Abstract
Objective:
We calculate body size-specific organ and effective doses for a total of 23,734 participants in the National Lung Screening Trial (NLST) using a CT dose calculator.
Methods:
We collected participant-specific technical parameters from 23,734 CT participants in the clinical trial. For each participant, we calculated two sets of organ dose using two methods. First, we computed body size-specific organ and effective doses using the National Cancer Institute dosimetry system for CT (NCICT), which is based on dose coefficients derived from a library of body size-dependent adult male and female computational phantoms. We then recalculated organ and effective doses using dose coefficients from reference size phantoms for all examinations to investigate potential errors caused by the lack of body size consideration in dose calculation.
Results:
The underweight participants (BMI < 18.5) received 1.3-fold greater lung dose (4.93 mGy) than the obese participants (BMI > 30) (3.90 mGy). Thyroid doses were about 1.3 – 1.6-fold greater than the lung doses (6.3 – 6.5 mGy). The reference phantom-based dose calculation underestimates the body size-specific lung dose by up to 50% for the underweight participants and overestimates that value by up to 200% for the overweight participants. The median effective dose ranges from 2.01 mSv (obese participants BMI>30) to 2.8 mSv (underweight participants BMI<18.5).
Conclusion:
Body size-specific organ and effective doses were computed for 23,734 NLST participants undergoing low-dose CT screening. Use of reference-size phantoms can lead to significant errors in organ dose when body size is not considered in the dose assessment.
Keywords: NLST, low dose CT scan, body size, organ dose, effective dose
1. Introduction
The National Lung Screening Trial (NLST) was a multi-center randomized, controlled trial comparing helical computerized tomography (CT) to a posteroanterior chest radiographic (CXR) examination in screening of older, current, and former heavy smokers for early detection of lung cancer. Enrollment began in September 2002 and ended in April 2004 when 53,454 participants had been randomized at 33 screening centers and screened by either CT or CXR in equal proportions. The NLST was a collaborative effort of the National Cancer Institute (NCI)’s Division of Cancer Prevention which funds and administers the NLST/Lung Screening Study (LSS), and the American College of Radiology Imaging Network (ACRIN), which administers the NLST/ACRIN. The primary endpoint of the NLST is lung cancer mortality. Participants agreed to a baseline imaging procedure for lung cancer screening plus two annual follow-ups. The participants ranged in age from 55 to 74 years at the date of entry to the study. Participants had to have had a significant smoking history, 30 or more pack-years of cigarette smoking (former smokers must have quit within the previous 15 years). The NLST enrolled 26,722 participants in the low-dose CT screening arm and 26,732 participants in the chest radiographic screening arm of the trial. During the trial, 26,722 participants in the CT screening arm underwent as many as three low-dose CT examinations. Trial results demonstrated that CT screening reduced cancer mortality by 20% in high-risk participants when compared to chest radiography (1). Exam data were obtained from CT examinations performed using all the NLST site CT imaging systems.
Cody et al. (2) reported normalized CT Dose Index (CTDI) of the CT scanners used in the NLST. They collected a total of 247 measurements on 96 multi-detector CT scanners and found that average normalized CTDI values varied by a factor of almost two across all scanners when the NLST lung screening exam acquisition parameters were used. Larke et al. (3) estimated organ and effective doses for the NLST participants by using a CT dose calculator, CT-Expo (4), and the CTDIvol data reported by the NLST screening center physicist. However, they noted that the calculation of organ and effective doses is for average-size participants and the size of individual participants was not taken into account.
The current study was intended to calculate absorbed doses to major organs within the chest region for NLST CT participants. These calculations utilized CT scan parameters as well as the height and weight of participants coupled with a CT dose calculator based on body size-dependent computational human phantoms. We compared the body size-specific organ dose with the data based on ICRP-compliant reference computational phantoms (nominally 50th percentile height and weight).
2. Materials and Methods
2.1. Patient cohort
In the current study, we collected a total of 48,849 participant CT examinations from the LSS arm and simulated only participant CT examinations where key technical parameters required for dose calculations were all available: the age, gender, height, and weight of the participants, scanner model, kVp, mAs, and pitch. A total of 23,734 participants (14,327 males and 9,407 females), whose height (mean: 173 cm, range: 147 – 193 cm) and weight (mean: 83 kg, range: 39 – 144 kg) are available, were included in the current study.
