Abstract
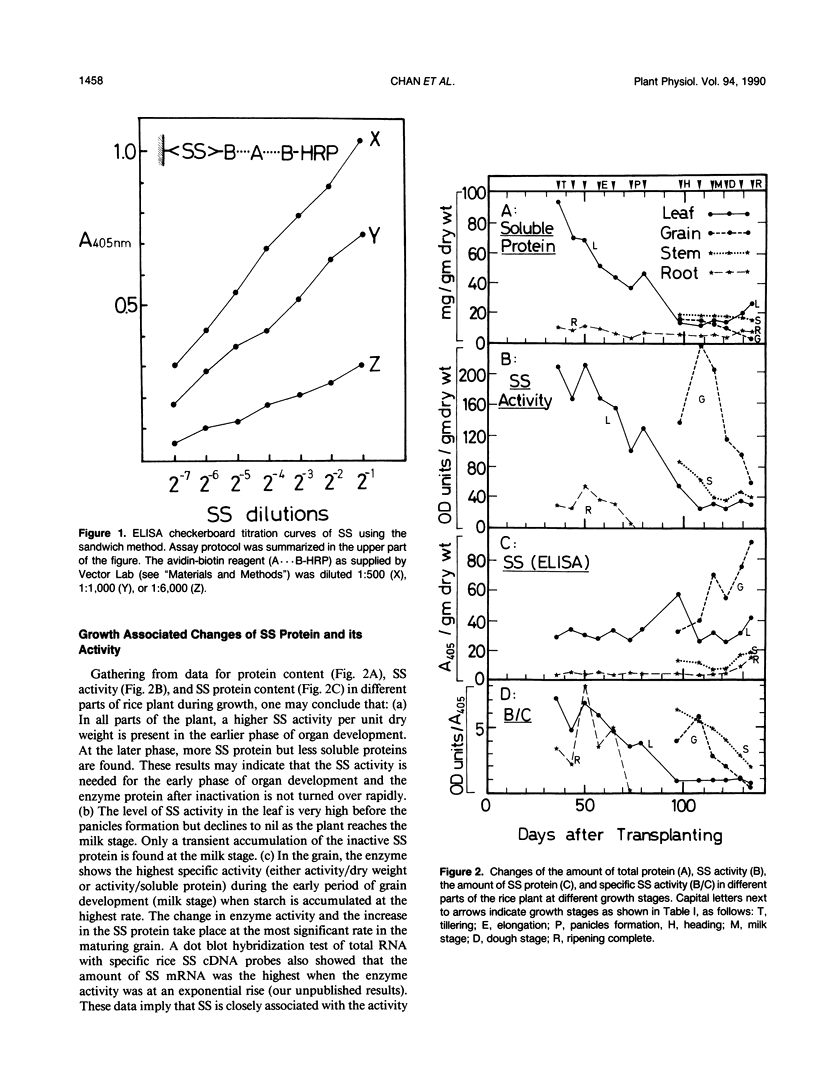
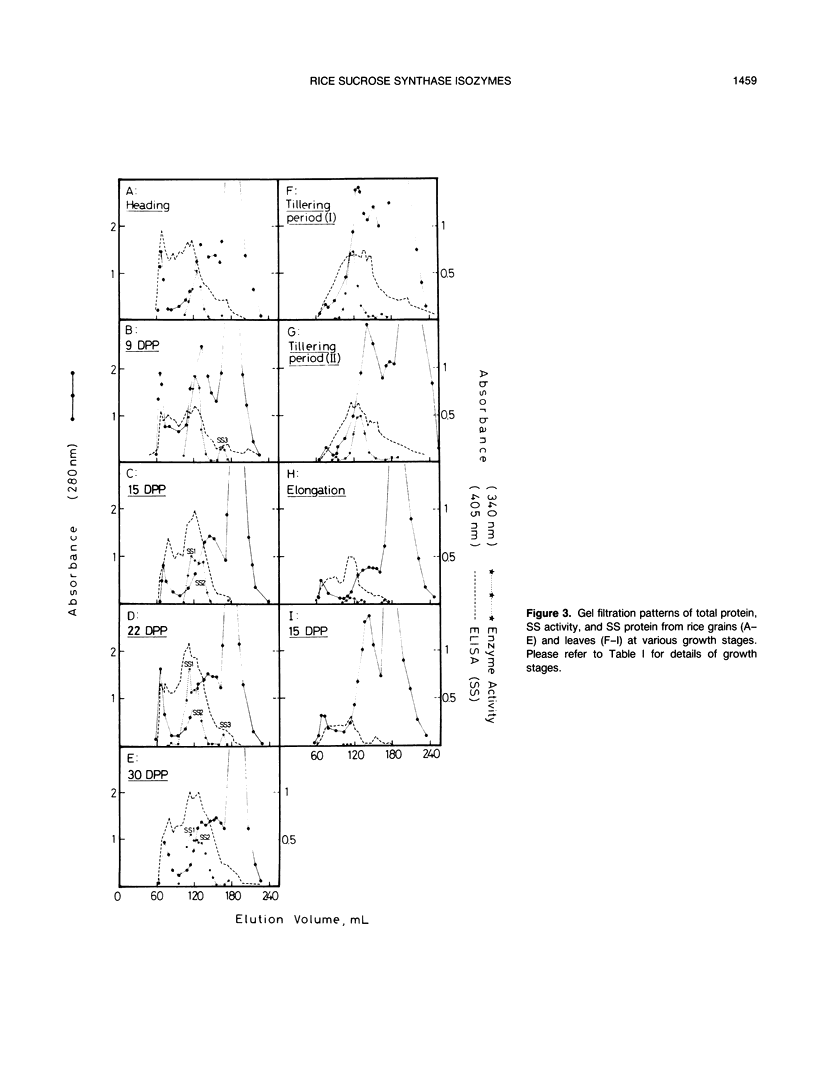
Different parts of the rice (Oryza sativa L.) plant at different growth stages were analyzed for sucrose synthase (SS) by enzyme activity assay and enzyme-linked immunosorbent assay directly on the extracts or on the eluates from a gel filtration column. On a dry matter basis, the amount of soluble protein and SS activity decreased significantly, but the amount of enzyme protein changed little in growing leaves. In the grain, the SS activity was the highest at the early ripening stage and decreased later, but the amount of SS protein increased with the increase in maturity. In the root, a low activity of SS was detectable only in the tillering but not in other stages. Immunoblotting of SS protein extracted from different parts of rice showed two bands. Elution patterns of crude extracts from a gel filtration column showed the presence of several types of SS protein. Among them, two to three types with larger elution volumes had the SS activity but others with smaller elution volumes (considered as the aggregated forms) had no activity. The SS purified from different parts of the plant showed similar but distinctly different electrophoretic mobilities in a native gel. It has been concluded that different isozymes are expressed in different tissues at different growth stages.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Diezel W., Kopperschläger G., Hofmann E. An improved procedure for protein staining in polyacrylamide gels with a new type of Coomassie Brilliant Blue. Anal Biochem. 1972 Aug;48(2):617–620. doi: 10.1016/0003-2697(72)90117-0. [DOI] [PubMed] [Google Scholar]
