ABSTRACT
Neonatal tetanus persists as a public health problem in many developing countries including Ethiopia. Maternal tetanus toxoid vaccination is a cornerstone to prevent neonatal tetanus. However, its prevalence is low in Ethiopia, and little has been devoted to its spatial epidemiology and associated factors. Hence, this study aimed to explore the spatial pattern and factors affecting tetanus-unprotected births in Ethiopia. A further analysis of the 2016 Ethiopia Demographic and Health Survey data was conducted, and a weighted sample of 7590 women was used for analysis. Spatial analysis was done using ArcGIS and SaTScan software. A binary logistic regression model was fitted to identify factors and variables with a p-value <.05 were considered as statistically significant. About 54.13% (95% CI: 53.01, 55.25) of births were not protected against neonatal tetanus, and spatial clustering of tetanus unprotected births was observed (Moran’s I = 0.144, p-value = .028). The primary and secondary SaTScan clusters were detected in Northeastern Tigray, Eastern Amhara, and almost the entire Afar (RR = 1.34 & LLR = 66.5, p < .01), and in the Somali region, and the western border of Gambela (RR = 1.44 & LLR = 31.3, p < .01), respectively. Tetanus unprotected births were higher among women without formal education (AOR = 1.63; 95% CI: 1.29, 2.04), came from poor households (AOR = 1.27; 95% CI: 1.12, 1.45), who had no ANC contact (AOR = 6.97; 95% CI: 6.21, 7.88), and who were not exposed to the media (AOR = 1.26; 95% CI: 1.09, 1.47). Hence, tetanus-unprotected birth hotspots require priority interventions, and it is good if the targeted interventions consider the identified factors.
KEYWORDS: Associated factors, Ethiopia, newborns, spatial pattern, tetanus-unprotected birth
Introduction
Tetanus is a non-communicable bacterial disease acquired via infection of a cut or wound with the spores of Clostridium tetani, which is characterized by spasms and autonomic nervous system dysfunction.1,2 Since Clostridium tetani is found in soil and animal digestive systems all over the world, it contaminates a variety of surfaces and materials,3 putting women are risk for acquiring the infection during unhygienic delivery practice.4,5 Even though tetanus can occur worldwide and in all age groups, neonates are most at risk, particularly when the mothers have insufficient antitoxins due to no or suboptimal immunization to protect themselves and their newborn babies.
Tetanus, especially in low-income countries like Ethiopia, continues to be a major cause of neonatal mortality with an 80%–100% case-fatality rate among neonates born from women who had insufficient vaccination and unhygienic delivery.6 Globally, children face the highest risk of dying in their first month of life at an average global rate of 18 deaths per 1,000 live births in 2021.7 The contribution of neonatal tetanus to those newborn deaths is not trivial; the World Health Organization (WHO)/United Nations Children’s Fund (UNICEF) Joint Reporting Form on Immunization report states that in 2019, 2173, 1151, and 107 neonatal tetanus cases were reported globally, from the African region, and Ethiopia, respectively.8 Even though the elimination of Maternal and Neonatal Tetanus (MNT) was achieved in most of the countries, by the end of 2015, there were still 21 countries including Ethiopia that had not yet attained the elimination of maternal and neonatal tetanus.9
To overcome such problems, World Health Assembly (WHA) sets strategies to eliminate maternal and neonatal tetanus. Vaccinating more than 80% of reproductive age women through routine and supplementary immunization activities (SIAs) was the first one since immunizing a mother during pregnancy with at least two doses of tetanus toxoid vaccine can reduce the risk of newborn tetanus by 94%.2,10 Tetanus protection for the newborn is provided by maternal immunization-induced antibodies that cross the placenta and reach the fetus.3 Consistently, the Ethiopian government had also set a goal to reach 90% coverage of tetanus protected birth by 2016 and 2020 at national and district level, respectively.11
Globally, tetanus protected birth increased from 74% in 2000 to 86% in 2020.12 However, 2016 Ethiopian Demographic and Health Survey (EDHS) report indicated that only 49% of pregnant women received at least two doses of tetanus toxoid vaccine. In addition, between 2016 and 2019, neonatal mortality increased from 29 to 30 per 1,000 live births; of those deaths, 9% were attributed to tetanus, which is 4.5 per 1,000 live births.13 Though the prevalence of tetanus-unprotected birth has varied across regions, the entire previous series of studies conducted in Ethiopia were prevalence studies,14–18 which failed to address the geographic variation of the problem. Thus, the identification of geographic areas with a high prevalence of tetanus-unprotected birth has become crucial to design targeted interventions with minimal cost. Therefore, this study aimed to explore the spatial pattern and factors affecting tetanus-unprotected births in Ethiopia.
Methods
Data source, study design and setting
A cross-sectional study was undertaken using the nationally representative 2016 Ethiopian Demographic and Health Survey dataset, which is only available (www.dhsprogram.com) through site requests. Ethiopia, where this study was conducted, is situated in the Horn of Africa (30–150 N latitude and 330–480 E longitude). The country occupies an area of 1.1 million square kilometers with an altitude that ranges from the highest peak at Ras Dashen (4,620 m above sea level) down to the Dallol Depression, about 148 m below sea level. It has none regional states (Afar, Amhara, Benishangul Gumuz, Gambela, Hareri, Oromia, Somali, Southern Nations, Nationalities, and People Region (SNNPR), and Tigray regions) and two city administrations (Addis Ababa and Dire Dawa) (Figure 1).
Figure 1.
Map of study area, Ethiopia.
Sampling procedure and sample size
The EDHS sample was derived using a stratified, two-stage cluster design, where enumeration areas (645 clusters from the total 84,915 enumeration areas) and households (28 households per cluster) were selected at the first stage and second stage, respectively. The detailed methodologies of the surveys are presented in the full EDHS report.19 In this survey 15,683 women aged 15–49 were interviewed, and for our study, a total sample of 7193 (weighted sample = 7590) women who gave birth within the five years preceding the survey was used.
