Abstract
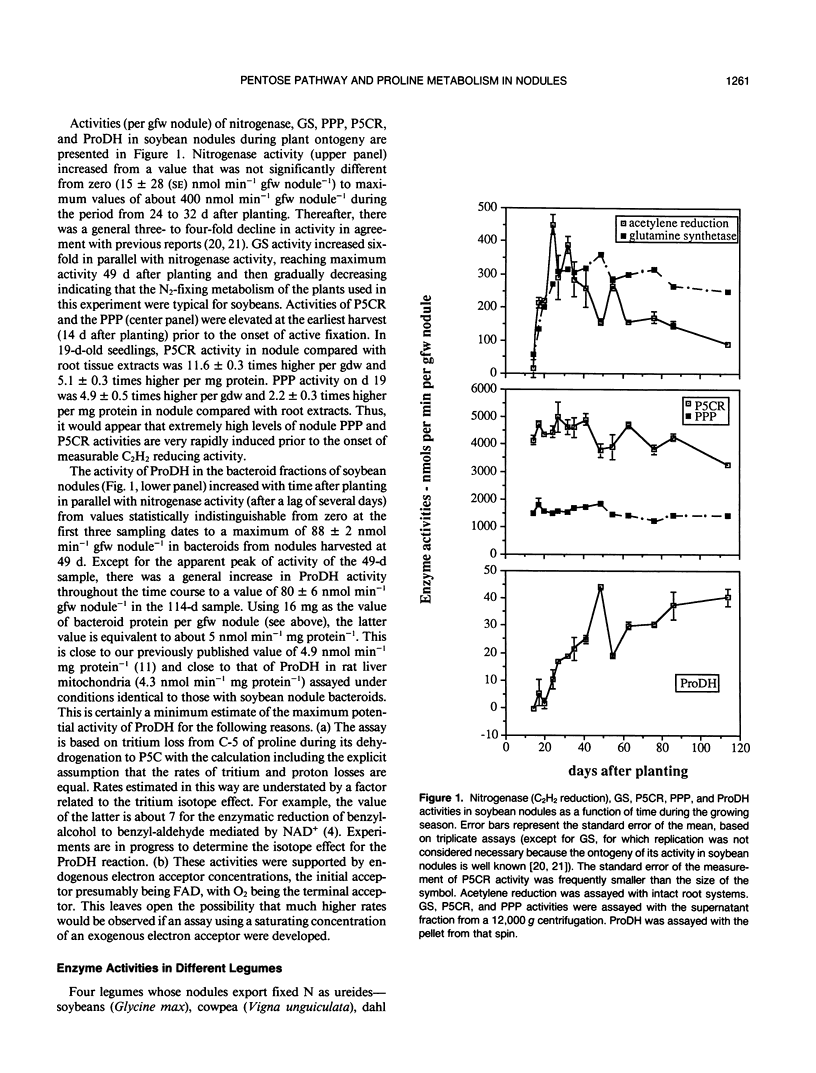
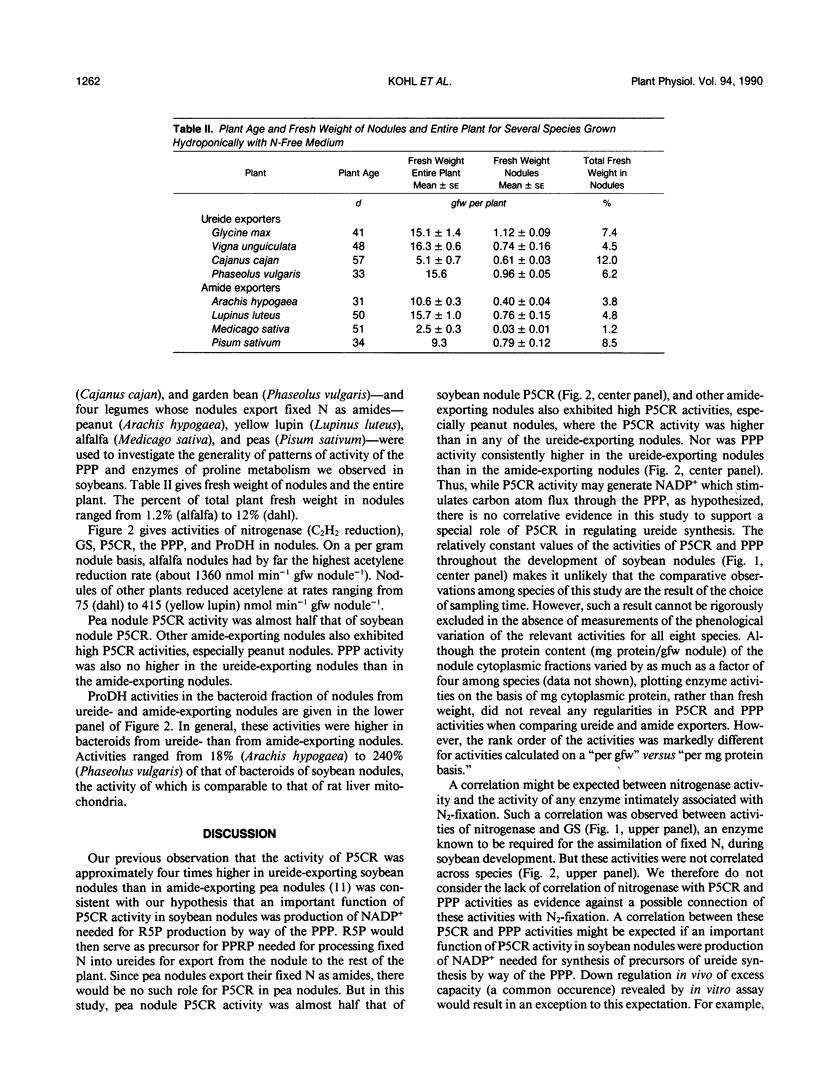
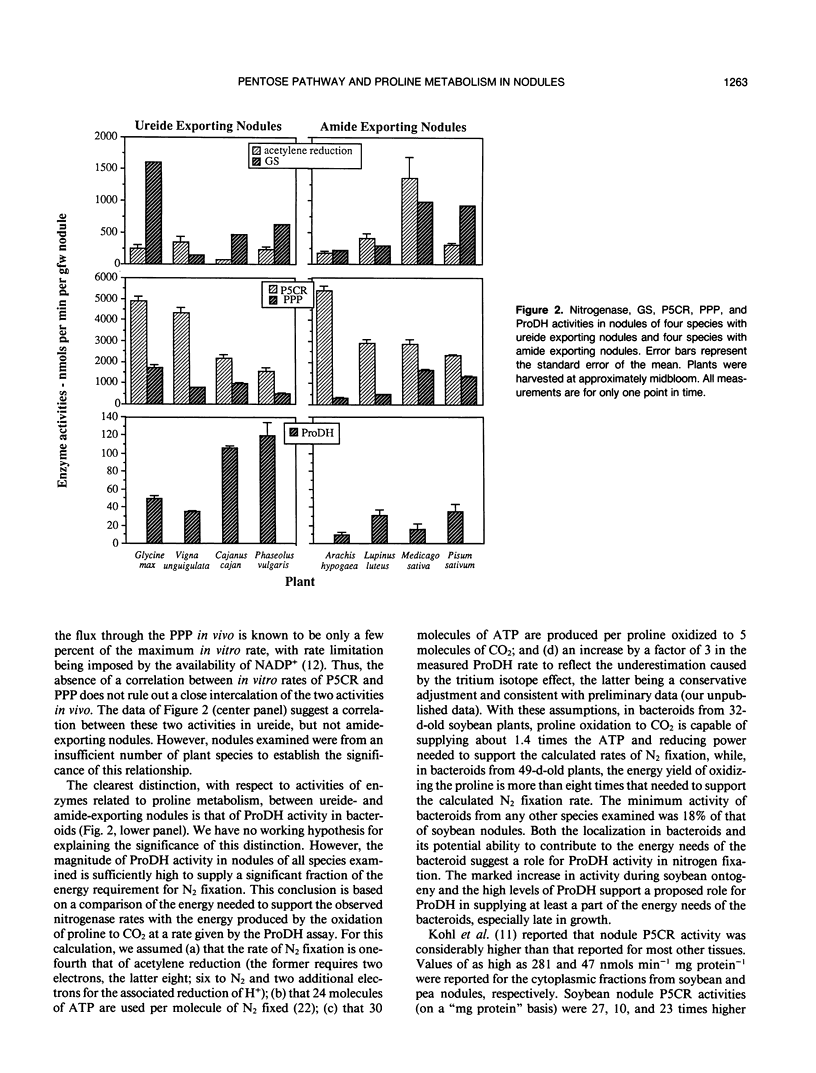
Based on localization and high activities of pyrroline-5-carboxylate reductase and proline dehydrogenase activities in soybean nodules, we previously suggested two major roles for pyrroline-5-carboxylate reductase in addition to the production of the considerable quantity of proline needed for biosynthesis; namely, transfer of energy to the location of biological N2 fixation, and production of NADP+ to drive the pentose phosphate pathway. The latter produces ribose-5-phosphate which can be used in de novo purine synthesis required for synthesis of ureides, the major form in which biologically fixed N2 is transported from soybean root nodules to the plant shoot. In this paper, we report rapid induction (in soybean nodules) and exceptionally high activities (in nodules of eight species of N2-fixing plants) of pentose phosphate pathway and pyrroline-5-carboxylate reductase. There was a marked increase in proline dehydrogenase activity during soybean (Glycine max) ontogeny. The magnitude of proline dehydrogenase activity in bacteroids of soybean nodules was sufficiently high during most of the time course to supply a significant fraction of the energy requirement for N2 fixation. Proline dehydrogenase activity in bacteroids from nodules of other species was also high. These observations support the above hypothesis. However, comparison of pentose phosphate pathway and pyrroline-5-carboxylate reductase