2.2. Scanner technical parameters
During the NLST screening period of 2002–2007, CT dose data from 97 Multi-Detector CT (MDCT) scanners at NLST screening sites nationwide were collected annually. As summarized in Table 1, four major CT vendors (i.e., GE Healthcare, Philips Healthcare, Siemens Healthcare, and Toshiba Medical Systems) and various scanner models from each vendor were represented in the trial. Two physicist quality assurance groups provided oversight for and monitored the NLST/LSS and the NLST/ACRIN administrative arms of the study. In consultation with NLST radiologists, they established a common set of protocol specifications and parameter ranges that were considered suitable for producing acceptable images from CT examinations.
Table 1.
CT Scanners Used in the National Lung Screening Trial (NLST) cohort selected in this study with corresponding normalized CTDIw (nCTDIw) (3).
| Manufacturer | Model | No of Patients (%) | nCTDIw (mGy/mAs) | |
|---|---|---|---|---|
| GE | HiSpeed QX/i | 2686 | 11.3 | 0.100 |
| LightSpeed QX/i | 3091 | 13.0 | 0.100 | |
| LightSpeed Plus | 2251 | 9.5 | 0.090 | |
| LighSpeed 16 | 3354 | 14.1 | 0.090 | |
| LightSpeed Pro 16 | 1070 | 4.5 | 0.090 | |
| Philips | MX8000 | 1609 | 6.8 | 0.080 |
| Siemens | Emotion 16 | 12 | 0.1 | NA |
| Sensation 16 | 3426 | 14.4 | 0.080 | |
| VolumeZoom | 5834 | 24.6 | 0.100 | |
| Toshiba | Aquilion | 401 | 1.7 | 0.130 |
We abstracted a list of technical parameters from Digital Imaging and Communication in Medicine (DICOM) image headers including scan length (cm), scanner model, tube current-time product (mAs), tube potential (kVp), and pitch. We also abstracted participant-specific parameters such as gender, height (cm), and weight (kg). Tube current modulation was in general not utilized during the NLST; instead the majority of participants were scanned with a manual technique (5).
2.3. CT dosimetry methods
We adopted the National Cancer Institute dosimetry system for CT (NCICT) program (6) for organ dose calculations. NCICT is based on a comprehensive library of pre-computed dose coefficients for a series of computational human phantoms (7) coupled with Monte Carlo radiation transport techniques. The dose coefficients (mGy/mGy), which is organ absorbed dose (mGy) normalized to the CTDIvol (mGy) of the reference scanner, were calculated for different phantom sizes, scan locations and CT x-ray spectra. When multiplied by the reported CTDIvol (mGy) for each CT scanner of interest, the result is the absolute organ doses (mGy). The detailed algorithms used in the organ dose calculations are found in Lee et al. (6) To conduct the body size-specific organ dose calculations in the current study, the original dose coefficients derived from a series of reference size computational phantoms were extended to the comprehensive set of body size-dependent computational human phantoms as reported by Geyer et al. (7). CTDIvol was derived from normalized CTDIw values (3), as tabulated in Table 1, and abstracted values of both pitch and mAs. Table 1 also shows the list of scanners and the number of participants imaged by each scanner make and model.
Figure 1 shows the distribution of the height and weight of participants involved in our study. The coverage of the height and weight of the body size-dependent phantoms available in NCICT is color-coded for four different Body Mass Index (BMI) groups defined by World Health Organization1: underweight (BMI < 18.5), normal (BMI 18.5 – 25), overweight (BMI 25 – 30), and obese (BMI > 30). The phantoms in NCICT covered about 71% and 53% of the male and female participants, respectively. Most of the female outliers are taller than the phantom coverage and the male outliers are shorter than the phantoms. However, the weight of the participants is relatively well covered by the phantom library. We forced the height and weight of the participants to match those of the phantoms if the body size of the participants was outside the phantom coverage. For example, we used the adult female phantom with the height and weight of 175 cm and 85 kg for the participant with the height and weight of 195 cm and 85 kg. Figure 2 illustrates the frontal views of selected computational human phantoms in different weights at the same heights: 165 cm and 175 cm, best representing the reference heights of adult female and male, respectively. The phantoms were selected based on the median weights of the male and female participants in the four BMI groups (<18.5, 18.5–25, 25–30, and >30).
Figure 1.
