Abstract
Importance:
Multicenter clinical trials play a critical role in the translational processes that enable new treatments to reach all people and improve public health. However, conducting multicenter randomized controlled trials (mRCT) presents challenges. The Trial Innovation Network (TIN), established in 2016 to partner with the Clinical and Translational Science Award (CTSA) Consortium of academic medical institutions in the implementation of mRCTs, consists of three Trial Innovation Centers (TICs) and one Recruitment Innovation Center (RIC). This unique partnership has aimed to address critical roadblocks that impede the design and conduct of mRCTs, in expectation of accelerating the translation of novel interventions to clinical practice. The TIN’s challenges and achievements are described in this paper, along with examples of innovative resources and processes that may serve as useful models for other clinical trial networks providing operational and recruitment support.
Observations:
The TIN has successfully integrated over 60 CTSA institution program Hubs into a functional network for mRCT implementation and optimization. A unique support system for investigators has been created that includes the development and deployment of novel tools, operational and recruitment services, consultation models, and rapid communication pathways designed to reduce delays in trial start-up, enhance recruitment, improve engagement of diverse research participants and communities, and streamline processes that improve the quality, efficiency, and conduct of mRCTs. These resources and processes span the clinical trial spectrum and enable the TICs and RIC to serve as coordinating centers, data centers, and recruitment specialists to assist trials across the NIH and other agencies. The TIN’s impact has been demonstrated through its response to both historical operational challenges and emerging public health emergencies, including the national opioid public health crisis and the COVID-19 pandemic.
Conclusions and Relevance:
The TIN has worked to reduce barriers to implementing mRCTs and to improve mRCT processes and operations by providing needed clinical trial infrastructure and resources to CTSA investigators. These resources have been instrumental in more quickly and efficiently translating research discoveries into beneficial patient treatments.
Introduction
The National Center for Advancing Translational Sciences (NCATS) established the Trial Innovation Network (TIN) in 2016 to transform the Clinical and Translational Science Award (CTSA) network’s ability to implement multicenter trials.1 Randomized controlled trials are widely recognized as the gold standard of evidence-based clinical care,2 with multicenter trials promising more rapid recruitment, larger sample sizes and greater generalizability.3 However, planning, launching, and conducting a multicenter randomized controlled trial (mRCT) presents myriad challenges to researchers, including study design; site selection and start-up; contracting and single IRB approvals; recruitment and retention of a diverse, generalizable participant population; and data management and reporting.4–7
NCATS funded three Trial Innovation Centers (TICs) and one Recruitment Innovation Center (RIC) to organize and support trial opportunities across the CTSA Consortium (over 60 institutions and their affiliates).8 Collectively, the TIN has created innovative tools, operational and recruitment services, trial-focused consultation models, and rapid communication pathways designed to reduce delays in trial start-up, achieve meaningful engagement of research participants from diverse communities, and harmonize processes to improve the quality and efficiency of conduct for mRCTs. By promoting partnerships among stakeholders, including CTSA researchers, industry, clinicians, and trial participants, the TIN has provided process improvements and infrastructure to the CTSA consortium to more quickly and efficiently translate research discoveries into beneficial patient treatments.
The TIN launched a basic framework in 2016 that connected the TIC and RIC institutions and laid the groundwork for expansion. By 2017, individual CTSA institutions had joined the TICs and RIC to form a virtual network where local investigators could submit clinical trial proposals and every CTSA institution could receive opportunities to participate as a site.8 TIN trial consultations were active within six months of TIC and RIC funding and by the end of the first year, the TIN had successfully launched a national scale single Institutional Review Board (sIRB) system, standard agreements, quality-by-design approaches to data management and monitoring, and novel recruitment and retention methods, including participant-centric community engagement studios.9 Over its first six years, the TIN has developed a system of support for investigators that includes design and implementation consultations, operational tools, and multicenter-based processes to accelerate and support trial implementation. The TIN has leveraged the expertise, diversity, and wide reach of the CTSA institutions and their CTSA Hub-appointed TIN liaisons, to inform and support clinical trial process innovation, operational excellence, and collaboration.1,8
As the network matured, the TIN created resources to aid investigators across the spectrum of clinical trial activities, and developed processes that enabled the TICs and RIC to serve as coordinating centers, data centers, and recruitment specialists to assist trials across the NIH and other agencies. This infrastructure has empowered the TIN to respond rapidly to the COVID-19 and national opioid public health emergencies.10–12 The TIN has also formed unique partnerships to support investigator-initiated trials at CTSA institutions nationwide, informing site selection, enhancing study design, streamlining trial initiation, and implementing decentralized approaches to trials. The TIN’s first six years as a network are described in this paper, along with examples of innovative resources and support processes it has developed and disseminated.
Foundational Resources and Process Development
The TICs13 are charged with facilitating CTSA Hub clinical trial implementation through the development and dissemination of innovative methods to improve the quality and efficiency of mRCTs across a broad range of disease states. The RIC’s mission is “to improve participant recruitment into clinical trials by using innovative means to assess the availability of potential participants and to enroll them in a timely manner.”14,15 In standing up the network, the TICs and RIC worked collaboratively to create resources with potential to reduce trial start-up delays, enhance recruitment, harmonize trial processes across CTSA Hubs, establish template agreements and foster collaborations between CTSA investigators and NIH Institutes, Centers, and Offices. CTSA Hub Liaison Teams (HLTs), composed of experts from each CTSA organization and dedicated to working with the TIN, were critical for launch and continuous optimization of the network.16 Additional key areas of focus are outlined below:
TIN Website and Webinars:
The TIN website was established to inform potential trialists, funders, HLTs, and other stakeholders about TIN services, share best practices, and serve as a portal for studies requesting and receiving support from the TIN. The public website is continuously updated and includes dynamic reporting of high-level TIN metrics. The website advertises and archives regular training event webinars on a wide variety of topics relevant to clinical trial recruitment and operations.
Trial Intake, Consultation Process, and Operational Dashboards:
Investigators register requests for TIN engagement via an online submission form. The subsequent consultation process includes a series of meetings between relevant experts coordinated by the TICs, RIC, and the investigative team to understand the overall goals of the trial and offer guidance in a written report customized to optimize trial design and conduct. The TIN then makes a collaborative determination on whether the consult concludes with the recommendations report, continues with local Hub resources, receives TIN resources (e.g., recruitment material development, sIRB support, recruitment and retention planning, master contracting, study design labs), or proceeds to a comprehensive consultation where the TIN may serve as a data or clinical coordinating center. Resources developed and offered by the TICs and RIC were initially focused on supporting clinical trial challenges identified in the original Request for Funding Announcement,17 and have evolved over time in response to the emerging landscape (Figure 1).
