Abstract
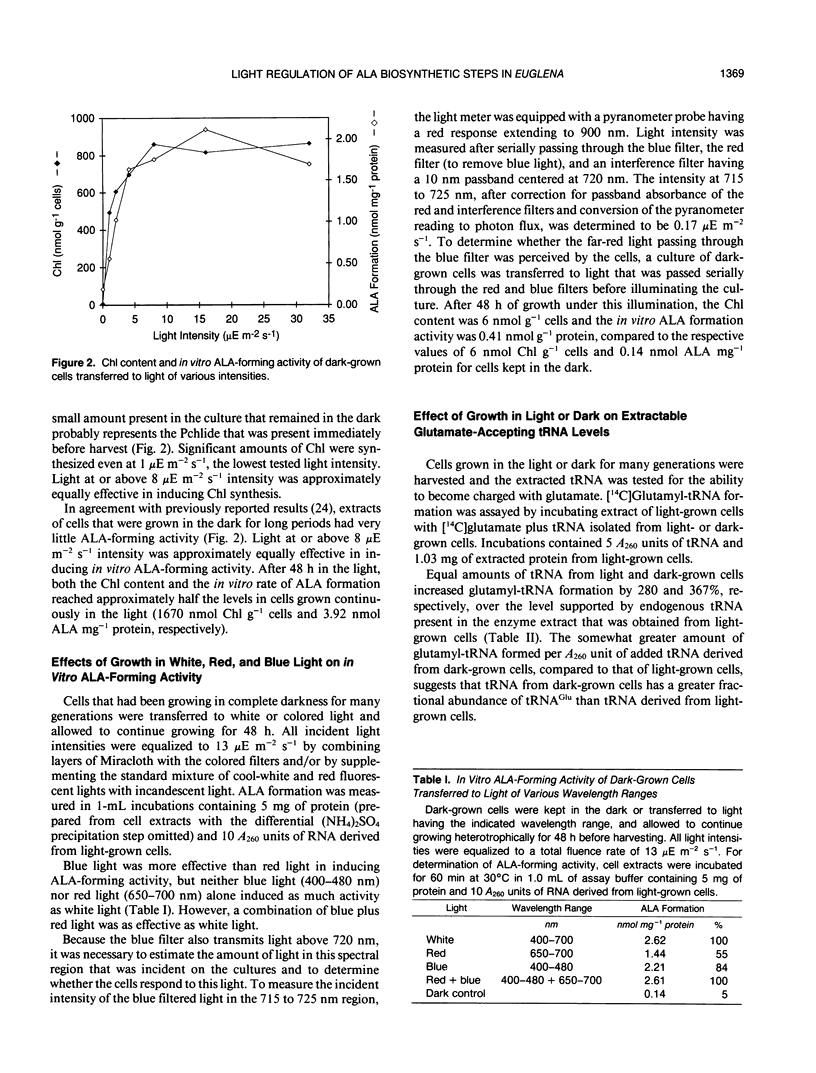
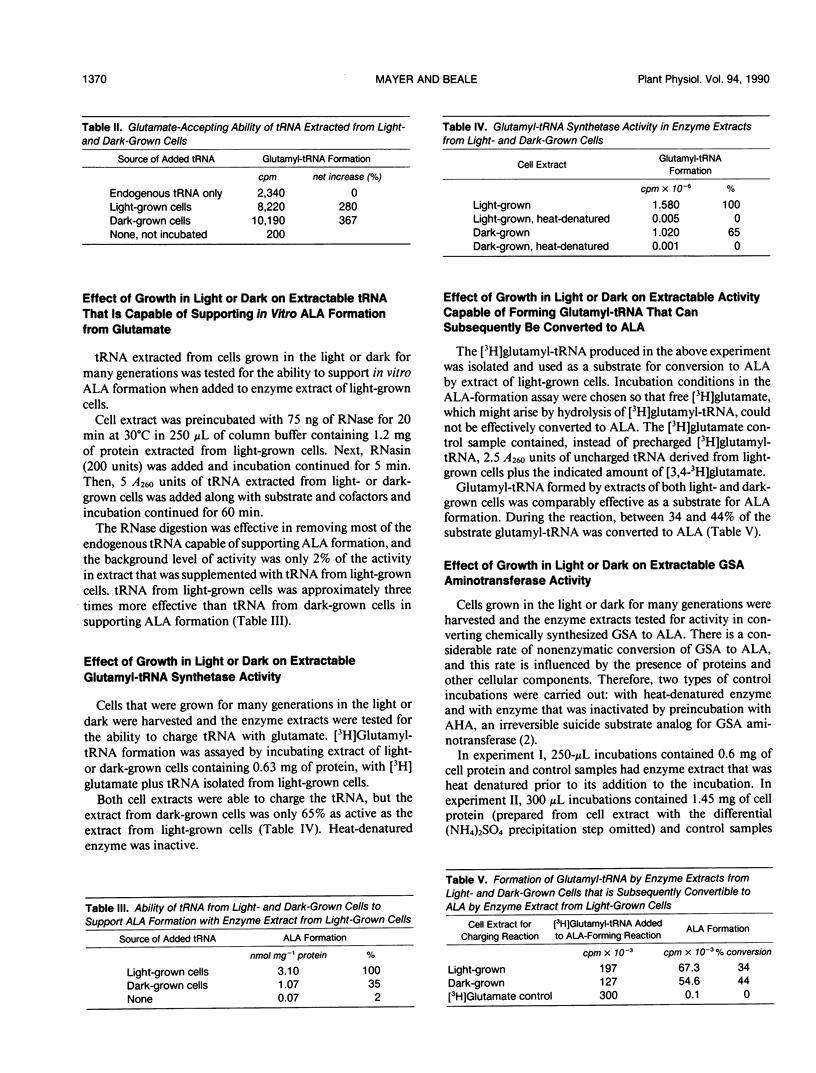
Chlorophyll synthesis in Euglena, as in higher plants, occurs only in the light. The key chlorophyll precursor, δ-aminolevulinic acid (ALA), is formed in Euglena, as in plants, from glutamate in a reaction sequence catalyzed by three enzymes and requiring tRNAGlu. ALA formation from glutamate occurs in extracts of light-grown Euglena cells, but activity is very low in dark-grown cell extracts. Cells grown in either red (650-700 nanometers) or blue (400-480 nanometers) light yielded in vitro activity, but neither red nor blue light alone induced activity as high as that induced by white light or red and blue light together, at equal total fluence rates. Levels of the individual enzymes and the required tRNA were measured in cell extracts of light- and dark-grown cells. tRNA capable of being charged with glutamate was approximately equally abundant in extracts of light- and dark-grown cells. tRNA capable of supporting ALA synthesis was approximately three times more abundant in extracts of light-grown cells than in dark-grown cell extracts. Total glutamyl-tRNA synthetase activity was nearly twice as high in extracts of light-grown cells as in dark-grown cell extracts. However, extracts of both light- and dark-grown cells were able to charge tRNAGlu isolated from light-grown cells to form glutamyl-tRNA that could function as substrate for ALA synthesis. Glutamyl-tRNA reductase, which catalyzes pyridine nucleotide-dependent reduction of glutamyl-tRNA to glutamate-1-semialdehyde (GSA), was approximately fourfold greater in extracts of light-grown cells than in dark-grown cell extracts. GSA aminotransferase activity was detectable only in extracts of light-grown cells. These results indicate that both the tRNA and enzymes required for ALA synthesis from glutamate are regulated by light in Euglena. The results further suggest that ALA formation from glutamate in dark-grown Euglena cells may be limited by the absence of GSA aminotransferase activity.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avissar Y. J., Beale S. I. Biosynthesis of Tetrapyrrole Pigment Precursors : Formation and Utilization of Glutamyl-tRNA for delta-Aminolevulinic Acid Synthesis by Isolated Enzyme Fractions from Chlorella Vulgaris. Plant Physiol. 1988 Nov;88(3):879–886. doi: 10.1104/pp.88.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett W. E., Pennington C. J., Jr, Fairfield S. A. Induction of euglena transfer RNA's by light. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1261–1268. doi: 10.1073/pnas.63.4.