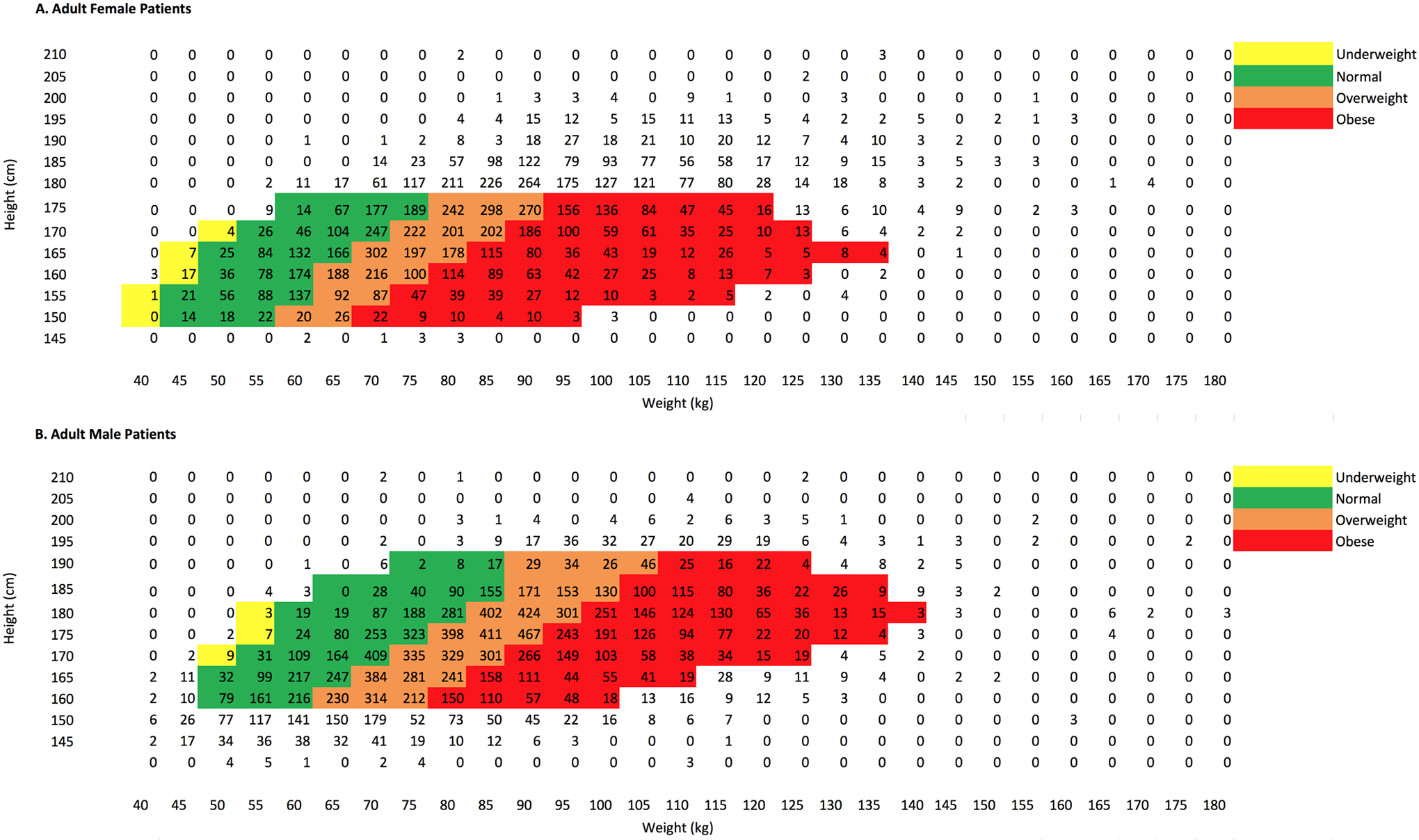
Distribution of the height and weight of NLST male participants (n=31,362) superimposed on the BMI distribution map of the UF/NCI size-dependent computational phantoms
Figure 2.