Variables of the study
The outcome variable was tetanus-unprotected birth, in which births are categorized as tetanus-protected and tetanus-unprotected. A birth was said to be tetanus-protected only if women had at least two Tetanus Toxoid (TT) doses during the indexed pregnancy or had received at least TT2 (the last within 3 years of the indexed birth), TT3 (the last within 5 years of the indexed birth), TT4 (the last within 10 years of the indexed birth), or TT5 at any time before the indexed birth; otherwise, it was considered as a tetanus-unprotected birth.20
The explanatory variables for this study were maternal age, residence, maternal education, marital status, working condition, household wealth status, media exposure, perception of distance from the health facility, health insurance coverage, wanted last-child, and Antenatal Care (ANC) contact for the indexed birth. The three variables of reading newspapers, listening to the radio, and watching television were combined to form the composite variable called “media exposure.” The response was then recoded as exposed (if a woman had been exposed to one of the three media sources at least once a week) or not exposed (if a woman had not had exposure to all three media sources at least once a week). The EDHS data drove wealth index using the principal component analysis (PCA) based on the number and kinds of household goods they own, ranging from a television to a bicycle or car, in addition to housing characteristics such as the source of drinking water, toilet facilities, and flooring materials. After conducting the PCA, five quintiles that divide the household into five groups (poorest, poorer, medium, richer, and richest) were constructed using the first principal component.
Data management and statistical analysis
Variables were managed accordingly using STATA version 17.0 software and Microsoft Excel to better suit our analysis. Throughout the analysis, sample weighting was done to adjust for non-proportional sample selection and for nonresponses as well to restore the representativeness of the data. Participant characteristics were described using frequency with percent.
Spatial analysis
Both ArcGIS version 10.7 and Kuldorff’s SaTScan version 9.6 software were used to explore the spatial distribution and identify significant hotspot areas of tetanus-unprotected births. The first step in conducting spatial analysis is determining whether the outcome variable was dispersed, clustered, or randomly distributed in the study area. Hence, the global spatial autocorrelation (Global Moran’s I) statistic was calculated, and we declared that the pattern of tetanus-unprotected births in Ethiopia was clustered with a statistically significant positive Moran’s I value (Moran’s Index = 0.144475 with a p-value of 0.028160). In addition, Incremental Spatial Autocorrelation was assessed to calculate an appropriate distance threshold for identifying spatial processes that promote clustering and to use this maximum peak distance value as the threshold distance for hotspot analysis.
Given these circumstances, a Hot Spot Analysis using local Getis-Ord Gi* statistics was conducted in ArcGIS version 10.7 by seating the maximum peak distance value during incremental spatial autocorrelation as the threshold distance. In relative to the mean tetanus-unprotected birth rate nationwide, it maps certain notable hot spot areas (areas with higher rates of tetanus-unprotected birth) and cold spot areas (areas with lower rates of tetanus-unprotected birth). Moreover, Bernoulli-based purely spatial scan statistics was performed using Kuldorff’s SaTScan version 9.6 software to identify the primary, secondary, tertiary, ….etc. most significant clusters for tetanus-unprotected birth. The maximum spatial cluster size was set to 50% of the population at risk. The most likely clusters were identified using p-values and log likelihood ratio (LLR) tests based on 999 Monte Carlo replications. Identified clusters were ranked using their LLR, and the cluster with the maximum LLR constitutes the primary cluster, the cluster that is least likely to have occurred by chance. Relative risk (RR) was also reported for each identified cluster, indicating how much risk a birth in a specified spatial window has to be tetanus-unprotected as compared to those births outside that window.
Finally, the geostatistical ordinary Kriging method of spatial interpolation was applied using ArcGIS 10.7 to predict the proportion of tetanus-unprotected birth for un-sampled/unmeasured location based on the neighborhood sampled/measured clusters.
Binary logistic regression analysis
Both bi-variable and multivariable binary logistic regression model was fitted using STATA version 17.0 software to see the association between tetanus-unprotected birth and covariates in Ethiopia. Variables that had a p-value less than or equal to 0.2 in the bi-variable analysis were taken into the multivariable model. In multivariable logistic regression model, significant associations between tetanus-unprotected birth and covariates were declared at alpha 0.05. Both the crude odds ratio (COR) and adjusted odds ratio (AOR) with its 95% confidence interval were reported. Actually, ordinary least squares (OLS) and geographically weighted regressions (GWR) had been considered before going to the binary logistic regression model. Nevertheless, the OLS regression model did not satisfy all of the assumptions that are required by this method. The model residuals were not normally distributed (significant Jarque-Bera statistic and Shapiro-Wilk test with p < .01) and revealed a spatial pattern with a spatial autocorrelation result of 0.138713 Moran’s Index and a significant p-value at alpha 0.05. In addition, the Koenker statistics were not statistically significant (p > .05), which indicates the relationship between each explanatory variable and tetanus-unprotected birth is stationary (the strength of the association did not vary) across the country and there is no need for a geographically weighted regression. Consequently, the GWR model also showed an almost similar adjusted R2 and Akaike’s Information Criterion (AIC) value to the OLS model. That is why we finally considered a global binary logistic regression model to identify factors associated with tetanus-unprotected birth in Ethiopia.
Results
Characteristics of respondents
Of the total weighted sample of 7590 women included in the analysis, 6621 (87.23%) were rural dwellers. The median age of the women were 28 years with Inter Quartile Range (IQR) of 10 (24–34). The majority of women (n = 4791, 63.12%) had no formal education and were from households with poor wealth status (n = 3306, 43.55%). Only 1486 (19.58%) and 3183 (41.94%) of the women had media exposure and perceived distance from the health facility as not a big problem. Majority 4757 (62.67%) and 5574 (73.43%) of women had ANC follow-up and wanted last child (Table 1).
Table 1.
