Abstract
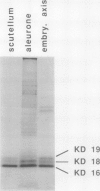
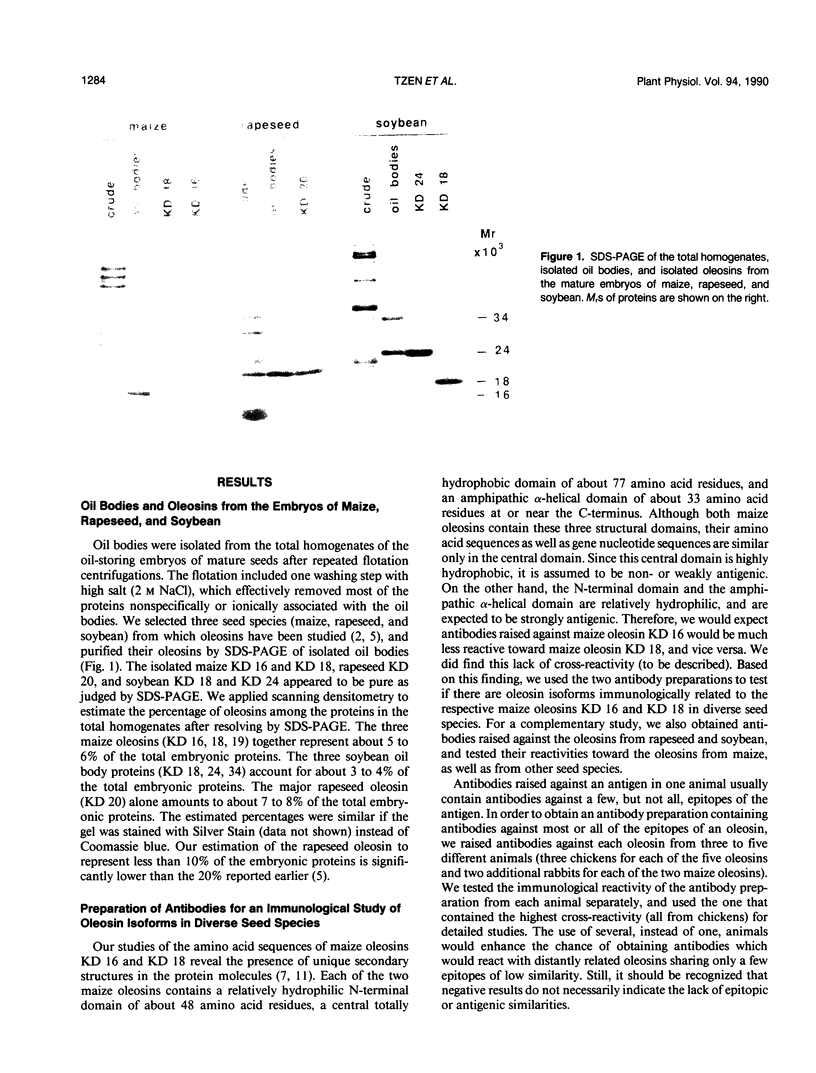
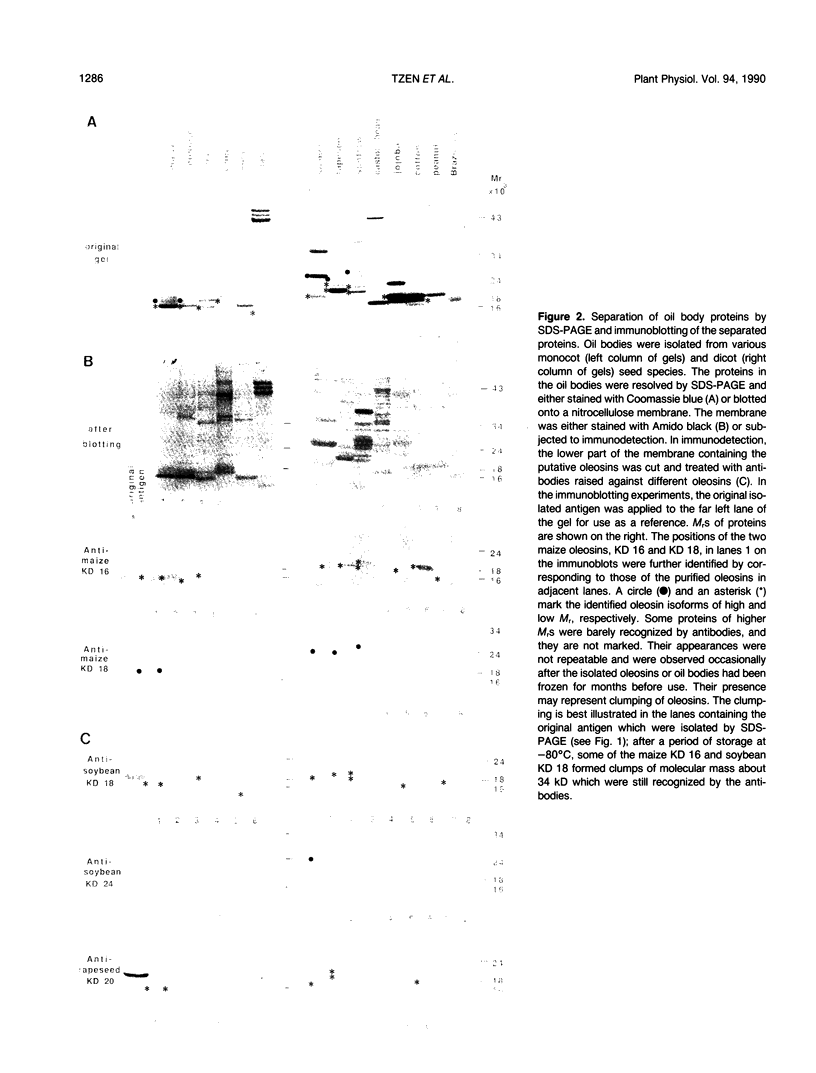
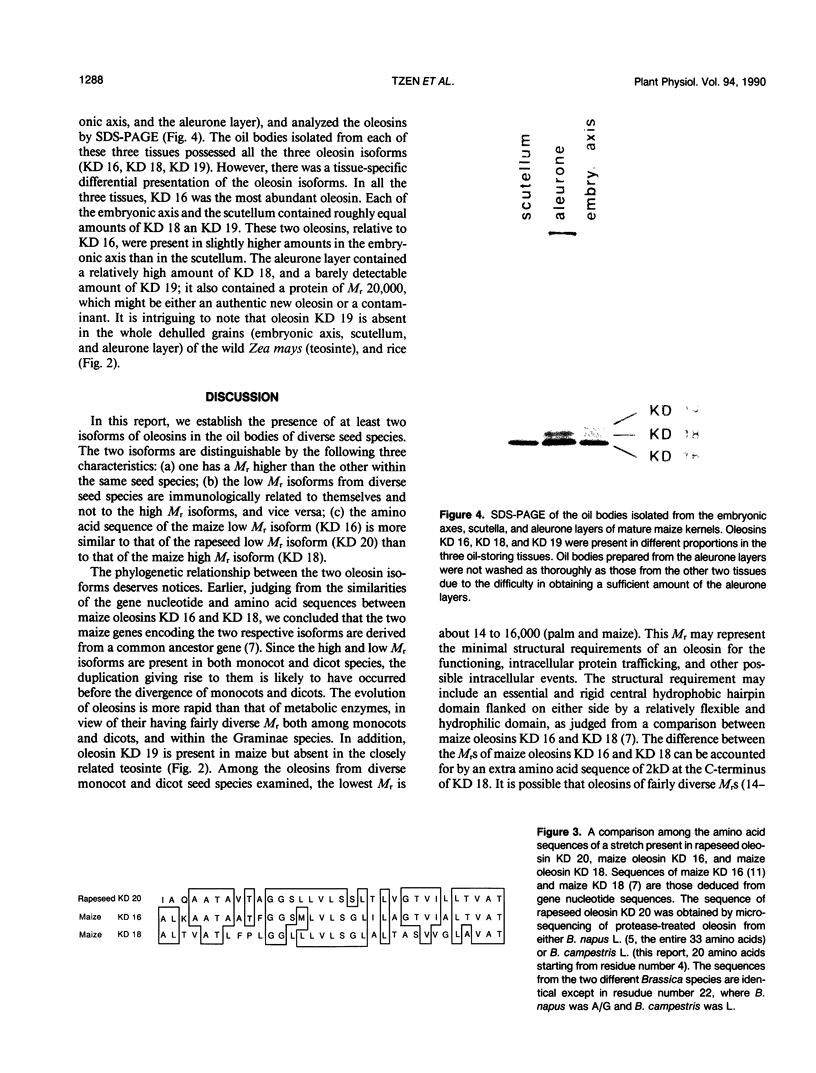
Oleosins are unique and major proteins localized on the surface of oil bodies in diverse seed species. We purified five different oleosins (maize [Zea mays L.] KD 16 and KD 18, soybean [Glycine max L.] KD 18 and KD 24, and rapeseed [Brassica campestris L.] KD 20), and raised chicken antibodies against them. These antibodies were used to test for immunological cross-reactivity among oleosins from diverse seed species. Within the same seed species, antibodies raised against one oleosin isoform did not cross-react with the other oleosin isoform (i.e. between maize oleosins KD 16 and KD 18, and between soybean oleosins KD 18 and KD 24). However, the respective antibodies were able to recognize oleosins from other seed species. Where interspecies cross-reactivity occurred, the results suggest that there are at least two immunologically distinct isoforms of oleosins present in diverse seed species, one of lower Mr, and another one