Figure 1.
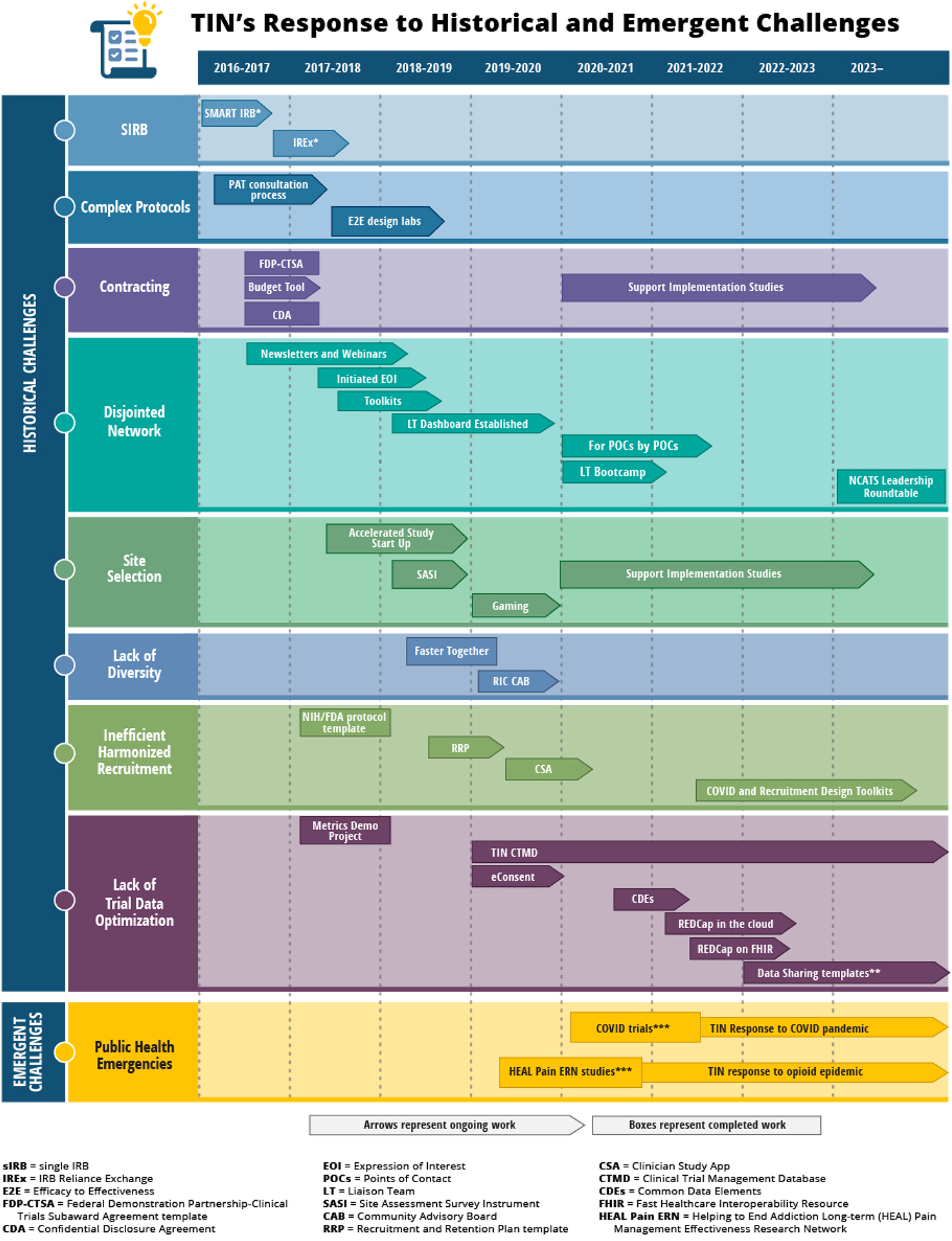
TIN’s response to historical operational challenges and emerging landscape
CTSA Hub Expression of Interest:
The opportunity to serve as a site for a specific clinical trial is communicated to CTSA HLTs through the TIN’s Expression of Interest (EOI) process. The EOI is a streamlined, centrally managed process that enables rapid dissemination of study opportunities across the network. This resource allows the CTSA program to function as a network while using locally established processes to review and respond to TIN study opportunities. To date, 59 EOI requests have been shared with CTSA Hubs and their affiliates. Of these, 30 were disseminated to support grant submissions prior to funding and 29 were shared after funding was awarded, during the study’s planning period, or to add additional study sites. CTSA Hub response rate to invitations was 64% with average response time of 18 days.
Single SIRB (sIRB):
In response to the 2016 mandate for federally funded mRCTs to use sIRBs,18 the TIN implemented the Streamlined, Multisite, Accelerated Resources for Trials (SMART) IRB master reliance agreement through network consultations and demonstration projects.19 To date, the TIN sIRBs have supported over 80 multicenter studies via more than 1,000 reliance arrangements. Additionally, the IRB Reliance Exchange (IREx) was created to provide a unified, transparent process of transitioning to the SMART IRB agreement across institutions and to document reliance and local considerations in a web-based sIRB platform.20
Contracting/Standard Agreements:
In August 2016, representatives from the CTSA Hubs and Federal Demonstration Partnership (FDP) Member Institutions approved a subaward standard agreement template created by a CTSA-supported working group to reduce the need for tedious contracting negotiations. During the first year of the TIN, two major agreements were developed to streamline master contracting efforts across the TIN.8 The first agreement, a Network FDP-CTSA Standard Agreement template, is used with federally funded, fixed price, domestic clinical trials; over 60 CTSA institutions and their affiliates have registered to use this agreement. The FDP-CTSA was used for six TIN-supported studies and resulted in median contract negotiations of 41–46 days, compared to 100-day historical averages.21 The second agreement, the TIN Network Umbrella Confidential Disclosure Agreement (CDA), allows sharing of study and protocol information across TIN sites. To date, over 60 CTSA sites and 21 affiliates have executed the Network Umbrella CDA. Additionally, a VA-specific Network Umbrella CDA was developed for CTSA affiliate VA sites.