activities of ureide versus amide-exporting nodules offers no support. The hypothesis predicts that pyrroline-5-carboxylate and pentose phosphate pathway activities should be higher in ureide-exporting nodules than in amide-exporting nodules. This predicted distinction was not observed in the results of in vitro assays of these activities.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cha Y., Murray C. J., Klinman J. P. Hydrogen tunneling in enzyme reactions. Science. 1989 Mar 10;243(4896):1325–1330. doi: 10.1126/science.2646716. [DOI] [PubMed] [Google Scholar]
- Coker G. T., Schubert K. R. Carbon Dioxide Fixation in Soybean Roots and Nodules: I. CHARACTERIZATION AND COMPARISON WITH N(2) FIXATION AND COMPOSITION OF XYLEM EXUDATE DURING EARLY NODULE DEVELOPMENT. Plant Physiol. 1981 Apr;67(4):691–696. doi: 10.1104/pp.67.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagedorn C. H., Phang J. M. Catalytic transfer of hydride ions from NADPH to oxygen by the interconversions of proline and delta 1-pyrroline-5-carboxylate. Arch Biochem Biophys. 1986 Jul;248(1):166–174. doi: 10.1016/0003-9861(86)90413-3. [DOI] [PubMed] [Google Scholar]
- Hardy R. W., Holsten R. D., Jackson E. K., Burns R. C. The acetylene-ethylene assay for n(2) fixation: laboratory and field evaluation. Plant Physiol. 1968 Aug;43(8):1185–1207. doi: 10.1104/pp.43.8.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON A. B., STRECKER H. J. The interconversion of glutamic acid and proline. IV. The oxidation of proline by rat liver mitochondria. J Biol Chem. 1962 Jun;237:1876–1882. [PubMed] [Google Scholar]