Socio-demographic and economic characteristics of women in Ethiopia.
| Variables | Weighted frequency | Percent |
|---|---|---|
| Residence | ||
| Urban | 969 | 12.77 |
| Rural | 6621 | 87.23 |
| Age of the women | ||
| 15–24 | 1804 | 23.77 |
| 25–34 | 3827 | 50.42 |
| ≥35 | 1959 | 25.82 |
| Marital status | ||
| Married | 7020 | 92.49 |
| Not married | 570 | 7.51 |
| Educational status of women | ||
| No education | 4791 | 63.12 |
| Primary education | 2150 | 28.32 |
| Secondary & above education | 649 | 8.55 |
| Working condition of women | ||
| Working | 2172 | 28.62 |
| Not working | 5418 | 71.38 |
| Wealth status of the household | ||
| Poor | 3306 | 43.55 |
| Medium | 1588 | 20.93 |
| Rich | 2696 | 35.52 |
| Media exposure | ||
| Exposed | 1486 | 19.58 |
| Not exposed | 6104 | 80.42 |
| Perception of distance from the health facility | ||
| Big problem | 4407 | 58.06 |
| Not big problem | 3183 | 41.94 |
| Need of indexed birth status | ||
| Wanted | 5574 | 73.43 |
| Not wanted | 2016 | 26.57 |
| ANC follow up | ||
| Yes | 4757 | 62.67 |
| No | 2833 | 37.33 |
| Health insurance coverage | ||
| Insured | 317 | 4.18 |
| Not insured | 7273 | 95.82 |
Prevalence and spatial pattern of tetanus-unprotected births
The overall prevalence of tetanus-unprotected birth in Ethiopia was 54.13% (95% CI: 53.01, 55.25). More specifically, the prevalence of tetanus-unprotected birth was 71.08%, 63.73%, 60.02%, and 55.03% in the Afar, Somali, Amhara, and Oromia regions, respectively. Whereas, its prevalence in SNNPR (52.46%), Benishangul (48.84%), Gambela (47.48%), Tigray (43.65%), Harari (32.19%), Dire Dawa (31.28%), and Addis Ababa (22.99%) was below the national average. The global test for spatial autocorrelation revealed that tetanus-unprotected birth has a clustered pattern across the country (Moran’s I = 0.144, p-value = .028) (Figure 2). According to the incremental spatial Autocorrelation result, the maximum distance at which clustering of tetanus-unprotected birth rate peaked was at 153.2 km (Figure 3).
Figure 2.
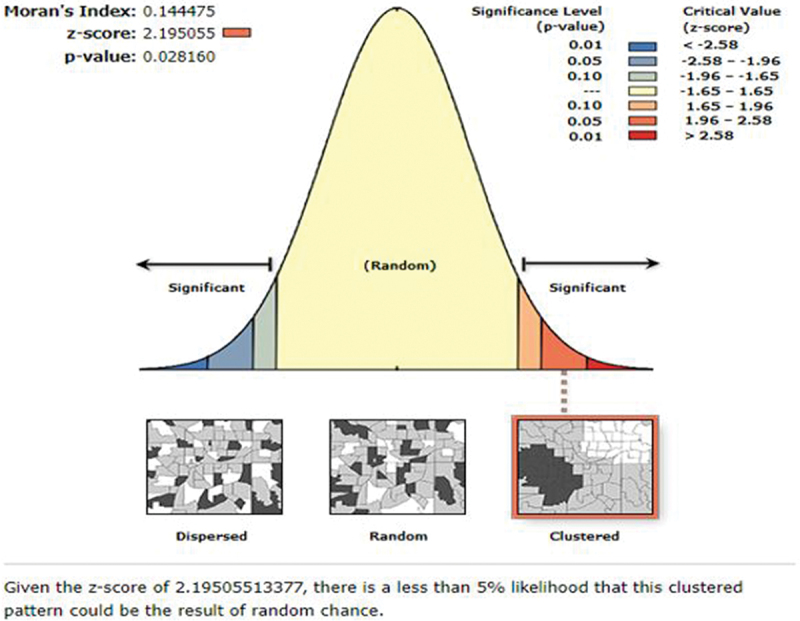
The global spatial autocorrelation analysis result to evaluate the spatial pattern of tetanus-unprotected births in Ethiopia.
Figure 3.
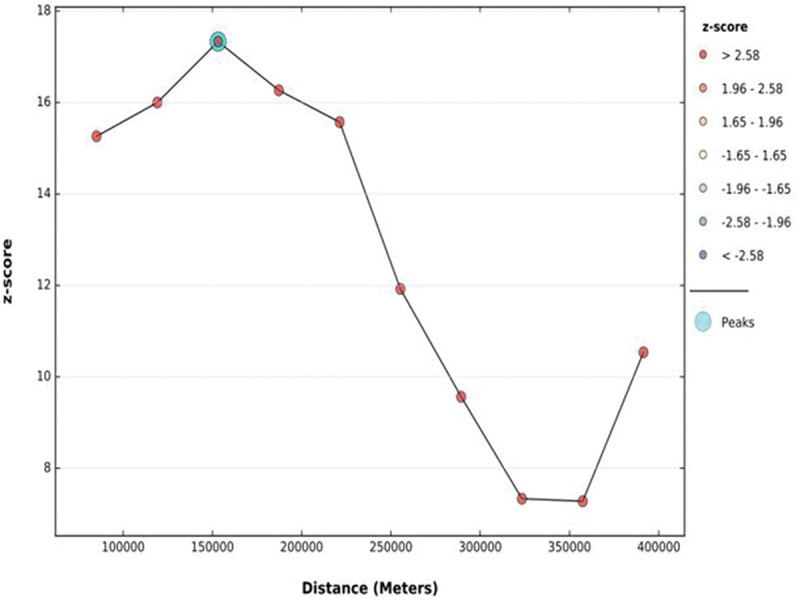
The incremental spatial autocorrelation of tetanus-unprotected births in Ethiopia by a function of distance.
The Hot Spot (Getis Ord Gi*) statistical analysis identified significant hot spot areas of tetanus-unprotected birth in the Amhara, Afar, and southeastern Tigray and some part of Somali regions and cold spot areas in the central Oromia, Addis Ababa, Dire Dawa, and Harari regions (Figure 4).
Figure 4.