Recruitment and Retention Planning:
To improve clinical trial recruitment planning and satisfy NIH grant submission recruitment planning requirements, the RIC developed a comprehensive Recruitment and Retention Plan (RRP) template. Following a literature review of best practices, and consultations with recruitment specialists, the template was finalized and posted to the publicly accessible TIN Toolbox in January 2019, garnering over 3,500 views and usage in over 82 studies22 to date.
TIN Areas of Impact
Researcher-Focused Consultations and Trial Support Services:
As of April 2023, the TIN has received 401 proposals requesting support. Trial focal areas have been diverse, with 76 therapeutic disease areas across 20 NIH Institutes and Centers (ICs). CTSA Hub participation was strong with submitting PIs representing 62 CTSA organizations (94% of total submissions). Faculty status of submitting PIs were: Professor (N=224; 55.9%), Associate Professor (N=102; 25.6%), Assistant Professor (N=65; 16.2%), Instructor (N=9, 2.3%), and Post-Doctoral Fellows (N=1, 0.3%).
Applicants seeking guidance and resources for existing multicenter trials receive an initial consultation and may work collaboratively with TIN coordinators to determine support services that best meet the needs of the trial. Applicants planning new multicenter trials are eligible for a comprehensive consultation which includes experts from a TIC and the RIC to guide proposal development prior to submitting projects to funders. TIC consultative and operational services include assistance with protocol development, study operations enhancement, trial budgeting guidance, regulatory agreements, data coordination and management, and data safety and monitoring. RIC consultative and operational services include community engagement studios,23 recruitment planning and feasibility assessment, recruitment materials, and selecting EHR-based recruitment strategies.24 Joint TIC/RIC responsibilities include information gathering and synthesis related to identification and selection of trial sites. Since 2019, 65 initial consultations completed before application submission have resulted in 37 successfully funded grant applications. RIC services available to ongoing trials have supported an additional 62 trials. Figures 2–3 provide additional TIN consultation metrics.
Figure 2.
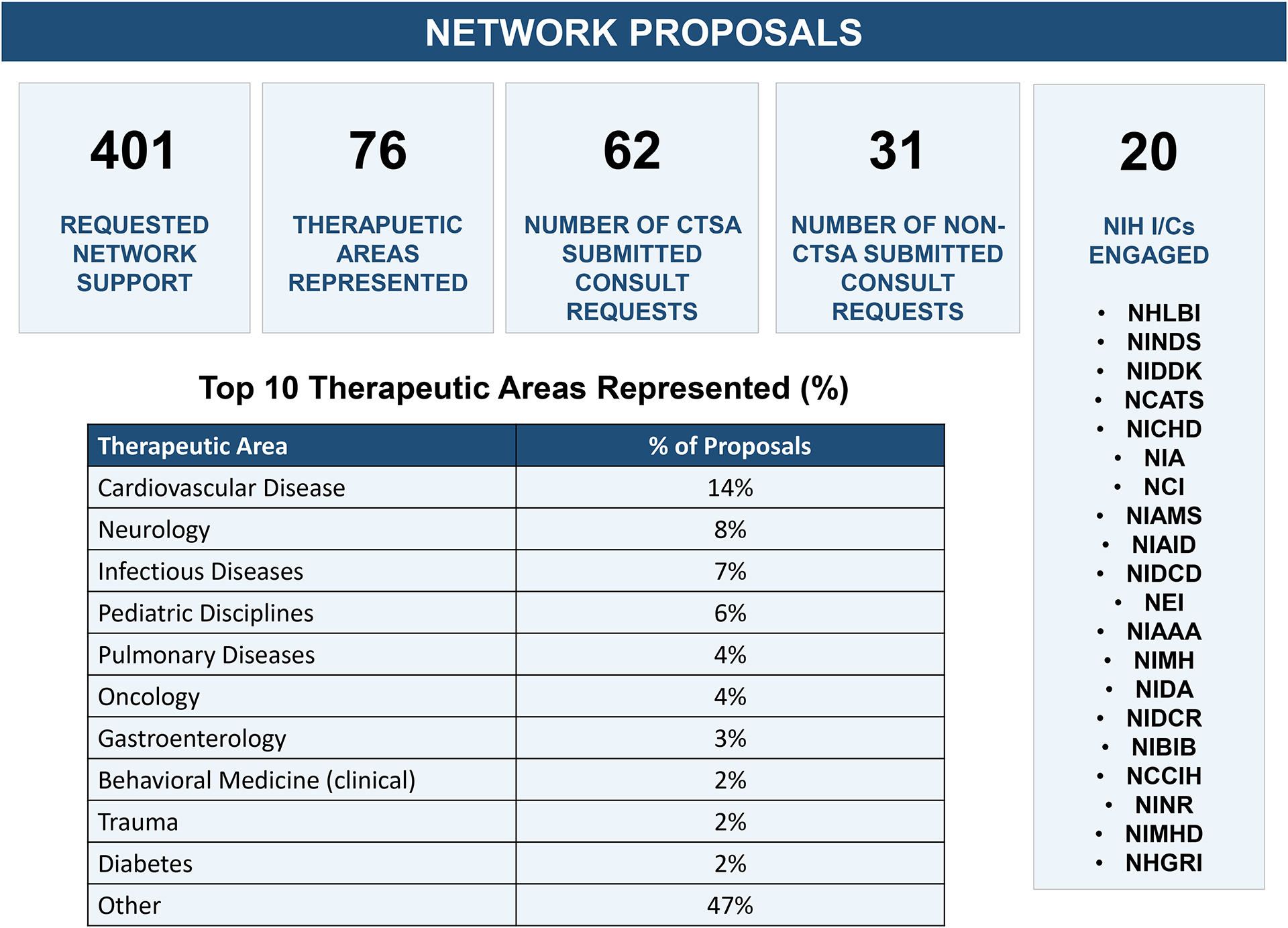
Summary of TIN’s consultations and network proposals
Figure 3.