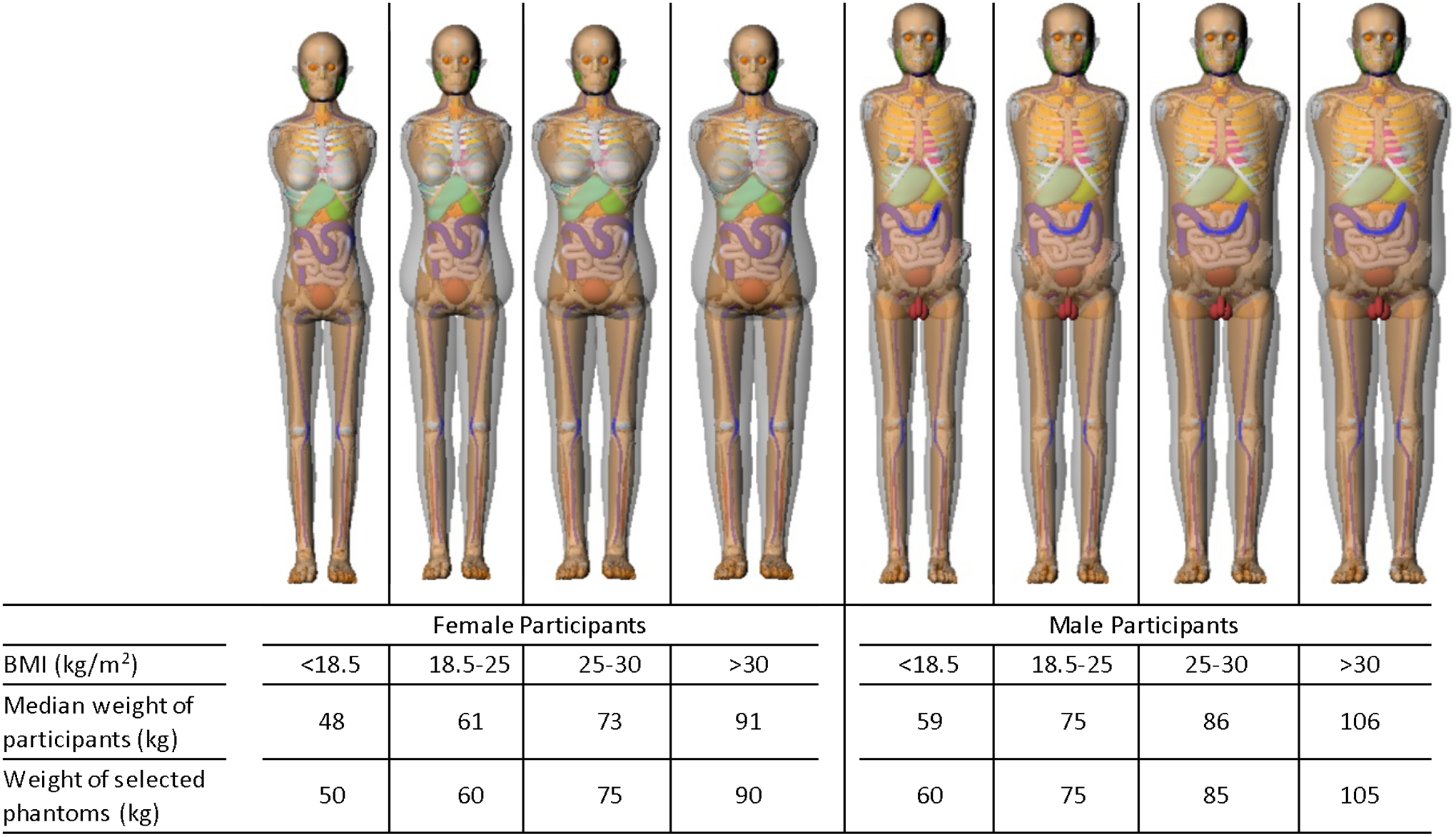
The median weight of the female and male participants and the weight of the corresponding computational phantoms used for dose calculations in four different BMI groups. The frontal views of the selected phantoms at the reference heights (165 cm for females and 175 cm for males) corresponding to each weight category are presented at the top row.
Scan length was only available for a subset of the cohort. We derived the following regression equation from the relationship between scan length and participant height:
where H is the height of a given participant in cm. Because the location of scan start and end was not available, we mapped the scan start location of participants to the level of the clavicles of the computational phantoms. The scan start was then at 29 cm and 28 cm for male and female participants, respectively, from the top of the head. We then added the overrange length (cm) to the scan start and end based on the measured values from Molen et al. (8): 3.3 cm for General Electric (GE), 4.4 cm for Phillips, 5.4 cm for Siemens, and 6.4 cm for Toshiba at the pitch of 1.5, which was the mean value of pitch found in the study.
Using the methods explained above, we calculated absorbed doses to major organs within the chest region, for which the tissue weighting factor of the ICRP Publication 103 is assigned, for NLST CT participants. We also calculated absorbed dose to the brain, lens, and salivary glands. We compared the body size-specific dose to the major organs with the data based on ICRP-compliant reference computational phantoms (nominally 50th percentile height and weight).