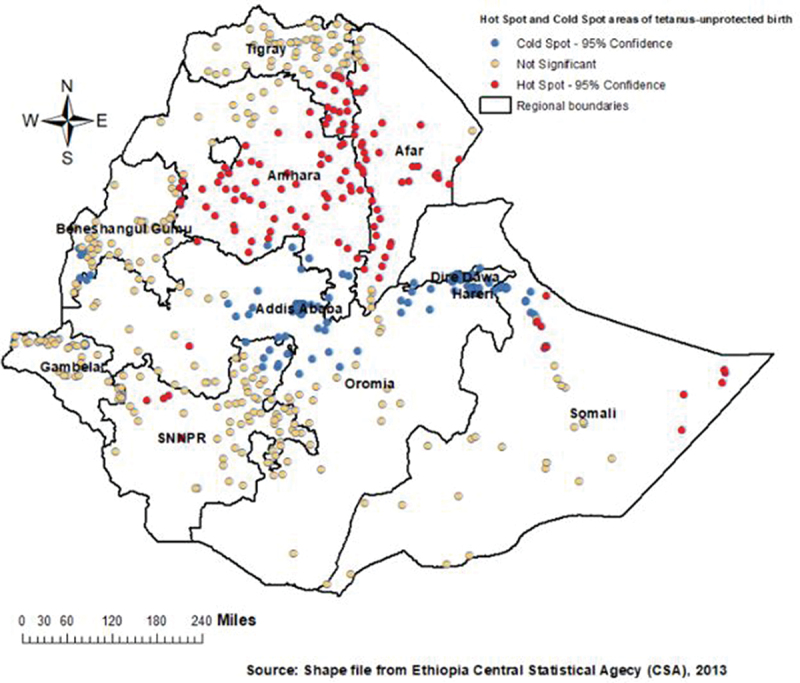
Hot spot analysis of tetanus-unprotected birth in Ethiopia.
The SaTScan analysis detected 216 statistically significant clusters with a high rate of tetanus-unprotected birth. Of these, 127 clusters were the most likely (primary) clusters and 23 were secondary clusters. The primary clusters’ spatial window (the most likely cluster with maximum LLR) was located in southeastern Tigray, most areas of Amhara, and almost the entire Afar regions, centered at 11.868414 N, 40.088208 E of geographic location with 267.27 km radius, and LLR of 66.5, at p < .01. Births within this spatial window had 1.34 times higher risk of being tetanus-unprotected than births outside the window. The secondary clusters’ scanning window was located at the border of eastern Oromia and northwestern Somali (LLR = 31.3, RR = 1.44, p < .01). In addition, the third most likely SaTScan cluster was also detected in the western border of Gambela region (LLR = 26.8, RR = 1.56, p < .01) (Figure 5).
Figure 5.
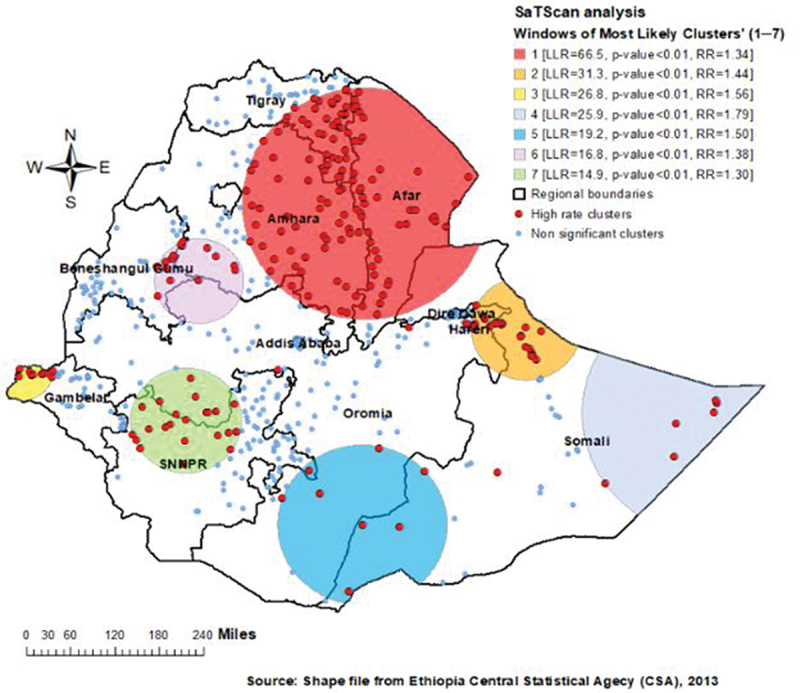
SaTScan analysis of tetanus-unprotected births in Ethiopia, map produced using ArcGIS.
Figure 6 shows the spatial interpolation of tetanus-unprotected births, which predicts the percentage of tetanus-unprotected birth for the unmeasured location across the country from the limited number of sample data points. More than 64.3% of the births were tetanus-unprotected in most part of Amhara and almost entire Afar region (Figure 6).
Figure 6.
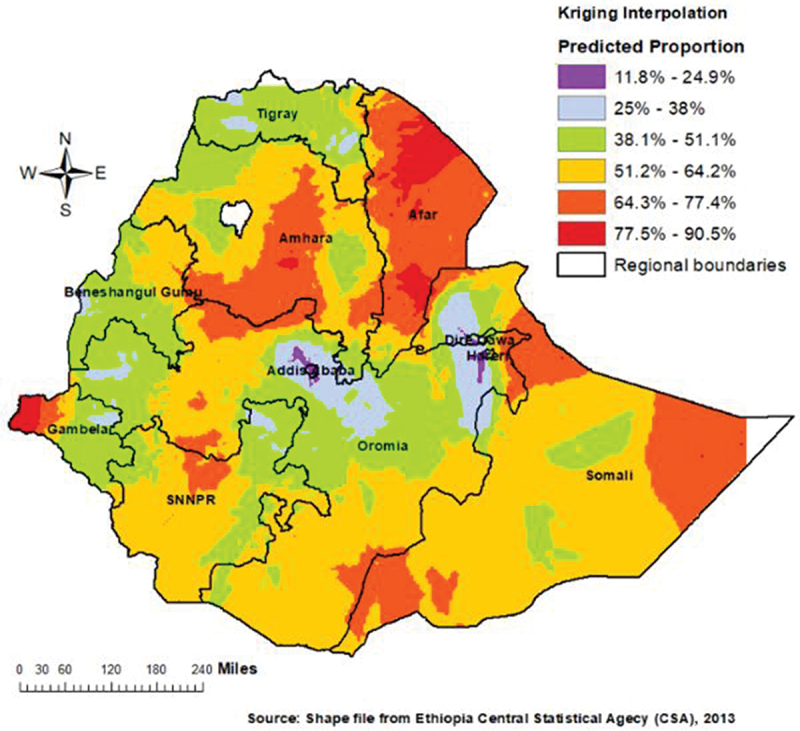
A spatial interpolation of tetanus-unprotected births in Ethiopia.