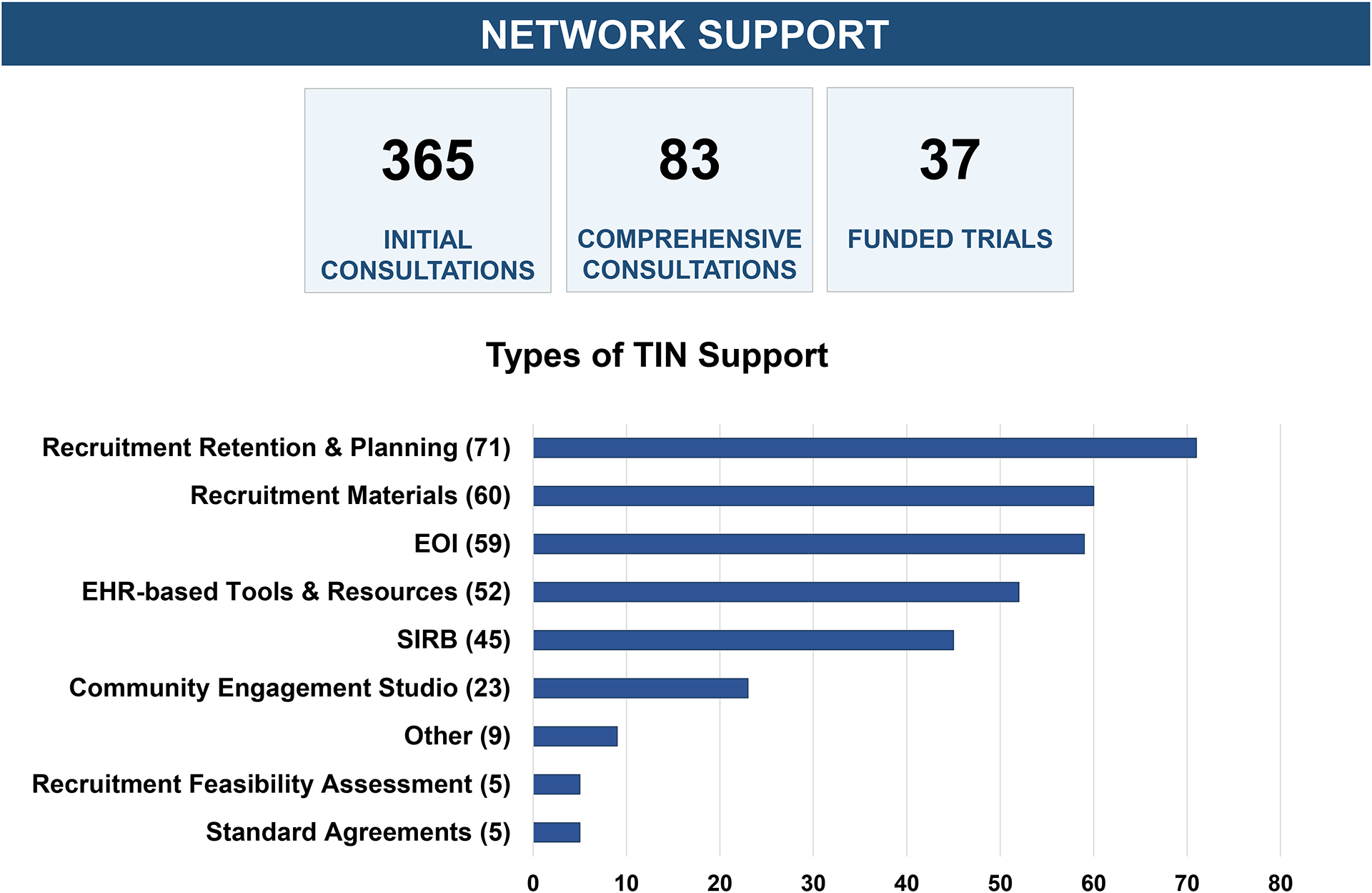
Summary of TIN’s consultations and provided support
Design, Deployment, and Dissemination of Research Infrastructure:
During a TIN consultation, the TICs and RIC present recommendations to research teams that often include trial-specific tools previously developed and disseminated by the TIN. Consultations occasionally result in ideation for new innovations, which are first piloted at the individual trial level, and if generalizable, may be considered for further evaluation, iterative development, and eventual broad dissemination as new TIN assets. In some cases, these assets are informational and disseminated via the online TIN Toolbox (e.g., Gamification Guide,25 Recruitment and Retention Planning Template22). In other cases, innovations are embedded as new features in existing technical platforms for wider dissemination (e.g., IREx for single IRB,20 ResearchMatch for recruitment and/or expert advice,26,27 FasterTogether Massive Open Online Course28 for guidance on engaging underrepresented communities in clinical research, and REDCap for Data Collection,29 eConsent,30 EHR-to-EDC data sourcing31). The TICs and RIC have established an innovations catalog to ensure new resources are proactively considered, aligned across the network, and evaluated for impact when possible (see examples in Table 1).
Table 1.
Innovations successfully implemented in studies
| Innovation | Description | Implementation and Impact |
|---|---|---|
| Study Design | ||
| Design Labs | A multi-stakeholder gathering that takes a systems approach to designing and catalyzing important advancements. |
|
| IRB including Informed Consent | ||
| IRB Reliance Exchange (IREx)19 | A web-based portal supporting single IRB documentation and coordination. |
|
| Consent Builder | A tool that uses technology systems (e.g., REDCap, Latex) to create and update multiple consent forms for trial sites simultaneously. |
|
| REDCap eConsent (electronic consent)28 | Offers study teams the opportunity to create centralized, personalized consent documents which can be sent to potential research participants electronically. |
|
| Site Selection and Start-up | ||
| Site Assessment Survey Instrument (SASI) | A two-part survey assessing the suitability and readiness of centers leading to the selection of teams with the best potential to achieve the aims and recruitment targets of a trial with good clinical practice and precision. |
|
| Accelerated Site Start Up (ASU) | A reorganization of the site start-up process into monthly, contingent, time-sensitive objectives to accelerate the time spent in start-up and to site activation within 90 days. |
|
| GEMS (Global Electronic Management System) | Electronic clinical trial workflow manager that allows coordinating centers and trial PIs to interactively track trial start-up productivity, timelines, and milestone metrics |
|
| CTSA FDP Standard Agreements | CTSA Hubs and TIC contracting experts review and approve standardized language for key elements of a clinical trial subaward agreement. |
|
| sIRB Two-Part Consent Documentation | A consent format designed when local IRBs rely on a single sIRB. The front part, or master consent, contains common language required across multiple sites and a second part contains site-specific consent information (SSCI). |
|
| Streamlined Local Context Review | A study-specific template and workflow algorithm to assist multicenter sites document relying sIRB requirements for local HRRP approvals and to verify that local IRB responsibilities are in place, as outlined in IRB reliance agreements. |
|
| Enrollment | ||
| ResearchMatch 24,25 | A disease-neutral, web-based recruitment registry to help match individuals seeking clinical trial participation with research studies actively recruiting volunteers (Harris, 2012). |
|
| Faster Together Massive Open Online Course 26 | This course aims to teach people how to enhance the recruitment of racial and ethnic minorities in clinical trials. |
|
| Gaming and Incentives 23 | Incorporating gaming innovations into clinical trial operations for networks to enhance site engagement, using competition and team building to establish trust and rapport and ultimately improve site team and trial performance. |
|
| Clinician Study App (CSA) | Customizable study apps that serve as an electronic replacement to the traditional Study Information Card. |
|
| Study Conduct | ||
| MyCap | A participant-facing mobile application for survey data collection and automated administration of mobile-sensing tasks. |
|
| REDCap on FHIR 29 | A REDCap data EMR aggregator that supports automated transfer of data from EMR to trial data base. |
|
TIN Toolbox Dissemination:
The TIN Toolbox is publicly available on the TIN website and includes access to innovative tools and informational resources from across the network (see eTable 1). The Toolbox also serves as an engagement vehicle as CTSA Hubs are invited to contribute local innovations for awareness and dissemination. Collectively, Toolbox resources have received over 10,000 views to date.
