinator will run a new search in September 2016 to re‐assess whether an update is needed.
Acknowledgements
We truly thank our data manager (Dr Marta Briarava) for her help in retrieving scientific articles and for setting up the database we used to collect and analyse the data from clinical trials. We also thank the Cochrane Skin Group, and in particular Prof Hywel Williams, Miss Laura Prescott, and Dr Finola Delamere for their assistance.
The Cochrane Skin Group editorial base wishes to thank Dedee Murrell who was the Key Editor for this review; Jo Leonardi‐Bee and Philippa Middleton who were the Statistical and Methods Editors, respectively; the clinical referees, Eleni Linos and Rubeta Matin; and the consumer referee, Kathie Godfrey.
Appendices
Appendix 1. CENTRAL (Cochrane Library) search strategy
#1 (melanoma*) #2 (interferon*) or (ifn alpha) or (ifn alfa) #3 MeSH descriptor Interferons explode all trees #4 MeSH descriptor Interferon‐alpha explode all trees #5 MeSH descriptor Melanoma explode all trees #6 (#1 OR #5) #7 (#2 OR #3 OR #4) #8 (#6 AND #7)
Appendix 2. MEDLINE (OVID) search strategy
1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized.ab. 4. placebo.ab. 5. clinical trials as topic.sh. 6. randomly.ab. 7. trial.ti. 8. 1 or 2 or 3 or 4 or 5 or 6 or 7 9. (animals not (human and animals)).sh. 10. 8 not 9 11. exp Melanoma/ 12. melanoma$.mp. 13. 11 or 12 14. interferon$.mp. 15. exp Interferons/ or exp Interferon‐alpha/ 16. (interferon alpha or interferon alfa).mp. 17. 14 or 15 or 16 18. 10 and 13 and 17
Appendix 3. EMBASE (OVID) search strategy
1. random$.mp. 2. factorial$.mp. 3. (crossover$ or cross‐over$).mp. 4. placebo$.mp. or PLACEBO/ 5. (doubl$ adj blind$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer] 6. (singl$ adj blind$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer] 7. (assign$ or allocat$).mp. 8. volunteer$.mp. or VOLUNTEER/ 9. Crossover Procedure/ 10. Double Blind Procedure/ 11. Randomized Controlled Trial/ 12. Single Blind Procedure/ 13. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 14. exp MELANOMA/ 15. melanoma$.mp. 16. 14 or 15 17. interferon$.mp. 18. (interferon alpha or interferon alfa).mp. 19. exp INTERFERON/ 20. exp alpha interferon/ 21. 17 or 18 or 19 or 20 22. 13 and 16 and 21
Appendix 4. AMED (OVID) search strategy
1. randomized controlled trial$/ 2. random allocation/ 3. double blind method/ 4. single blind method.mp. 5. exp Clinical trials/ 6. (clin$ adj25 trial$).mp. [mp=abstract, heading words, title] 7. ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$ or dummy)).mp. [mp=abstract, heading words, title] 8. (placebo$ or random$).mp. [mp=abstract, heading words, title] 9. research design/ or clinical trials/ or comparative study/ or double blind method/ or random allocation/ 10. prospective studies.mp. 11. cross over studies.mp. 12. Follow up studies/ 13. control$.mp. 14. (multicent$ or multi‐cent$).mp. [mp=abstract, heading words, title] 15. ((stud or design$) adj25 (factorial or prospective or intervention or crossver or cross‐over or quasi‐experiment$)).mp. [mp=abstract, heading words, title] 16. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 17. exp Melanoma/ 18. melanoma$.mp. [mp=abstract, heading words, title] 19. 17 or 18 20. exp Interferons/ 21. interferon$.mp. [mp=abstract, heading words, title] 22. 20 or 21 23. 16 and 19 and 22
Appendix 5. LILACS search strategy
melanoma$ and (interferon$ or ifn$)
Data and analyses
Comparison 1. Interferon alpha versus any other comparator.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Disease‐free survival (DFS) | 17 | Hazard Ratio (Fixed, 95% CI) | 0.83 [0.78, 0.87] | |
| 2 Overall Survival (OS) | 15 | Hazard Ratio (Fixed, 95% CI) | 0.91 [0.85, 0.97] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Agarwala 2011.
| Methods | 553 centres enrolled participants Accrual period: 1998 to 2010 Design: phase III randomised controlled trial (RCT) |
|
| Participants | Number of participants: 1150 Observation N = 569 (group A); interferon alpha 2b N = 581 (group B) Participants randomised: 1150 Participants evaluable: 1150 Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
|
|
| Outcomes |
Subgroup analysis: not performed |
|
| Notes | Median follow‐up: not known Participants with lymph node metastasis: histopathological status of lymph node was known in 1035 participants (90%). Sentinel node biopsy and elective lymph node dissection were performed in 955 (83%) and 122 (11%), respectively. Lymph node metastasis were detected in 101 (20%) and 95 (18%) in the observation and interferon arms, respectively Dose reduction/treatment discontinuation: no available data Quality of life: According to the protocol, it was assessed before treatment at day 22, every 3 months for 2 years, and then every 6 months for 3 years (results not reported) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | There was insufficient information about the sequence generation process to permit judgment |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | There was no blinding, but we judged that lack of blinding was not likely to influence the outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | There was no blinding, but we judged that lack of blinding was not likely to influence the outcome |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There was insufficient reporting of attrition/exclusions to permit judgment |
| Selective reporting (reporting bias) | Unclear risk | There was insufficient reporting of attrition/exclusions to permit judgment |
| Other bias | Low risk | There was insufficient information to assess whether an important risk of bias existed, but we judged this study to be free of other sources of bias |
Cameron 2001.
| Methods | 9 centres enrolled participants Accrual period: not reported Design: phase III randomised controlled trial (RCT) |
|
| Participants | Number of participants: 96 Interferon alpha 2b N = 46 (group A); observation N = 49 (group B) Participants randomised: 96 Participants evaluable: 94 Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
|
|
| Outcomes |
Subgroup analysis: not performed |
|
| Notes | Median follow‐up: 78 months Participants with lymph node metastasis: not reported Dose reduction/treatment discontinuation: 3 participants (7%) required dose reduction (2 for neutropenia and 1 for drug‐related fever); treatment was stopped in 13 (28%) cases following disease recurrence Quality of life: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomized" Comment: There was insufficient information about the sequence generation process to permit judgment |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | There was no blinding, but we judged that lack of blinding was not likely to influence the outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | There was no blinding, but we judged that lack of blinding was not likely to influence the outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data were balanced in numbers across intervention groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports include all expected outcomes |
| Other bias | Low risk | The study appeared to be free of other sources of bias |
Cascinelli 2001.
| Methods | 23 centres belonging to the World Health Organization (WHO) Melanoma Programme enrolled participants Accrual period: 1990 to 1993 Design: phase III RCT |
|
| Participants | Number of participants: 444 Interferon alpha 2a N = 225 (group A); observation N = 219 (group C) Randomised: 444 Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
|
|
| Outcomes |
Subgroup analyses: not reported |
|
| Notes | Median follow‐up: 88 Lymph node dissection was performed following the guidelines produced by the education project of the WHO Melanoma Programme Participants with lymph node metastasis: 100% Dose reduction/treatment discontinuation: not reported Quality of life: Interferon has no negative effect on quality of life or daily activities of treated participants; fatigue and anxiety were more frequent in the interferon alpha 2a group than in those who had surgery alone |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "eligible patients were randomly assigned" Comment: There was insufficient information about the sequence generation process to permit judgment |
| Allocation concealment (selection bias) | Low risk | The trial used central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | There was no blinding, but we judged that lack of blinding was not likely to influence the outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | There was no blinding, but we judged that lack of blinding was not likely to influence the outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data were balanced in numbers across intervention groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports include all expected outcomes |
| Other bias | Low risk | The study appeared to be free of other sources of bias |
Creagan 1995.
| Methods | The North Central Cancer Treatment Group and the Mayo Clinic conducted this study Accrual period: 1984 to 1990 Design: phase III RCT |
|
| Participants | Number of participants: 266 High‐dose interferon alpha 2b N = 132 (group A); observation N = 132 (group B) Participants randomised: 264 Participants evaluable: 264 Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
|
|
| Outcomes |
Subgroup analysis: There was a significant disease‐free survival benefit for participants with lymph node metastasis |
|
| Notes | Median follow‐up: 73 months Lymph node dissection: Lymphadenectomy was performed in case of clinically positive lymph node Participants with lymph node metastasis: 62% Dose reduction/treatment discontinuation: More than half of participants (no quantification has been provided) required dose modification Quality of life: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients randomized" Comment: There was insufficient information about the sequence generation process to permit judgment |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | There was no blinding, but we judged that lack of blinding was not likely to influence the outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | There was no blinding, but we judged that lack of blinding was not likely to influence the outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data were balanced in numbers across intervention groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports include all expected outcomes |
| Other bias | Low risk | The study appeared to be free of other sources of bias |
Eggermont 2005.