Factors associated with tetanus-unprotected birth
After adjusting for different variables, women education level, wealth status, media exposure, and ANC follow-up were significantly associated with tetanus-unprotected birth in Ethiopia. The odds of giving tetanus-unprotected birth was 1.63 [AOR = 1.63; 95% CI: 1.29, 2.04, p = .001] times higher among women who had no formal education as compared to those with secondary and above educational level. Women who were not exposed to media had 1.26 [AOR = 1.26; 95% CI: 1.09, 1.47, p < .001] times higher odds of giving tetanus-unprotected birth than their counterparts. The odds of giving tetanus-unprotected birth was also 1.27 [AOR = 1.27; 95% CI: 1.12, 1.45, p < .001] and 1.21 [AOR = 1.21; 95% CI: 1.04, 1.41, p = .012] times higher among women from households with poor and medium wealth status than those form the rich households, respectively. Women who did not have at least one ANC contact during the indexed pregnancy were 6.99 [AOR = 6.97; 95% CI: 6.21, 7.88, p < .001] times more likely to give tetanus-unprotected birth than those who had at least one ANC contact (Table 2).
Table 2.
Multivariable binary logistic regression analysis of factors associated with tetanus-unprotected birth in Ethiopia.
| Variables | Tetanus-unprotected birth |
COR (95% CI) | AOR (95% CI) | p-value | |
|---|---|---|---|---|---|
| Yes (%) | No (%) | ||||
| Residence | |||||
| Urban | 321 (33.1) | 648 (66.9) | 1 | 1 | |
| Rural | 3788 (57.2) | 2833(42.8) | 2.70 (2.35, 3.12) | 1.05 (0.86, 1.27) | .821 |
| Age of the women | |||||
| 15–24 | 937 (52.0) | 867 (48.0) | 1 | 1 | |
| 25–34 | 1998 (52.2) | 1829(47.8) | 1.01 (0.90, 1.13) | 0.89 (0.78, 1.02) | .267 |
| ≥35 | 1174 (59.9) | 785 (40.1) | 1.38 (1.21, 1.57) | 0.93 (0.79, 1.09) | .725 |
| Educational status of women | |||||
| No education | 2944 (61.5) | 1847(38.5) | 4.15 (3.46, 4.98) | 1.63 (1.29, 2.04)* | .001 |
| Primary education | 984 (45.8) | 1166(54.2) | 2.19 (1.81, 2.66) | 1.21 (0.97, 1.50) | .206 |
| Secondary & above | 180 (27.7) | 469 (72.3) | 1 | 1 | |
| Working condition | |||||
| Working | 1039 (47.8) | 1133(52.2) | 1 | 1 | |
| Not working | 3070 (56.7) | 2348(43.3) | 1.43 (1.29, 1.57) | 1.12 (0.99, 1.26) | .102 |
| Wealth status | |||||
| Poor | 2061 (62.3) | 1245(37.7) | 2.21 (1.99, 2.45) | 1.27 (1.12, 1.45)* | <.001 |
| Medium | 893 (56.3) | 695 (43.7) | 1.72 (1.51, 1.95) | 1.21 (1.04, 1.41)* | .012 |
| Rich | 1155 (42.8) | 1541(57.2) | 1 | 1 | |
| Media exposure | |||||
| Exposed | 559 (37.6) | 927 (62.4) | 1 | 1 | |
| Not exposed | 3549 (58.1) | 2555(41.9) | 2.30 (2.05, 2.59) | 1.26 (1.09, 1.47)* | <.001 |
| Perception of distance from the health facility | |||||
| Big problem | 2576 (58.5) | 1831(41.5) | 1.52 (1.38, 1.66) | 0.92 (0.82, 1.03) | .389 |
| Not big problem | 1532 (48.1) | 1651(51.9) | 1 | 1 | |
| ANC follow up | |||||
| Yes | 1767 (37.1) | 2990(62.9) | 1 | 1 | |
| No | 2341 (82.6) | 492 (17.4) | 8.05 (7.19, 9.02) | 6.99 (6.21, 7.88)* | <.001 |
| Need of indexed birth status | |||||
| Wanted | 2981 (53.5) | 2593(46.5) | 1 | 1 | |
| Unwanted | 1127 (55.9) | 889 (44.1) | 1.10 (0.99, 1.22) | 0.96 (.85, 1.08) | .631 |
*significant at p-value < .05.
COR = Crude Odds Ratio; AOR = Adjusted Odds Ratio; CI = Confidence Interval.