- Guesdon J. L., Ternynck T., Avrameas S. The use of avidin-biotin interaction in immunoenzymatic techniques. J Histochem Cytochem. 1979 Aug;27(8):1131–1139. doi: 10.1177/27.8.90074. [DOI] [PubMed] [Google Scholar]
- Juang R. H., Chang Y. D., Sung H. Y., Su J. C. Oven-drying method for polyacrylamide gel slab packed in cellophane sandwich. Anal Biochem. 1984 Sep;141(2):348–350. doi: 10.1016/0003-2697(84)90053-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lambin P. Reliability of molecular weight determination of proteins by polyacrylamide gradient gel electrophoresis in the presence of sodium dodecyl sulfate. Anal Biochem. 1978 Mar;85(1):114–125. doi: 10.1016/0003-2697(78)90281-6. [DOI] [PubMed] [Google Scholar]
- Su J. C., Preiss J. Purification and properties of sucrose synthase from maize kernels. Plant Physiol. 1978 Mar;61(3):389–393. doi: 10.1104/pp.61.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J. C. Purification and Characterization of Sucrose Synthetase from the Shoot of Bamboo Leleba oldhami. Plant Physiol. 1977 Jul;60(1):17–21. doi: 10.1104/pp.60.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassey T. L. Light/Dark profiles of sucrose phosphate synthase, sucrose synthase, and Acid invertase in leaves of sugar beets. Plant Physiol. 1989 Jan;89(1):347–351. doi: 10.1104/pp.89.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]