| Methods | The trial was performed on behalf of the European Organization for Research and Treatment of Cancer (EORTC). 85 institutions in 22 countries enrolled participants Accrual period: 1996 to 2000 Design: phase III RCT |
|
| Participants | Number of participants: 1418 13‐month interferon alpha 2b N = 553 (group A); 25‐month interferon alpha 2b N = 556 (group B); observation N = 279 (group C) Participants randomised: 1388 Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
|
|
| Outcomes |
Subgroup analysis: node‐negative participants (AJCC stage IIb) treated with interferon alpha 2b for 25 months had a better distant metastasis‐free interval and survival than participants enrolled in the observation arm |
|
| Notes | Median follow‐up: 56 months Lymph node dissection: Regional lymph node dissection had to contain more than 5 nodes (inguinal), more than 10 nodes (axillary), or more than 15 nodes (neck) Participants with lymph node metastasis: 75% Dose reduction/treatment discontinuation: 16% of participants treated with 13‐months of interferon and 20% of participants treated with 25‐months of interferon Quality of life: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We enrolled and randomly assigned..." Quote: "randomisation was done with minimisation techniques" |
| Allocation concealment (selection bias) | Low risk | Quote: "randomisation was done centrally from the EORTC data centre" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | There was no blinding, but we judged that lack of blinding was not likely to influence the outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | There was no blinding, but we judged that lack of blinding was not likely to influence the outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data were balanced in numbers across intervention groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports include all expected outcomes, including those that were prespecified |
| Other bias | Low risk | The study appeared to be free of other sources of bias |
Eggermont 2008.
| Methods | The trial was performed on behalf of the EORTC. 99 centres in 17 countries (mainly in Europe) enrolled participants Accrual period: information not reported Design: phase III RCT |
|
| Participants | Number of participants: 1256 Pegylated interferon alpha 2b N = 627 (group A); observation N = 629 (group B) Participants randomised: 1256 Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
|
|
| Outcomes |
Subgroup analyses: Pegylated interferon alpha seemed effective in prolonging disease‐free survival for participants with microscopic lymph node involvement (i.e. participants with positive sentinel node biopsy) |
|
| Notes | Median follow‐up: 46 months Lymph node dissection: Complete regional lymphadenectomy must have occurred 70 days or less before randomisation Participants with lymph node metastasis: 100% Dose reduction/treatment discontinuation: The study protocol specified stepwise dose adjustments (6 μg/kg per week to 3, 2, and 1 μg/kg per week during the induction phase, and from 3 μg/kg per week to 2 and 1 μg/kg per week during the maintenance phase) to adjust for toxicity and to maintain an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1 for each participant. Treatment could be interrupted for surgery for local or regional recurrence of melanoma, then resumed after surgery Quality of life: Quality of life analysis, which has been reported elsewhere (Bottomley 2009), showed that pegylated interferon worsened patient quality of life. In particular, there were important differences for 5 scales used to assess quality of life: 2 functioning scales (social and role functioning) and 3 symptom scales (appetite loss, fatigue, and dyspnea), with the pegylated interferon alpha 2b arm being most impaired |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomly assigned" Quote: "randomisation was done with minimisation techniques; the sequence was generated by computer" |
| Allocation concealment (selection bias) | Low risk | Quote: "randomisation was done centrally at the EORTC data centre" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | There was no blinding, but we judged that lack of blinding was not likely to influence the outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | There was no blinding, but we judged that lack of blinding was not likely to influence the outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data were balanced in numbers across intervention groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports include all expected outcomes, including those that were prespecified |
| Other bias | Low risk | The study appeared to be free of other sources of bias |
Garbe 2008.
| Methods | 42 centres belonging to the Dermatologic Cooperative Oncology Group (DeCOG) network in Germany and Switzerland enrolled participants Accrual period: 1997 to 2001 Design: phase III RCT |
|
| Participants | Number of participants: 444 Interferon alpha 2a N = 148 (group A); interferon alpha 2a and dacarbazine (DTIC) N = 148 (group B); observation N = 148 (group C) Randomised: 444 Evaluable: 441 Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
|
|
| Outcomes |
Subgroup analyses: not reported |
|
| Notes | Median follow‐up: 47 months Lymph node dissection: This was carried out before randomisation, according to the German guidelines on the treatment of melanoma Participants with lymph node metastasis: 100% Dose reduction/treatment discontinuation: 21 participants withdrew their informed consent Quality of life: EORTC QLQ‐C30 questionnaire scores at baseline and 6 months after randomisation were compared in 238 participants. Participants under adjuvant treatment had a better outcome for 'physical functioning' (group A versus C: P = 0.007), 'role functioning' (group A versus C: P = 0.008), and 'emotional functioning' (group A versus C: P = 0.048) in comparison to the participants treated with surgery only |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomly assigned" Quote: "a permuted block randomization list" |
| Allocation concealment (selection bias) | Low risk | The trial used central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | There was no blinding, but we judged that lack of blinding was not likely to influence the outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | There was no blinding, but we judged that lack of blinding was not likely to influence the outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data were balanced in numbers across intervention groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports include all expected outcomes |
| Other bias | Low risk | The study appeared to be free of other sources of bias |
Grob 1998.
| Methods | 31 centres in France enrolled participants Accrual period: 1990 to 1994 Design: phase III RCT |
|
| Participants | Number of participants: 499 Interferon alpha 2a N = 244 (group A); observation N = 243 (group B) Participants randomised: 499 Participants evaluable: 487 Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
|
|
| Outcomes |
Subgroup analyses: not reported |
|
| Notes | Median follow‐up: 60 months Lymph node dissection: not reported Participants with lymph node metastasis: Lymph node metastases were detected in 68 (28%) and 78 participants in the interferon and observation groups, respectively Dose reduction/treatment discontinuation: Treatment was discontinued in 89 participants (36%): 43 relapsed; 7 refused further treatment; 3 were lost to follow‐up; and 1 stopped because of a wrong diagnosis. 35 participants withdrew because of 1 or several adverse events Quality of life: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned" Quote: "The random‐allocation list was computer generated" |
| Allocation concealment (selection bias) | Low risk | The trial used central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | There was no blinding, but we judged that lack of blinding was not likely to influence the outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | There was no blinding, but we judged that lack of blinding was not likely to influence the outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data were balanced in numbers across intervention groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports include all expected outcomes |
| Other bias | Low risk | The study appeared to be free of other sources of bias |
Hancock 2004.
| Methods | Participants were enrolled in the UK Accrual period: 1995 to 2000 Design: phase III RCT |
|
| Participants | Number of participants: 674 Interferon alpha 2a N = 338 (group A); observation N = 336 (group B) Participants randomised: 674 Participants evaluable: 633 Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
|
|
| Outcomes |
Subgroup analysis: Younger participants (age < 50 years) treated with interferon had a better DFS than younger participants untreated |
|
| Notes | Median follow‐up: 36 months Lymph node dissection: No description of the adopted surgical protocol was reported Participants with lymph node metastasis: 85 participants (13%) had lymph node metastasis at the time of primary melanoma diagnosis Dose reduction/treatment discontinuation: There were 50 withdrawals (14.8%) from interferon therapy due to toxicity; 24 participants stopped therapy for reasons other than toxicity, recurrence, or death from unrelated causes; dose reductions for toxicity were recorded for 21 participants (5 subsequently withdrew from therapy); 15 participants (3 subsequently withdrew) had breaks in therapy due to toxicity Quality of life: Interferon has significant effects on quality of life and symptomatology and is unlikely to be cost‐effective (Dixon 2006) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned" Quote: "Random assignments were balanced by minimization" |
| Allocation concealment (selection bias) | Low risk | The trial used central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | There was no blinding, but we judged that lack of blinding was not likely to influence the outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | There was no blinding, but we judged that lack of blinding was not likely to influence the outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data were balanced in numbers across intervention groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports include all expected outcomes |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Hansson 2011.
| Methods | Participants were enrolled by 35 centres in Nordic countries: 2 in Denmark, 5 in Finland, 6 in Norway, and 22 in Sweden. randomisation was done centrally at the data management centre (Karolinska University Hospital, Stockholm, Sweden) Design: phase III RCT |
|
| Participants | Number of participants: 855 Observation N = 284 (group A); 1‐year treatment with interferon alpha 2b N = 285 (group B); 2‐year treatment with interferon alpha 2b N = 286 (group C) Participants randomised: 855 Participants evaluable: 855 Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
|
|
| Outcomes |
Subgroup analysis: Participants without ulcerated primary tumours and treated with interferon had benefit in terms of both DFS and OS. Participants with AJCC stage III disease with clinically palpable lymph node metastases had improved DFS if treated with interferon (P = 0.015) |
|
| Notes | Median follow‐up: 72.4 months Lymph node dissection: Sentinel node biopsy (SNB) was not performed in all enrolled participants as it was not routinely practised in enrolling countries. Elective lymph node dissection was performed in the majority of participants, whereas few participants were managed with sentinel node biopsy and completion lymph node dissection Participants with lymph node metastasis: 80.6% Dose reduction/treatment discontinuation: Dose reductions were reported in 164 (28.7%) of the 571 participants randomly assigned to interferon alpha 2b therapy: 77 (27.0%) in group B and 87 (30.4%) in group C. Interferon alpha 2b therapy was interrupted or discontinued prematurely in 147 participants (25.7%): 75 (26.3%) in group B and 72 (25.2%) in group C Quality of life: assessed with the EORTC QLQ‐C30 questionnaire at 9 time points from before randomisation to up to 2 years, with focus on the 6‐month and 2‐year assessments. It was significantly negatively affected in almost all areas during treatment, but levels similar to those in the control group were restored after the end of treatment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned" Quote: "block randomisation, with block size of 15"; "The allocation sequence was computer‐generated" |
| Allocation concealment (selection bias) | Low risk | The trial used central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | There was no blinding, but we judged that lack of blinding was not likely to influence the outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | There was no blinding, but we judged that lack of blinding was not likely to influence the outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data were balanced in numbers across intervention groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | The study protocol was available, and all of the study's prespecified (primary and secondary) outcomes that were of interest in the review were reported in the prespecified way |
| Other bias | Low risk | The study appeared to be free of other sources of bias |
Kirkwood 1996.