Discussion
Since tetanus-unprotected birth is highly related to child and maternal health, exploring the spatial distribution of tetanus-unprotected birth provides evidence for the need to target intervention in high-risk areas where tetanus-unprotected birth is most likely to occur. Thus, this is the first study that attempted to assess the spatial variation of tetanus-unprotected birth and its associated factor in Ethiopia using the national-level data. Accordingly, significant hotspot areas of tetanus-unprotected birth were observed in Afar, Amhara, southeastern Tigray and limited part of Somali regions. Almost similar areas were also identified as a primary clusters in the SaTScan analysis. Safest regions that had lower proportion of tetanus-unprotected birth were central Oromia, Addis Ababa, Dire Dawa, and Hareri. A study conducted to assess the inequalities in immunization against maternal and neonatal tetanus in seventy-six countries also pointed out the significant regional variations of tetanus-unprotected birth.21
The long-lasting humanitarian crises, including poor political will, competing priorities, and continuous armed conflicts, in the border of Amara, Afar, and Tigray regions may be to blame for the highest proportion of tetanus-unprotected birth in this geographic location. These crises led to the destruction of health facilities, the displacement of people, including health workers, and decreased access to healthcare for the women. This variation might also attributed to the low educational status of women in Afar and Somali regions.19 It is true that uneducated women are less likely to actively participate in knowledge-enhancing activities including peer conversations, service promotion, and reading materials, which raises awareness of the negative consequence of giving tetanus-unprotected birth.22 The geographical variation might be explained as the mobile pastoralist community in Afar and Somali regions lacks access to health information and services23 and had poor health-seeking behavior.24 The widespread impacts of the COVID-19 pandemic also caused the reallocation of resources away from already-existing programs and put the continuous coverage, service access, and delivery of routine vaccinations under question.25 A study indicated that Tdap (Tetanus, Diphtheria, and Pertussis) vaccination uptake of pregnant women was 85% in the pre-pandemic, significantly decreasing to 76% during the pandemic.26
The overall prevalence of tetanus-unprotected births (54.13%) in Ethiopia was also high, which may be due to the shortage of vaccines in health institutions. In a study from Sidama, Ethiopia, a shortage of vaccines in health institutions (32.5%) was one of the significant reasons for delayed vaccination.27 Actually, there are very few vaccine manufacturers to meet the global demand for vaccines. This leads to a shortage and a high cost of vaccines for use, especially in developing countries where the need is very dire. In the Ethiopian context, only minimal experience in producing bacterial and viral vaccines has been recorded.28 Accordingly, the immunization coverage of other vaccines was also low in Ethiopia. Only 61%, 60%, and 59% of children aged 12–23 months receive the recommended three doses of pentavalent, all three doses of the polio, and measles vaccines, respectively.13
In this study, women with low education level, poor wealth status, who were not exposed to media, and did not attend ANC follow-up were more likely to have tetanus-unprotected birth. The odds of giving tetanus-unprotected birth was 1.63 times higher among mothers who did not have formal education compared with those who attend secondary and above educational. This is consistent with studies conducted in five African countries,29 Nigeria,30 Sudan,31 and Ethiopia.17,32 The possible explanation might be that education can improve the uptake of tetanus vaccination as educated women may have a higher level of health awareness, greater knowledge of available health services, improved ability to afford the cost of medical health care, and greater autonomy in making health-related decisions, including the freedom to travel outside the home to seek healthcare.33,34
Women from households with poor wealth status had higher odds of poor TT immunization as compared to those from rich households. The finding from ten East African countries,35 Kenya,36 Sudan,31 and Ethiopia,37 supports the finding of this study regarding the association between wealth status and tetanus-unprotect birth occurrence. Even with the affordability of the vaccines and being free of charge, in the condition of inaccessible healthcare facilities women with low economic status could not afford the high transportation costs. Additionally, women with poor socioeconomic position could be preoccupied with other tasks to meet their basic needs, which prevents them from taking advantage of health care services.
Another important factor that significantly associated with the occurrence of tetanus-unprotected birth was the media exposure status of women, which is consistent with other studies conducted elsewhere.17,32,35,38 The findings revealed that the odds of giving a tetanus-unprotected birth were higher among women with no media exposure than their counterparts. Possibly, it could be due to the fact that women without access to media exposure may not be exposed to health service-related messages, including immunization that are conveyed through the media. In turn, it will cause women to have poor knowledge about TT immunization, which might decrease the odds of women being vaccinated against tetanus and giving tetanus protected birth.
Antenatal care follow-up was also found to be an independent predictor of tetanus-unprotected birth, with women who had no ANC contact was around seven times more likely to give tetanus-unprotected birth who had at least one ANC contact. This finding is also congruent with the study conducted in ten East African countries35 and different parts of Ethiopia.15,17,38 This might be because tetanus immunization is one of the ANC service package and ANC contact is the best opportunity for women to take the immunization. The ANC contact also offers opportunities to reach pregnant women with information on several health practices. Thus, women who had no ANC follow-up might lose these opportunities and are less likely to be vaccinated against tetanus, which in turn results in tetanus-unprotected births.
The following strengths and limitations of this study should be considered for the interpretation and conclusion of the results: The main strength of this study is the use of nationally representative EDHS data, which can be generalized to the whole Ethiopia and will provide good evidence for policymakers at the national level. Furthermore, we used an advanced spatial analysis that allows the understanding of geographic variation in the occurrence of tetanus-unprotected births. However, the study was not free of limitations. The study might have had recall bias since the immunization status was ascertained through maternal reports for the question “how many tetanus doses did you take during your lifetime and your indexed pregnancy,” and the cross-sectional nature of the EDHS data does not allow us to confirm the cause-and-effect relationship. Because of the secondary nature of the data, some important factors, like the knowledge and attitude of women toward TT immunization, were not measured and included in the analysis. In addition, SaTScan analysis only picks up circular and/or elliptical clusters; therefore, it is possible that irregularly shaped clusters go undetected.
Conclusion
In Ethiopia, tetanus-unprotected birth had a clustered spatial pattern. The statistically significant primary clusters and hotspot areas of tetanus-unprotected birth were detected in eastern Amhara, southeastern Tigray, and the entire Afar regions. Tetanus-unprotected births were more likely to occur in women without formal education, came from poor households, who had no ANC contact, and who were not exposed to the media. Therefore, location-based interventional strategies that are designed based on these identified factors are required to eliminate tetanus-unprotected birth in a more cost-effective way than simply implementing the interventions randomly. Strengthening ANC service, making health facilities physically accessible, particularly for the poor, and empowering women through education and media exposure could increase the proportion of mothers who are protected from tetanus at birth. It is also better to consider the supplementary TT immunization campaigns/activities in high-risk clusters and integrate the routine immunization activity with other maternal healthcare programs to reduce missed opportunities for vaccination. Furthermore, researchers are advised to conduct community-based implementation research by collecting primary data and assessing tetanus protection using antibody testing.