- Kohl D. H., Schubert K. R., Carter M. B., Hagedorn C. H., Shearer G. Proline metabolism in N2-fixing root nodules: energy transfer and regulation of purine synthesis. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2036–2040. doi: 10.1073/pnas.85.7.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A., Eggleston L. V. The regulation of the pentose phosphate cycle in rat liver. Adv Enzyme Regul. 1974;12:421–434. doi: 10.1016/0065-2571(74)90025-9. [DOI] [PubMed] [Google Scholar]
- Krueger R., Jäger H. J., Hintz M., Pahlich E. Purification to homogeneity of pyrroline-5-carboxylate reductase of barley. Plant Physiol. 1986 Jan;80(1):142–144. doi: 10.1104/pp.80.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson C. Regulatory volume increase in Ehrlich ascites tumor cells. Biochim Biophys Acta. 1990 Jan 15;1021(1):1–8. doi: 10.1016/0005-2736(90)90375-x. [DOI] [PubMed] [Google Scholar]
- Mezl V. A., Knox W. E. Properties and analysis of a stable derivative of pyrroline-5-carboxylic acid for use in metabolic studies. Anal Biochem. 1976 Aug;74(2):430–440. doi: 10.1016/0003-2697(76)90223-2. [DOI] [PubMed] [Google Scholar]
- Phang J. M., Downing S. J., Valle D. A radioisotopic assay for delta1-pyrroline-5-carboxylate reductase. Anal Biochem. 1973 Sep;55(1):266–271. doi: 10.1016/0003-2697(73)90312-6. [DOI] [PubMed] [Google Scholar]
- Phang J. M., Downing S. J., Yeh G. C., Smith R. J., Williams J. A., Hagedorn C. H. Stimulation of the hexosemonophosphate-pentose pathway by pyrroline-5-carboxylate in cultured cells. J Cell Physiol. 1982 Mar;110(3):255–261. doi: 10.1002/jcp.1041100306. [DOI] [PubMed] [Google Scholar]
