Abstract
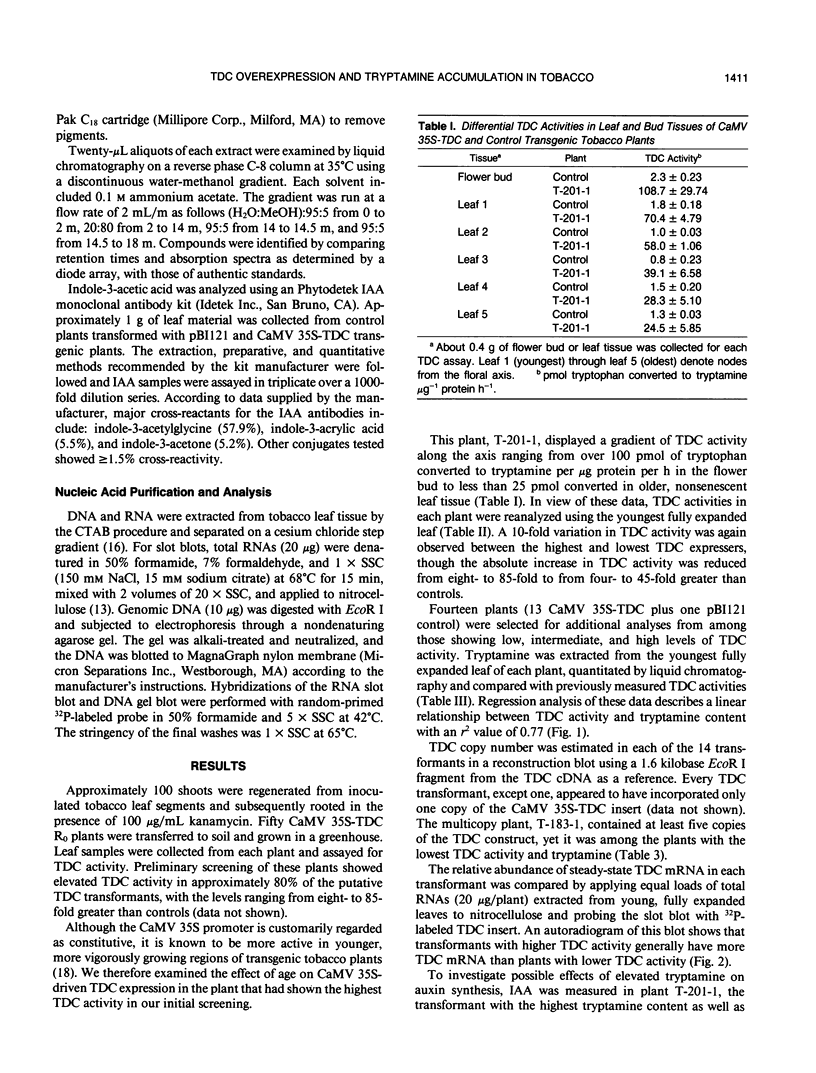
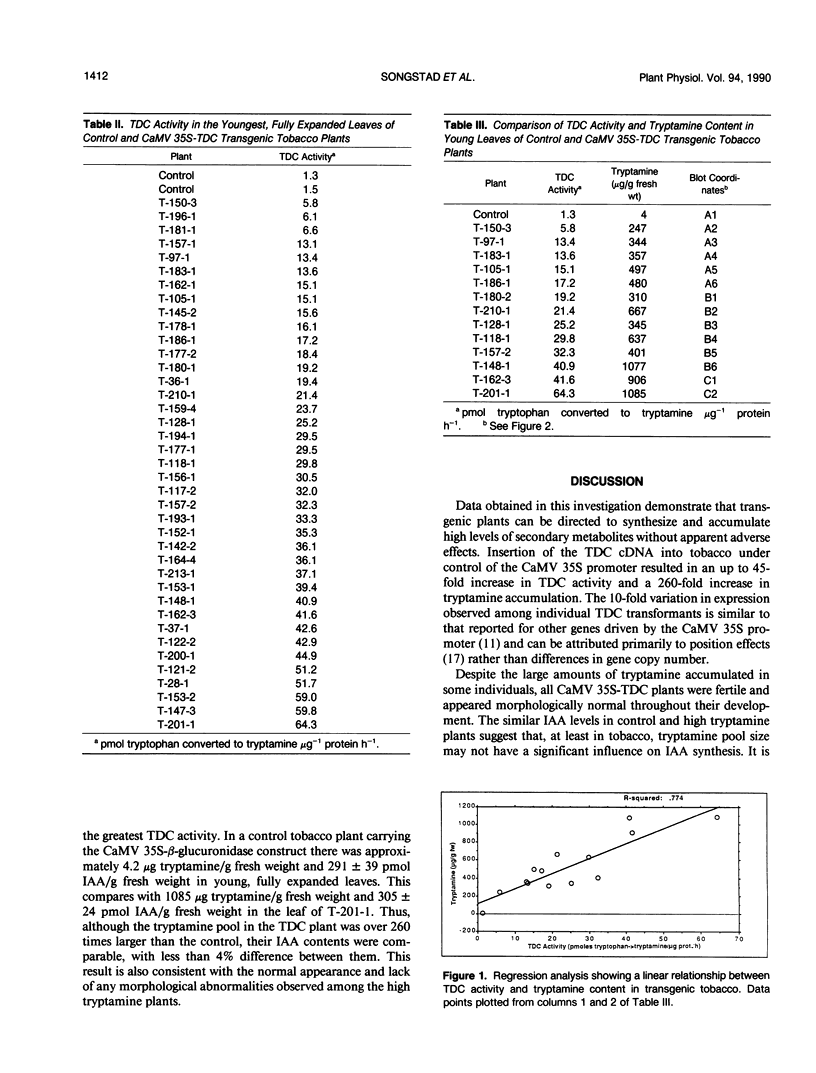
A full-length complementary DNA clone encoding tryptophan decarboxylase (TDC; EC 4.1.1.28) from Catharanthus roseus (De Luca V, Marineau C, Brisson N [1989] Proc Natl Acad Sci USA 86: 2582-2586) driven by the CaMV 35S promoter was introduced into tobacco (Nicotiana tabacum) to direct the synthesis of the protoalkaloid tryptamine from endogenous tryptophan. Young, fully expanded leaves of CaMV 35S-TDC transformed plants had from four to 45 times greater TDC activity than did controls. Tryptamine accumulated in transgenic plants to levels that were directly proportional to their TDC specific activity. Despite their increased tryptamine content, the growth and development of the CaMV 35S-TDC plants appeared normal with no significant differences in indole-3-acetic acid levels between high tryptamine and control plants. Plants with the highest TDC activity contained more than 1 milligram of tryptamine per gram fresh weight, a 260-fold increase over controls.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bevan M. Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res. 1984 Nov 26;12(22):8711–8721. doi: 10.1093/nar/12.22.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- De Luca V., Fernandez J. A., Campbell D., Kurz W. G. Developmental Regulation of Enzymes of Indole Alkaloid Biosynthesis in Catharanthus roseus. Plant Physiol. 1988 Feb;86(2):447–450. doi: 10.1104/pp.86.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca V., Marineau C., Brisson N. Molecular cloning and analysis of cDNA encoding a plant tryptophan decarboxylase: comparison with animal dopa decarboxylases. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2582–2586. doi: 10.1073/pnas.86.8.2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein E., Cohen J. D., Bandurski R. S. Concentration and Metabolic Turnover of Indoles in Germinating Kernels of Zea mays L. Plant Physiol. 1980 Mar;65(3):415–421. doi: 10.1104/pp.65.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson R. A., Kavanagh T. A., Bevan M. W. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987 Dec 20;6(13):3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutchan T. M. Expression of enzymatically active cloned strictosidine synthase from the higher plant Rauvolfia serpentina in Escherichia coli. FEBS Lett. 1989 Oct 23;257(1):127–130. doi: 10.1016/0014-5793(89)81802-2. [DOI] [PubMed] [Google Scholar]
- Kutchan T. M., Hampp N., Lottspeich F., Beyreuther K., Zenk M. H. The cDNA clone for strictosidine synthase from Rauvolfia serpentina. DNA sequence determination and expression in Escherichia coli. FEBS Lett. 1988 Sep 12;237(1-2):40–44. doi: 10.1016/0014-5793(88)80167-4. [DOI] [PubMed] [Google Scholar]
- Lagrimini L. M., Bradford S., Rothstein S. Peroxidase-Induced Wilting in Transgenic Tobacco Plants. Plant Cell. 1990 Jan;2(1):7–18. doi: 10.1105/tpc.2.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weising K., Schell J., Kahl G. Foreign genes in plants: transfer, structure, expression, and applications. Annu Rev Genet. 1988;22:421–477. doi: 10.1146/annurev.ge.22.120188.002225. [DOI] [PubMed] [Google Scholar]
- Williamson J. D., Hirsch-Wyncott M. E., Larkins B. A., Gelvin S. B. Differential Accumulation of a Transcript Driven by the CaMV 35S Promoter in Transgenic Tobacco. Plant Physiol. 1989 Aug;90(4):1570–1576. doi: 10.1104/pp.90.4.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]



