Abstract
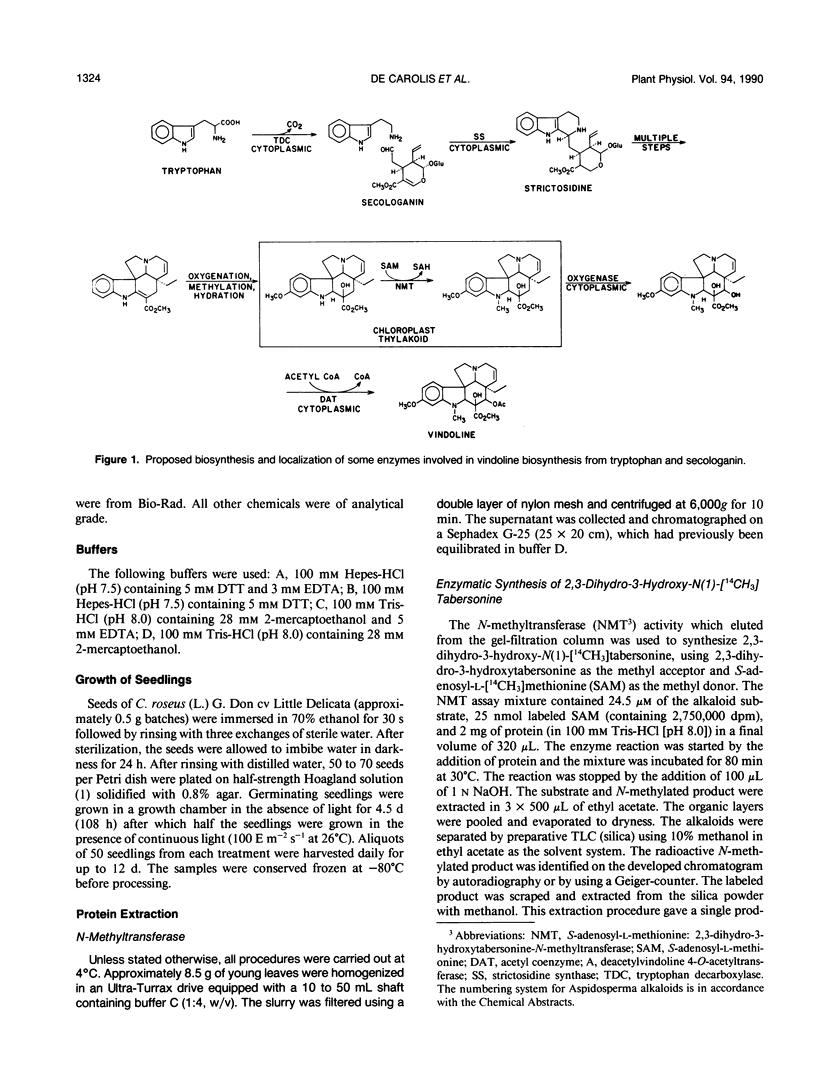
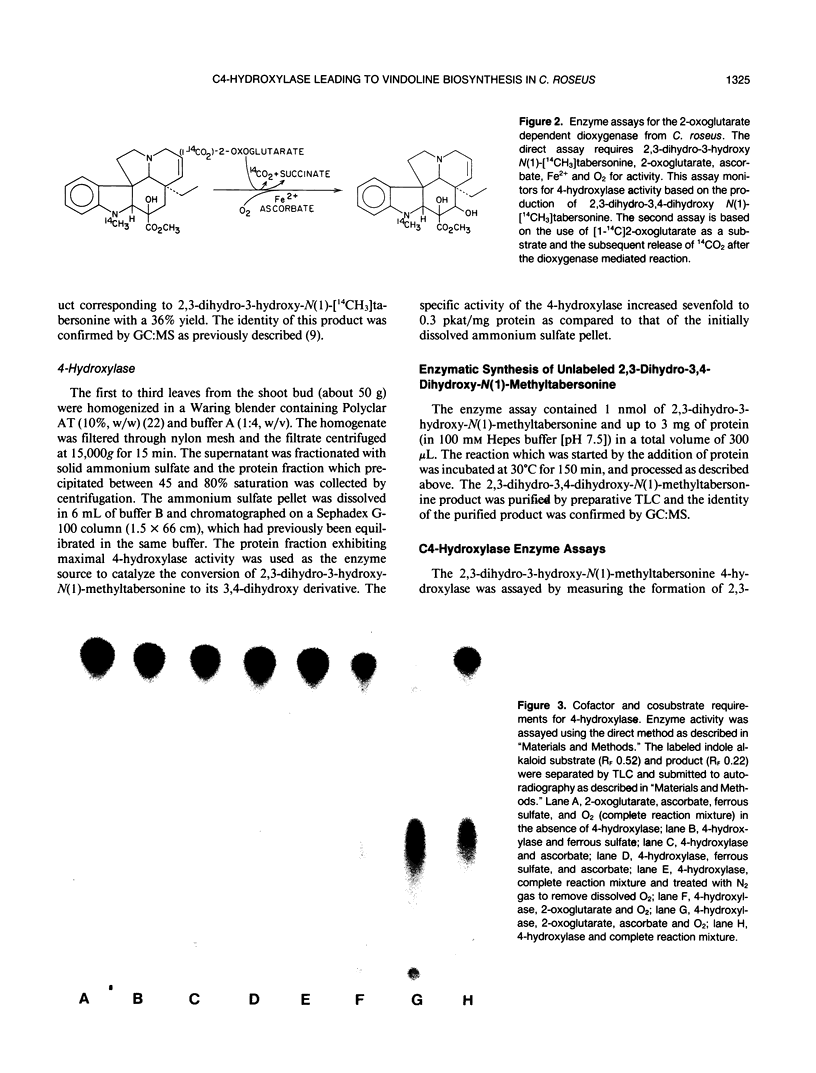
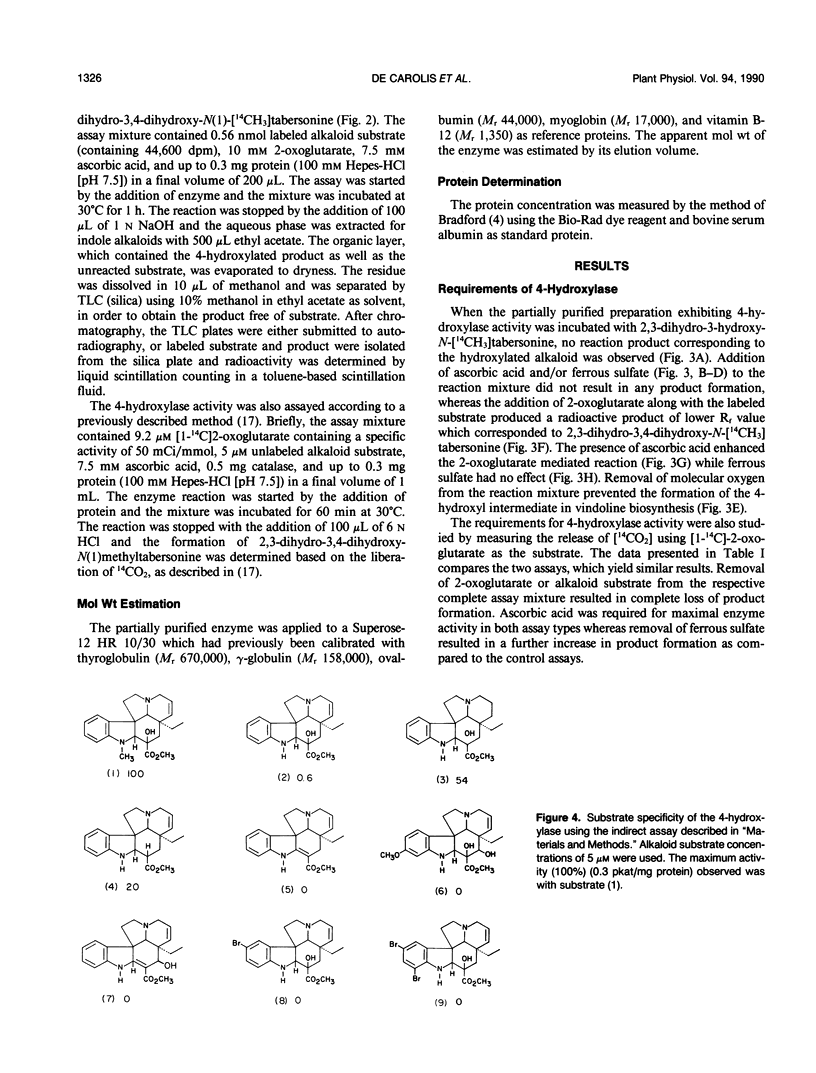
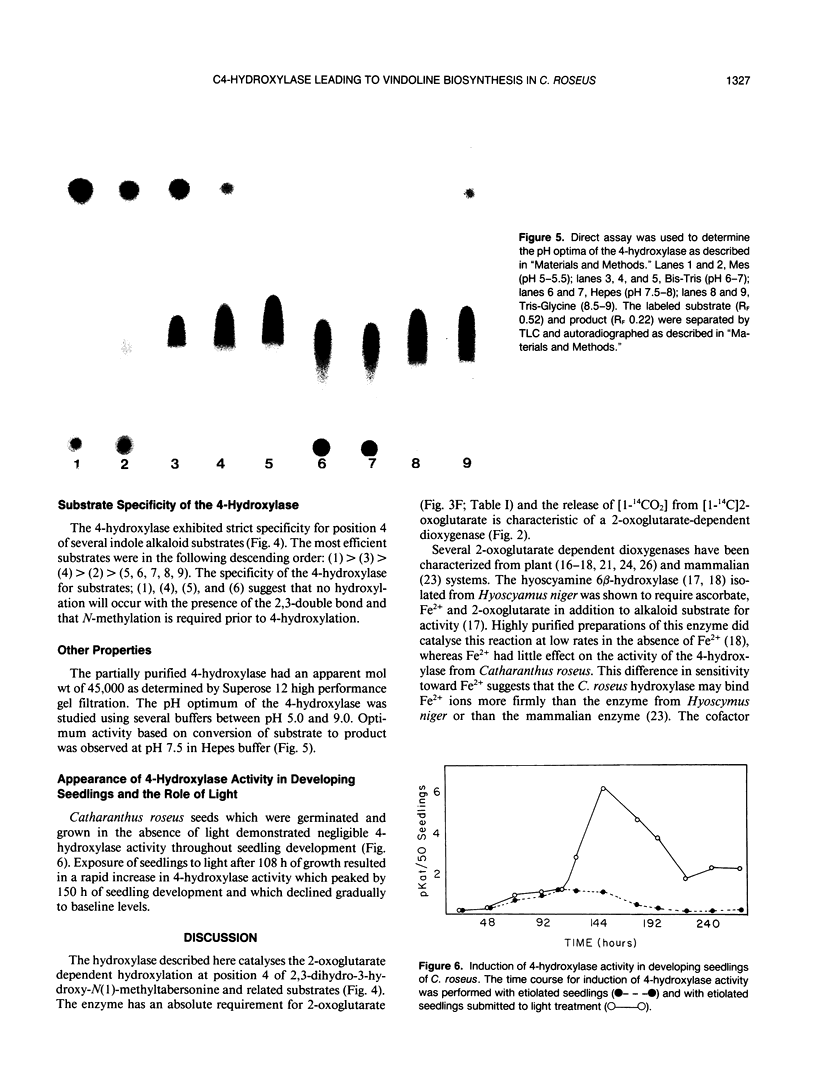
Young leaves from Catharanthus roseus plants contain the enzymes which convert the monoterpenoid indole alkaloid, tabersonine by three hydroxylations, two methylations, and one acetylation step to vindoline. A novel direct enzyme assay has been developed for a hydroxylase involved in vindoline biosynthesis, which catalyzes the C4-hydroxylation of 2,3-dihydro-3-hydroxy-N(1)-methyltabersonine to the 3,4-dihydroxy derivative. The enzyme showed an absolute requirement for 2-oxoglutarate and enzymatic activity was enhanced by ascorbate, establishing it as a 2-oxoglutarate-dependent dioxygenase (EC 1.14.11.-). The hydroxylase exhibited specificity for position 4 of various alkaloid substrates. The enzyme exhibited a pH optima between 7 and 8 and an apparent molecular weight of 45,000. The appearance of 4-hydroxylase