- Reynolds P. H., Boland M. J., Blevins D. G., Schubert K. R., Randall D. D. Enzymes of amide and ureide biogenesis in developing soybean nodules. Plant Physiol. 1982 Jun;69(6):1334–1338. doi: 10.1104/pp.69.6.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert K. R. Enzymes of Purine Biosynthesis and Catabolism in Glycine max: I. COMPARISON OF ACTIVITIES WITH N(2) FIXATION AND COMPOSITION OF XYLEM EXUDATE DURING NODULE DEVELOPMENT. Plant Physiol. 1981 Nov;68(5):1115–1122. doi: 10.1104/pp.68.5.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiono T., Kador P. F., Kinoshita J. H. Stimulation of the hexose monophosphate pathway by pyrroline-5-carboxylate reductase in the lens. Exp Eye Res. 1985 Dec;41(6):767–775. doi: 10.1016/0014-4835(85)90185-x. [DOI] [PubMed] [Google Scholar]
- Smith R. J., Downing S. J., Phang J. M. Enzymatic synthesis and purification of L-pyrroline-5-carboxylic acid. Anal Biochem. 1977 Sep;82(1):170–176. doi: 10.1016/0003-2697(77)90145-2. [DOI] [PubMed] [Google Scholar]
- WENNER C. E., HACKNEY J. H., MOLITERNO F. The hexose monophosphate shunt in glucose catabolism in ascites tumor cells. Cancer Res. 1958 Oct;18(9):1105–1114. [PubMed] [Google Scholar]
- Yeh G. C., Phang J. M. Stimulation of phosphoribosyl pyrophosphate and purine nucleotide production by pyrroline 5-carboxylate in human erythrocytes. J Biol Chem. 1988 Sep 15;263(26):13083–13089. [PubMed] [Google Scholar]