Acknowledgments
We would like to thank the measure DHS program for the on-request open access to its dataset.
Funding Statement
The author(s) reported there is no funding associated with the work featured in this article.
Abbreviations
- AOR
Adjusted Odds Ratio
- AIC
Akaike’s Information Criterion
- COR
Crude Odds Ratio
- EDHS
Ethiopian Demographic and Health Survey
- GWR
Geographically Weighted Regressions
- LLR
Log Likelihood Ratio
- MNT
Maternal and Neonatal Tetanus
- OLS
Ordinary Least Squares
- PCA
Principal Component Analysis
- RR
Relative Risk
- SNNPR
Southern Nations Nationalities and People Region
- SSA
Sub-Saharan Africa
- SIAs
Supplementary Immunization Activities
- TT
Tetanus Toxoid
- UNICEF
United Nations Children’s Fund
- WHA
World Health Assembly
- WHO
World Health Organization
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The managed raw data supporting the conclusions of this article will be made available upon reasonable request by the corresponding authors without undue reservation.
Authors’ contribution
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Ethical approval and consent to participate
Ethical approval and participant consent were not necessary since it was a secondary data analysis of publicly available survey data from the measure DHS program. However, the proposal was acknowledged and given full approval by the research and community service coordinator of the College of Health Science, Debre Tabor University (Ref. No: CHS/1951/2023). In addition, we requested DHS Program data archivists and permission be granted to download and use the data for this study from www.measuredhs.com. At all, all the methods were performed in accordance with Helsinki Declaration of 1975.
References
- 1.Guerrant RL, Walker DH, Weller PF.. Tropical infectious diseases: principles, pathogens, & practice, 2-volume set with CD-ROM. Amsterdam, Netherlands: Elsevier; 2011. p. 1–11. [Google Scholar]
- 2.Blencowe H, Lawn J, Vandelaer J, Roper M, Cousens S. Tetanus toxoid immunization to reduce mortality from neonatal tetanus. Int J Epidemiol. 2010 April 1;39(suppl_1):i102–109. doi: 10.1093/ije/dyq027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roper MH, Vandelaer JH, Gasse FL. Maternal and neonatal tetanus. Lancet. 2007;370(9603):1947–1959. doi: 10.1016/S0140-6736(07)61261-6. [DOI] [PubMed] [Google Scholar]
- 4.Mehanna AA. Recommendation of tetanus toxoid vaccination for pregnant females in a country that achieved elimination of maternal and neonatal tetanus. A commentary on the study: knowledge and health beliefs of reproductive-age women in Alexandria about tetanus toxoid immunization. Arch Obstet Gynaecol. 2021;2:5–8. [Google Scholar]
- 5.Burgess C, Gasse F, Steinglass R, Yakubu A, Raza AA, Johansen K. Eliminating maternal and neonatal tetanus and closing the immunity gap. Lancet. 2017;389(10077):1380–1381. doi: 10.1016/S0140-6736(17)30635-9. [DOI] [PubMed] [Google Scholar]
- 6.WHO . Tetanus vaccines: WHO position paper – February 2017. Wkly Epidemiol Rec. 2017;92(6):53–76. [PubMed] [Google Scholar]
- 7.Unicef: neonatal mortality. 2023. https://data.unicef.org/topic/child-survival/neonatal-mortality/.
- 8.WHO: Tetanus reported cases and incidence. 2023. https://immunizationdata.who.int/pages/incidence/ttetanus.html?CODE=Global+AFR+ETH&DISEASE=NTETANUS&YEAR=.
- 9.WHO: protecting all against tetanus: guide to sustaining maternal and neonatal tetanus elimination (MNTE) and broadening tetanus protection for all populations. 2019.
- 10.Vandelaer J, Birmingham M, Gasse F, Kurian M, Shaw C, Garnier S. Tetanus in developing countries: an update on the maternal and neonatal tetanus elimination initiative. Vaccine. 2003;21:3442–5. [DOI] [PubMed] [Google Scholar]
- 11.Health FMo: Ethiopia national expanded programme on immunization. BMJ Publishing Group FMOE Addis Ababa; 2015. [Google Scholar]
- 12.Kanu FA, Yusuf N, Kassogue M, Ahmed B, Tohme RA. Progress toward achieving and sustaining maternal and neonatal tetanus elimination—worldwide, 2000–2020. Morb Mort Wkly Rep. 2022;71:406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Csace I: Ethiopia mini demographic and health survey 2019. Addis Ababa, Ethiopia, and Rockville, Maryland, USA: CSA and ICF;2019. p. 1–551. [Google Scholar]
- 14.Mengesha MB, Weldegeorges DA, Assefa NE, Gebremeskel SG, Hidru HD, Teame H, Hailesilassie Y. Tetanus toxoid immunization status and associated factors among mothers in Hawzen, Eastern zone of Tigray, Ethiopia, 2019. Open Public Health J. 2020;13(1):281–288. doi: 10.2174/1874944502013010281. [DOI] [Google Scholar]
- 15.Dubale Mamoro M, Kelbiso Hanfore L. Tetanus toxoid immunization status and associated factors among mothers in Damboya Woreda, Kembata Tembaro Zone, SNNP, Ethiopia. J Nutr Metabol. 2018;22(2839579):1–9. doi: 10.1155/2018/2839579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mihret MS, Limenih MA, Gudayu TW. The role of timely initiation of antenatal care on protective dose tetanus toxoid immunization: the case of northern Ethiopia post natal mothers. BMC Pregnancy Childbirth. 2018;18(1):1–10. doi: 10.1186/s12884-018-1878-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teshale AB, Tesema GA, Das JK. Determinants of births protected against neonatal tetanus in Ethiopia: a multilevel analysis using EDHS 2016 data. PLoS One. 2020;15(12):e0243071. doi: 10.1371/journal.pone.0243071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gebremedhin TS, Welay FT, Mengesha MB, Assefa NE, Werid WM. Tetanus toxoid vaccination uptake and associated factors among mothers who gave birth in the last 12 months in Errer district, Somali Regional State, Eastern Ethiopia. Biomed Res Int. 2020;2020:1–8. doi: 10.1155/2020/4023031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Csa I: Central statistical agency (CSA)[Ethiopia] and ICF . Ethiopia demographic and health survey. Addis Ababa, Ethiopia and Calverton, Maryland, USA; 2016. p. 1. [Google Scholar]