3. Results
3.1. CT scan parameters
Table 2 summarizes major technical parameters obtained from the 23,734 participants. The NLST specification is also included for comparison. We found all parameters are within the range of the NLST specification. We found that 65% of all participants were scanned by CT scanners with 4 channels while 35% were scanned by CT scanners with 16-channel scanners. A tube voltage of 120 kVp was used for 94% of participants although the NLST specified a range of kVp, 120 – 140. Mean pitch is 1.5 with the range between 0.75 and 2. Effective mAs ranged from 16 to 79 with the average of 37. Total scan time varied from 6 to 37 seconds but the mean was 19 seconds which is less than the 25 seconds in the NLST specifications.
Table 2.
Summary of technical parameters in the cohort selected in this study and the protocol specifications used in the National Lung Screening Trial (NLST).
| Scanning Parameters | This Study | NLST Specifications |
|---|---|---|
| No. of channels | 4 (65%) or 16 (35%) | 4 (Minimum) |
| Peak kilovoltage (kVp) | 120 (94%) | 120 – 140 |
| Pitch | 1.5 (0.75 – 2) | 1.25 – 2.00 |
| Effective mAs* | 37 (16 – 79) | 20 – 60 |
| Total Scan Time (seconds) | 19 (6 – 37) | 25 (Maximum) |
Effective mAs = mAs / pitch
3.2. Body size-specific organ dose
The results of CTDIvol and body size-specific organ doses for a single CT screening are tabulated in Table 3 by BMI group. About 71% of the participants were overweight (43%) or obese (29%). We found that smaller CTDIvol was used for the underweight participant group compared to the obese group. The CTDIvol (3.02 mGy) for the underweight group (BMI < 18.5) was 79% of the CTDIvol (3.81 mGy) for the obese participant group. The mean CTDIvol for the whole participant cohort was 3.6 mGy (median 3.4 mGy, 1.26 – 8.45).
Table 3.
Median dose (mGy) for major organs by gender and BMI group.
| BMI (Kg/m2) | Underweight | Normal | Overweight | Obese | |
|---|---|---|---|---|---|
| <18.5 | 18.5–25 | 25–30 | > 30 | ||
| Number of Participants | 215 | 6550 | 10182 | 6787 | |
| Median CTDIvol (mGy) | 3.02 | 3.10 | 3.40 | 3.81 | |
| Organ dose (mGy) based on participant body size | Brain | 0.06 | 0.06 | 0.06 | 0.06 |
| Lens | 0.04 | 0.04 | 0.05 | 0.07 | |
| Salivary glands | 0.59 | 0.60 | 0.66 | 0.74 | |
| Thyroid | 6.42 | 6.41 | 6.48 | 6.34 | |
| Esophagus | 3.93 | 3.60 | 3.21 | 2.95 | |
| Thymus | 4.70 | 4.35 | 4.08 | 3.81 | |
| Lungs | 4.93 | 4.56 | 4.19 | 3.90 | |
| Breasts | 4.56 | 4.21 | 4.31 | 4.28 | |
| Red bone marrow | 1.79 | 1.46 | 1.29 | 1.25 | |
| Effective dose (mSv) | 2.80 | 2.37 | 2.19 | 2.10 | |
| Organ dose (mGy) based on reference body size | Thyroid | 6.17 | 6.18 | 6.68 | 7.12 |
| Esophagus | 3.40 | 3.59 | 3.73 | 4.15 | |
| Thymus | 4.07 | 4.33 | 4.45 | 4.99 | |
| Lungs | 4.28 | 4.33 | 4.70 | 5.03 | |
| Breasts | 3.65 | 3.69 | 4.32 | 4.78 | |
| Red bone marrow | 1.48 | 1.48 | 1.60 | 1.72 | |
| Effective dose (mSv) | 2.29 | 2.31 | 2.52 | 2.71 | |
Underweight participants tended to have received greater organ doses as compared to those for overweight participants. The underweight participant group (BMI < 18.5) received roughly a 1.3-fold greater lung dose (4.93 mGy) than the obese participant group (BMI > 30) (3.90 mGy). The esophagus and thymus of the underweight participant group also received about a 1.3-fold greater dose than the overweight participant group. The brain and lens received less than 2% of the lung dose. The thyroid received approximately 1.3 – 1.6-fold greater dose than for the lungs, which was in the range of 6.3 to 6.5 mGy. The red bone marrow received 30 – 35% of the lung dose, which was in the range 1.25 – 1.79 mGy.