of higher Mr. This suggestion is also supported by the relative similarities between the amino acid sequence of a small portion of rapeseed oleosin KD 20 and those of maize oleosins KD 16 and KD 18. In maize kernel, there was a tissue-specific differential presentation of the three oleosins, KD 16, KD 18, and KD 19, in the oil-storing scutellum, embryonic axis, and aleurone layer. The phylogenetic relationship between the high and low Mr isoforms within the same, and among diverse, seed species is discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Polson A., Coetzer T., Kruger J., von Maltzahn E., van der Merwe K. J. Improvements in the isolation of IgY from the yolks of eggs laid by immunized hens. Immunol Invest. 1985 Aug;14(4):323–327. doi: 10.3109/08820138509022667. [DOI] [PubMed] [Google Scholar]
- Qu R. D., Huang A. H. Oleosin KD 18 on the surface of oil bodies in maize. Genomic and cDNA sequences and the deduced protein structure. J Biol Chem. 1990 Feb 5;265(4):2238–2243. [PubMed] [Google Scholar]
- Qu R., Wang S. M., Lin Y. H., Vance V. B., Huang A. H. Characteristics and biosynthesis of membrane proteins of lipid bodies in the scutella of maize (Zea mays L.). Biochem J. 1986 Apr 1;235(1):57–65. doi: 10.1042/bj2350057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick D., Sartoris D. J. Diagnosing internal derangement of the knee with magnetic resonance imaging. West J Med. 1989 Jun;150(6):682–683. [PMC free article] [PubMed] [Google Scholar]
- Slack C. R., Bertaud W. S., Shaw B. D., Holland R., Browse J., Wright H. Some studies on the composition and surface properties of oil bodies from the seed cotyledons of safflower (Carthamus tinctorius) and linseed (Linum ustatissimum). Biochem J. 1980 Sep 15;190(3):551–561. doi: 10.1042/bj1900551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance V. B., Huang A. H. The major protein from lipid bodies of maize. Characterization and structure based on cDNA cloning. J Biol Chem. 1987 Aug 15;262(23):11275–11279. [PubMed] [Google Scholar]
- Yatsu L. Y., Jacks T. J. Spherosome membranes: half unit-membranes. Plant Physiol. 1972 Jun;49(6):937–943. doi: 10.1104/pp.49.6.937. [DOI] [PMC free article] [PubMed] [Google Scholar]