- 20.Croft TN, Marshall AM, Allen CK, Arnold F, Assaf S, Balian S. Guide to DHS statistics. Rockville: ICF; 2018. p. 645. [Google Scholar]
- 21.Johns NE, Cata-Preta BO, Kirkby K, Arroyave L, Bergen N, Danovaro-Holliday MC, Santos TM, Yusuf N, Barros AJD, Hosseinpoor AR. Inequalities in immunization against maternal and neonatal tetanus: a cross-sectional analysis of protection at birth coverage using household health survey cata from 76 countries. Vaccines. 2023;11(4):752. doi: 10.3390/vaccines11040752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yusuf N, Raza AA, Chang-Blanc D, Ahmed B, Hailegebriel T, Luce RR, Tanifum P, Masresha B, Faton M, Omer MD, et al. Progress and barriers towards maternal and neonatal tetanus elimination in the remaining 12 countries: a systematic review. Lancet Glob Health. 2021;9(11):e1610–e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jebena MG, Tesfaye M, Abashula G, Balina S, Jackson R, Assefa Y, Kifle Y, Tesfaye C, Yilma M, Hiruy A, et al. Barriers and facilitators of maternal health care services use among pastoralist women in Ethiopia: systems thinking perspective. Pastoralism. 2022;12(1):27. doi: 10.1186/s13570-022-00236-6. [DOI] [Google Scholar]
- 24.Gammino VM, Diaz MR, Pallas SW, Greenleaf AR, Kurnit MR, Werneck GL. Health services uptake among nomadic pastoralist populations in Africa: a systematic review of the literature. PLoS Negl Trop Dis. 2020;14(7):e0008474. doi: 10.1371/journal.pntd.0008474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saxena S, Skirrow H, Bedford H. Routine vaccination during COVID-19 pandemic response. Vol. 369. London: British Medical Journal Publishing Group; 2020. [DOI] [PubMed] [Google Scholar]
- 26.Cotter SB, Taylor LL, Grace R, Miao D, Coleman MA, Ratan BM. Effects of the COVID-19 pandemic on routine maternal vaccine uptake [A176]. Obstet Gynecol. 2022;139(1):51S–51S. doi: 10.1097/01.AOG.0000825980.61496.81. [DOI] [Google Scholar]
- 27.Negash BT, Tediso Y, Yoseph A. Predictors of timeliness of vaccination among children of age 12–23 months in Boricha district, Sidama region Ethiopia, in 2019. BMC Pediatr. 2023;23(1):409. doi: 10.1186/s12887-023-04234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.EPHI: Vaccine & Diagnostics Production . https://ephi.gov.et/research/vaccine-diagnostics-production/.
- 29.Yeshaw Y, Jemere T, Dagne H, Andualem Z, Akalu Y, Dewau R, Teshale AB, Tesema GA, Dagnew B, Todd CS. Factors associated with births protected against neonatal tetanus in Africa: evidences from demographic and health surveys of five African countries. PLoS One. 2021;16(6):e0253126. doi: 10.1371/journal.pone.0253126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gabriel-Job N, Yaguo Ide EL. Tetanus toxoid status and determinants of uptake among women in etche local government area, Rivers State, Nigeria: a community based study. Asian J Med Health. 2020;17(4):1–7. doi: 10.9734/ajmah/2019/v17i430171. [DOI] [Google Scholar]
- 31.Mohamed SOO, Ahmed EM. Prevalence and determinants of antenatal tetanus vaccination in Sudan: a cross-sectional analysis of the multiple indicator cluster survey. Trop Med Health. 2022;50(1):022–00398. doi: 10.1186/s41182-022-00398-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nigussie J, Girma B, Molla A, Mareg M. Tetanus toxoid vaccination coverage and associated factors among childbearing women in Ethiopia: a systematic review and meta-analysis. BioMed Res Int. 2021;8(5529315):1–0. doi: 10.1155/2021/5529315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang H, Frasco E, Takesue R, Tang K. Maternal education level and maternal healthcare utilization in the Democratic Republic of the Congo: an analysis of the multiple indicator cluster survey 2017/18. BMC Health Serv Res. 2021;21(1):850. doi: 10.1186/s12913-021-06854-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amwonya D, Kigosa N, Kizza J. Female education and maternal health care utilization: evidence from Uganda. Reprod Health. 2022;19(1):142. doi: 10.1186/s12978-022-01432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Belay AT, Fenta SM, Agegn SB, Muluneh MW, Ortega JA. Prevalence and risk factors associated with rural women’s protected against tetanus in East Africa: evidence from demographic and health surveys of ten East African countries. PLoS ONE. 2022;17(3):e0265906. doi: 10.1371/journal.pone.0265906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haile ZT, Chertok IR, Teweldeberhan AK. Determinants of utilization of sufficient tetanus toxoid immunization during pregnancy: evidence from the Kenya Demographic and Health Survey, 2008-2009. J Commun Health. 2013;38:492–9. [DOI] [PubMed] [Google Scholar]
- 37.Liyew AM, Ayalew HG. Individual and community-level determinants of poor tetanus toxoid immunization among pregnant women in Ethiopia using data from 2016 Ethiopian demographic and health survey; multilevel analysis. Arch Public Health. 2021;79(1):021–00622. doi: 10.1186/s13690-021-00622-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gashaw A, Hunie M, Amare E, Zewdie A, Abebe M, Demeke M, Kefelegn S, Yehualashet D, Alemu A, Adamu Y, et al. Proportion of births protected against neonatal tetanus and its associated factors among mothers who gave birth within the past 6 months in Gozamn district, Northwest Ethiopia, 2022. Hum Vaccin Immunother. 2023;14(2):2223066. doi: 10.1080/21645515.2023.2223066. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The managed raw data supporting the conclusions of this article will be made available upon reasonable request by the corresponding authors without undue reservation.