| Methods | The study was conducted on behalf of the Eastern Cooperative Oncology Group (ECOG) and United States (US) intergroup adjuvant studies Accrual period: 1984 to 1990 Design: phase III RCT |
|
| Participants | Number of participants: 287 High‐dose interferon alpha 2b N = 147 (group A); observation N = 140 (group B) Participants randomised: 287 Participants evaluable: 287 Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
|
|
| Outcomes |
Subgroup analysis: not performed |
|
| Notes | Median follow‐up: 83 months Lymph node dissection: not available Participants with lymph node metastasis: 89% Dose reduction/treatment discontinuation: A dose reduction was observed in 35% of participants; treatment discontinuation was necessary in 34% and 41% of participants during induction and maintenance phases, respectively Quality of life: Participants who received interferon alpha had more quality‐of‐life‐adjusted survival than the observation group, regardless of the relative valuations placed on toxicity and relapse (Cole 1996) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomized by permuted blocks" |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | There was no blinding, but we judged that lack of blinding was not likely to influence the outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | There was no blinding, but we judged that lack of blinding was not likely to influence the outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data were balanced in numbers across intervention groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports include all expected outcomes |
| Other bias | Low risk | The study appeared to be free of other sources of bias |
Kirkwood 2000.
| Methods | The study was conducted on behalf of the Eastern Cooperative Oncology Group (ECOG) and United States (US) intergroup adjuvant studies Accrual period: 1991 to 1995 Design: phase III RCT |
|
| Participants | Number of participants: 642 High‐dose interferon alpha 2b N = 215 (group A); low‐dose interferon alpha 2b N = 215 (group B); observation N = 212 (group C) Participants randomised: 642 Participants evaluable: 608 Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
|
|
| Outcomes |
Subgroup analysis: High‐dose interferon alpha 2b was associated with a survival benefit in participants with 2 to 3 positive lymph nodes |
|
| Notes | Median follow‐up: 52 months Lymph node dissection: Participants with tumour depth > 4 mm and no clinical evidence of lymph node metastasis were not required to undergo lymphadenectomy Participants with lymph node metastasis: 75% Dose reduction/treatment discontinuation: 58% of participants treated with high‐dose interferon alpha 2b required delay or dose reduction (44% due to toxicity). A significantly larger proportion of relapsed participants from the observation arm (31%) received an interferon‐containing salvage regimen compared with only 15% of participants from the high‐dose interferon alpha 2b arm Quality of life: 77% of participants would experience a benefit in quality‐of‐life‐adjusted‐survival from interferon alpha, and 23% would experience a decrease in quality‐of‐life‐adjusted‐survival, although these effects did not reach statistical significance (Kilbridge 2002) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomized by permuted blocks" |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | There was no blinding, but we judged that lack of blinding was not likely to influence the outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | There was no blinding, but we judged that lack of blinding was not likely to influence the outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data were balanced in numbers across intervention groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports include all expected outcomes |
| Other bias | Low risk | The study appeared to be free of other sources of bias |
Kirkwood 2001.
| Methods | The study was conducted by Eastern Cooperative Oncology Group (ECOG) and United States (US) intergroup adjuvant studies Accrual period: 1996 to 1999 Design: phase III RCT |
|
| Participants | Number of participants: 880 GMK N = 389 (group A); interferon alpha 2b N = 385 (group B) Participants randomised: 774 Participants evaluable: 774 Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
|
|
| Outcomes |
Subgroup analyses: Node‐negative participants had a statistically significant DFS and OS benefit compared with participants treated with GMK |
|
| Notes | Median follow‐up: 16 months Lymph node dissection: It was performed in case of clinically positive lymph node or positive sentinel lymph node Participants with lymph node metastasis: Lymph node involvement was documented clinically or pathologically in 77% of participants Dose reduction/treatment discontinuation: 45 participants in the interferon alpha 2b arm (10%) discontinued treatment because of adverse events, while no participants experienced treatment discontinuation, dose reduction, or both, in the GMK arm Quality of life: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomized" There was insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Low risk | The trial used central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | There was no blinding, but we judged that lack of blinding was not likely to influence the outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | There was no blinding, but we judged that lack of blinding was not likely to influence the outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data were balanced in numbers across intervention groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports include all expected outcomes |
| Other bias | Low risk | The study appeared to be free of other sources of bias |
Kirkwood 2001a.
| Methods | The study was conducted on behalf of the Eastern Cooperative Oncology Group (ECOG) and United States (US) intergroup adjuvant studies Accrual period: ‐ Design: phase II RCT |
|
| Participants | Number of participants: 107 Interferon alpha 2b and GMK vaccine starting on day 1 N = 36 (group A); GMK vaccine starting from day 1 and interferon alpha 2b starting from week 5 N = 36 (group B); GMK vaccine alone N = 35 (group C) Participants randomised: 107 Participants evaluable: 107 Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
|
|
| Outcomes |
Subgroup analysis: not performed |
|
| Notes | Median follow‐up: 24 months Lymph node dissection: not reported Participants with lymph node metastasis: 89% Dose reduction/treatment discontinuation: 17 participants (16%) discontinued treatment (12 because of toxicity, and 5 withdrew consent) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were assigned randomly" There was insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Low risk | The trial used central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | There was no blinding, but we judged that lack of blinding was not likely to influence the outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | There was no blinding, but we judged that lack of blinding was not likely to influence the outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data were balanced in numbers across intervention groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports include all expected outcomes |
| Other bias | Low risk | The study appeared to be free of other sources of bias |
Kleeberg 2004.
| Methods | Participants were randomised by 45 institutions in 13 countries within the frame of the EORTC 18871 3‐arm trial (comparing observation versus interferon alpha 2b versus interferon gamma) and the DKG‐80‐1 4‐arm trial (comparing observation versus interferon alpha versus interferon gamma versus Iscador‐M) Accrual period: 1988 to 1996 Design: phase III RCT |
|
| Participants | Number of participants: 830 EORTC 18871 3‐arm trial (N = 423): observation N = 142; interferon alpha 2b N = 139; interferon gamma N = 142 DKG‐80‐1 4‐arm trial (N = 407): observation N = 102; interferon alpha 2b N = 101; interferon gamma N = 102; Iscador‐M N = 102 In order to ascertain interferon's role in the adjuvant treatment of high‐risk melanoma participants, the results of these 2 trials were pooled together: Overall, there were 244 participants in the observation arm, 240 in the interferon alpha 2b arm, and 244 in the interferon gamma arm Participants randomised: 830 Participants evaluable: 793 Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Median follow‐up: not reported Lymph node dissection: Clinically lymph node‐positive participants received radical lymph node dissection, while clinically node‐negative participants were treated with elective lymph node dissection or followed by observation based upon each institution's policy Participants with lymph node metastasis: 58% Dose reduction/treatment discontinuation: 11 participants (4.6%) treated with interferon alpha 2b, 19 (7.8%) treated with interferon gamma, and 5 (4.9%) in the Iscador‐M arm due to toxicity Quality of life: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomized" There was insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Low risk | The trial used central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | There was no blinding, but we judged that lack of blinding was not likely to influence the outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | There was no blinding, but we judged that lack of blinding was not likely to influence the outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data were balanced in numbers across intervention groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports include all expected outcomes |
| Other bias | Low risk | The study appeared to be free of other sources of bias |
McMasters 2008.
| Methods | The Sunbelt Melanoma Trial involved 79 centres across North America Accrual period: 1997 to 2003 Design: phase III RCT |
|
| Participants | Number of participants: 774 Protocol A: Participants with only 1 pathologically positive sentinel lymph node (SLN) who underwent completion lymph node dissection (CLND) were randomised to observation (group A) versus high‐dose interferon therapy (group B) Protocol B: Histologically negative SLN were analysed by reverse transcription polymerase chain reaction (PCR); participants with PCR‐positive SLN were randomised to 3 treatment arms: observation (group C), CLND (group D), or CLND plus interferon (group E) Participants randomised: 774 Participants evaluable: 774 Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions | Protocol A (participants with 1 positive sentinel lymph node submitted to CLND)
Protocol B (participants with histologically negative but PCR‐positive SLN)
|
|
| Outcomes |
Subgroup analysis: A paper reporting a subgroup analysis from this trial investigated the association between primary tumour ulceration and regional lymph node status, showing that interferon treatment was associated with improved disease‐free survival in participants with ulcerated primaries and lymph node metastasis (McMasters 2010) |
|
| Notes | Median follow‐up: 64 months Lymph node dissection: All participants underwent SLN biopsy; CLND was performed according to the above reported criteria Participants with lymph node metastasis: 18% Dose reduction/treatment discontinuation: not reported Quality of life: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | There was insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | There was no blinding, but we judged that lack of blinding was not likely to influence the outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | There was no blinding, but we judged that lack of blinding was not likely to influence the outcome |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There was insufficient reporting of attrition or exclusions to permit judgment |
| Selective reporting (reporting bias) | Unclear risk | There was insufficient information to permit judgment |
| Other bias | Low risk | There was insufficient information to assess whether an important risk of bias exists, but we judged this study to be free of other sources of bias |
Pehamberger 1998.
| Methods | The Austrian Melanoma Cooperative Group (7 Institutions) conducted the study Accrual period: 1990 to 1994 Design: phase III RCT |
|
| Participants | Number of participants: 311 Interferon alpha 2a N = 154 (group A); observation N = 157 (group B) Participants randomised: 311 Participants evaluable: 293 Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
|
|
| Outcomes |
Subgroup analysis: No significant subgroup of enrolled participants had a benefit after interferon treatment |
|
| Notes | Median follow‐up: 41 months Lymph node dissection: Elective lymph node dissection was not performed Participants with lymph node metastasis: 0% Dose reduction/treatment discontinuation: A dose reduction was necessary in 8 treated participants and a discontinuation of treatment in 12 Quality of life: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly assigned" There was insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to permit judgment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | There was no blinding, but we judged that lack of blinding was not likely to influence the outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | There was no blinding, but we judged that lack of blinding was not likely to influence the outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data were balanced in numbers across intervention groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports include all expected outcomes |
| Other bias | Low risk | The study appeared to be free of other sources of bias |
Rusciani 1997.