TIN Training Events:
The TIN webinar series, free and open to the public, features presentations from experts in the field of clinical research. The webinars cover a broad range of topics, including patient recruitment, data sharing, regulatory compliance, and more. To date, the TIN has hosted 100 collaboration webinars with presenters from 122 research organizations, including 40 from the TICs and the RIC. Collectively, these training events have served over 5,900 individuals from 394 organizations.
TIN Publications:
The TIN also disseminates its methods, models, tools, case studies, and commentaries on emerging trends in clinical research though peer-reviewed journal publications. These articles enable the TIN to share research findings and network accomplishments with a broad audience, and to promote the adoption of best practices in clinical research. The TIN has published approximately 90 peer-reviewed papers covering the entire clinical trial lifecycle (see eFigure 1 and eTable 2).
Advising and Thought Leadership:
The TIN supports some investigators through the design, grant application, and implementation of a multicenter trial. Such support can include study design and protocol development, statistical analysis planning, insights into operationalizing a multicenter trial, and advice on creating a suitable budget. For funded UG3-UH3 programs, the TIN provides expert advice and coaching on the roles of the clinical coordinating center, the data coordinating center, DSMBs, regulatory requirements, sIRB, site agreements, recruitment strategies, and medical monitors. After six years of providing consultations, the TIN recently published a summary guide in the TIN Toolbox describing ten recommendations for investigators desiring to progress from single-center to multicenter trials.32
Responding to Public Health Emergencies:
The value of the TIN’s leadership and collaborative structure has also been demonstrated through its rapid response to both the national opioid public health crisis and the COVID-19 pandemic. As part of the NIH Helping to End Addiction Long-term® (HEAL) Initiative to speed scientific solutions to address the national opioid crisis, NCATS leveraged the TIN to implement clinical trials to evaluate methods of enhancing pain management in ways that might reduce opioid use. The TICs and RIC serve as a consolidated unit supporting five NIH HEAL Pain Management Effectiveness Research Network (ERN) trials using a multi-institutional platform to operationalize project management, recruitment planning, clinical coordination, data coordination, and safety/statistical support. This collaboration provided experienced, tested methods to investigators and sites across the HEAL ERN program.
The TIN also rapidly pivoted to respond to the COVID-19 pandemic. Between April and July 2020, the TIN received a growing number of consultation requests for assistance with trials testing COVID-19 therapeutics, and accelerated the rate of TIN EOI requests distributed to CTSA Hubs to accommodate the increased number of trial submissions. To date, the TIN has accepted 36 COVID proposals, resulting in 11 COVID-19 EOIs. The TIN has served as a coordinating center on 9 COVID-19 trials, with three convalescent plasma trials launched throughout the U.S. via the TIN and the CTSA Program Hubs and their affiliates. These TIN-coordinated trials were ready to enroll on average 35–50 days after site receipt of protocol. The TIN supported two additional COVID trials within the NIH Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) program, a public-private partnership to develop a coordinated research strategy for prioritizing and speeding development of promising treatments and vaccines.33 The TIN identified recruiting sites for ACTIV-1, a global inpatient trial, with the first patient enrolled within three weeks of awarding the site coordinating center contract, and enrollment was completed in 14 months.
The TIN provided rapid assessment and support for a university-sponsored, statewide hydroxychloroquine trial early in the pandemic. The study’s first enrollment occurred within three weeks of submission to the TIN portal, and as the REDCap eConsent module was adopted, enrollment continued despite COVID-19 shutdowns. The study enrolled 368 participants and answered a key question about the use of hydroxychloroquine when, based on preliminary evidence, speculation was rampant about the drug’s efficacy against COVID.
The TIN supported the ABC Science Collaborative that used community data to help a partnership of school and community leaders make informed decisions about safety during the pandemic.34 As a result, public policy changes were incorporated into North Carolina state legislation regarding school closures and re-opening. The Test To Stay program was estimated to have resulted in 90% fewer missed school days.35
Other activities addressing the COVID-19 pandemic include a coordinated response to the Federal Register Request for Information: Clinical Research Infrastructure and Emergency Clinical Trials where the TIN provided advice regarding critical randomized trial resources needed for a national emergency response program. Additionally, a RIC COVID-19 Recruitment and Retention Toolkit was developed to provide investigators with strategies and resources on recruitment and retention planning for COVID research.36
mRCT Challenges and TIN Responses
The TIN is a unique inter-institutional initiative providing resources to improve mRCTs across the CTSA consortium. As a new program, several challenges were encountered:
TIN Awareness:
To communicate to CTSA Hub PIs and NIH ICs that the TIN was fully operational and ready to assist trialists, the TIN employed several communication strategies, including webinars, informational meetings with HLTs, communication with CTSA PIs, internal NIH networking, and participation in scientific conferences.
Institutional Inclusiveness and CTSA Hub Autonomy:
CTSA Hubs are diverse in their local capacity, research platforms, and expertise. The TIN created a successful distributed process for EHR site-based cohort estimation based on sharing phenotype algorithms that could be run on any data warehousing platform.37 While the large number of CTSA Hubs and their nation-wide distribution has been a strength for the TIN, only a finite set of sites are interested in or needed for each trial. Because the PI maintains final site selection authority, not all interested sites will be selected to participate in every trial.
Defining Reasonable Outcomes for Research Teams:
Outcomes from investigator applications for TIN support varied across studies due to diverse investigator needs as well as TIN scope and priorities. A significant number of TIN initial consultations concluded with a TIN recommendation not to move forward to a comprehensive consultation and application for external funding, including requests from inexperienced investigators with impracticable goals. Other investigators preferred only an initial consultation or discrete resources, rather than more in-depth support the TIN can provide through comprehensive consultations.