3.3. Comparison with reference size-based organ dose
Organ doses calculated from fixed size computational phantoms are also tabulated following the body size-specific organ doses in Table 3. Opposite to the trend in body size-specific organ dose mentioned above, the underweight participant group based on reference size phantoms tended to show smaller doses than the overweight participant group. The lung dose (4.28 mGy) in the group of BMI < 18.5 was 85% of the lung dose (5.03 mGy) in the group of BMI > 30. It was observed that organ dose based on body size-specific computational phantoms can be underestimated for the underweight participants and overestimated for the overweight participants when participant body size is not incorporated in the dose calculation.
To further investigate the potential dosimetric errors in using reference size phantoms for different size participants, we plotted the ratio of reference phantom-based lung dose to body size-specific lung dose in Figure 3. It is clear that the reference phantom-based dose calculation underestimates the body size-specific lung dose by up to 50% for the underweight participant group and overestimates by up to 200% for the overweight participant group. For normal weight patients, however, the reference size phantoms do not under- or over-estimate the body size-specific lung doses because the ratio is close to 1 in the normal BMI participant group.
Figure 3.
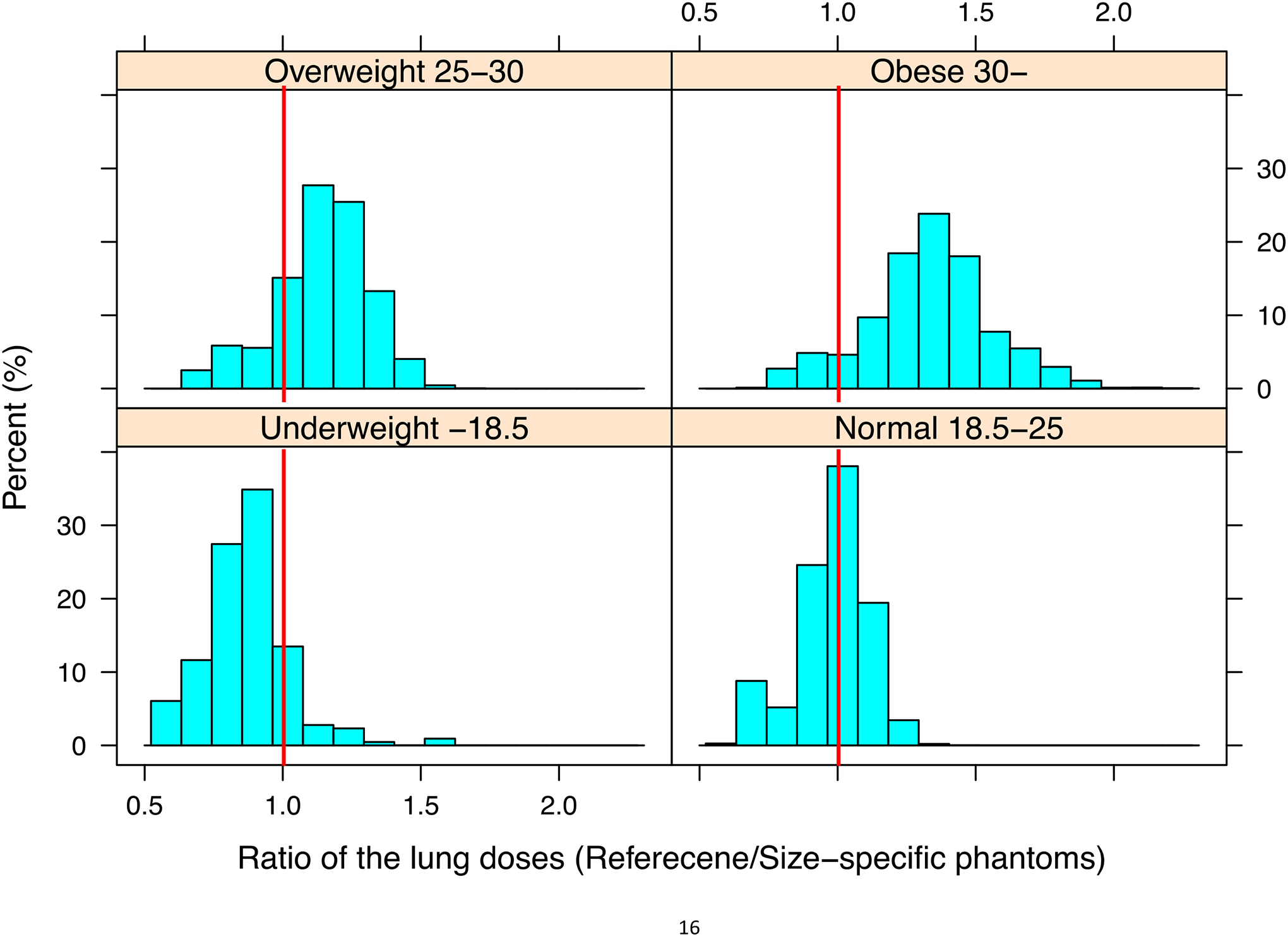
Ratio of the lung doses from participant size-specific phantoms to those from reference phantoms
Median values of body size-specific effective doses are tabulated in Table 3, with the results based on reference size phantoms shown in the lower portion of the table. As for the underweight participant group (BMI<18.5), the median effective dose (2.29 mSv) based on the reference phantom is 80% of the value given for body size-specific phantoms (2.8 mSv). For the obese participant group (BMI>30), the median effective dose (2.71 mSv) based on the reference size phantoms was 1.3-fold greater than the its value based on the body size-specific phantoms (2.1 mSv).