| Methods | The study was conducted by the Catholic University and Sapienza University, Rome, Italy Accrual period: not available Design: phase III RCT |
|
| Participants | Number of participants: 154 Interferon alpha 2b N = 84 (group A); observation N = 70 (group B) Participants randomised: 154 Participants evaluable: 154 Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions |
|
|
| Outcomes | The crude incidence of recurrence was lower for participants treated with interferon alpha than participants enrolled in the control arm. The analysis were not performed following a methodology suitable to investigate survival |
|
| Notes | Median follow‐up: not reported Lymph node dissection: not performed Participants with lymph node metastasis: 0% Dose reduction/treatment discontinuation: Treatment suspension or discontinuation was never required because of adverse events Quality of life: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "The authors have started a randomized clinical trial", "concomitant patients" Comment: We contacted the authors of the study by telephone, and they confirmed the randomised design of the study There was no information about the sequence generation process |
| Allocation concealment (selection bias) | High risk | There was no information about allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | There was no blinding, but we judged that lack of blinding was not likely to influence the outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | There was no blinding, but we judged that lack of blinding was not likely to influence the outcome |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There was insufficient reporting of attrition/exclusions to permit judgement of 'low risk' or 'high risk' (e.g. number randomised not stated; no reasons for missing data provided) |
| Selective reporting (reporting bias) | High risk | Outcomes of interest in the review were reported incompletely, so we could not enter them in a meta‐analysis. The study report fails to include results for disease‐free survival, overall survival, or both, which would be expected to have been reported for such a study |
| Other bias | Low risk | The study appeared to be free of other sources of bias |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anaya 2008 | This was a non‐randomised study |
| Chiarion‐Sileni 2011 | As this trial compared 2 different schedules/dosages of adjuvant interferon alpha (i.e. intensified dose of interferon alpha versus standard high dose), it was not suitable for investigating whether or not interferon is superior to any comparator other than interferon for the adjuvant treatment of high‐risk melanoma participants |
| Dillman 2003 | Participants enrolled in this study received interferon gamma and not interferon alpha, which is the drug under investigation in this review |
| Grob 2010 | As this trial compared 2 different schedules and types of interferon (36 months of pegylated interferon versus 18 months of low‐dose interferon), it was not suitable for investigating whether or not interferon is superior to any comparator other than interferon for the adjuvant treatment of high‐risk melanoma participants |
| Hauschild 2003 | This study randomised participants to low‐dose interferon in a combination regimen with low‐dose interleukin‐2 or to observation. The presence of interleukin‐2 (an active drug in metastatic melanoma) in the treatment schedule might affect interferon efficacy, which precluded inclusion of this trial |
| Hauschild 2009 | As this trial compared 2 different schedules/dosages of adjuvant interferon alpha (i.e. low‐dose interferon alpha 2b with or without a modified high‐dose interferon alpha 2b induction phase), it was not suitable for investigating whether or not interferon is superior to any comparator other than interferon for the adjuvant treatment of high‐risk melanoma participants |
| Hauschild 2010 | As this trial compared 2 different schedules/dosages of adjuvant interferon alpha (i.e. low‐dose interferon alpha 2a for 18 versus 60 months), it was not suitable for investigating whether or not interferon is superior to any comparator other than interferon for the adjuvant treatment of high‐risk melanoma participants |
| Kerin 1995 | As this trial compared interferon combined with dacarbazine versus observation, it was not suitable for investigating whether or not interferon is superior to any comparator other than interferon for the adjuvant treatment of high‐risk melanoma participants |
| Kim 2009 | This was a 2‐arm randomised trial comparing biochemotherapy (including interferon) versus interferon in high‐risk melanoma participants. As interferon was included in both treatment arms, this trial was not suitable for inclusion in this review |
| Kokoschka 1990 | The randomisation of the trial was unclear. Even though the authors mentioned that participants were randomly selected to receive interferon alpha therapy, there was no mention of a corresponding randomised control arm |
| Mao 2011 | This phase II randomised study enrolled participants with acral melanoma and compared different interferon schedules (i.e. 1 month versus 1 year high‐dose interferon adjuvant treatment), so it did not meet the inclusion criteria of this review |
| Meyskens 1995 | Participants enrolled in this study received interferon gamma and not interferon alpha, which is the drug under investigation in this review |
| Mitchell 2007 | Participants of this study were randomly assigned to receive low‐dose interferon alpha plus specific immunotherapy with allogeneic melanoma lysates or high‐dose interferon alpha. As interferon was included in both treatment arms, this trial was not suitable for investigating whether or not interferon is superior to any comparator other than interferon for the adjuvant treatment of high‐risk melanoma participants |
| Mohr 2008 | As this trial compared 2 different schedules/dosages of adjuvant interferon alpha (i.e. intensified dose of interferon alpha versus standard high dose), it was not suitable for investigating whether or not interferon is superior to any comparator other than interferon for the adjuvant treatment of high‐risk melanoma participants |
| Pectasides 2009 | As this trial compared 2 different schedules of adjuvant interferon alpha (1 month versus 1 year), it was not suitable for investigating whether or not interferon is superior to any comparator other than interferon for the adjuvant treatment of high‐risk melanoma participants |
| Richtig 2005 | Participants with AJCC stage II melanoma were randomly assigned to receive interferon alpha at a dosage of 3 megaunits 3 times each week for 2 years, plus isotretinoin at a dose of 20 mg for participants < or = 73 kg, 30 mg for participants greater than 73 kg, versus interferon alpha at the same dosage plus placebo. As interferon alpha was scheduled in both arms, it was not eligible for inclusion |
| Rusciani 2007 | This was a non‐randomised study |
Characteristics of ongoing studies [ordered by study ID]
ECOG E1609.
| Trial name or title | A Phase III Randomized Study of Adjuvant Ipilimumab Anti‐CTLA4 Therapy Versus High‐Dose Interferon Alpha‐2b for Resected High‐Risk Melanoma (clinicaltrial.gov identifier: NCT01274338) |
| Methods | This is a phase III randomised study |
| Participants |
|
| Interventions |
|
| Outcomes |
Primary outcomes of the trial
Secondary outcomes of the trial
|
| Starting date | May 2011 |
| Contact information | Principal Investigator: Ahmad Tarhini, Eastern Cooperative Oncology Group |
| Notes | Expected primary completion date: May 2018 |
EORTC 18081.
| Trial name or title | Adjuvant Pegylated‐Interferon‐alpha2b (SylatronTM) for 2 Years Versus Observation in Patients With an Ulcerated Primary Cutaneous Melanoma With T(2‐4)bN0M0: a Randomized Phase III Trial of the EORTC Melanoma Group (clinicaltrial.gov identifier: NCT01502696) |
| Methods | This is a phase III randomised study |
| Participants |
|
| Interventions |
|
| Outcomes |
Primary outcomes of the trial
Secondary outcomes of the trial
|
| Starting date | October 2012 |
| Contact information | Alexander Eggermont, Institut Gustave Roussy, Paris, France |
| Notes | Expected primary completion date: April 2020 |
NCT01782508.
| Trial name or title | A Phase II Randomized Study of Imatinib Versus High Dose Interferon as Adjuvant Therapy in KIT‐mutated Patients With Resected Melanoma |
| Methods | This is a phase II randomised study |
| Participants |
|
| Interventions |
|
| Outcomes |
Primary outcomes of the trial
Secondary outcomes of the trial
|
| Starting date | August 2012 |
| Contact information | Jun Guo, Peking University Cancer Hospital |
| Notes | Expected primary completion date: December 2014 |
Differences between protocol and review
The final version of this review differs from the original protocol for a number of reasons:
First of all, the critical revision of two authors (VCS (oncologist) and ML (dermatologist)) suggested a number of changes in order to make the text clearer to non‐melanoma experts. This required the rephrasing of many sentences throughout the text. Moreover, a new author (VCS), who was not involved at the stage of writing the protocol, has joined the team of this work at the review stage and has brought her viewpoint (she is an oncologist) on several aspects regarding this subject.
Secondly, all authors have added new references and replaced others in order to provide readers with the best and most updated information on this subject.
Contributions of authors
SM was the contact person for the editorial base, co‐ordinated the contributions from the co‐authors, analysed the data, and wrote the final draft of the review. ML and SP contributed to the methods section of the protocol and inputted the data into RevMan. PP checked the protocol for readability, clarity, and for relevance to consumers. SM and VCS are the guarantors of the final version of the review.
Sources of support
Internal sources
University of Padova, Padova, Italy.
External sources
No sources of support supplied
Declarations of interest
Sandro Pasquali, Marko B Lens, Pierluigi Pilati, and Simone Mocellin have all declared they have no conflicts of interest.