Researcher Willingness to Evaluate Operational Innovations:
Identifying feasible studies with investigative teams and funders that are willing to embed and evaluate TIN tools and processes within their mRCT poses a continuing challenge.
Long Cycle Times:
Another critical mRCT challenge is reducing planning and start-up time while increasing time devoted to recruitment. On average, TIN-supported multicenter trials took 3.5 years from initial consultation to receipt of NIH funding, with most trials lasting five years. To offset such extended planning timelines, standardized mRCT activation methods have reduced site-related study start-up time to on average 90 days from receipt of approved study protocol and consent to activation to enrollment.
Defining Meaningful Measures of Operational Efficiency:
Measuring and evaluating operational efficiency requires standardization and assessment across multiple trials. We have identified useful measures of efficiency in trial operation processes and have utilized these in 18 trials. Collection of these measures requires persistence and long-term commitment from the innovation centers as well as study teams. Disseminating the value of these measures will be important for the future of the TIN.
‘Rescue’ Recruitment and Retention:
Enrolling and retaining a sufficient, representative participant population for clinical trials is especially challenging when trialists come to the TIN for help with ongoing trials already enrolling and in ‘rescue’ mode. In many cases, inadequate time or remaining budget forestalls opportunities to institute processes for effecting meaningful change.
Recruitment of Historically Underrepresented Populations:
Enhancing participation in clinical trials among historically underrepresented populations is a TIN challenge and commitment. Based on numerous TIN consultations, the RIC has developed recommendations to engage diverse communities in clinical trials, including proactively assessing recruitment and retention barriers, minimizing burden while returning value to participants, prioritizing data-driven site selection, and establishing multi-stage stakeholder engagement with minoritized communities.38
Conclusions
The TIN has integrated over 60 CTSA Program Hubs into an efficient and reliable network capable of initiating and completing mRCTs. The TIN’s response to the opiate and COVID-19 pandemics demonstrate the network’s ability to create trial operational efficiencies under emergency conditions.
Conducting efficient, evidence-informed clinical trials requires a trained and robust translational science workforce. The TIN has assembled such a workforce via its CTSA partners, TIN executive committee, innovation centers, and working groups closely aligned with the HLTs across the CTSA consortium. The TIN’s stakeholder engagement and consultative process, aided by the diversity within the CTSA network, stimulates development of study-specific solutions to support research teams representing a wide array of disease areas and study methodologies. The TIN’s focus on innovations that support many trials, rather than single investigations, has improved the larger clinical trial ecosystem. The TIN has provided solutions to several major mRCT challenges and succeeded in reducing trial barriers particularly related to site selection, study start up, and recruitment.
Many challenges remain, including reducing burdens and barriers for inexperienced sites and non-academic medical center sites, fully engaging participants in study design processes, ensuring equitable inclusion criteria and outcome measures, shortening the timespan from trial inception to trial completion and improving data management, harmonization, and sharing. Addressing these needs will encourage future TIN innovations, enabling the network to conduct more efficient trials and speed the translation of results into clinical practice.
Supplementary Material
Acknowledgements
The authors wish to acknowledge the contributions of the following TIN team members who support the day-to-day operations of the TIN, including network consultations, development and dissemination of innovations, and data tracking and evaluation: from the Duke/Vanderbilt TIC: Rachel Greenberg MD, Kanecia Zimmerman MD, Brian Smith MD, Frank Rockhold PhD, Lori Poole BA, Jesse Hickerson MBA, Eilene Pham BA, Eve Marion MA, Helen Boyle MPH, Vincent Miller MMCi, Sonya Sutton MA, Michelle Jones MEd, Princess Abbott-Grimes MPH; from the Johns Hopkins University/Tufts University TIC: Nichol McBee MPH, Andrew Mould MPH, Lindsay Eyzaguirre MS, Megan Singleton JD, Janelle Maddox-Regis MS, Shannon Hillery BS, Angeline Nanni MBA, Meghan Hildreth MS, Cecilia Pessoa-Gingerich BS, Emily Bartlett BA, Theodora Cohen PhD, Cortney Wieber MS, Krista Vermillion MS; from the Utah TIC: Erin Rothwell PhD, Kevin Watt MD PhD, Kathy Sward PhD, John M. VanBuren PhD, Mary Pautler MPH, Marie Kay BA, Jordan Bridges BS, Krista Ellis MS, Frances Sebahar BS, Eun Hea Unsicker MPH, Valeriya Vasenina MS, Talmage Morris MS, Michelle Aponte BS, Ann Johnson PhD MPA, Annie Risenmay MPA, Lisa Rigtrup BS, Gary Henderson MPA, Ammon Leon Pate BS, Monse Lopez BS; from the Vanderbilt RIC: Leslie Boone MPH, Loretta Byrne MS, Tara Helmer MPH, Maeve Tischbein PhD, Leah Dunkel MPH, Michelle Jones MEd, Stephanie Mayers MEd, Kaysi Quarles BS, Jasmine Bell MPH, Bridget Swindell RN, Jabari Ichimura BS, Devan Ray BA, Caitlin Rantala BA, Jessica Eidenmuller BA, Emily Serdoz MPA, Natalie Dilts MPH, David Crenshaw MSW, Brooklyn Henderson RN, Meghan Vance BBA, Delicia Burts, MPH, Dionne Grant RN, Jahi Hamilton BS, Matt Schorr BA, Colleen Lawrence PhD, Meredith Bernui MBA, Taylor Budine MS, Amna Baig MPH, Joseph Christodoulou MPH, Jodie Cohen MA, Tiffany Chen MPH, Julia Dunagan MBA Wendy Lloyd BA.
The authors also express gratitude and thanks to the RIC Community Advisory Board (CAB) who represent a broad spectrum of diverse communities for offering their perspective and guidance related to TIN initiatives, consultations, service lines, and toolbox offerings, and to Mrs. E. Hill De Loney MA and Mr. Ed Napia PhD, the community collaborators of the Patients, Providers and Public Working Group who provided thought leadership and contributed to the development of guiding principles and recommendations to operationalize community engagement in clinical trials.