4. Discussion
We compared our results with the previous dosimetry publication from the NLST medical physics working group. Larke et al. (2) reported the organ and effective doses calculated from the NLST scan parameters coupled with CT-Expo dose calculator (4). The lung and thymus doses from this earlier study were 4.7 and 4.4 mGy, respectively, which are very close to the results given in this study: 4.4 and 4.2 mGy, respectively. However, the previously reported esophagus dose (4.4 mGy) is much greater than our result (3.4 mGy). These differences may be due to the anatomical differences between the stylized phantoms (9) of the CT-Expo software and the more anatomically realistic voxel phantoms (7) utilized in NCICT. The previous study also reported an effective dose of 2 mGy calculated from CT-Expo, which is slightly smaller than our size-specific effective dose result of 2.33 mSv. The other effective dose, 1.4 mSv, was also reported in the Larke et al. (2) based on a Dose Length Product (DLP)-based conversion factor (also known as k factor) (10), which is based on standard size stylized phantoms. The range of body size-specific effective dose, 2.1–2.8 mSv, is only 30–40% of the average effective dose range of a standard chest CT examination (11).
Our study reports the first calculation of body size-specific organ and effective dose for the NLST CT participants. NCICT dose calculator based on a comprehensive set of body size-dependent computational human phantoms (7) and Monte Carlo radiation transport techniques was used for dose calculation of a large number of participants. The organ and effective doses are presented in different BMI groups. The results are then compared with reference size phantom-based organ doses. This comparison highlights that potential errors up to 2-fold can occur with the use of single-sized reference phantoms, a method adopted in most of CT calculation tools (4,12). Kruger et al. (13) provides participant and organ specific dose calculations for the NLST chest radiographic screening arm of the trial using similar methods. It must be noted that the dose reported in the current study is for one of the three low-dose CT examinations that the trial participants received.
As with all computational dosimetry studies, our study has several limitations. First, scan length and scan start/stop landmarks were not available for the full cohort so this parameter was derived from participant height using a regression equation developed from a subset of the participants. The derived scan length may provide uncertainty in the dose to organs near or outside the scan coverage (e.g., salivary gland, thyroid). Scan start position is expected to vary by facility and by operator. Second, we forced the height and weight of participants to match those of the body size-dependent phantoms because our phantoms do not completely cover the range of body sizes in the participant cohort. We observed that the height of about 30% of the female participants was greater than the height range of the phantoms as shown in Figure 1a. The scan length derived from participant height was applied to the phantoms that were actually used for dose calculations, which could be shorter than the participants by up to 20 cm. This approach may have led to an overestimate of the red bone marrow dose and effective dose, which may explain the slightly higher effective dose in our study compared to Larke et al. (2), where protocol-based fixed scan length was used. However, these limitations do not affect the dependency of organ doses on body size as the weight of the participant was fully covered by the phantom library. Finally, body size-dependent effective dose computed in the current study may deviate from its original definition reported in the ICRP Publication 103 (14), which is defined for reference size individual averaged over ages and genders. The body size-dependent effective dose in Table 3 would be only for comparison with other data.
5. Conclusion
We report body size-specific organ and effective doses for participants undergoing low-dose lung screening CT examination. We found underweight participants tended to receive greater organ and effective doses as compared to overweight participants. Study results indicated that the dosimetry approach based on reference size phantoms tends to underestimate organ doses to underweight participants and to overestimate organ doses to overweight participants by a factor of 2. The body size-dependent dosimetry method used in the current study will be useful in future studies of organ doses for participants undergoing CT exams.