Vanna Chiarion‐Sileni was reimbursed for consulting activity as a melanoma expert on advisory boards for GSK, Roche, BMS, and SMD. This was always done with the approval of her institution and outside her work time. She received compensation for advisory board participation from Merck, Roche Genentech, Bristol‐Meyers Squibb, GlaxoSmithKline MSD for the drugs ipilimumab, dabrafenib, vemurafenib, trametinib, and anti‐Pd1. She was the medical leader of the following excluded study:
Chiarion‐Sileni V, Guida M, Romanini A, Ridolfi R, Mandala M, Del Bianco P, et al. Intensified high‐dose intravenous interferon alpha 2b (IFNa2b) for adjuvant treatment of stage III melanoma: A randomized phase III Italian Melanoma Intergroup(IMI) trial [ISRCTN75125874]. [Abstract 8506] ASCO Annual Meeting 2011. Journal of Clinical Oncology 2011;29(15 Suppl 1):8506.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Agarwala 2011 {published data only}
- Agarwala SS, Lee SJ, Flaherty LE, Smylie M, Kefford RF, Carson WE, et al. Randomized phase III trial of high‐dose interferon alfa‐2b (HDI) for 4 weeks induction only in patients with intermediate‐ and high‐risk melanoma (Intergrouptrial E 1697). ASCO Annual Meeting, 3‐7 June 2011, Chicago, US. Journal of Clinical Oncology 2011;29(15 Suppl 1):8505. [Google Scholar]
Cameron 2001 {published data only}
- Cameron DA, Cornbleet MC, Mackie RM, Hunter JA, Gore M, Hancock B, et al. Adjuvant interferon alpha 2b in high risk melanoma ‐ the Scottish study. British Journal of Cancer 2001;84(9):1146‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cascinelli 2001 {published data only}
- Cascinelli N, Belli F, MacKie RM, Santinami M, Bufalino R, Morabito A. Effect of long‐term adjuvant therapy with interferon alpha‐2a in patients with regional node metastases from cutaneous melanoma: a randomised trial. Lancet 2001;358(9285):866‐9. [PUBMED: 11567700] [DOI] [PubMed] [Google Scholar]
Creagan 1995 {published data only}
- Creagan ET, Dalton RJ, Ahmann DL, Jung SH, Morton RF, Langdon RM, et al. Randomized, surgical adjuvant clinical trial of recombinant interferon alfa‐2a in selected patients with malignant melanoma. Journal of Clinical Oncology 1995;13(11):2776‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Eggermont 2005 {published data only}
- Eggermont AM, Suciu S, MacKie R, Ruka W, Testori A, Kruit W, et al. Post‐surgery adjuvant therapy with intermediate doses of interferon alfa 2b versus observation in patients with stage IIb/III melanoma (EORTC 18952): randomised controlled trial. Lancet 2005;366(9492):1189‐96. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Eggermont 2008 {published data only}
- Eggermont AM, Suciu S, Santinami M, Testori A, Kruit WH, Marsden J, et al. Adjuvant therapy with pegylated interferon alfa‐2b versus observation alone in resected stage III melanoma: final results of EORTC 18991, a randomised phase III trial. Lancet 2008;372(9633):117‐26. [PUBMED: 18620949] [DOI] [PubMed] [Google Scholar]
Garbe 2008 {published data only}
- Garbe C, Radny P, Linse R, Dummer R, Gutzmer R, Ulrich J, et al. Adjuvant low‐dose interferon {alpha}2a with or without dacarbazine compared with surgery alone: a prospective‐randomized phase III DeCOG trial in melanoma patients with regional lymph node metastasis. Annals of Oncology 2008;19(6):1195‐201. [PUBMED: 18281266] [DOI] [PubMed] [Google Scholar]
Grob 1998 {published data only}
- Grob JJ, Dreno B, Salmoniere P, Delaunay M, Cupissol D, Guillot B, et al. Randomised trial of interferon alpha‐2a as adjuvant therapy in resected primary melanoma thicker than 1.5 mm without clinically detectable node metastases. French Cooperative Group on Melanoma. Lancet 1998;351(9120):1905‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hancock 2004 {published data only}
- Hancock BW, Wheatley K, Harris S, Ives N, Harrison G, Horsman JM, et al. Adjuvant interferon in high‐risk melanoma: the AIM HIGH Study‐‐United Kingdom Coordinating Committee on Cancer Research randomized study of adjuvant low‐dose extended‐duration interferon Alfa‐2a in high‐risk resected malignant melanoma. Journal of Clinical Oncology 2004;22(1):53‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hansson 2011 {published data only}
- Hansson J, Aamdal S, Bastholt L, Brandberg Y, Hernberg M, Nilsson B, et al. Two different durations of adjuvant therapy with intermediate‐dose interferon alfa‐2b in patients with high‐risk melanoma (Nordic IFN trial): a randomised phase 3 trial. Lancet Oncology 2011;12(2):144‐52. [PUBMED: 21256809] [DOI] [PubMed] [Google Scholar]
Kirkwood 1996 {published data only}
- Kirkwood JM, Strawderman MH, Ernstoff MS, Smith TJ, Borden EC, Blum RH. Interferon alfa‐2b adjuvant therapy of high‐risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. Journal of Clinical Oncology 1996;14(1):7‐17. [PUBMED: 8558223] [DOI] [PubMed] [Google Scholar]
Kirkwood 2000 {published data only}
- Kirkwood JM, Ibrahim JG, Sondak VK, Richards J, Flaherty LE, Ernstoff MS, et al. High‐ and low‐dose interferon alfa‐2b in high‐risk melanoma: first analysis of intergroup trial E1690/S9111/C9190. Journal of Clinical Oncology 2000;18(12):2444‐58. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kirkwood 2001 {published data only}
- Kirkwood JM, Ibrahim JG, Sosman JA, Sondak VK, Agarwala SS, Ernstoff MS, et al. High‐dose interferon alfa‐2b significantly prolongs relapse‐free and overall survival compared with the GM2‐KLH/QS‐21 vaccine in patients with resected stage IIB‐III melanoma: results of intergroup trial E1694/S9512/C509801. Journal of Clinical Oncology 2001;19(9):2370‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kirkwood 2001a {published data only}
- Kirkwood JM, Ibrahim J, Lawson DH, Atkins MB, Agarwala SS, Collins K, et al. High‐dose interferon alfa‐2b does not diminish antibody response to GM2 vaccination in patients with resected melanoma: results of the Multicenter Eastern Cooperative Oncology Group Phase II Trial E2696. Journal of Clinical Oncology 2001;19(5):1430‐6. [PUBMED: 11230488] [DOI] [PubMed] [Google Scholar]
Kleeberg 2004 {published data only}
- Kleeberg UR, Suciu S, Brocker EB, Ruiter DJ, Chartier C, Lienard D, et al. Final results of the EORTC 18871/DKG 80‐1 randomised phase III trial. rIFN‐alpha2b versus rIFN‐gamma versus ISCADOR M versus observation after surgery in melanoma patients with either high‐risk primary (thickness >3 mm) or regional lymph node metastasis. European Journal of Cancer 2004;40(3):390‐402. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
McMasters 2008 {published data only}
- McMasters KM, Edwards MJ, Ross MI, Reintgen DS, Martin RC, Urist MM, et al. Ulceration as a predictive marker for response to adjuvant interferon therapy in melanoma. Annals of Surgery 2010;252(3):460‐5; discussion 465‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- McMasters KM, Ross MI, Reintgen DS, Edwards MJ, Noyes RD, Urist M, et al. Final results of the Sunbelt Melanoma Trial [Abstract 9003]. 2008 ASCO Annual Proceedings. Journal of Clinical Oncology 2008;26(15 Suppl):15s. [Google Scholar]
Pehamberger 1998 {published data only}
- Pehamberger H, Soyer HP, Steiner A, Kofler R, Binder M, Mischer P, et al. Adjuvant interferon alfa‐2a treatment in resected primary stage II cutaneous melanoma. Austrian Malignant Melanoma Cooperative Group. Journal of Clinical Oncology 1998;16(4):1425‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rusciani 1997 {published data only}
- Rusciani L, Petraglia S, Alotto M, Calvieri S, Vezzoni G. Postsurgical adjuvant therapy for melanoma. Evaluation of a 3‐year randomized trial with recombinant interferon‐alpha after 3 and 5 years of follow‐up. Cancer 1997;79(12):2354‐60. [PUBMED: 9191523] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Anaya 2008 {published data only}
- Anaya DA, Xing Y, Feng L, Huang X, Camacho LH, Ross MI, et al. Adjuvant high‐dose interferon for cutaneous melanoma is most beneficial for patients with early stage III disease. Cancer 2008;112(9):2030‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chiarion‐Sileni 2011 {published data only}
- Chiarion‐Sileni V, Guida M, Romanini A, Ridolfi R, Mandala M, Bianco P, et al. Intensified high‐dose intravenous interferon alpha 2b (IFNa2b) for adjuvant treatment of stage III melanoma: A randomized phase III Italian Melanoma Intergroup(IMI) trial [ISRCTN75125874]. [Abstract 8506] ASCO Annual Meeting 2011. Journal of Clinical Oncology 2011;29(15 Suppl 1):8506. [Google Scholar]
Dillman 2003 {published data only}
- Dillman RO, Wiemann M, Nayak SK, DeLeon C, Hood K, DePriest C. Interferon‐gamma or granulocyte‐macrophage colony‐stimulating factor administered as adjuvants with a vaccine of irradiated autologous tumor cells from short‐term cell line cultures: a randomized phase 2 trial of the cancer biotherapy research group. Journal of Immunotherapy 2003;26(4):367‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Grob 2010 {published data only}