activity was developmentally regulated and was shown to be inducible by light treatment of seedlings. Substrate specificity studies of this enzyme for indole alkaloid substrate suggested that hydroxylation at position 3 and N-methylation occur prior to hydroxylation at position 4. This is in agreement with previous studies which suggest that C4-hydroxylation is the second to last step in vindoline biosynthesis in Catharanthus roseus.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- De Luca V., Cutler A. J. Subcellular Localization of Enzymes Involved in Indole Alkaloid Biosynthesis in Catharanthus roseus. Plant Physiol. 1987 Dec;85(4):1099–1102. doi: 10.1104/pp.85.4.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca V., Fernandez J. A., Campbell D., Kurz W. G. Developmental Regulation of Enzymes of Indole Alkaloid Biosynthesis in Catharanthus roseus. Plant Physiol. 1988 Feb;86(2):447–450. doi: 10.1104/pp.86.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deus-Neumann B., Stöckigt J., Zenk M. H. Radioimmunoassay for the quantitative determination of catharanthine. Planta Med. 1987 Apr;53(2):184–188. doi: 10.1055/s-2006-962668. [DOI] [PubMed] [Google Scholar]
- Hashimoto T., Yamada Y. Hyoscyamine 6beta-hydroxylase, a 2-oxoglutarate-dependent dioxygenase, in alkaloid-producing root cultures. Plant Physiol. 1986 Jun;81(2):619–625. doi: 10.1104/pp.81.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T., Yamada Y. Purification and characterization of hyoscyamine 6 beta-hydroxylase from root cultures of Hyoscyamus niger L. Hydroxylase and epoxidase activities in the enzyme preparation. Eur J Biochem. 1987 Apr 15;164(2):277–285. doi: 10.1111/j.1432-1033.1987.tb11055.x. [DOI] [PubMed] [Google Scholar]
- Sadava D., Chrispeels M. J. Hydroxyproline biosynthesis in plant cells. Peptidyl proline hydroxylase from carrot disks. Biochim Biophys Acta. 1971 Feb 10;227(2):278–287. doi: 10.1016/0005-2744(71)90060-x. [DOI] [PubMed] [Google Scholar]