Authors acknowledge the partnership and engagement of the Hub Liaison Teams from across the CTSA Program Hubs, particularly the Liaison Team Medical Directors, and the Points of Contacts (POCs). We also acknowledge contributions from specific contributing CTSA Institutions and past and present representative members of the Liaison Team Working Group: Albert Einstein College of Medicine, Ilija Atanasovski BS, Zoe Tsagaris MS; Children’s National Hospital, Jurran Wilson BA, Rachel Walega MS; Mayo Clinic, Laura Meiners MBA; Medical College of Wisconsin, Amit Gode MPH; Medical University of South Carolina, Amanda Cameron MPH, Signe Denmark MS, Kristen Clason MEd; New York University, Patricia Corby DDS; Northwestern University, Keith Herzog MPPA; Oregon Health & Science University, Kitt Swartz MPH; The Scripps Research Institute, Shelly Meese MHA, Emily Spencer PhD; Stanford University, Maya Berdichesky DMD; University of Arkansas Medical Sciences, Amy Jo Jenkins MS, David Avery BS, Monica Smith BA; University of California-San Francisco, Carmela Lomonaco PhD, Nimit Ruparel MPP; University of Colorado-Denver, Ben Echalier MBA, Aaron Mobley PhD, Maya Koch; University of Michigan-Ann Arbor, Santinio Jones MBA; University of New Mexico Health Sciences Center, George Garcia BS, Susan Tigert BA BS; University of North Carolina-Chapel Hill, Laura Viera MA; University of Rochester, Yonjong Choi MS; University of Texas Health Sciences-San Antonio; Patricia Dahia MD, Shweta Bansal MD; Yale University, Helen Seow PhD.
Lastly, the authors want to collectively acknowledge and thank NCATS program staff, PI Advisors, CTSA PIs, and investigative teams that have collaborated with the TIN.
Funding:
This work is supported by the National Institutes of Health Center for Advancing Translational Sciences (NCATS) and the National Institute on Aging (NIA), in support of the Trial Innovation Network under grant numbers U24TR001579 (Vanderbilt University), U24TR001597 (University of Utah), U24TR001608 (Duke/Vanderbilt Universities), and U24TR001609 (Johns Hopkins/Tufts Universities).
NCATS participated in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Research from the Johns Hopkins-Tufts Trial Innovation Center was partially supported by National Institutes of Health, National Institute on Aging (NIA), grant U24 TR001609. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health’s National Institute on Aging. NIA did not participate in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Conflict of Interest Disclosures:
Drs Harris, Benjamin, Bernard, Dean, Dwyer, Ford, Selker, Wilkins, Huvane, Palm, Kimberly, Reilly, and Hanley; Mss Cook, Burr, Edwards, Kennedy, Lane, Nelson, Stroud, Thompson, and Busacca; and Mr Majkowski received support from grants from the National Center for Advancing Translational Sciences. Drs Ford, Selker, Palm, and Hanley; Ms Lane; and Mr Majkowski received support from grants from the National Institute on Aging. Dr Benjamin reported personal fees from Allergan, Melinta Therapeutics, and Syneos Health outside the submitted work. Dr Bernard reported grants from the National Institutes of Health Trial Innovation Center during the conduct of the study. Dr Dean reported grants from the National Institutes of Health during the conduct of the study. Dr Dwyer reported grants from Cincor and AstraZeneca; personal fees from Acuta, Akebia, Applied Therapeutics, Aurinia, Ardelyx, AstraZeneca, Bayer, Boehringer Ingelheim, Lilly, BioRasi, BioVie, Botanix, Caladrius, Cincor, ES Therapeutics, Fibrogen, Genentech, GSK, Inversago, Icon, Ionis, Keros, Medpace, MicRx, Novo Nordisk, ProKidney, PSI CRO, Rarestone, Reata, RenalytixAI, Sanofi, Tricida, ValenzaBio, and Worldwide Clinical Trials outside the submitted work; holds stock or options in Venostent, PathEx, Acelyrin, BioRasi, and Innovative Renal Care; and serves as President of the Collaborative Study Group and a Trustee of The Bolles School. Dr Wilkins reported grants from National Institutes of Health during the conduct of the study. Dr Huvane reported grants from the National Institutes of Health during the conduct of the study. Dr Elkind reported nonfinancial support from BMS-Pfizer and grants from Roche outside the submitted work. Dr Reilly reported personal fees from Novartis outside the submitted work. No other disclosures were reported.
References
- 1.Shah MR, Culp MA, Gersing KR, et al. Early Vision for the CTSA Program Trial Innovation Network: A Perspective from the National Center for Advancing Translational Sciences. Clin Transl Sci. 2017;10(5):311–313. doi: 10.1111/cts.12463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akobeng AK. Understanding randomised controlled trials. Arch Dis Child 2005;90:840–844. https://adc.bmj.com/content/90/8/840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Das MK. Multicenter Studies: Relevance, Design and Implementation. Indian Pediatr. 2022;59:571–579. doi: 10.1007/s13312-022-2561-y [DOI] [PubMed] [Google Scholar]
- 4.Cernik C, Shergina E, Thompson JA, et al. Non-cancer clinical trials start-up metrics at an academic medical center: Implications for advancing research. Contemp Clin Trials Commun. 2021;22:100774. doi: 10.1016/j.conctc.2021.100774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marquis-Gravel G, Robertson H, Jones WS, et al. Streamlining the institutional review board process in pragmatic randomized clinical trials: challenges and lessons learned from the Aspirin Dosing: A Patient-centric Trial Assessing Benefits and Long-Term Effectiveness (ADAPTABLE) trial. Trials. 2021;22(1):90. doi: 10.1186/s13063-021-05026-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Psotka MA, Ammon SE, Fiuzat M, et al. Heart Failure Site-Based Research in the United States: Results of the Heart Failure Society of America Research Network Survey. JACC Heart Fail. 2019;7(5):431–438. doi: 10.1016/j.jchf.2019.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlisle B, Kimmelman J, Ramsay T, MacKinnon N. Unsuccessful trial accrual and human subjects protections: an empirical analysis of recently closed trials. Clin Trials. 2015;12(1):77–83. doi: 10.1177/1740774514558307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernard G, Harris P, Pulley J, et al. A collaborative, academic approach to optimizing the national clinical research infrastructure: The first year of the Trial Innovation Network. J Clin Transl Sci. 2018;2(4):187–192. doi: 10.1017/cts.2018.319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson AR, Pautler M, Burr JS, et al. Using single IRB consultations to meet the educational needs of investigative teams. Contemp Clin Trials Commun. 2022;29:100971 doi: 10.1016/j.conctc.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.NIH HEAL Initiative. https://healpain-ern.org/