- Grob JJ, Jouary T, Dreno B, Gutzmer R, Hauschild A, Leccia MT, et al. Adjuvant therapy with pegylated interferon alfa‐2b (36 months) versus low‐dose interferon alfa‐2b (18 months) in melanoma patients without macro‐metastatic nodes: EADO trial [Abstract LBA8506]. 2010 ASCO Annual Meeting Proceedings. Journal of Clinical Oncology 2010;18(Suppl):e291‐e311. [Google Scholar]
Hauschild 2003 {published data only}
- Hauschild A, Weichenthal M, Balda BR, Becker JC, Wolff HH, Tilgen W, et al. Prospective randomized trial of interferon alfa‐2b and interleukin‐2 as adjuvant treatment for resected intermediate‐ and high‐risk primary melanoma without clinically detectable node metastasis. Journal of Clinical Oncology 2003;21(15):2883‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hauschild 2009 {published data only}
- Hauschild A, Weichenthal M, Rass K, Linse R, Ulrich J, Stadler R, et al. Prospective randomized multicenter adjuvant dermatologic cooperative oncology group trial of low‐dose interferon alfa‐2b with or without a modified high‐dose interferon alfa‐2b induction phase in patients with lymph node‐negative melanoma. Journal of Clinical Oncology 2009;27(21):3496‐502. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hauschild 2010 {published data only}
- Hauschild A, Weichenthal M, Rass K, Linse R, Berking C, Bottjer J, et al. Efficacy of low‐dose interferon {alpha}2a 18 versus 60 months of treatment in patients with primary melanoma of >= 1.5 mm tumor thickness: results of a randomized phase III DeCOG trial. Journal of Clinical Oncology 2010;28(5):841‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kerin 1995 {published data only}
- Kerin MJ, Gillen P, Monson JR, Wilkie J, Keane FB, Tanner WA. Results of a prospective randomized trial using DTIC and interferon as adjuvant therapy for stage I malignant melanoma. European Journal of Surgical Oncology 1995;21(5):548‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kim 2009 {published data only}
- Kim KB, Legha SS, Gonzalez R, Anderson CM, Johnson MM, Liu P, et al. A randomized phase III trial of biochemotherapy versus interferon‐alpha‐2b for adjuvant therapy in patients at high risk for melanoma recurrence. Melanoma Research 2009;19(1):42‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kokoschka 1990 {published data only}
- Kokoschka EM, Trautinger F, Knobler RM, Pohl Markl H, Micksche M. Long‐term adjuvant therapy of high‐risk malignant melanoma with interferon alpha 2b. Journal of Investigative Dermatology 1990;95(6 Suppl):193S‐7S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mao 2011 {published data only}
- Mao L, Si L, Chi Z, Cui C, Sheng X, Li S, et al. A randomised phase II trial of 1month versus 1year of adjuvant high‐dose interferon alpha‐2b in high‐risk acral melanoma patients. European Journal of Cancer 2011;47(10):1498‐503. [PUBMED: 21493058] [DOI] [PubMed] [Google Scholar]
Meyskens 1995 {published data only}
- Meyskens FL, Kopecky KJ, Taylor CW, Noyes RD, Tuthill RJ, Hersh EM, et al. Randomized trial of adjuvant human interferon gamma versus observation in high‐risk cutaneous melanoma: a Southwest Oncology Group study. Journal of the National Cancer Institute 1995;87(22):1710‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mitchell 2007 {published data only}
- Mitchell MS, Abrams J, Thompson JA, Kashani‐Sabet M, DeConti RC, Hwu WJ, et al. Randomized trial of an allogeneic melanoma lysate vaccine with low‐dose interferon Alfa‐2b compared with high‐dose interferon Alfa‐2b for Resected stage III cutaneous melanoma. Journal of Clinical Oncology 2007;25(15):2078‐85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mohr 2008 {published data only}
- Mohr P, Hauschild A, Enk A, Trefzer U, Rass K, Grabbe S, et al. Intermittent high‐dose intravenous interferon alpha 2b (IFNa2b) for adjuvant treatment of stage III malignant melanoma: An interim analysis of a randomized phase III study (NCT00226408) [Abstract 9040]. ASCO Annual Meeting 2008. Journal of Clinical Oncology 2008;26(15 Suppl 1):492. [Google Scholar]
Pectasides 2009 {published data only}
- Pectasides D, Dafni U, Bafaloukos D, Skarlos D, Polyzos A, Tsoutsos D, et al. Randomized phase III study of 1 month versus 1 year of adjuvant high‐dose interferon alfa‐2b in patients with resected high‐risk melanoma. Journal of Clinical Oncology 2009;27(6):939‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Richtig 2005 {published data only}
- Richtig E, Soyer HP, Posch M, Mossbacher U, Bauer P, Teban L, et al. Prospective, randomized, multicenter, double‐blind placebo‐controlled trial comparing adjuvant interferon alfa and isotretinoin with interferon alfa alone in stage IIA and IIB melanoma: European Cooperative Adjuvant Melanoma Treatment Study Group. Journal of Clinical Oncology 2005;23(34):8655‐63. [PUBMED: 16260701] [DOI] [PubMed] [Google Scholar]
Rusciani 2007 {published data only}
- Rusciani L, Proietti I, Paradisi A, Rusciani A, Guerriero G, Mammone A, et al. Recombinant interferon alpha‐2b and coenzyme Q10 as a postsurgical adjuvant therapy for melanoma: a 3‐year trial with recombinant interferon‐alpha and 5‐year follow‐up. Melanoma Research 2007;17(3):177‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ECOG E1609 {published data only}
- ECOG E1609. A phase III randomized study of adjuvant ipilimumab anti‐CTLA4 therapy versus high‐dose interferon alpha‐2b for resected high‐risk melanoma. http://www.eortc.be/clinicaltrials/details.asp?protocol=18081.
EORTC 18081 {unpublished data only}
- EORTC 18081. Adjuvant peginterferon alpha‐2b for 2 years vs observation in patients with an ulcerated primary cutaneous melanoma with T(2‐4)bN0M0: a randomized phase III trial of the EORTC Melanoma Group. http://www.eortc.be/clinicaltrials/details.asp?protocol=18081. [NCT01502696]
NCT01782508 {published data only}
- NCT01782508. A phase II randomized study of imatinib versus high dose interferon as adjuvant therapy in KIT‐mutated patients with resected melanoma. http://clinicaltrials.gov/show/NCT01782508.
Additional references
Altman 1999
- Altman DG, Andersen PK. Calculating the number needed to treat for trials where the outcome is time to an event. BMJ 1999;319(7223):1492‐5. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ascierto 2008
- Ascierto PA, Kirkwood JM. Adjuvant therapy of melanoma with interferon: lessons of the past decade. Journal of Translational Medicine 2008;6:62. [PUBMED: 18954464] [DOI] [PMC free article] [PubMed] [Google Scholar]
Australian Cancer Network 2008
- Australian Cancer Network Melanoma Guidelines Revision Working Party. Clinical practice guidelines for the management of melanoma in Australia and New Zealand. Cancer Council Australia and Australian Cancer Network, Sydney and New Zealand Guidelines Group. Wellington, 2008. [Google Scholar]
Bajetta 2008
- Bajetta E. Adjuvant use of interferon alpha 2b is not justified in patients with stage IIb/III melanoma. Nature Clinical Practice Oncology 2008;5(1):4‐5. [PUBMED: 18043604] [DOI] [PubMed] [Google Scholar]
Balch 2009
- Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, et al. Final version of 2009 AJCC melanoma staging and classification. Journal of Clinical Oncology 2009;27(36):6199‐206. [PUBMED: 19917835] [DOI] [PMC free article] [PubMed] [Google Scholar]
Borden 2007
- Borden EC, Sen GC, Uze G, Silverman RH, Ransohoff RM, Foster GR, et al. Interferons at age 50: past, current and future impact on biomedicine. Nature Reviews. Drug Discovery 2007;6(12):975‐90. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Borenstein 2009
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Multiple comparisons within a study. Introduction to Meta Analysis. Chichester: Wiley, 2009:239‐242. [Google Scholar]
Bottomley 2009
- Bottomley A, Coens C, Suciu S, Santinami M, Kruit W, Testori A, et al. Adjuvant therapy with pegylated interferon alfa‐2b versus observation in resected stage III melanoma: a phase III randomized controlled trial of health‐related quality of life and symptoms by the European Organisation for Research and Treatment of Cancer Melanoma Group. Journal of Clinical Oncology 2009;27(18):2916‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Brandberg 2012
- Brandberg Y, Aamdal S, Bastholt L, Hernberg M, Stierner U, Maase H, et al. Health‐related quality of life in patients with high‐risk melanoma randomised in the Nordic phase 3 trial with adjuvant intermediate‐dose interferon alfa‐2b. European Journal of Cancer (Oxford, England: 1990) 2012;48(13):2012‐9. [PUBMED: 22196968] [DOI] [PubMed] [Google Scholar]
Brown 2006
- Brown L, Roda J, Terrell C, Chaudhury AR, Crespin T, Carson WE, et al. Interferon alpha and CPG oligodeoxynucleotides elicit additive immunostimulatory and antitumor effects. Surgery 2006;140(2):297‐306. [PUBMED: 16904983] [DOI] [PubMed] [Google Scholar]
Caraceni 1998