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beale S. I., Castelfranco P. A. The Biosynthesis of delta-Aminolevulinic Acid in Higher Plants: I. Accumulation of delta-Aminolevulinic Acid in Greening Plant Tissues. Plant Physiol. 1974 Feb;53(2):291–296. doi: 10.1104/pp.53.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beale S. I., Foley T., Dzelzkalns V. delta-Aminolevulinic acid synthase from Euglena gracilis. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1666–1669. doi: 10.1073/pnas.78.3.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Elich T. D., Lagarias J. C. 4-amino-5-hexynoic Acid-a potent inhibitor of tetrapyrrole biosynthesis in plants. Plant Physiol. 1988 Nov;88(3):747–751. doi: 10.1104/pp.88.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett W. F., Dunn M. F. Exopolysaccharides Produced by Phytopathogenic Pseudomonas syringae Pathovars in Infected Leaves of Susceptible Hosts. Plant Physiol. 1989 Jan;89(1):5–9. doi: 10.1104/pp.89.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett W. F., Dunn M. F. Exopolysaccharides Produced by Phytopathogenic Pseudomonas syringae Pathovars in Infected Leaves of Susceptible Hosts. Plant Physiol. 1989 Jan;89(1):5–9. doi: 10.1104/pp.89.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley T., Dzelzkalns V., Beale S. I. delta-Aminolevulinic Acid Synthase of Euglena gracilis: Regulation of Activity. Plant Physiol. 1982 Jul;70(1):219–226. doi: 10.1104/pp.70.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricke U. Tritosol: a new scintillation cocktail based on Triton X-100. Anal Biochem. 1975 Feb;63(2):555–558. doi: 10.1016/0003-2697(75)90379-6. [DOI] [PubMed] [Google Scholar]
- Harel E., Ne'eman E. Alternative Routes for the Synthesis of 5-Aminolevulinic Acid in Maize Leaves : II. Formation from Glutamate. Plant Physiol. 1983 Aug;72(4):1062–1067. doi: 10.1104/pp.72.4.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holowinsky A. W., Schiff J. A. Events surrounding the early development of Euglena chloroplasts. I. Induction by preillumination. Plant Physiol. 1970 Mar;45(3):339–347. doi: 10.1104/pp.45.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk J. T., Allen R. L. Dependence of chloroplast pigment synthesis on protein synthesis: effect of actidione. Biochem Biophys Res Commun. 1965 Dec 21;21(6):523–530. doi: 10.1016/0006-291x(65)90516-4. [DOI] [PubMed] [Google Scholar]
- Mayer S. M., Beale S. I., Weinstein J. D. Enzymatic conversion of glutamate to delta-aminolevulinic acid in soluble extracts of Euglena gracilis. J Biol Chem. 1987 Sep 15;262(26):12541–12549. [PubMed] [Google Scholar]
