Abstract
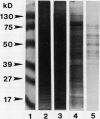
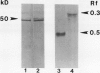
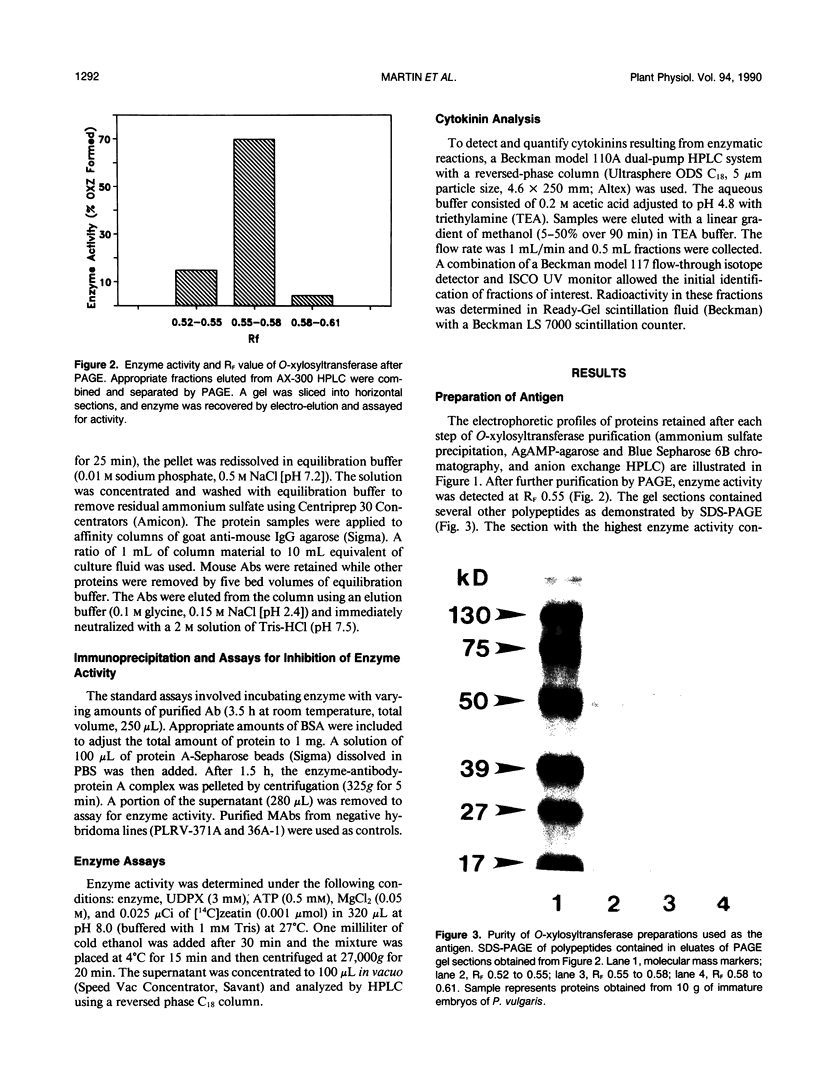
Zeatin O-xylosyltransferase and zeatin O-glucosyltransferase occur in immature embryos of Phaseolus vulgaris and P. lunatus, respectively. Purified preparations of the xylosyltransferase were used as antigen to elicit the formation of antibodies in mice. Hybridoma clones were produced by fusion of mouse spleen cells with myeloma cell line Fox-NY. A clone secreting monoclonal antibody (MAb), XZT-1, capable of immunoprecipitating both enzymes was obtained. The MAb detected a unique protein band from crude embryo extracts of each species with the correct molecular mass (50 kilodaltons) and relative charge (RF = 0.5 and 0.3) of the respective enzymes. Competition experiments with substrates indicated that the glycosyl dinucleotide binding sites of the enzymes are probably not involved in MAb-enzyme recognition. Western blotting of samples from vegetative tissues of P. vulgaris detected a low level of O-glucosyltransferase but not O-xylosyltransferase, in leaves. These findings suggest the occurrence of two genes in P. vulgaris coding for O-glycosylation enzymes with tissue-specific expression. The MAb will be used to screen expression libraries and to obtain pure enzymes for amino acid sequencing and for the production of additional MAbs.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dixon S. C., Martin R. C., Mok M. C., Shaw G., Mok D. W. Zeatin Glycosylation Enzymes in Phaseolus: Isolation of O-Glucosyltransferase from P. lunatus and Comparison to O-Xylosyltransferase from P. vulgaris. Plant Physiol. 1989 Aug;90(4):1316–1321. doi: 10.1104/pp.90.4.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Lee Y. H., Mok M. C., Mok D. W., Griffin D. A., Shaw G. Cytokinin metabolism in phaseolus embryos : genetic difference and the occurrence of novel zeatin metabolites. Plant Physiol. 1985 Mar;77(3):635–641. doi: 10.1104/pp.77.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. C., Mok M. C., Shaw G., Mok D. W. An enzyme mediating the conversion of zeatin to dihydrozeatin in phaseolus embryos. Plant Physiol. 1989 Aug;90(4):1630–1635. doi: 10.1104/pp.90.4.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok M. C., Mok D. W., Armstrong D. J. Differential cytokinin structure-activity relationships in phaseolus. Plant Physiol. 1978 Jan;61(1):72–75. doi: 10.1104/pp.61.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J. E., Mok D. W., Mok M. C., Shaw G. Isolation and partial purification of an enzyme catalyzing the formation of O-xylosylzeatin in Phaseolus vulgaris embryos. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3714–3717. doi: 10.1073/pnas.84.11.3714. [DOI] [PMC free article] [PubMed] [Google Scholar]