- Caraceni A, Gangeri L, Martini C, Belli F, Brunelli C, Baldini M, et al. Neurotoxicity of interferon‐alpha in melanoma therapy: results from a randomized controlled trial. Cancer 1998;83(3):482‐9. [PUBMED: 9690541] [DOI] [PubMed] [Google Scholar]
Chapman 2011
- Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. New England Journal of Medicine 2011;364(26):2507‐16. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cole 1996
- Cole BF, Gelber RD, Kirkwood JM, Goldhirsch A, Barylak E, Borden E. Quality‐of‐life‐adjusted survival analysis of interferon alfa‐2b adjuvant treatment of high‐risk resected cutaneous melanoma: an Eastern Cooperative Oncology Group study. Journal of Clinical Oncology 1996;14(10):2666‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cormier 2011
- Cormier JN, Askew RL. Assessment of patient‐reported outcomes in patients with melanoma. Surgical Oncology Clinics of North America 2011;20(1):201‐13. [PUBMED: 21111967] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cormier 2012
- Cormier JN, Cromwell KD, Ross MI. Health‐related quality of life in patients with melanoma: overview of instruments and outcomes. Dermatologic clinics 2012;30(2):245‐54, viii. [PUBMED: 22284139] [DOI] [PubMed] [Google Scholar]
Crosby 2000
- Crosby T, Fish R, Coles B, Mason MD. Systemic treatments for metastatic cutaneous melanoma. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD001215] [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [PUBMED: 3802833] [DOI] [PubMed] [Google Scholar]
Dixon 2006
- Dixon S, Walters SJ, Turner L, Hancock BW. Quality of life and cost‐effectiveness of interferon‐alpha in malignant melanoma: results from randomised trial. British Journal of Cancer 2006;94(4):492‐8. [PUBMED: 16449995] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dummer 2009
- Dummer R, Hauschild A, Pentheroudakis G. Cutaneous malignant melanoma: ESMO clinical recommendations for diagnosis, treatment and follow‐up. Annals of Oncology 2009;20 Suppl 4:129‐31. [PUBMED: 19454433] [DOI] [PubMed] [Google Scholar]
Duval 2000
- Duval S, Tweedie R. Trim and fill: a simple funnel‐plot‐based method of testing and adjusting for publication bias in meta‐analysis. Biometrics 2000;56(2):455‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Eggermont 2008a
- Eggermont AM, Voit C. Management of melanoma: a European perspective. Surgical Oncology Clinics of North America 2008;17(3):635‐48, x. [PUBMED: 18486887] [DOI] [PubMed] [Google Scholar]
Eggermont 2009
- Eggermont AM, Testori A, Marsden J, Hersey P, Quirt I, Petrella T, et al. Utility of adjuvant systemic therapy in melanoma. Annals of Oncology 2009;20 Suppl 6:30‐4. [PUBMED: 19617295] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eggermont 2011
- Eggermont AM, Suciu S, Testori A, Kruit WH, Marsden J, Punt CJ, et al. Ulceration and stage are predictive of interferon efficacy in melanoma: Results of the phase III adjuvant trials EORTC 18952 and EORTC 18991. European Journal of Cancer 2011;48(2):218‐25. [PUBMED: 22056637] [DOI] [PubMed] [Google Scholar]
Fecher 2009
- Fecher LA, Flaherty KT. Where are we with adjuvant therapy of stage III and IV melanoma in 2009?. Journal of the National Comprehensive Cancer Network 2009;7(3):295‐304. [PUBMED: 19401062] [DOI] [PubMed] [Google Scholar]
Ferlay 2010
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International Journal of Cancer 2010;127(12):2893‐917. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Foser 2006
- Foser S, Redwanz I, Ebeling M, Heizmann CW, Certa U. Interferon‐alpha and transforming growth factor‐beta co‐induce growth inhibition of human tumor cells. Cellular & Molecular Life Sciences 2006;63(19‐20):2387‐96. [PUBMED: 16988787] [DOI] [PMC free article] [PubMed] [Google Scholar]
Friedlander 2010
- Friedlander P, Hodi FS. Advances in targeted therapy for melanoma. Clinical Advances in Hematology & Oncology 2010;8(9):619‐27. [MEDLINE: ] [PubMed] [Google Scholar]
Garbe 2010
- Garbe C, Peris K, Hauschild A, Saiag P, Middleton M, Spatz A, et al. Diagnosis and treatment of melanoma: European consensus‐based interdisciplinary guideline. European Journal of Cancer 2010;46(2):270‐83. [PUBMED: 19959353] [DOI] [PubMed] [Google Scholar]
Gogas 2007
- Gogas HJ, Kirkwood JM, Sondak VK. Chemotherapy for metastatic melanoma: time for a change?. Cancer 2007;109(3):455‐64. [PUBMED: 17200963] [DOI] [PubMed] [Google Scholar]
Hauschild 2008
- Hauschild A, Gogas H, Tarhini A, Middleton MR, Testori A, Dréno B, et al. Practical guidelines for the management of interferon‐alpha‐2b side effects in patients receiving adjuvant treatment for melanoma: expert opinion. Cancer 2008;112(5):982‐94. [PUBMED: 18236459] [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. [updated March 2011]. The Cochrane Collaboration 2011. Available from www.cochrane‐handbook.org. The Cochrane Collaboration. Available from www.cochrane‐handbook.org.
Hodi 2010
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. New England Journal of Medicine 2010;363(8):711‐23. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Janku 2010
Jemal 2010
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA: a Cancer Journal for Clinicians 2010;60(5):277‐300. [PUBMED: 20610543] [DOI] [PubMed] [Google Scholar]
Kilbridge 2001
- Kilbridge KL, Weeks JC, Sober AJ, Haluska FG, Slingluff CL, Atkins MB, et al. Patient preferences for adjuvant interferon alfa‐2b treatment. Journal of Clinical Oncology: official journal of the American Society of Clinical Oncology 2001;19(3):812‐23. [PUBMED: 11157035] [DOI] [PubMed] [Google Scholar]
Kilbridge 2002
- Kilbridge KL, Cole BF, Kirkwood JM, Haluska FG, Atkins MA, Ruckdeschel JC, et al. Quality‐of‐life‐adjusted survival analysis of high‐dose adjuvant interferon alpha‐2b for high‐risk melanoma patients using intergroup clinical trial data. Journal of Clinical Oncology 2002;20(5):1311‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kirkwood 2004
- Kirkwood JM, Manola J, Ibrahim J, Sondak V, Ernstoff MS, Rao U. A pooled analysis of eastern cooperative oncology group and intergroup trials of adjuvant high‐dose interferon for melanoma. Clinical Cancer Research 2004;10(5):1670‐7. [PUBMED: 15014018] [DOI] [PubMed] [Google Scholar]
Kirkwood 2008
- Kirkwood JM, Tarhini AA, Moschos SJ, Panelli MC. Adjuvant therapy with high‐dose interferon alpha 2b in patients with high‐risk stage IIB/III melanoma. Nature Clinical Practice Oncology 2008;5(1):2‐3. [PUBMED: 18030300] [DOI] [PubMed] [Google Scholar]
Kirkwood 2009
- Kirkwood JM, Tawbi HA, Tarhini AA, Moschos SJ. Does pegylated interferon alpha‐2b confer additional benefit in the adjuvant treatment of high‐risk melanoma?. Nature Clinical Practice Oncology 2009;6(2):70‐1. [PUBMED: 19092800] [DOI] [PubMed] [Google Scholar]
Kumar 2007
- Kumar KG, Liu J, Li Y, Yu D, Thomas‐Tikhonenko A, Herlyn M, et al. Raf inhibitor stabilizes receptor for the type I interferon but inhibits its anti‐proliferative effects in human malignant melanoma cells. Cancer Biology & Therapy 2007;6(9):1437‐41. [PUBMED: 17873516] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lens 2002
- Lens MB, Dawes M. Interferon alfa therapy for malignant melanoma: a systematic review of randomized controlled trials. Journal of Clinical Oncology 2002;20(7):1818‐25. [PUBMED: 11919239] [DOI] [PubMed] [Google Scholar]
Lens 2004
- Lens MB, Dawes M. Global perspectives of contemporary epidemiological trends of cutaneous malignant melanoma. British Journal of Dermatology 2004;150(2):179‐85. [PUBMED: 14996086] [DOI] [PubMed] [Google Scholar]
Lens 2006
- Lens M. Cutaneous melanoma: interferon alpha adjuvant therapy for patients at high risk for recurrent disease. Dermatologic Therapy 2006;19(1):9‐18. [PUBMED: 16405565] [DOI] [PubMed] [Google Scholar]
Lesinski 2008
- Lesinski GB, Raig ET, Guenterberg K, Brown L, Go MR, Shah NN, et al. IFN‐alpha and bortezomib overcome Bcl‐2 and Mcl‐1 overexpression in melanoma cells by stimulating the extrinsic pathway of apoptosis. Cancer Research 2008;68(20):8351‐60. [PUBMED: 18922907] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lesinski 2010
- Lesinski GB, Zimmerer JM, Kreiner M, Trefry J, Bill MA, Young GS, et al. Modulation of SOCS protein expression influences the interferon responsiveness of human melanoma cells. BMC Cancer 2010;10:142. [PUBMED: 20398276] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lui 2007
- Lui P, Cashin R, Machado M, Hemels M, Corey‐Lisle PK, Einarson TR. Treatments for metastatic melanoma: synthesis of evidence from randomized trials. Cancer Treatment Reviews 2007;33(8):665‐80. [PUBMED: 17919823] [DOI] [PubMed] [Google Scholar]
McMasters 2010
- McMasters KM, Edwards MJ, Ross MI, Reintgen DS, Martin RC 2nd, Urist MM, et al. Ulceration as a predictive marker for response to adjuvant interferon therapy in melanoma. Annals of Surgery 2010;252(3):460‐5; discussion 465‐6. [PUBMED: 20739846] [DOI] [PubMed] [Google Scholar]
Mocellin 2006