- Nadler K., Granick S. Controls on chlorophyll synthesis in barley. Plant Physiol. 1970 Aug;46(2):240–246. doi: 10.1104/pp.46.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthier B., Krauspe R., Samtleben S. Light-stimulated synthesis of aminoacyl-tRNA synthetases in greening Euglena gracilis. Biochim Biophys Acta. 1972 Aug 25;277(2):335–341. doi: 10.1016/0005-2787(72)90415-7. [DOI] [PubMed] [Google Scholar]
- Richard F., Nigon V. La syntheèse de l'acide delta-aminolévulinique et de la chlorophylle lors de l'éclairement d'Euglena gracilis étiolées. Biochim Biophys Acta. 1973 Jun 20;313(1):130–149. [PubMed] [Google Scholar]
- Schneegurt M. A., Beale S. I. Characterization of the RNA Required for Biosynthesis of delta-Aminolevulinic Acid from Glutamate : Purification by Anticodon-Based Affinity Chromatography and Determination That the UUC Glutamate Anticodon Is a General Requirement for Function in ALA Biosynthesis. Plant Physiol. 1988 Feb;86(2):497–504. doi: 10.1104/pp.86.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schön A., Kannangara C. G., Gough S., Söll D. Protein biosynthesis in organelles requires misaminoacylation of tRNA. Nature. 1988 Jan 14;331(6152):187–190. doi: 10.1038/331187a0. [DOI] [PubMed] [Google Scholar]
- Stern A. I., Schiff J. A., Epstein H. T. Studies of Chloroplast Development in Euglena. V. Pigment Biosynthesis, Photosynthetic Oxygen Evolution and Carbon Dioxide Fixation during Chloroplast Development. Plant Physiol. 1964 Mar;39(2):220–226. doi: 10.1104/pp.39.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein J. D., Beale S. I. Enzymatic conversion of glutamate to delta-aminolevulinate in soluble extracts of the unicellular green alga, Chlorella vulgaris. Arch Biochem Biophys. 1985 Mar;237(2):454–464. doi: 10.1016/0003-9861(85)90299-1. [DOI] [PubMed] [Google Scholar]
- Weinstein J. D., Beale S. I. Separate physiological roles and subcellular compartments for two tetrapyrrole biosynthetic pathways in Euglena gracilis. J Biol Chem. 1983 Jun 10;258(11):6799–6807. [PubMed] [Google Scholar]
- Weinstein J. D., Mayer S. M., Beale S. I. Formation of delta-Aminolevulinic Acid from Glutamic Acid in Algal Extracts : Separation into an RNA and Three Required Enzyme Components by Serial Affinity Chromatography. Plant Physiol. 1987 Jun;84(2):244–250. doi: 10.1104/pp.84.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein J. D., Mayer S. M., Beale S. I. Stimulation of delta-Aminolevulinic Acid Formation in Algal Extracts by Heterologous RNA. Plant Physiol. 1986 Dec;82(4):1096–1101. doi: 10.1104/pp.82.4.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]