- 11.Boutzoukas AE, Olson R, Sellers MA, et al. Mechanisms to expedite pediatric clinical trial site activation: The DOSE trial experience. Contemp Clin Trials. 2023;125:107067. doi: 10.1016/j.cct.2022.107067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenberg RG, Poole L, Ford DE, et al. Response of the trial innovation network to the COVID-19 pandemic. J Clin Transl Sci. 2021;5(1):e100. doi: 10.1017/cts.2021.782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Department of Health and Human Services. Part 1. Overview Information CTSA Network - Trial Innovation Centers (TICs)(U24). 2015. https://grants.nih.gov/grants/guide/rfa-files/RFA-TR-15-002.html [Google Scholar]
- 14.Department of Health and Human Services. Part 1. Overview Information Clinical and Translational Science Award (CTSA) Network - Recruitment Innovation Centers (RICs)(U24). 2015. https://grants.nih.gov/grants/guide/rfa-files/rfa-tr-15-004.html [Google Scholar]
- 15.Wilkins CH, Edwards TL, Stroud M, et al. The Recruitment Innovation Center: Developing novel, person-centered strategies for clinical trial recruitment and retention. J Clin Transl Sci. 2021;5(1):e194. doi: 10.1017/cts.2021.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palm ME, Thompson DD, Edwards T, et al. The Trial Innovation Network Liaison Team: Building a National Clinical and Translational Community of Practice. [Under review - J Clin Transl Sci] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burr JS, Johnson A, Risenmay A, et al. Demonstration Project: Transitioning a Research Network to New Single IRB Platforms. Ethics Hum Res. 2022;44(6):32–38 doi: 10.1002/eahr.500149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.NIH. Final NIH policy on the use of a Single Institutional Review Board for Multi-site research. 2016. https://grants.nih.gov/grants/guide/notice-files/NOT-OD-16-094.html
- 19.Cobb N, Witte E, Cervone M, et al. The SMART IRB platform: A national resource for IRB review for multisite studies. J Clin Transl Sci. 2019;3(4):129–39 doi: 10.1017/cts.2019.394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Serdoz ES, Edwards T, Pulley J, et al. The IRB Reliance Exchange (IREx): A national web-based platform for operationalizing single IRB review. J Clin Transl Sci. 2022;6(1):e39 doi: 10.1017/cts.2022.376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawrence C, Bruce VM, Salberg LD, Edwards T, Morales C, Palm M, Bernard GR. Quantitative assessment of the impact of standard agreement templates on multisite clinical trial start up time. [under review - J Clin Trans Sci] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yazici C, Dyer AM, Conwell DL, et al. Type 1 Diabetes in Acute Pancreatitis Consortium (T1DAPC). Recruitment and Retention Strategies for the Diabetes Related to Acute Pancreatitis and Its Mechanisms Study: From the Type 1 Diabetes in Acute Pancreatitis Consortium. Pancreas. 2022;51(6):598–603. doi: 10.1097/MPA.0000000000002072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joosten YA, Israel TL, Head A, Vaughn Y, Villalta-Gil V, Mouton C, Wilkins CH. Enhancing translational researchers’ ability to collaborate with community stakeholders: Lessons from the Community Engagement Studio. J Clin Transl Sci. 2018. Aug;2(4):201–207. doi: 10.1017/cts.2018.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grout RW, Hood D, Nelson SJ, Harris PA, Embí PJ. Selecting EHR-driven recruitment strategies: An evidence-based decision guide. J Clin Transl Sci. 2022. Aug 8;6(1):e108. doi: 10.1017/cts.2022.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lane K, Majkowski R, Gruber J, et al. Using gamification to enhance clinical trial start-up activities. J Clin Transl Sci. 2022;6(1):e75. doi: 10.1017/cts.2022.405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris PA, Scott KW, Lebo L, Hassan N, Lightner C, Pulley J. ResearchMatch: a national registry to recruit volunteers for clinical research. Acad Med. 2012;87(1):66–73. doi: 10.1097/ACM.0b013e31823ab7d2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Byrne LM, Cook SK, Kennedy N, et al. Opening doors to clinical trial participation among Hispanics: Lessons learned from the Spanish translation of ResearchMatch. J Clin Transl Sci. 2020;5(1):e46. doi: 10.1017/cts.2020.539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kusnoor SV, Villalta-Gil V, Michaels M, et al. Design and implementation of a massive open online course on enhancing the recruitment of minorities in clinical trials - Faster Together. BMC Med Res Methodol. 2021;21(1):44. doi: 10.1186/s12874-021-01240-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lawrence CE, Dunkel L, McEver M, et al. A REDCap-based model for electronic consent (eConsent): Moving toward a more personalized consent. J Clin Transl Sci. 2020;4(4):345–353. doi: 10.1017/cts.2020.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng AC, Duda SN, Taylor R, et al. REDCap on FHIR: Clinical Data Interoperability Services. J Biomed Inform. 2021;121:103871. doi: 10.1016/j.jbi.2021.103871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lane J, Palm M, Marion E, et al. Approaches for enhancing the informativeness and quality of clinical trials: Innovations and principles for implementing multicenter trials from the Trial Innovation Network. 2023. [under review at J Clin Transl Sci.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Institutes of Health. Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV). https://www.nih.gov/research-training/medical-researchinitiatives/activ#overview
- 34.Boutzoukas AE, Zimmerman KO, Inkelas M, et al. School Masking Policies and Secondary SARS-CoV-2 Transmission. Pediatrics. 2022;149(6):e2022–056687. doi: 10.1542/peds.2022056687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Campbell MM, Benjamin DK, Mann T, et al. Test-to-Stay After Exposure to SARS-CoV-2 in K-12 Schools. Pediatrics. 2022;149(5):e2021056045. doi: 10.1542/peds.2021-056045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kennedy N, Dunagan J, Boone LR, et al. The RIC COVID-19 Recruitment & Retention Toolkit: A community-informed resource of recruitment tools and strategies for clinical trial investigators. J Clin Transl Sci. 2022;6(1):e94. doi: 10.1017/cts.2022.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nelson SJ, Drury B, Hood D, et al. EHR-based cohort assessment for multicenter RCTs: a fast and flexible model for identifying potential study sites. JAMIA. 2022;29(4): 652–659. doi: 10.1093/jamia/ocab265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cook SK, Kennedy N, Boone L, et al. What we wish every investigator knew: Top 4 recruitment and retention recommendations from the Recruitment Innovation Center. J Clin Transl Sci. 2022;6(1):e31. doi: 10.1017/cts.2022.370 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