Acknowledgement
This research was supported by contracts from the Division of Cancer Prevention, National Cancer Institute, NIH, DHHS, and by grants (U01 CA80098 and CA79778) to the American College of Radiology Imaging Network (ACRIN) under a cooperative agreement with Cancer Imaging Program, Division of Cancer Treatment and Diagnosis, NCI. This work was partly supported by the intramural research program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology and Genetics. The authors thank the Screening Center investigators and staff of the National Lung Screening Trial (NLST). Most importantly, we acknowledge the study participants, whose contributions made this study possible. The online staff listing can be found at the website identified below. http://www.nejm.org/doi/suppl/10.1056/NEJMoa1102873/suppl_file/nejmoa1102873_appendix.pdf
Footnotes
Body Mass Index classification by World Health Organization (http://apps.who.int/bmi/index.jsp?introPage=intro_3.html)
References
- 1.National Lung Screening Trial Research Team, Aberle DR, Berg CD, Black WC, Church TR, Fagerstrom RM, et al. The National Lung Screening Trial: overview and study design. Radiology. Radiological Society of North America, Inc; 2011. Jan;258(1):243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larke FJ, Kruger RL, Cagnon CH, Flynn MJ, McNitt-Gray MM, Wu X, et al. Estimated Radiation Dose Associated With Low-Dose Chest CT of Average-Size Participants in the National Lung Screening Trial. American Journal of Roentgenology. 2011. Nov;197(5):1165–9. [DOI] [PubMed] [Google Scholar]
- 3.Cody DD, Kim H-J, Cagnon CH, Larke FJ, McNitt-Gray MM, Kruger RL, et al. Normalized CT dose index of the CT scanners used in the National Lung Screening Trial. American Journal of Roentgenology. 2010. Jun;194(6):1539–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stamm G, Nagel HD. CT-expo--a novel program for dose evaluation in CT. R\“oFo: Fortschritte auf dem Gebiete der R\”ontgenstrahlen und der Nuklearmedizin. 2002;174(12):1570. [DOI] [PubMed] [Google Scholar]
- 5.Cagnon CH, Cody DD, McNitt-Gray MF, Seibert JA, Judy PF, Aberle DR. Description and Implementation of a Quality Control Program in an Imaging-Based Clinical Trial. Academic Radiology. 2006. Nov;13(11):1431–41. [DOI] [PubMed] [Google Scholar]
- 6.Lee C, Kim KP, Bolch WE, Moroz BE, Folio Les. NCICT: a computational solution to estimate organ doses for pediatric and adult patients undergoing CT scans. Journal of Radiological Protection. IOP Publishing; 2015. Nov 25;35(4):891–909. [DOI] [PubMed] [Google Scholar]
- 7.Geyer AM, O’Reilly S, Lee C, Long DJ, Bolch WE. The UF/NCI family of hybrid computational phantoms representing the current US population of male and female children, adolescents, and adults--application to CT dosimetry. Physics in Medicine and Biology. 2014. Sep 21;59(18):5225–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Molen AJ, Geleijns J. Overranging in Multisection CT: Quantification and Relative Contribution to Dose—Comparison of Four 16-Section CT Scanners 1. Radiology. 2007. Jan;242(1):208–16. [DOI] [PubMed] [Google Scholar]
- 9.Cristy M Mathematical phantoms representing children of various ages for use in estimates of internal dose. Oak Ridge, TN: Oak Ridge National Laboratory; 1980. [Google Scholar]
- 10.McCollough C, Cody D, Edyvean S, Geise R, Gould R, Keat N, et al. The measurement, reporting, and management of radiation dose in CT. Report of AAPM Task Group #23. 2008;:1–34. [Google Scholar]
- 11.Mettler FA, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology. 2008. Jul;248(1):254–63. [DOI] [PubMed] [Google Scholar]
- 12.ImPACT. ImPACT CT Patient Dosimetry Calculator [Internet]. London, UK; 2011. Available from: http://www.impactscan.org/ctdosimetry.htm [Google Scholar]
- 13.Kruger R, Flynn MJ, Judy PF, Cagnon CH, Seibert JA. Effective Dose Assessment for Participants in the National Lung Screening Trial Undergoing Posteroanterior Chest Radiographic Examinations. American Journal of Roentgenology. 2013. Jul;201(1):142–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ICRP. The 2007 Recommendations of the International Commission on Radiological Protection. ICRP publication 103, Ann ICRP. 1st ed. Oxford; Pergamon Press: International Commission on Radiological Protection; 2007;37(2–4):1–332. [DOI] [PubMed] [Google Scholar]