- Mocellin S, Hoon D, Ambrosi A, Nitti D, Rossi CR. The prognostic value of circulating tumor cells in patients with melanoma: a systematic review and meta‐analysis. Clinical Cancer Research 2006;12(15):4605‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mocellin 2007
- Mocellin S, Hoon DS, Pilati P, Rossi CR, Nitti D. Sentinel lymph node molecular ultrastaging in patients with melanoma: a systematic review and meta‐analysis of prognosis. Journal of Clinical Oncology 2007;25(12):1588‐95. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mocellin 2010
- Mocellin S, Pasquali S, Rossi CR, Nitti D. Interferon alpha adjuvant therapy in patients with high‐risk melanoma: a systematic review and meta‐analysis. Journal of the National Cancer Institute 2010;102(7):493‐501. [PUBMED: 20179267] [DOI] [PubMed] [Google Scholar]
Mocellin 2010a
- Mocellin S, Shrager J, Scolyer R, Pasquali S, Verdi D, Marincola FM, et al. Targeted Therapy Database (TTD): a model to match patient's molecular profile with current knowledge on cancer biology. PLoS ONE 2010;5(8):e11965. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mocellin 2011
- Mocellin S, Nitti D. Cutaneous melanoma in situ: translational evidence from a large population‐based study. Oncologist 2011;16(6):896‐903. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mocellin 2011a
- Mocellin S, Pasquali S, Nitti D. The impact of surgery on survival of patients with cutaneous melanoma: revisiting the role of primary tumor excision margins. Annals of Surgery 2011;253(2):238‐43. [PUBMED: 21173691] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta‐Analyses: The PRISMA Statement. PLoS Medicine 2009;6(7):e1000097. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morton 2006
- Morton DL, Thompson JF, Cochran AJ, Mozzillo N, Elashoff R, Essner R, et al. Sentinel‐node biopsy or nodal observation in melanoma. New England Journal of Medicine 2006;355(13):1307‐17. [PUBMED: 17005948] [DOI] [PubMed] [Google Scholar]
Moschos 2006
- Moschos SJ, Edington HD, Land SR, Rao UN, Jukic D, Shipe‐Spotloe J, et al. Neoadjuvant treatment of regional stage IIIB melanoma with high‐dose interferon alfa‐2b induces objective tumor regression in association with modulation of tumor infiltrating host cellular immune responses. Journal of Clinical Oncology 2006;24(19):3164‐71. [PUBMED: 16809739] [DOI] [PubMed] [Google Scholar]
Moschos 2007
- Moschos S, Kirkwood JM. Present role and future potential of type I interferons in adjuvant therapy of high‐risk operable melanoma. Cytokine & Growth Factor Reviews 2007;18:451‐8. [PUBMED: 17693125] [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints [Erratum appears in Stat Med 2004 23(11):1817]. Statistics in Medicine 1998;17(24):2815‐34. [PUBMED: 9921604] [DOI] [PubMed] [Google Scholar]
Pasquali 2010
- Pasquali S, Mocellin S. The anticancer face of interferon alpha (IFN‐alpha): from biology to clinical results, with a focus on melanoma. Current Medicinal Chemistry 2010;17(29):3327‐36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pasquali 2010a
- Pasquali S, Mocellin S, Campana LG, Bonandini E, Montesco MC, Tregnaghi A, et al. Early (sentinel lymph node biopsy‐guided) versus delayed lymphadenectomy in melanoma patients with lymph node metastases : personal experience and literature meta‐analysis. Cancer 2010;116(5):1201‐9. [PUBMED: 20066719] [DOI] [PubMed] [Google Scholar]
Pestka 2007
- Pestka S. Purification and cloning of interferon alpha. Current Topics in Microbiology & Immunology 2007;316:23‐37. [PUBMED: 17969442] [DOI] [PubMed] [Google Scholar]
Pirard 2004
- Pirard D, Heenen M, Melot C, Vereecken P. Interferon alpha as adjuvant postsurgical treatment of melanoma: a meta‐analysis. Dermatology 2004;208(1):43‐8. [PUBMED: 14730236] [DOI] [PubMed] [Google Scholar]
Rao 2010
- Rao UN, Lee SJ, Luo W, Mihm MC Jr, Kirkwood JM. Presence of tumor‐infiltrating lymphocytes and a dominant nodule within primary melanoma are prognostic factors for relapse‐free survival of patients with thick (t4) primary melanoma: pathologic analysis of the e1690 and e1694 intergroup trials. American Journal of Clinical Pathology 2010;133(4):646‐53. [PUBMED: 20231618] [DOI] [PMC free article] [PubMed] [Google Scholar]
Saiag 2007
- Saiag P, Bosquet L, Guillot B, Verola O, Avril MF, Bailly C, et al. Management of adult patients with cutaneous melanoma without distant metastasis. 2005 update of the French Standards, Options and Recommendations guidelines. Summary report. European Journal of Dermatology 2007;17(4):325‐31. [PUBMED: 17540641] [DOI] [PubMed] [Google Scholar]
Sasse 2007
- Sasse AD, Sasse EC, Clark LG, Ulloa L, Clark OA. Chemoimmunotherapy versus chemotherapy for metastatic malignant melanoma. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD005413.pub2] [DOI] [PubMed] [Google Scholar]
Sladden 2009
- Sladden MJ, Balch C, Barzilai DA, Berg D, Freiman A, Handiside T, et al. Surgical excision margins for primary cutaneous melanoma. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD004835.pub2; PUBMED: 19821334] [DOI] [PubMed] [Google Scholar]
Sterne 2000
- Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta‐analysis: power of statistical tests and prevalence in the literature. Journal of Clinical Epidemiology 2000;53(11):1119‐29. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sutton 2000
- Sutton A, Abrams K, Jones D, Sheldon T, Song F. Methods for Meta‐analysis in Medical Research. Chichester: Wiley, 2000. [Google Scholar]
Tawbi 2007
- Tawbi HA, Kirkwood JM. Management of metastatic melanoma. Seminars in Oncology 2007;34(6):532‐45. [PUBMED: 18083377] [DOI] [PubMed] [Google Scholar]
Theofilopoulos 2005
- Theofilopoulos AN, Baccala R, Beutler B, Kono DH. Type I interferons (alpha/beta) in immunity and autoimmunity. Annual Review of Immunology 2005;23:307‐36. [PUBMED: 15771573] [DOI] [PubMed] [Google Scholar]
Thirlwell 2008
- Thirlwell C, Nathan P. Melanoma‐‐part 2: management. BMJ 2008;337:a2488. [PUBMED: 19047194] [DOI] [PubMed] [Google Scholar]
Thompson 2005
- Thompson JF, Scolyer RA, Kefford RF. Cutaneous melanoma. Lancet 2005;365(9460):687‐701. [PUBMED: 15721476] [DOI] [PubMed] [Google Scholar]
Thompson 2009
- Thompson JF, Scolyer RA, Kefford RF. Cutaneous melanoma in the era of molecular profiling. Lancet 2009;374(9687):362‐5. [PUBMED: 19647595] [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;8:16. [PUBMED: 17555582] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tsao 2004
- Tsao H, Atkins MB, Sober AJ. Management of cutaneous melanoma. New England Journal of Medicine 2004;351(10):998‐1012. [PUBMED: 15342808] [DOI] [PubMed] [Google Scholar]
Verma 2006
- Verma S, Quirt I, McCready D, Bak K, Charette M, Iscoe N. Systematic review of systemic adjuvant therapy for patients at high risk for recurrent melanoma. Cancer 2006;106(7):1431‐42. [PUBMED: 16511841] [DOI] [PubMed] [Google Scholar]
Wang 2007
- Wang SQ, Halpern AC. Management of cutaneous melanoma: a public health and individual patient care perspective. Advances in Dermatology 2007;23:81‐98. [PUBMED: 18159897] [DOI] [PubMed] [Google Scholar]
Wang 2008
- Wang W, Edington HD, Rao UN, Jukic DM, Radfar A, Wang H, et al. Effects of high‐dose IFNalpha2b on regional lymph node metastases of human melanoma: modulation of STAT5, FOXP3, and IL‐17. Clinical Cancer Research 2008;14(24):8314‐20. [PUBMED: 19088050] [DOI] [PubMed] [Google Scholar]
Wheatley 2003
- Wheatley K, Ives N, Hancock B, Gore M, Eggermont A, Suciu S. Does adjuvant interferon‐alpha for high‐risk melanoma provide a worthwhile benefit? A meta‐analysis of the randomised trials. Cancer Treatment Reviews 2003;29(4):241‐52. [PUBMED: 12927565] [DOI] [PubMed] [Google Scholar]
Wheatley 2007
- Wheatley K, Ives N, Eggermont A, Kirkwood J, Cascinelli N, Markovic SN, et al. Interferon alpha as adjuvant therapy for melanoma: an individual patient data meta‐analysis of randomised trials [Abstract 8526]. 2007 ASCO Annual Meeting Proceedings. Journal of Clinical Oncology 2007;25(18S):8526. [Google Scholar]
Winstanley 2013
- Winstanley JB, Saw R, Boyle F, Thompson J. The FACT‐Melanoma quality‐of‐life instrument: comparison of a five‐point and four‐point response scale using the Rasch measurement model. Melanoma research 2013;23(1):61‐9. [PUBMED: 23262441] [DOI] [PubMed] [Google Scholar]
Yurkovetsky 2007a
- Yurkovetsky ZR, Kirkwood JM, Edington HD, Marrangoni AM, Velikokhatnaya L, Winans MT, et al. Multiplex analysis of serum cytokines in melanoma patients treated with interferon‐alpha2b. Clinical Cancer Research 2007;13(8):2422‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ziefle 2011a
- Ziefle S, Egberts F, Heinze S, Volkenandt M, Schmid‐Wendtner M, Tilgen W, et al. Health‐related quality of life before and during adjuvant interferon‐alpha treatment for patients with malignant melanoma (DeCOG‐trial). Journal of Immunotherapy 2011;34(4):403‐8. [PUBMED: 21499123] [DOI] [PubMed] [Google Scholar]


